Capsule Summary:
Lower plasma levels of the oxysterol cholestenoic acid associate with increased mortality and organ failure in septic patients with acute respiratory distress syndrome (ARDS). Cholestenoic acid warrants further validation as a novel ARDS biomarker.
Keywords: Acute Respiratory Distress Syndrome, Acute Lung Injury, Cholestenoic Acid, Oxysterol, Biomarker, Sterol 27-hydroxylase, Mitochondria, Macrophage, Innate Immunity
To the Editor:
Despite improvements in supportive care, mortality from acute respiratory distress syndrome (ARDS) remains high (~30–40%).1 In recent years, several biomarkers of inflammation, epithelial damage, endothelial damage, and coagulation have been identified that show promise for identifying ARDS endotypes with different prognosis and therapeutic responsiveness.1
Unfortunately, few lung-specific biomarkers have been validated, and none yet have been adopted into routine clinical practice.
Cholestenoic acid (CA; 3β-hydroxy-5-cholestenoic acid) is an oxysterol produced by the action of the mitochondrial-localized enzyme sterol 27-hydroxylase (CYP27A1) upon cholesterol.2 In humans, the plasma concentration of CA is reported to derive virtually quantitatively from biosynthesis in the lung.3 Remarkably, plasma CA falls by ~50% following pneumonectomy, and by 31% within 60 minutes of cardiopulmonary bypass.3 Alveolar macrophages (AMs) express much higher CYP27A1 and secrete much higher CA than all other cells surveyed,2 and are thought to be the major source of circulating CA.2,3 Delivery of cholesterol substrate to CYP27A1 at the inner mitochondrial membrane – the rate-limiting step for CYP27A1 function – requires respiring mitochondria.4
We hypothesized that plasma CA is a biomarker of AM functional integrity, and that it would decline with increasing disease severity in ARDS, perhaps due to mitochondrial dysfunction and/or other cytotoxicity. Reported methods for CA quantitation in plasma have generally used a starting volume of ≥0.5 mL and have required reverse phase and ion exchange solid phase extractions, methylation, and trimethylsilylation prior to chromatographic separation.3 Here, we developed a simplified and robust methodology to measure CA from 4 μL of plasma, as detailed in this article’s Online Repository at www.jacionline.org (Tables E1 and E2; Figure E1).
First, we confirmed that AMs freshly collected from normal, healthy volunteers (N=5) by bronchoalveolar lavage (BAL) had robust expression of CYP27A1 (Figure 1A). AM lysates had a mean CA content of ~6.4 ng (SD, 1.6) per million cells, but no substantial quantity of the CA metabolites 3β,7α-dihydroxy-5-cholestenoic acid or 7α-hydroxy-3-oxo-4-cholestenoic acid, suggesting that CA is the primary C27 carboxylic acid synthesized by human AMs. CA was also detected in BAL fluid (N=7; mean, 0.90 +/− 0.73 ng/mL). Ex vivo treatment of human AMs with lipopolysaccharide for 4h did not modify CYP27A1 expression (Figure 1B). These data neither rule out a change in CYP27A1 function nor changes in CYP27A1 expression at later time points. Indeed, a more prolonged time course of CYP27A1 expression in mice following in vivo lipopolysaccharide inhalation interestingly revealed a transient downregulation on day 3 in CD64+F4/80+CD11chighCD11low resident AMs, whereas CD64+F4/80+CD11clowCD11bhigh recruited (i.e., blood monocyte-derived) AMs exhibited a progressive, time-dependent upregulation (Figure 1C).E1
Figure 1. Relationship of alveolar macrophage and monocyte CYP27A1 to ARDS severity.
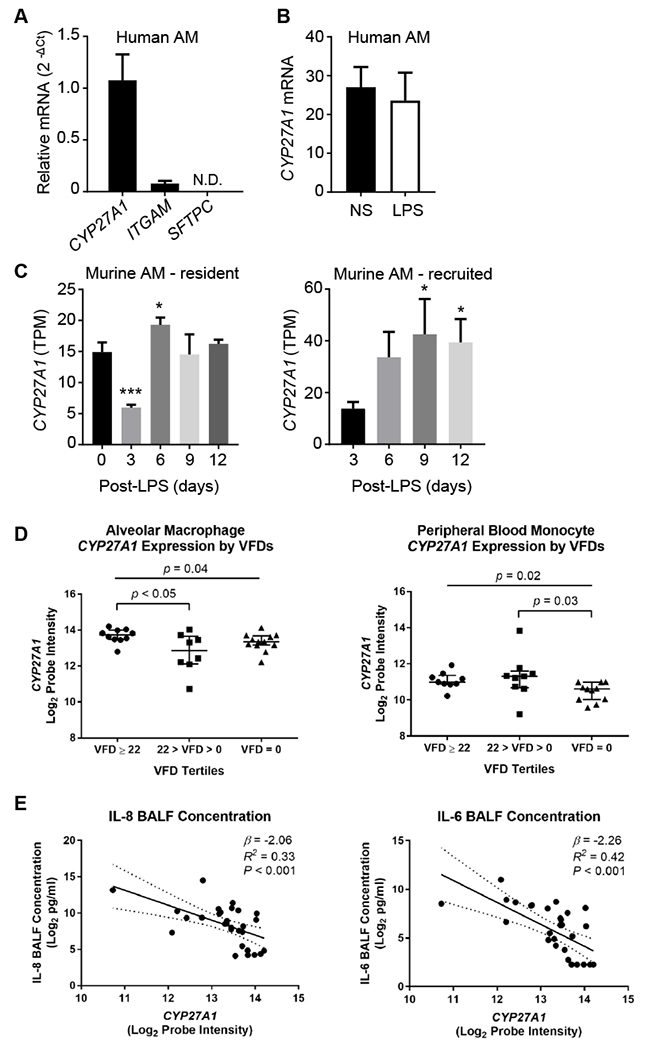
(A) Expression of indicated transcripts in alveolar macrophages (AMs) from normal, nonsmoking volunteers (N=5). Integrin subunit alpha M (ITGAM [CD11b]) is a positive control; SFTPC (surfactant protein C), a negative control. (B) AMs from volunteers (N=4) were non-stimulated (NS) or treated with 10 ng/ml lipopolysaccharide (LPS) for 4h, and CYP27A1 mRNA quantified. (C) C57BL/6 mice were exposed i.t. to 20 μg LPS. Resident (CD64+F4/80+CD11chighCD11blow) and recruited (CD64+F4/80+CD11clowCD11bhigh) AMs were purified from bronchoalveolar lavage at indicated times, and CYP27A1 transcripts per million (TPM) quantified by RNA-Sequencing. Data are mean +/− S.D. *, P<0.05; ***, P<0.001 (comparison: day 0 for resident; day 3 for recruited). (D) AMs (N=30, left) and peripheral blood monocytes (N=29, right) were isolated from patients <48h after development of ARDS. The relationship between normalized log2 CYP27A1 expression and ventilator-free days (VFDs) was tested. Only comparisons with P<0.05 are displayed. Individual values, median, and interquartile ranges are depicted. (E) AMs as in panel D were plotted in relation to bronchoalveolar lavage fluid (BALF) IL-8 and IL-6. Depicted are regression lines and 95% confidence intervals (solid and dashed lines), β coefficients, R2 values, and P values.
Next, we measured plasma CA on day 1 of study enrollment in 144 ARDS patients with sepsis who participated in the NHLBI ARDS Network ALVEOLI trial of low vs. high positive end-expiratory pressure5 (Table E3). Regression analyses adjusted for body mass index, gender, race/ethnicity, APACHE III score, ARDS risk factor, diabetes, plasma bilirubin, and plasma albumin revealed that each 1 ng/ml increase in plasma CA was associated with a nearly 1% reduction in 90-day mortality (Table 1). Albumin and bilirubin were included in the model given that CA is carried on serum albumin and likely hepatically cleared.3,6 Each 1 ng/ml increase in plasma CA was also associated with an increase in adjusted ventilator-free days (VFDs) of 0.016 days (p=0.04), and with an increase in adjusted organ-failure-free days of 0.025 days (p<0.001). Upon stratification, plasma CA was associated with statistically significant increases in organ failure-free days within multiple individual organ systems.
Table 1.
Relationship of plasma cholestenoic acid to mortality and adverse clinical outcomes in acute respiratory distress syndrome patients with sepsis.
| n3 | Odds Ratio1/β2 | 95% CI | p | |
|---|---|---|---|---|
| Mortality1 | 124 | 0.994 | 0.989-0.998 | 0.01 |
| Ventilator-free days2 | 124 | 0.016 | 0.001-0.030 | 0.04 |
| Organ failure-free days2 | 123 | 0.025 | 0.012-0.039 | <0.001 |
| Cardiovascular | 123 | 0.022 | 0.009-0.034 | 0.001 |
| Respiratory | 123 | 0.020 | 0.004-0.037 | 0.014 |
| Coagulation | 123 | 0.013 | 0.001-0.026 | 0.03 |
| Renal | 123 | 0.025 | 0.010-0.039 | 0.001 |
| Hepatic | 121 | 0.020 | 0.005-0.036 | 0.012 |
Logistic regression of plasma cholestenoic acid concentration to death at 90 days. Odds ratio is adjusted for body mass index, gender, race/ethnicity, APACHE III score, ARDS risk factor, diabetes mellitus, plasma bilirubin, and plasma albumin.
Linear regression of plasma cholestenoic acid concentration to ventilator-free days, organ failure-free days, and organ system-specific organ failure-free days, adjusted for the same covariates used in the logistic regression for mortality. β coefficient designates the mean change in ventilator-free or organ failure-free days per unit (1 ng/ml) increase in plasma cholestenoic acid concentration.
The n is smaller than in Table E3 study population because only those subjects with data for all model variables were included.
CI=confidence interval.
We examined CYP27A1 expression in AMs (N=30 patients) and peripheral blood monocytes (N=29 patients) purified within 48h of ARDS diagnosis from patients in the Fish Oil Phase-II Randomized Placebo-Controlled Trial, a study of enteral fish oil vs. saline placebo treatment in ARDS patients (Table E4).7 Lower CYP27A1 mRNA in AMs and monocytes was observed in patients with lower VFDs (i.e., higher illness severity) (Figure 1D). This suggests that the reduced plasma CA in patients with severe ARDS may derive, at least in part, from CYP27A1 downregulation. An inverse relationship was also observed between AM CYP27A1 mRNA and BAL fluid cytokines (Figure 1E). There was no relationship of PaO2/FIO2 ratio to either AM or monocyte CYP27A1 levels (not depicted), suggesting that CYP27A1 expression does not correlate with oxygenation.
Similar to other oxysterols, CA is an agonist of Liver X Receptor (LXR), a nuclear receptor that induces cholesterol efflux genes and also suppresses nuclear factor (NF)-κB-dependent pro-inflammatory genes.8 Given this, we speculated that CA might attenuate pro-inflammatory gene expression in human AMs. However, at concentrations that induced LXR target genes in human AMs ex vivo, CA had no effect on lipopolysaccharide-induced cytokines (Figure E2). This suggests that although declining CA may serve as a readout of AM dysfunction, local reduction of CA in the alveolus is unlikely itself to feedback on AM pro-inflammatory functions during ARDS.
Within AMs and other cells, CYP27A1-dependent synthesis of CA is rate-limited by delivery of cholesterol substrate to the inner mitochondrial membrane, a pathway that requires functional, polarized mitochondria.4 Mitotoxic stressors may set a vicious cycle in motion, given that CYP27A1 plays a role in detoxification of oxidized lipids and prevention of mitotoxic cholesterol overload in macrophages.4 As increased numbers of cholesterol-laden macrophage ‘foam’ cells are found in a wide range of lung diseases,8 it is intriguing to consider that insufficient CYP27A1 product flux, perhaps arising from mitochondrial dysfunction, could be a unifying and perhaps targetable event in human lung disease.
We propose plasma CA as a novel biomarker of AM mitochondrial function during ARDS. However, future studies will be required to verify this postulate. CYP27A1 product flux is expected to also be sensitive to expression and function of the intracellular proteins that transfer cholesterol into mitochondria. In addition, given that macrophage efflux of sterols other than CA may potentially be favored in the presence of extracellular lipoprotein acceptors,2 it is conceivable that leakage of plasma lipoproteins into the airspace during lung injury could modify CA flux from AMs.
CA has several features of a robust biomarker. Plasma CA concentration displays little diurnal variation,9 and, unlike other oxysterols, CA is not substantially produced by artefactual autoxidation.6 Given that the plasma samples we analyzed had been stored at −80°C for ~10 years, it appears that CA analysis is robust to long-term storage. Unlike other sterols, CA is not carried on plasma lipoproteins and has no significant correlation with plasma cholesterol,3 suggesting it is not substantially confounded by dyslipidemia. CA also has little-to-no renal excretion under normal conditions,3 and thus may potentially be little affected during renal failure. Glucocorticoids reportedly upregulate CYP27A1 activity in cell culture,E2 whereas the sedative dexmedetomidine inhibits it;E3 whether this translates to changes in plasma CA in vivo is uncertain. Given that ARDS is commonly associated with multiple comorbidities and therapeutic exposures, future studies will need to carefully address the specificity of changes in plasma CA to ARDS pathophysiology.
Taken together, we identify CA as a novel ARDS biomarker. We speculate that plasma CA may represent a valuable new peripheral blood indicator of AM mitochondrial function, and propose that it should now be measured in a wider range of lung diseases, and, potentially, in emerging studies of cell-based mitochondrial rescue in the lung.
Supplementary Material
Acknowledgments
Sources of Funding: This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES102005).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no relevant conflicts to disclose.
References
- 1.Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am J Respir Crit Care Med 2017; 195:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babiker A, Andersson O, Lund E, Xiu RJ, Deeb S, Reshef A, et al. Elimination of cholesterol in macrophages and endothelial cells by the sterol 27-hydroxylase mechanism. Comparison with high density lipoprotein-mediated reverse cholesterol transport. J Biol Chem 1997; 272:26253–61. [DOI] [PubMed] [Google Scholar]
- 3.Babiker A, Andersson O, Lindblom D, van der Linden J, Wiklund B, Lutjohann D, et al. Elimination of cholesterol as cholestenoic acid in human lung by sterol 27-hydroxylase: evidence that most of this steroid in the circulation is of pulmonary origin. J Lipid Res 1999; 40:1417–25. [PubMed] [Google Scholar]
- 4.Allen AM, Taylor JM, Graham A. Mitochondrial (dys)function and regulation of macrophage cholesterol efflux. Clin Sci (Lond) 2013; 124:509–15. [DOI] [PubMed] [Google Scholar]
- 5.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004; 351:327–36. [DOI] [PubMed] [Google Scholar]
- 6.Babiker A, Diczfalusy U. Transport of side-chain oxidized oxysterols in the human circulation. Biochim Biophys Acta 1998; 1392:333–9. [DOI] [PubMed] [Google Scholar]
- 7.Stapleton RD, Martin TR, Weiss NS, Crowley JJ, Gundel SJ, Nathens AB, et al. A phase II randomized placebo-controlled trial of omega-3 fatty acids for the treatment of acute lung injury. Crit Care Med 2011; 39:1655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fessler MB. A New Frontier in Immunometabolism. Cholesterol in Lung Health and Disease. Ann Am Thorac Soc 2017; 14:S399–S405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lund E, Andersson O, Zhang J, Babiker A, Ahlborg G, Diczfalusy U, et al. Importance of a novel oxidative mechanism for elimination of intracellular cholesterol in humans. Arterioscler Thromb Vasc Biol 1996; 16:208–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


