Abstract
Background
Near infrared spectroscopy (NIRS) derived hemoglobin difference (HbD: oxygenated [HbO2] − reduced hemoglobin [Hb]) and/or total hemoglobin (HbT: HbO2+Hb) have been used as surrogate measures of cerebral blood flow and volume, respectively. Statistically, a lack of HbD-blood pressure (BP) or negative HbT-BP association is regarded as a state of intact cerebral pressure autoregulation (CPA). In contrast, a co-variation of HbD/HbT and systemic blood pressure (BP) in the same direction is thought of as a failure of CPA. If the quality of one (NIRS/BP) or both signals is compromised, the reliability of the results may be adversely affected. In this work, we develop an analytic approach to assess the quality of the NIRS signals.
New Method
Given that cardiac pulses cause hemodynamic changes that are transmitted through the peripheral vasculature, cerebral NIRS signals should exhibit cyclical changes at the pulse frequency. Therefore, we propose that an association between HbD/HbT and electrocardiogram (EKG) signals would be an indicator of NIRS quality. We demonstrate the application of this approach with data collected from six newborns undergoing therapeutic hypothermia for neonatal encephalopathy.
Results
We observed an intermittent lack of association between NIRS signals and EKG data over the course of several hours of continuous records, indicating a loss in the strength in NIRS signals.
Comparison with Existing Method
Existing CPA characterization suffers from Type-II error which the current preprocessing approach can mitigate.
Conclusions
The proposed approach will allow for real-time assessment of NIRS signal quality that is essential for accurate CPA monitoring.
Keywords: Near infrared spectroscopy, Electrocardiogram, Cerebral pressure autoregulation, Spectral coherence
1. Introduction
Near infrared spectroscopy (NIRS) has become a widely used clinical and research tool for monitoring cerebral hemodynamics and oxygenation during intensive care of critically ill patients. After validation in animal models, the NIRS-derived variables of hemoglobin difference(HbD = oxygenated hemoglobin [HbO2] reduced hemoglobin [Hb]), and total hemoglobin levels (HbT = HbO2 + Hb), entered clinical and research practice as surrogate markers for cerebral blood flow and cerebral blood volume, respectively (Brady et al., 2008; Tsuji et al.,1998). In combination with continuous blood pressure measurements, NI has provided insight into intrinsic regulatory systems such as cerebral pressure autoregulation (CPA) which maintains a relatively steady cerebral blood flow during periods of BP instability. This in turn has provided the opportunity to perform long-term monitoring of CPA using NIRS technology (Wahr et al., 1996; Wolfberg and du Plessis, 2006). Based on these developments, several long-term continuous studies have supported the feasibility of NIRS-based techniques for detecting impaired CPA, or cerebral pressure passivity. Soul et al (Soul et al., 2007) demonstrated the fluctuating nature of CPA, while other studies have shown that pressure passivity is associated with higher risk for brain injury.(Lee et al.; Massaro et al., 2015; O’Leary et al., 2009; Soul et al., 2007) In previous studies, the fluctuating CPA was measured by the amount of time spent in a pressure passive state over a longer recording period (i.e., the pressure passive index; PPI) (O’Leary et al., 2009; Soul et al., 2007). However, perhaps more than most continuous monitoring techniques in intensive care, NIRS is prone to artefact and signal loss during long recordings, given its sensitivity to movement and ambient light. The loss of NIRS signal quality may not be readily evident to the untrained observer, and may therefore lead to false interpretation data of measures such as of NIRS-based CPA. For these reasons, a reliable continuous index of NIRS signal quality is needed. In this report, we describe a fundamental approach for ensuring that NIRS signals of suboptimal quality are readily identified during recordings of NIRS-based cerebral hemodynamics. Specifically, we use the coherence between NIRS signal change and the simultaneously measured R-waves (coinciding with cardiac systole) on the bedside electrocardiogram (EKG) signal. Since cardiac pulsations should produce changes in cerebral oxygenation, the coherence between NIRS changes and EKG should be high at the pulse frequency. Conversely, a lack of coherence at the pulse frequency indicates a lack of adequate power and signal content in the NIRS signals, and suggests that any signal based on NIRS during that period is likely to be compromised. As a demonstration of this technique we use a retrospective dataset containing simultaneously recorded and time-locked data from NIRS, continuous intra-arterial BP, and EKG recordings in term newborns undergoing hypothermia for neonatal encephalopathy (NE).
2. Materials and Methods
We collected data from six term newborns undergoing therapeutic hypothermia for NE following an established protocol (Shankaran et al., 2005). Cardiorespiratory and electroencephalogram monitoring was initiated within 10 – 26 hours of birth. Cerebral NIRS (NIRO 200, Hamamatsu Photonics, Hamamatsu, Japan) signals were measured from over the frontotemporal regions (forehead) of each hemisphere (See Figure 1). The distance between optodes was 40 mm. Cerebral NIRS signals included oxygenated hemoglobin (HbO2) and reduced hemoglobin (Hb). Electrocardiograms and blood pressure signals were retrieved from the analog port of bedside monitor (Philips IntelliVue MP70, MA, USA). All signals were acquired and digitized simultaneously using custom-made software developed in LabView (National Instruments, TX, USA) at a sampling rate of 1000 Hz. The native sampling frequencies of the (analog) signals were 5 Hz, 250 Hz, and 125 Hz for NIRS, EKG, and blood pressure, respectively.
Figure 1.
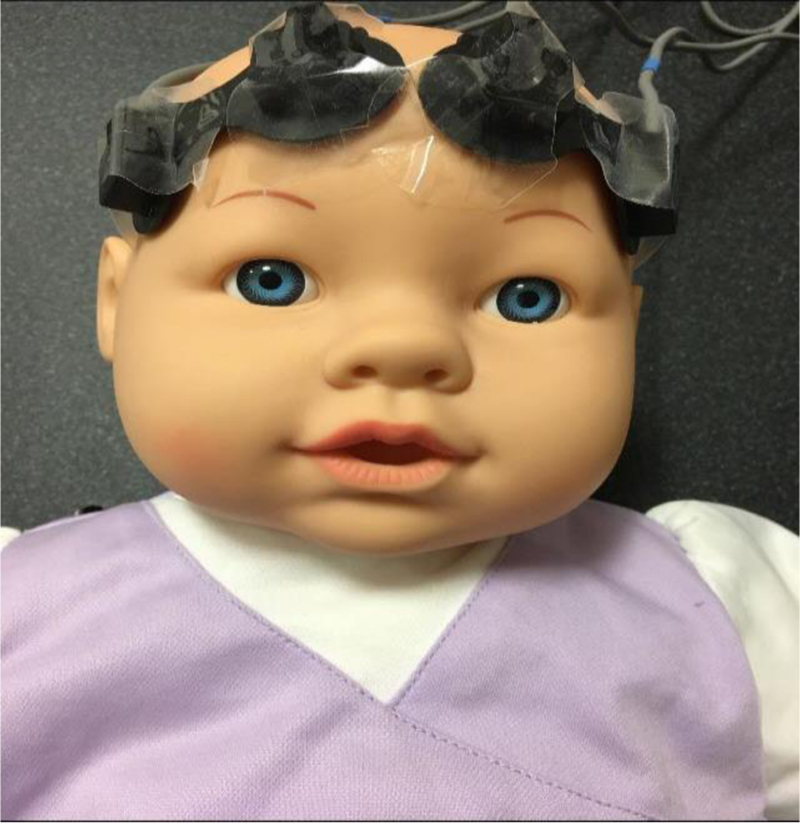
Placement of NIRS optodes in the frontotemporal region on both cerebral hemispheres is shown on a mannequin. In our clinical studies, we wrap the optodes using co-band and ensure that the optodes are firmly attached to the scalp.
Coherence Estimation:
To identify the presence of cardiac pulsations in cerebral hemodynamics, we calculated the spectral coherence between the NIRS signals and EKG. For coherence analysis, we partitioned the data into 10-minute epochs. Coherence between EKG (X) and NIRS (Y) signals in each epoch was calculated using the Welch periodogram approach (Halliday et al., 1995). In this approach, the signals were portioned into 30-second non-overlapping windows to capture the oscillations in a frequency resolution of 0.033 Hz (Halliday et al., 1995). In every 30-second window, periodograms of X, (SXX), and Y, (SYY) were calculated as the square of the magnitude of the Fourier transforms of the respective signals. Similarly, cross-spectrum (SXY) between X and Y was calculated as the product of the Fourier transform of X and the complex conjugate of the Fourier transform of Y. To this end, estimates of these spectral quantities were calculated as the average overall windows.
Spectral coherence was calculated as the ratio of the square of the magnitude of the cross-spectrum to the product of the auto-spectra as follows: C(ω) the overbar indicates an estimate of that quantity. The confidence limit of the coherence at α significance level is given by (Govindan et al., 2013; Halliday et al., 1995): where M is the number of windows involved in the estimation of the spectral quantities, which is 20 in our case. The significance level was set to 0.0001. Accordingly, the confidence limit for coherence was 0.3842. We studied the coherence between the cerebral NIRS signals (HbO2, Hb, HbD, and HbT) and EKG. The analysis was performed for NIRS signals from each cerebral hemisphere independently.
Quality Assessment:
To quantify periods of high-quality NIRS signals, we defined the quality index (QI) as the percentage of time over which the coherence between the NIRS and EKG signals reached significance over the entire recording period. To determine whether the Hb or HbO2 signal was the main contributor to decreased HbD and HbT quality, we evaluated the relationship between the QI of the Hb (QI-Hb) and HbO2 (QI-HbO2) components with the QI of HbD (QI-HbD) and HbT (QI-HbT) using a multiple regression analysis. The measures from both cerebral hemispheres were averaged for these analyses since we do not expect hemispheric differences in the results. A value of p<0.05 was considered significant.
For Infant1 and Infant2, we present the coherence spectra obtained for every 10-minute epoch as contour plots. For Infants 3 to 6, we present the maximum coherence between HbO2-EKG in the 0.8–2.5 Hz, a frequency band that encompasses heart rate of the newborns. Of note, the heart rate of the newborns is in the range of 0.8 – 2. 5 Hz and no other relevant physiological signals are known to fluctuate in this frequency band. Although this is the time constant for the delta power of electroencephalogram (EEG) (0.5 – 4 Hz), the brain electrical activity (EEG) is not part of the cerebral autoregulation characterization. Hence, in the chosen frequency band (0.8 – 2.5 Hz), the oscillations in the NIRS signals between 0.5 – 4 Hz can be safely regarded as those from cardiac pulses. The primary focus of this study is to develop a preprocessing technique that optimizes the quality of NIRS signals used for continuous cerebral monitoring. To demonstrate this approach, we used the pre-processing EKG-NIRS coherence to augment resolution of the NIRS-based cerebral pressure autoregulation monitoring, using the pressure-passive index (PPI; described above) (Govindan et al., 2013; Massaro et al., 2015). In the cases of Infant1 and Infant2 we demonstrate how the current approach helps to identify the poor signal quality NIRS can lead to a false conclusion of the state of intact autoregulation (Type-II error). In order to demonstrate the potential impact of poor quality NIRS signals on the calculation of autoregulation metrics, we calculated the pressure passivity index (PPI) (Govindan et al., 2013; Massaro et al., 2015) for two infants with contrasting outcomes (Infant1 with normal brain MRI and good outcome versus Infant2 with severe brain injury by MRI). The cerebral pressure autoregulation was quantified by calculating the coherence between HbD and mean arterial pressure with a frequency resolution of 0.033 Hz (Massaro et al., 2015) (i.e.) using the same procedure as described above. Of note, the mean arterial pressure was calculated using the continuous blood pressure signal acquired along with EKG. A significant coherence (>0.3842) in 0.05–0.25 Hz indicated the state of impaired autoregulation (Govindan et al., 2013). The percentage of coherent epochs in a six-hour window defined pressure passivity index (PPI).All analyses were performed off-line in MATLAB (Mathworks Inc, MA, USA).
3. Results
We studied data from six infants (Infant1 to Infant6). The studies were initiated at a median age of 14 hours of life (minimum = 11 hours; maximum = 25 hours). The median duration of the studies was 76 hours (minimum=64 hours; maximum=81 hours). The results of the coherence analyses for infant 1 and infant 2 are shown in Figures 2 and 3 as contour plots. In all figures, the coherence spectra are plotted as a function of frequency and time. The lower and upper limits of the contour plot were set to the coherence threshold (0.3842) and one, respectively (Govindan et al., 2013). For Infant1, all the NIRS signals exhibited significant coherence with EKG at the cardiac pulsation frequency (Figures 2a–h). However, the coherence was weaker between Hb and EKG (see Figures 2c and 2d). The PPI calculated for the right and left cerebral hemispheres are shown in Figures 2i and 2j, respectively. The PPI values were less than 0.5, consistent with the favorable outcome of the infant. The results obtained for Infant 2 are shown in Figure 3 in a similar arrangement as shown in Figure 2 for Infant1. The magnitude of the coherence between NIRS signals and EKG is weaker or insignificant for up to 50 hours, significant from 50 to 78 hours, and after that weaker/insignificant again (see Figures 3 a–h). The PPI results are shown in Figures 3i and 3j for the right and left cerebral hemispheres, respectively. The PPI was zero or less than 0.1 until 40 hours of life, and then it started to increase gradually. Note that the PPI results parallel the HbD-EKG coherences shown in Figures 3e and 3f. The PPI results alone would indicate that the baby had intact CPA (i.e., low coherence between NIRS and BP changes) until 40 hours of life. However, it is likely that the lack of coherence observed before 40 hours of life was related to poor NIRS signal quality giving a spuriously low coherence (i.e., apparently intact CPA) in this infant with an adverse outcome.
Figure 2.
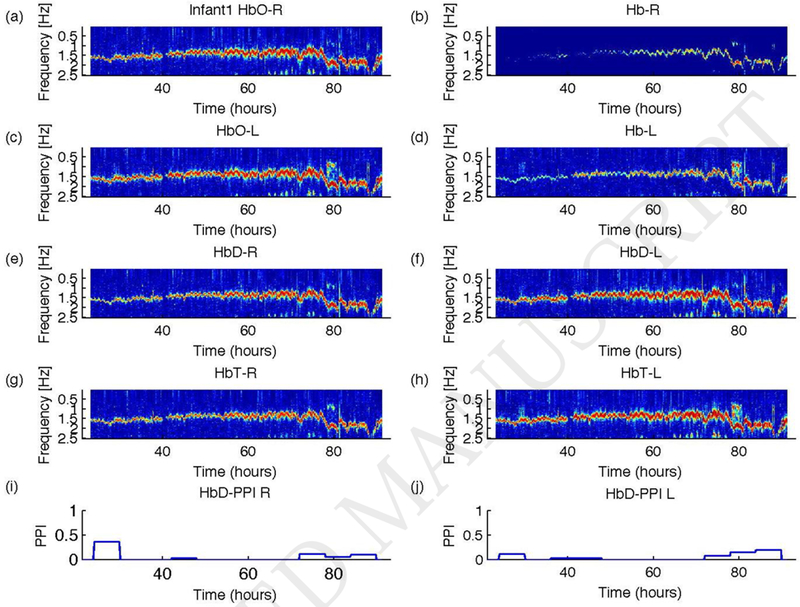
Results of coherence analysis of NIRS signals and EKG for Infant1. For signals from the right cerebral hemisphere: (a) HbO-EKG, (b) Hb-EKG, (e) HbD-EKG, and (g) HbT-EKG. For signal from the left cerebral hemisphere: (c) HbO-EKG, (d) Hb-EKG, (f) HbD-EKG, and (h) HbT-EKG. HbO indicates HbO2. The pressure passivity index (PPI) calculated from HbD-Blood pressure coherence for (i) right and (j) left cerebral NIRS are shown as a function of time. This infant had a favorable outcome. Coherence spectra are plotted as contour diagram as a function frequency in Hz (y-axis) and time since birth in hours (x-axis). The lowest limit of the color scale (blue) was set to the threshold value of coherence and the highest limit of the color scale was set to 1. At the cardiac pulse frequency, there is a significant coherence in almost all NIRS signals (see text for details).
Figure 3.
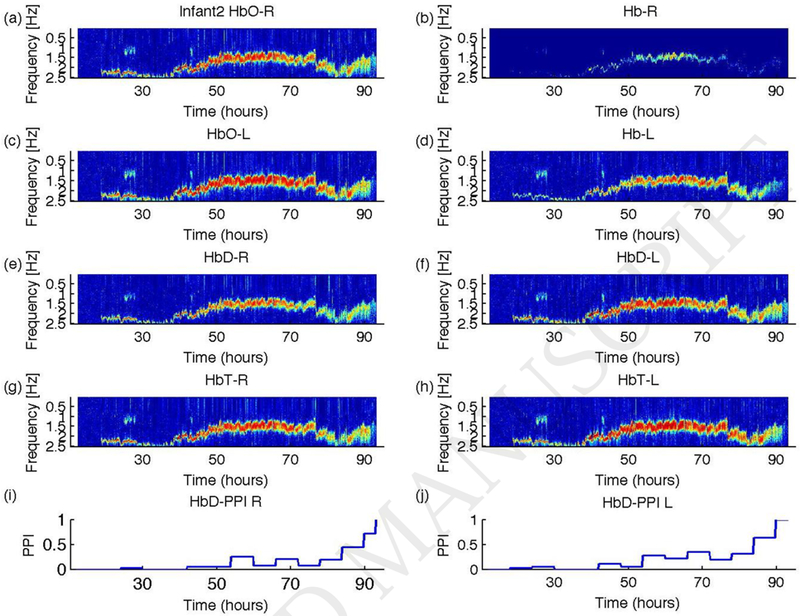
Results of coherence analysis of NIRS and EKG for Infant2. The variables plotted are the same as shown in Figure 1 but for Infant2. As in Figure 1, we have set the lowest color scale to the threshold and the highest value to one. Coherence between NIRS signals and EKG is insignificant at few segments. In all plots, coherence is insignificant during the early study period. The PPIs shown in (i) and (j) were zero indicating the state of autoregulation is intact during this period. This infant had adverse outcome. The PPI during the period of insignificant HbD-EKG coherence is not reliable.
The QI-HbD was significantly associated with the quality of the HbO2 signal (coefficient=0.88; p=0.0001), while not affected by the quality of the Hb signal (coefficient=−0.07; p=0.74). Similarly, the QI-HbT was also significantly associated with the quality of HbO2 (coefficient=1.07; p<0.0001) and not Hb (coefficient=−0.009; p=0.96). These results show that poor HbO2 signal quality (indicated by a loss in HbO2-EKG coherence) is a major determinant of HbD and HbT quality.
In Figure 4, the maximum coherence between HbO2 and EKG in the 0.8 – 2.5 Hz band is plotted for infants 3 through 6 over time. The significance threshold is shown via the dotted line, and tracings of individual patients demonstrate frequent episodes where HbO2 signal quality drops below the threshold, indicating poor quality during the recording period. In Infant3, there are only a few instances in both the left and right hemispheres where the EKG-HbO2 coherence was insignificant (see Figures 4 a–b); however, in infants 4, 5, and 6, the EKG-HbO2 coherence below significance over several periods (see Figures 4 c–h). In particular, in Infant4, the EKG-HbO2 coherence remained continuously below significance for about 20 hours between 30–50 hours (see Figure 4d).
Figure 4.
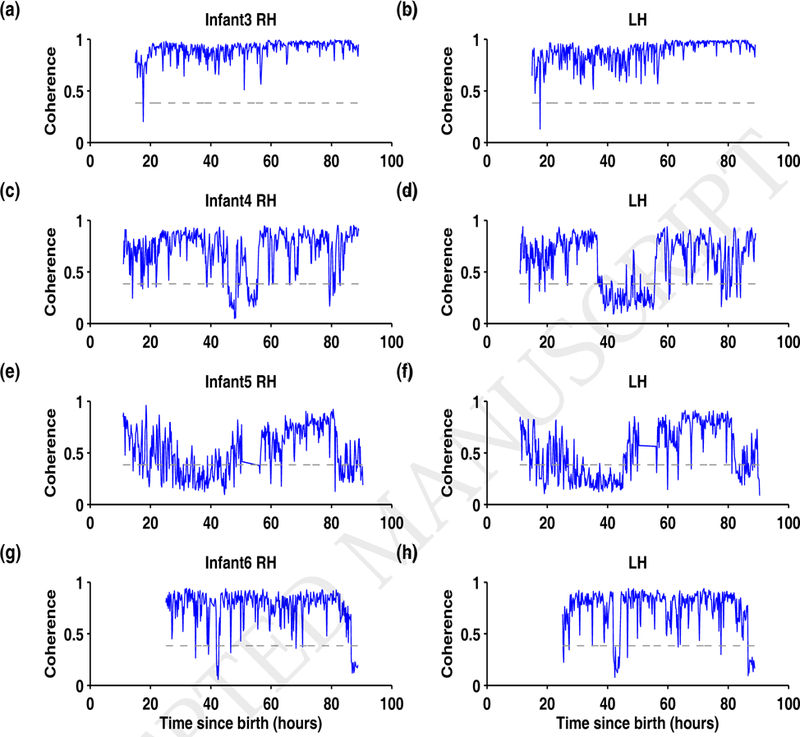
Summary of HbO2-EKG coherence for infants 3 through 6. For Infant3 to Infant6, the coherence calculated from the right cerebral hemisphere are plotted in (a), (c), (e), and (g) and for the left cerebral hemisphere in (b), (d), (f), and (h), respectively. In each plot, the maximum coherence between the HbO2-EKG in the frequency band 0.8–2.5 Hz is plotted as a function of time since birth (hours). The horizontal dashed line at coherence = 0.3842 is the threshold of significant coherence. In infants 4, 5, and 6 the coherence is insignificant for more than 5 hours either in left, right or both cerebral hemispheres.
4. Discussion
Spectral coherence between NIRS signals and EKG at the cardiac pulse frequency can be used to assess the quality of the NIRS signal. Since long recordings are at risk for loss of NIRS signal quality, the approach described here can be incorporated into computational algorithms to improve the accuracy of other NIRS-based signals such as cerebral pressure autoregulation. Furthermore, we demonstrate that the HbO2 signal is the optimal NIRS variable for determining the quality of the derived variables (HbD and HbT).
NIRS allows continuous monitoring of cerebral pressure autoregulation at the bedside of critically ill patients. Several studies have utilized NIRS-derived HbD (Bassan et al., 2005; Massaro et al., 2015; Soul et al., 2007; Tsuji et al., 1998; Tsuji et al., 2000) or HbT (Brady et al.; Brady et al., 2008; Brady et al., 2007; Lee et al., 2009) to monitor continuously cerebral pressure autoregulation. In these studies, a significant coherence between HbD and BP(Bassan et al., 2005; Massaro et al., 2015; Soul et al., 2007; Tsuji et al., 1998; Tsuji et al., 2000) or positive correlation between HbT and BP (Brady et al.; Brady et al., 2008; Brady et al., 2007; Lee et al., 2009) was interpreted as an impairment in cerebral pressure autoregulation. In contrast, the lack of HbD-BP coherence, as well as negative or lack of correlation between HbT and BP, was interpreted as the state of intact cerebral pressure autoregulation. However, poor quality or “noisy” NIRS signals may also show lack of correlation with BP and have no physiological basis as an indicator of autoregulation. Given that poor quality NIRS signals can serve as an important potential contributor of type II error affecting bedside CPA monitoring, this study suggests that the absence of coherence/correlation between the NIRS signals and BP should not be interpreted as intact autoregulation without an appropriate check on the quality of the NIRS signals.
Poor NIRS signal quality might be due to poor contact between the NIRS optodes and skin, malposition, and/or interference with the environmental light and other factors. Our approach can be applied to real time CPA monitoring by providing the means to identify time periods when NIRS quality is poor (i.e. when coherence between HbO2 and EKG is insignificant) in order to alert the user to check appropriate NIRS application and system integrity. It can also be used to filter poor NIRS signal data from use in the quantification of CPA metrics in order to improve accuracy.
An interesting finding of our study was the particular importance of HbO2 signal quality in quantifying CPA. This may be explained in part by the fact that cerebral venous compliance is about 20 times higher than cerebral arterial compliance (Heldt et al., 2012). Hence, most of the changes in the NIRS variables at the cardiac pulse frequency can be regarded as changes in the venous compartment. Venous oxygen saturation under normal cerebral oxygen extraction is about 65%. Thus HbO2 concentration will be higher than that of Hb (Heldt et al., 2012). As a result, the changes in HbO2 will be higher than the changes in Hb. This, taken together with the finding that coherence is a direct measure of signal-to-noise of the signals (Timmer et al., 1998), explains the higher magnitude of HbO2-EKG coherence compared to Hb-EKG coherence in our study.
In this study, the NIRS signals were collected from frontotemporal regions from both cerebral hemispheres. The NIRS measured from other cerebral regions will also work for signal quality assessment (HbO2-EKG coherence) and this generalizability is based on the following reason: for every cardiac pulse, the brain is perfused through major arteries, namely, left and right anterior cerebral arteries (ACA), mid-cerebral artery (MCA), posterior cerebral arteries (PCA), basilar, and vestibular arteries, which encompass the entire brain. Since NIRS measures regional oxygenation changes, the hemodynamic gradations caused by cardiac pulse should manifest globally across the cortex; this is established by the aforementioned major arteries. Hence, NIRS signals, if sampled with adequate rate from any region of the cortex, will contain cardiac oscillations and characterization of these oscillations helps to assess the signal quality of NIRS signals.
This work shows that loss of signal quality in HbO2 measured with NIRS significantly affects the signal quality of HbD and HbT signals. Cerebral pressure autoregulation is regarded as an upstream antecedent of brain injury. Hence it is important to characterize it reliably. Offline would be late, and hence any analysis approach should work in real-time.We have proposed a plan for the real-time characterization of cerebral pressure passivity while ensuring the signal quality of the NIRS signals in parallel.
In the real-time analysis module, we plan to include the current analysis regimen as follows: For coherence analysis, partition the data into 10-minute non-overlapping epochs. For data in each epoch, perform the coherence analysis between HbD and blood pressure (COHHbD-BP), HbT and blood pressure (COHHbT-BP) and HbO2 and EKG (COHHbO2-EKG). If COHHbO2-EKG is greater than the predefined threshold (indicates good NIRS quality), interpret COHHbD-BP and COHHbT-BP as the NIRS signal quality is reliable in this epoch, if not, disregard this epoch from further interpretation.
Although this study has several strengths, including the use of long continuous recordings with a duration of over 72 hours, the high rate of NIRS signal sampling, the simultaneously recorded EKG, the homogenous study population, and the advanced signal processing techniques to characterize the signals, it has certain limitations. Current NIRS technology allows sampling of the signals in an analog format at 5 Hz, and this rate is sufficient if the heart rate is less than 2.5 Hz. However, the heart rate might exceed 2.5 Hz (150 bpm) in some infants, and in those scenarios, the current technology might not yield reliable results. Of note, NIRS signals sampled at a higher rate can be retrieved through the serial port of the Hamamatsu device, which can be used in our analysis pipeline to control quality. Regarding the frequency resolution of NIRS-EKG coherence spectrum, an EKG is a broadband signal, and hence the proposed approach would work for any choice of Fourier transform window between 3–30 seconds. In our work, we used a 30-second window to obtain the spectral estimate at the same resolution as used in the characterization of the cerebral pressure autoregulation (Govindan et al., 2013; Massaro et al., 2015).
As the coupling between EKG and NIRS signals happens at a characteristic time scale (at the period of the heart functioning), the frequency domain approach, coherence, is used in this work to quantify their association between them. There are advanced frequency domain methods such as wavelet transform and phase synchronization approaches (Furdea et al., 2009; Pikovsky et al., 2002). Future work will also explore the use of these methods to quantify the coupling/cross-coupling between NIRS and EKG signals and assess NIRS signal quality.
Conclusion
We have proposed an elegant tool for assessing bedside NIRS signal quality that can be performed in real-time. Whether assessment of cerebral pressure autoregulation of critically ill infants in light of this new tool improves predictive ability for downstream brain injury warrants further investigation.
Highlights.
Quality of NIRS signal is important in quantifying cerebral autoregulation
Coherence between EKG and NIRS signals objectively determines NIRS signals’ quality
Our approach can be applied in real-time quantification of cerebral autoregulation
Acknowledgment
We would like to thank Ms. Sophie Wohlers for her editorial assistance and Dr. Gilbert Vezina for his neuroradiographic classification of outcomes for the subjects described in this work. This work was supported by the Clinical and Translational Science Institute at Children’s National (UL1TR000075, 1KL2RR031987-01), the Intellectual and Developmental Disabilities Research Consortium (NIH P30HD040677) and internal special purpose funds at Children’s National.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bassan H, Gauvreau K, Newburger JW, Tsuji M, Limperopoulos C, Soul JS, Walter G, Laussen PC, Jonas RA, du Plessis AJ. Identification of pressure passive cerebral perfusion and its mediators after infant cardiac surgery. Pediatr Res, 2005; 57: 35–41. [DOI] [PubMed] [Google Scholar]
- Brady K, Joshi B, Zweifel C, Smielewski P, Czosnyka M, Easley RB, Hogue CW Jr. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke; 41: 1951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KM, Lee JK, Kibler KK, Easley RB, Koehler RC, Shaffner DH. Continuous measurement of autoregulation by spontaneous fluctuations in cerebral perfusion pressure: comparison of 3 methods. Stroke, 2008; 39: 2531–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KM, Lee JK, Kibler KK, Smielewski P, Czosnyka M, Easley RB, Koehler RC, Shaffner DH. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke, 2007; 38: 2818–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furdea A, Eswaran H, Wilson JD, Preissl H, Lowery CL, Govindan RB. Magnetomyographic recording and identification of uterine contractions using Hilbert-wavelet transforms. Physiol Meas, 2009; 30: 1051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan RB, Massaro AN, Andescavage NN, Chang T, du Plessis A. Cerebral pressure passivity in newborns with encephalopathy undergoing therapeutic hypothermia. Front Hum Neurosci, 2013; 8: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data--theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol, 1995; 64: 237–78. [DOI] [PubMed] [Google Scholar]
- Heldt T, Kashif FM, Sulemanji M, O’Leary HM, du Plessis AJ, Verghese GC. Continuous quantitative monitoring of cerebral oxygen metabolism in neonates by ventilator-gated analysis of NIRS recordings. Acta Neurochir Suppl, 2012; 114: 177–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Brady KM, Chung SE, Jennings JM, Whitaker EE, Aganga D, Easley RB, Heitmiller K, Jamrogowicz JL, Larson AC, Lee JH, Jordan LC, Hogue CW, Lehmann CU, Bembea MM, Hunt EA, Koehler RC, Shaffner DH. A pilot study of cerebrovascular reactivity autoregulation after pediatric cardiac arrest. Resuscitation [DOI] [PMC free article] [PubMed]
- Lee JK, Kibler KK, Benni PB, Easley RB, Czosnyka M, Smielewski P, Koehler C, Shaffner DH, Brady KM. Cerebrovascular reactivity measured by near-infrared spectroscopy. Stroke, 2009; 40: 1820–6. [DOI] [PubMed] [Google Scholar]
- Massaro AN, Govindan RB, Vezina G, Chang T, Andescavage NN, Wang Y, Al-Shargabi T, Metzler M, Harris K, du Plessis AJ. Impaired cerebral autoregulation and brain injury in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. J Neurophysiol, 2015; 114: 818–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary H, Gregas MC, Limperopoulos C, Zaretskaya I, Bassan H, Soul JS, Di Salvo DN, du Plessis AJ. Elevated cerebral pressure passivity is associated with prematurity-related intracranial hemorrhage. Pediatrics, 2009; 124: 302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikovsky AS, Rosenblum MG, Kurths J. Synchronization: A Universal Concept in Nonlinear Sciences Cambridge University Press., 2002. [Google Scholar]
- Shankaran S, Laptook AR, Ehrenkranz RA, Tyson JE, McDonald SA, Donovan EF, Fanaroff AA, Poole WK, Wright LL, Higgins RD, Finer NN, Carlo WA, Duara S, Oh W, Cotten CM, Stevenson DK, Stoll BJ, Lemons JA, Guillet R, Jobe AH. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med, 2005; 353: 1574–84. [DOI] [PubMed] [Google Scholar]
- Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, Disalvo DN, Moore M, Akins P, Ringer S, Volpe JJ, Trachtenberg F, du Plessis AJ. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res, 2007; 61: 467–73. [DOI] [PubMed] [Google Scholar]
- Timmer J, Lauk M, Pfleger W, Deuschl G. Cross-spectral analysis of physiological tremor and muscle activity. I. Theory and application to unsynchronized electromyogram. Biol Cybern, 1998; 78: 349–57. [DOI] [PubMed] [Google Scholar]
- Tsuji M, duPlessis A, Taylor G, Crocker R, Volpe JJ. Near infrared spectroscopy detects cerebral ischemia during hypotension in piglets. Pediatr Res, 1998; 44: 591–5. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R, Volpe JJ. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics, 2000; 106: 625–32. [DOI] [PubMed] [Google Scholar]
- Wahr JA, Tremper KK, Samra S, Delpy DT. Near-infraredspectroscopy: theory and applications. J Cardiothorac Vasc Anesth, 1996; 10: 406–18. [DOI] [PubMed] [Google Scholar]
- Wolfberg AJ, du Plessis AJ. Near-infrared spectroscopy in the fetus and neonate. Clin Perinatol, 2006; 33: 707–28, viii. [DOI] [PubMed] [Google Scholar]


