Abstract
The function of acid ceramidase (ACDase), whose congenital deficiency leads to Farber disease, has been recognized to be vital to tumor cell biology, and inhibition of its activity may be beneficial in cancer therapy. Therefore, manipulation of the activity of this enzyme may have significant effect, especially on cancer cells. LCL521, Di-DMGB13, is a lysosomotropic inhibitor of ACDase. Here we define complexities in the actions of LCL521 on ACDase. Systematic studies in MCF7 cells showed dose and time divergent action of LCL521 on ACDase protein expression and sphingolipid levels. Low dose of LCL521 (1 μM) effectively inhibited ACDase in cells, but the effects were transient. A higher dose of LCL521 (10 μM) caused a profound decrease of sphingosine and increase of ceramide, but additionally affected the processing and regeneration of the ACDase protein, with biphasic and reversible effects on the expression of ACDase, which paralleled the long term changes of cellular sphingosine and ceramide. Finally, the higher concentrations of LCL521 also inhibited Dihydroceramide desaturase (DES-1). In summary, LCL521 exhibits significant effects on ACDase in a dose and time dependent manner, but dose range and treatment time need to be paid attention to specify its future exploration on ACDase targeted cancer treatment.
Keywords: LCL521, B13, lysosomes, acid ceramidase, sphingolipids, LC-MS /MS lipid analysis
1. INTRODUCTION
Bioactive sphingolipid (SL) metabolites, including specific ceramide species (Cn-Cer), sphingosine (Sph) and sphingosine 1-phosphate (S1P), are recognized as signaling molecules involved in regulation of survival, proliferation and cell death.1 Cer is known to be a key modulator of cancer cell growth and apoptosis; conversely, S1P acts as an anti-apoptotic tumor protective agent.2, 3 Therefore, the levels and balance of natural Cer-Sph-S1P must be well controlled.
The conversion of Cer to S1P involves ceramidases (CDs) and sphingosine kinases (SKs). In addition, because it is a central molecule in SL metabolism, Cer can be generated from various pathways, one of them is from the last step of the de novo pathway, which is from dihydroceramide (dhCer) under the catalysis of dihydroceramide desaturases (DES). Many of these enzymes are being recognized as therapeutic targets for cancer.4–6
Lysosomal acid ceramidase (ACDase) hydrolyzes Cer to Sph and free fatty acids at pH optimum ~ 4.5, which is also known to be the last step of lysosomal SL degradation by the stepwise action of specific hydrolases.7–9 ACDase was also discovered to be localized in the nucleus to influence lipid metabolism and gene expression there.10 Human ACDase is synthesized as a precursor with an apparent molecular weight of 53–55 kDa that is processed in the acidic compartments of the cells (late endosomes and /or lysosomes) into an un-glycosylated 13 kDa α-subunit (α-ACDase) and a glycosylated 40-kDa β-subunit (β-ACDase), both linked by a disulfide bridge, which could be dissociated under reducing conditions. ACDase cleavage is most likely an auto-proteolytic event, and a free Cys143 is required for both ACDase processing and activity. ACDase autocatalytic self-cleavage occurs most rapidly under acidic conditions, but also at neutral condition.7–10 The β subunit was shown to possess Cer N-Acyl hydrolase activity to produce lysosomal Sph. The detection of this subunit shows variable molecular weight possibly due to changes in N-glycosylation (up to 5 active sites), therefore, the presence of ACDase is mostly visualized via its α -subunit and/or ACDase precursor.7–13
The function of ACDase has been recognized to be vital to tumor cell biology, and inhibition of its activity may be beneficial in cancer therapy.14–17 However, a congenital deficiency of ACDase activity leads to Farber disease.18 Several studies have shown that ACDase overexpression could provoke tumor cell resistance to chemotherapy and radiation therapy.19–21 Thus, manipulation of the activity of this enzyme may have important effects for diseases.
Over the last decade, our group has been focusing on the development of ACDase inhibitors based on the B13 scaffold (1R, 2R)-1-(4’-nitrophenyl)-2-N-(tetradecanoylamino)-1, 3-propandiol.22–28 Our latest approach aimed at direct delivery of B13 to the lysosomes via its DMG modification. Among them, LCL521, (1R, 2R)-2-N-(tetradecanoylamino)-1-(4’-nitrophenyl)-propyl-1, 3 -O, O-(N,N-dimethylamino) acetate dihydrochloride, represents the most effective modification (Fig. 1A).29,30 LCL521 inhibits ACDase specifically among the ceramidases in vitro, which is reinforced by the lysosomal targeting. Moreover, inhibition of lysosomal ACDase results in dramatic decrease of endogenous Sph.28 Nevertheless, effects of LCL521 on ACDase protein expression and key SL metabolism are not fully described.
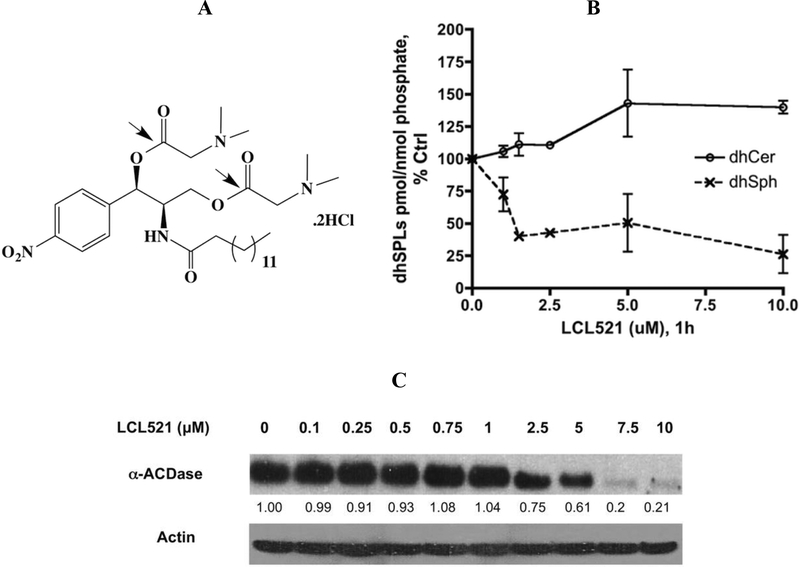
Figure 1. LCL521’s structure and its effects on dhSL metabolites and ACDase protein expression.
A. Structure of LCL521; B. Dose response of LCL521 on cellular dhSph and dhCer at 1h. MCF7 cells were treated with vehicle, or 1, 1.5, 2.5, 5 and 10μM LCL521 for 1h, then cell pellets were collected and lipids were extracted. Cellular levels of dhSph and dhCer were measured by LC-MS/MS. Results are expressed as pmol dh-SL/nmol Lipid phosphate, and presented as % Control (baseline for dhCer: 3.3151×10−2 ±4.21×10−4 pmol/nmol Lipid Phosphate; dhSph: 8.789×10−3±3.386×10−4pmol/nmol Lipid Phosphate). Results are presented as means ± st dev. of duplicates; C. Effect of LCL521 on ACDase protein expression at 1h. MCF7 cells were treated with vehicle, or with 0.1, 0.25, 0.5, 0.75, 1, 2.5, 5, 7.5 and 10μM LCL521 for 1h. α-ACDase protein expression was visualized by western blot as described; actin was utilized to monitor protein loading and transfer.
The present study concentrated on dose and time-dependent action of LCL521 on ACDase protein expressions and key SL metabolites. These studies were conducted in MCF7 cells where ACDase exhibits both high protein expression and gene expression, and predominant lysosomal localization as well.10 Thus, the action of LCL521 in MCF7 cells should be mainly associated with the inhibition of lysosomal ACDase.
2. RESULTS AND DISCUSSION
2.1. Early effects of LCL521 on dhSL metabolites and ACDase protein expression
Previous studies revealed that at 1h, LCL521 decreased Sph and S1P starting from 100 nM, but increased Cer became statistically significant only for a high dose (10 μM, p<0.05 vs Ctrl, ~19 pmols), and the amount was very close to the decrease of Sph (18 pmols).30 Our current studies revealed that at 1h, LCL521 also decreased dhSph with slightly increased dhCers (Fig. 1B), indicating LCL521 also affected dh-SLs. Meanwhile, we also observed dose dependent effects of LCL521 on ACDase protein expression. The results showed that low doses of LCL521 (≤1 μM) had no effects on ACDase. Starting at 2.5 μM, LCL521 induced a dose dependent loss of α-ACDase protein expression (Fig.1C).
Given the above results, it became of interest to investigate time dependent effects of LCL521 on SL metabolites at a low dose (1μM), which had no effects on ACDase protein expressions vs high dose (10μM), with the additional impact on ACDase protein (Fig.1C & S.Fig1).
2.2. Effects of 1μM LCL521
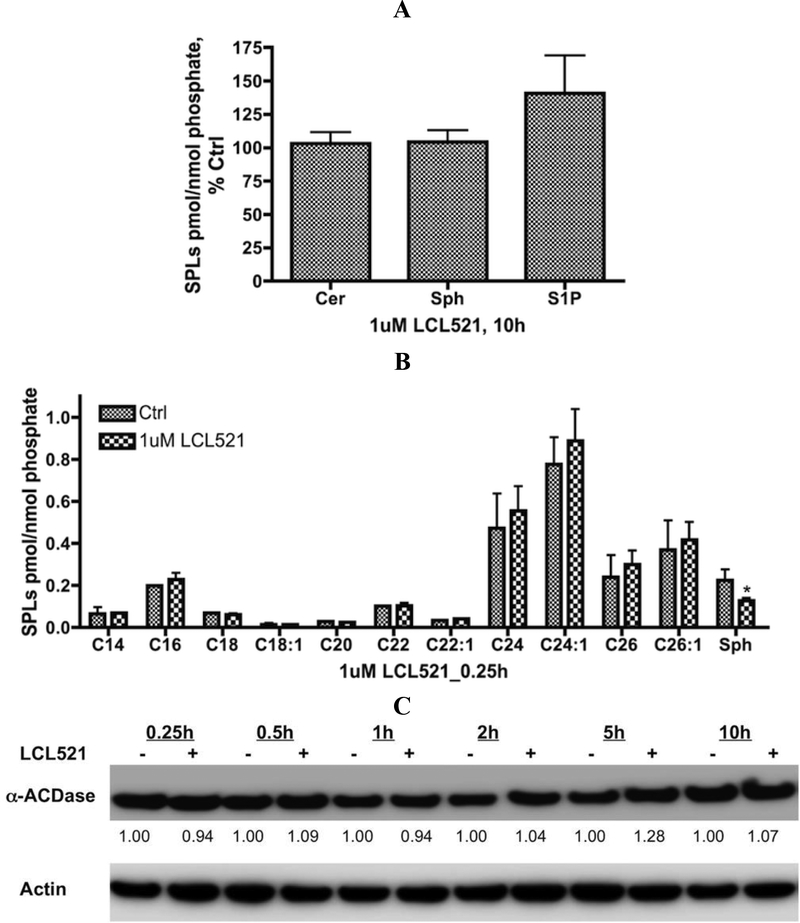
Our previous time course results of 1 μM LCL521 indicated a significant reduction of Sph level at 1h.30 But extended treatments revealed regeneration of cellular Sph and S1P, which were fully recovered at 10h (Fig. 2A). Surprisingly, we also observed little changes of total Cer irrespective of the length of acyl chain (15min, Fig. 2B). Over this time course, 1μM LCL521 did not affect the expression of α-ACDase (Fig. 2C). Taken together, these results demonstrate that LCL521 is a relatively potent and effective inhibitor of ACDase in cells (at 1μM), but the effects are transient. Therefore, to achieve ACDase long-lasting inhibition, multiple treatments of low dose are probably necessary.
Figure 2. Effects of 1μM LCL521 on SL metabolites and ACDase protein expression.
A. Effects of 1μM LCL521 on SL metabolites at 10h. Cells were treated with either vehicle, or 1μM LCL521 for 10h, then cell pellets were collected and lipids were extracted. Levels of Cer, Sph and S1P were measured by LC-MS/MS approach. Results are expressed as pmol SLs/nmol Lipid Phosphate, and presented as % Control (baseline for Cer: 2.647±0.808 pmol/nmol Lipid Phosphate; Sph: 2.789×10−1±7.606×10−2pmol/nmol Lipid Phosphate; S1P: 2.955×10−2 ±2.323×10−2pmol/nmol Lipid Phosphate) with means ± st dev. of 5x replicates; B. Effects of 1μM LCL521 on Cn-Cer and Sph at 0.25h. Cells were treated with vehicle or 1μM LCL521 for 0.25h (15min), then cell pellets were collected, lipids were extracted, and levels of Cn-Cer and Sph were measured by LC-MS/MS. Results are presented as pmol SL/nmol Lipid Phosphate with means ± st dev. of 5x replicates, * p value <0.05 (vs Control); C. Effects of 1μM LCL521 on ACDase protein expression. Cells were treated with either vehicle or 1 μM LCL521 for 0.25, 0.5, 1, 2, 5, 10h. α-ACDase protein expression was then visualized by western blotting as described. Actin was utilized to monitor protein loading and transfer.
2.3. Effects of 10 μM LCL521
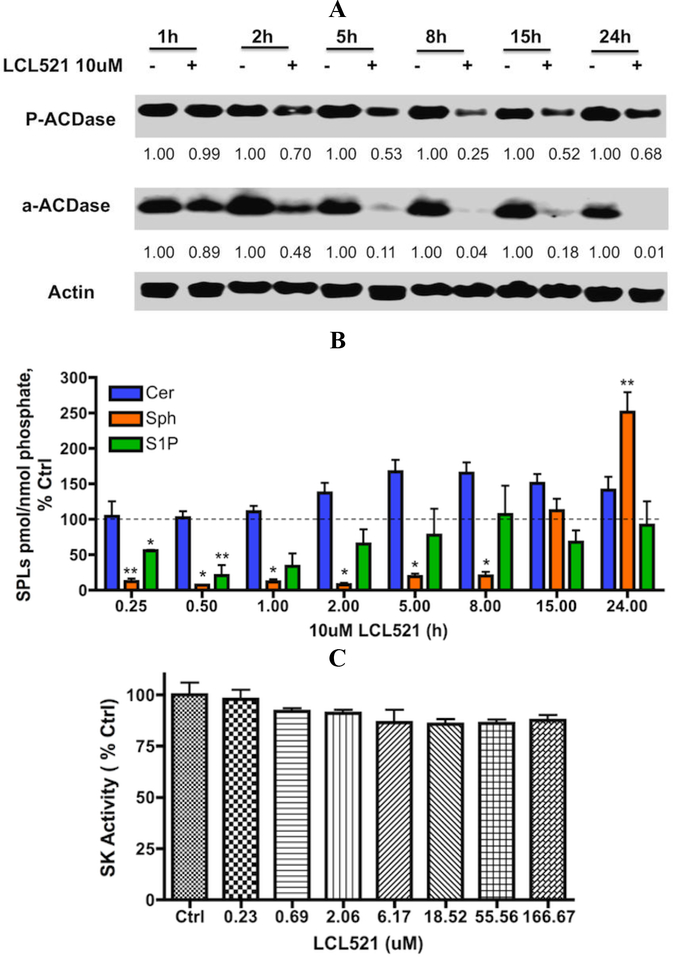
The effects of 10 μM LCL521on ACDase protein expression were investigated over the time course of 1–24h. LCL521 (10 μM) caused a decrease of α–ACDase expression starting from 2 h, persisting upto 24h. While the minimum level of both P-and α-ACDase was observed at 8h, P-ACDase was regenerated prior to α-ACDase and returned towards the control level at 24h (>70% Ctrl, Fig. 3A). In general, control samples showed relatively stable protein levels over the time course. These results suggest that although the higher dose of LCL521 causes degradation of P-ACDase, the effects are also reversible.
Figure 3. Effects of 10μM LCL521 on SL metabolites and ACDase protein expression over the time.
A. Effect of 10μM LCL521 on ACDase protein expression over time. Cells were treated with vehicle or 10μM LCL521 for 1, 2, 5, 8, 15 and 24h. ACDase protein expression (α-and P-ACDase) was then visualized by western blot as described; actin was utilized to monitor protein loading and transfer. B. Effects of 10μM CL521 on SL metabolites over time. Cells were treated with vehicle, or 10μM LCL521 for 0.25, 0.5, 1, 2, 5, 8, 15 and 24h, then cell pellets were collected and lipids were extracted. Levels of Cer, Sph and S1P were then measured by LC-MS/MS. Results are expressed as pmol SLs/nmol Phosphate, and presented as % Control (baseline for Cer: 2.419±0.919 pmol/nmol Lipid Phosphate; Sph: 6.656×10−2±2.820×10−2 pmol/nmol Lipid Phosphate; S1P: 5.330×10−3±1.144×10−3 pmol/nmol Lipid Phosphate) with means ± st dev. of 4x replicate, * p value <0.005, ** p value <0.05 (vs Control); C. Effects of LCL521 on SK activity. Equal amount of SK1 with either vehicle or indicated dose of LCL521, 5μM Sph, and 5μM ATP in reaction buffer for 1h at room temperature by plate shaker. After incubation, ATP detector was added and mixed before luminescence was recorded. Results are presented as % control with means ± st dev. of duplicate.
Time-course studies of 10μM LCL521 revealed early, profound, and persistent drop of cellular Sph, whereas the level of Cer became elevated starting at the 2h time point. The levels of Sph recovered and exceeded the control levels at 24 h, which was accompanied by a regeneration of P-ACDase (Fig. 3A & B). Interestingly, the level of S1P also dropped significantly, and the pattern of change was very close to changes in Sph but did not exceed the control level over the time course (Fig. 3B). Since LCL521 has no inhibitory effects on Sph kinases (SKs, Fig. 3C), these results imply that the later elevation in Sph was not utilized by SKs to generate S1P, suggesting that this pool of Sph might be trapped in the lysosomes.
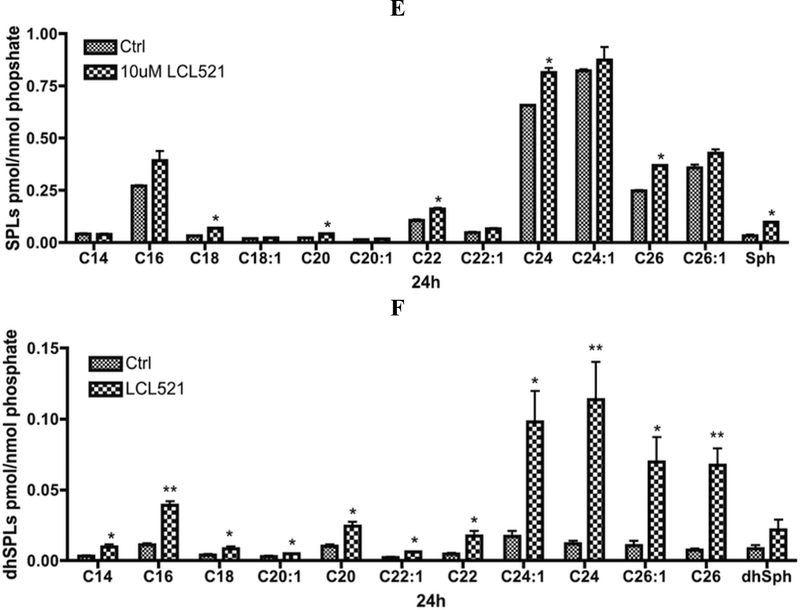
As for the specific species of Cn-Cer, early time (1h) results demonstrated that 10 μM LCL521 significantly increased C14 and C16-Cer (Fig. 4A). Interestingly, at the same time, LCL521 slightly increased dhC16-Cer as well (Fig. 4B). It should be noted that in MCF7 cells, the levels of Cn-Cers are about 40 times higher than those of Cn-dhCers. These results suggest significant inhibition of ACDase under these conditions. With prolonged LCL521 treatment, at 8h, when both P-and α -ACDase were down to the minimal levels, almost all Cer and dhCer species were significantly elevated with no preference to the fatty acyl chain length (Fig. 4C & D). After 24h treatment, while a-ACDase was still decreased but P-ACDase had partially recovered, most Cer species were still above the control levels but very slightly (Fig. 4E). In contrast and remarkably, dhCer species were markedly elevated, with more profound elevations in the very long chain species (≥dhC24:1, >5 fold, Fig. 4F). These results show that 10μM LCL521 causes sustained inhibition of ACDase with significant elevations across most Cer species, consistent with the ability of ACDase to hydrolyze various ceramides.31 These results also suggest that higher doses of LCL521 may have preferential effects on metabolism of dhCer (see below).
Figure 4. Effects of 10μM LCL521 on SL and dhSL metabolites at 1, 8 and 24h.
A. Effects of 10μM LCL521 on Cn-Cer and Sph at 1h. Cells were treated with vehicle or 10μM LCL521 for 1h, then cell pellets were collected, lipids were extracted, and levels of Cn-Cer and Sph were measured by LC-MS/MS. Results are presented as pmol SL/nmol Lipid Phosphate with means ± st dev. of 6x replicates, * p value <0.05, ** p value <0.01 (LCL vs Control); B. Effect of 10μM LCL521 on Cn-dhCer and dhSph at 1h. Cells were treated with vehicle or 10uM LCL521 for 1h then cell pellets were collected, lipids were extracted, and levels of Cn-dhCer and dhSph were measured by LC-MS/MS. Results are presented as pmol dhSL/nmol Lipid Phosphate with means ± st dev. of 6x replicates, * p value <0.05 (LCL vs Control); C. Effects of 10μM LCL521 on Cn-Cer and Sph at 8h. Cells were treated with vehicle or 10μM LCL521 for 8h, then cell pellets were collected and lipids were then extracted. Level of Cn-Cer and Sph were measured by LCMS/MS. Results are presented as pmol SL/nmol Lipid Phosphate, and are presented as means ± st dev. of 4x replicates, * p value <0.05, ** p value <0.005 (LCL vs Control); D. Effects of 10μM LCL521 on Cn-dhCer and dhSph at 8h. Cells were treated with vehicle or 10μM LCL521 for 8h, then cell pellets were collected and lipids were extracted. Level of Cn-dhCer and dhSph were measured by LC-MS/MS. Results are presented as pmol dhSL/nmol Lipid Phosphate, and are presented as means ± st dev. of 4x replicates, * p value <0.05, ** p value <0.005 (LCL vs Control); E. Effects of 10μM LCL521 on Cn-Cer and Sph at 24h. Cells were treated with vehicle or 10μM LCL521 for 24h, then cell pellets were collected and lipids were then extracted. Level of Cn-Cer and Sph were measured by LC-MS/MS. Results are presented as pmol SL/nmol Lipid Phosphate, and are presented as means ± st dev. of 4x replicates, * p value <0.05 (LCL vs Control); F. Effects of 10μM LCL521 on Cn-dhCer and dhSph at 24h. Cells were treated with vehicle or 10μM LCL521 for 24h, then cell pellets were collected and lipids were then extracted. Level of Cn-dhCer and dhSph were measured by LC-MS/MS. Results are presented as pmol dhSL/nmol Lipid Phosphate, and are presented as means ± st dev. of 4 x replicates, * p value <0.05, ** p value <0.01 (vs Control).
2.4. Higher dose of LCL521 additionally affects DES-1 activity
To determine how LCL521 affects this late dhCer accumulation, we pretreated MCF7 cells with Myriocin, an inhibitor of L-serine palmitoyltransferase, the enzyme that catalyzes the first step of de novo synthesis of SL.32 The results clearly indicated that pretreatment with Myriocin completely inhibited LCL521-induced late accumulation of dhCer species (Fig. 5A). To further investigate which enzyme from the de novo pathway could provide dhCer late accumulation, we evaluated the effects of the higher dose of LCL521 on dihydroceramide desaturase (DES-1), the enzyme that is responsible for converting dihydroceramide to ceramide.33 The results showed that LCL521 at 5 and 10 μM inhibited DES-1 activity as assayed in MCF7 cells (Fig. 5B & S.Fig2). Thus, LCL521 exerts additional effects at the higher concentrations that impact the SL network.
Figure 5. Higher dose of LCL521 induces dhCer late accumulation through inhibiting SL’s de novo pathway.
A. MCF-7 cells were pretreated with 100nM Myriocin (M) for 1h before in combination with either vehicle or 10μM LCL521 for additional 24h, then cell pellets were collected and lipids were then extracted. Level of Cn-dhCer was measured by LC-MS/MS. Results are presented as pmol Cn-dhCer/nmol Lipid Phosphate, and are presented as means ± st dev. of triplicates, * p value <0.01 (vs Control); ** p value <0.01 (LCL vs M+L); B. Higher dose of LCL521 inhibits DES-1 activity in intact MCF7 cells. MCF7 cells were treated with either vehicle as control or indicated dose of LCL521 for 24h before collected for activity assay, results are presented as means ± st dev. of triplicates, * p value <0.005 (vs 0μM).
2. 5. Effects of LCL521 on protein synthesis and stability of P-and α-ACDase.
To explore how LCL521 (10 μM) regulates the α-and P-ACDase, we employed cyclohexamide (CHX), an inhibitor of protein biosynthesis.34 Treatment with CHX showed no significant difference in either P-or α-ACDase, with the precursor persisting up to 24h, demonstrating that this enzyme is long lived (Fig. 6A). The half-life of the α-ACDase is estimated between 8–24h.
Figure 6. LCL521 has predominant action on decrease of P-ACDase expression and Sph formation.
A. Cells were pretreated with either vehicle or 40uM CHX 1h before vehicle, 10μM LCL521, or in combination with 10 μM LCL521 for 1, 8 and 24h. ACDase protein expression (α-and P-ACDase) was then visualized by western blot as described; actin was utilized to monitor protein loading and transfer. B. Matched SL-profile, which are presented as means ± st dev. of triplicates, * p value <0.005; ** p value <0.05 (vs Control).
In contrast, the combination of CHX and LCL521 had effects on P-ACDase that resembled those of LCL521 in that CHX (which on its own increased the levels of this subunit at 24h) was unable to induce levels in the presence of LCL521. Importantly, as viewed from the LCL521 action, LCL521 was still able to induce loss of both α-subunit and precursor. These results suggest that LCL521 primarily causes loss of P-ACDase, which is consistent with the predominant effects of LCL521 on decrease of Sph in the same period of time (Fig. 6B)
3. SUMMARY AND CONCLUSION
Recent studies suggest that ACDase could be an important target in cancer therapy, and its small molecule inhibitor, LCL521, exhibited significant additive effects on tumor proliferation and death.28–30, 35 However, the action of LCL521 on ACDase and key SL metabolism were not fully described. In this context, we focus here on the effects of LCL521’s dose response and time course on SL metabolism and ACDase protein expression. The results show that low dose of LCL521 clearly inhibits ACDase activity, but the inhibition was transient. Our results also demonstrate that single treatment of higher dose of LCL521 elevated total levels of both Cer and dhCer, decreased endogenous Sph and dhSph, and decreased S1P as well, but also temporarily disturbed ACDase protein expression, similar results were also observed in two other randomly picked cell lines, mouth squamous cell carcinoma, SCC14A, and prostate cancer cell line, PPC-1 (S.Fig5). We then evaluated inhibitors of proteolysis, and found that inhibiting Cathepsin B could prevent losing of a-ACDase protein expression (S.Fig7). Moreover, at 24h, we also observed marked increases in the amount of dhCer, which was then shown to be an effect of inhibiting of DES-1.
Taken together, our results suggest that higher dose of LCL521 induces MCF7 cells growth inhibition through dual actions of inhibiting both ACDase and DES-1, whereas only low dose is essential to achieve specific but not permanent inhibition of ACDase, thereby specifying the future exploration for the treatment of cancer.
4. EXPERIMENTAL SECTION
4.1. Chemicals
LCL521 was synthesized and characterized as previously described.28 All solvents and general reagents were purchased from Sigma-Aldrich, Fisher and VWR. Analytical standards were either purchased from Avanti Polar Lipids or were synthesized in the Synthetic Unit, Lipidomics Shared Resource, Medical University of South Carolina (MUSC).
4.2. Cell culture and reagents
MCF7 human breast adenocarcinoma cell line was obtained from ATCC (Manassas, VA), cultured in complete medium (RPMI 1640 medium supplemented with 10% FBS and 100μg/ml Normocin), and maintained under standard incubator condition (humidified atmosphere with 5% CO2 at 37°C). Cells in the exponential growth phase were harvested from the culture and used in all experiments. All experiments, if not specifically mentioned, will be with 1×106 cells seeded in 100 mm dishes overnight before each treatment.
4. 3. Acid ceramidase protein expression by Western Blot
ACDase protein expression was visualized by western blot and followed by the same protocol as previously described.23 The anti-ACDase antibody reagents were purchased from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA (sc-28486 and sc-136275) and from BD-Biosciences, San Jose, CA, USA (# 612302).
4.4. Sphingosine kinase activity assay
The activity of sphingosine kinases were quantified by using a commercial sphingosine Kinase activity assay kit (Echelon Biosciences Inc., K-3500, Salt Lake City, UT) as the manufacturer instructed. Briefly, equal amount of SK1 with either vehicle or indicated dose of LCL521, 5uM Sph, and 5uM ATP in reaction buffer for 1h at room temperature, mixed by plate shaker. After incubation, ATP detector was added and mixed before luminescence was recorded. Results are presented as % control with means± st dev. of duplicates.
4.5. Dihydroceramide desaturase activity assay
MCF7 cells were seeded in 150mm dish (3 × 106) overnight before treatment with indicated dose of LCL521 for 24h. Cells were then collected, pelleted, and activity of dihydroceramide desaturase was measured as previously described.33
4.6. Lipid extract preparation and UHPLC-MS/MS analysis of cellular SL.
Lipid extracts were prepared and advanced analyses of endogenous bioactive SL were performed as previously described.36
4.7. Statistical analysis
Where indicated, data were represented as mean ± SD. Statistical analysis was performed using Graphpad ANOVA, with p-value <0.05 considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENT
We thank Dr. Joe Cheng at the Department of Microbiology & Immunology, Medical University of South Carolina, for running the western blot of S.Fig.7.
Financial support was provided in part by the National Cancer Institute [PO1-CA097132 (YAH, AB)], National Cancer Institute [R41CA139637 (AB, SphingoGene Inc. subcontract to MUSC)], and National Institutes of Health-National Center for Research Resources [UL1TR000062 Voucher Pilot Program (APB)]. Research was supported in part by the Lipidomics Shared Resource, Hollings Cancer Center, Medical University of South Carolina [P30 CA138313] and the Lipidomics and Pathobiology COBRE, Department Biochemistry, MUSC [P20 RR017677]. We especially acknowledge the National Center for Research Resources and the Office of the Director of the National Institutes of Health for the funding [C06 RR018823], which provided laboratory space for Lipidomics Facility in MUSC’s Children’s Research Institute.
Abbreviation:
- B13
(1R, 2R)-2-N-(Tetradecanoylamino-1-(4’-nitrophenyl)-1, 3-propandiol
- LCL521
1,3-di-DMG-B13.2HCl
- (1R, 2R)-2-N-(Tetradecanoylamino)-1-(4’-nitrophenyl)-propyl-1,3-O,O-(N,N-dimethylamino) acetate dihydrochloride)
- DMG
dimethyl-glycine
- Sph
sphingosine
- dhSph
dihydrosphingosine
- Cer
ceramide
- Cn-Cer
ceramide species
- Cn-dhCer
dihydroceramide species
- S1P
sphingosine 1-phosphate
- ACDase
acid ceramidase
- SL
sphingolipids
- dhSL
dihydrosphingolipids
- α-ACDase
acid ceramidase α subunit
- β-ACDase
acid ceramidase β subunit
- P-ACDase
acid ceramidase precursor
- CHX
cyclohexamide
- DES-1
dihydroceramide desaturase
- SKs
sphingosine kinases
- CDs
ceramidases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Hannun YA; Obeid LM Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol 2018, 19(3), 175–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao C; Obeid LM Ceramidases: regulators of cellular responses mediated by ceramide, sphingosine, and sphingosine-1-phsophate. Biochim. Biophys. Acta 2008, 1781 (9), 424–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogretmen B Sphingolipid metabolism in cancer signaling and therapy. Nat. Rev. Cancer 2018, 18(1), 33–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coant N; Sakamoto W; Mao C; Hannun YA Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv, Biol, Regul. 2017, 63, 122–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis CS; Voelkel-Johnson C; Smith CD Targeting sphingosine kinases for the treatment of cancer. Adv. Cancer Res 2018, 140, 295–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casasampere M; Ordonez YF; Pou A; Casas J Inhibitors of dihydroceramidase desaturase 1: therapeutic agents and pharmacological tools to decipher the role of dihydroceramides in cell biology. Chem. Phys. Lipids 2016, 197, 33–44. [DOI] [PubMed] [Google Scholar]
- 7.Ferlinz K; Kopal G; Bernardo K; Linke T;Bar J; Breiden B; Neumann U; Lang F; Schuchman EH; Sandhoff K Human acid ceramidase: processing, glycosylation, and lysosomal targeting. J Biol Chem. 2001, 276(38), 35352–60. [DOI] [PubMed] [Google Scholar]
- 8.Schulze H; Schepers U; Sandhoff K Overexpression and mass spectrometry analysis of mature human acid ceramidase. Biol. Chem 2007, 388 (12), 1333–43. [DOI] [PubMed] [Google Scholar]
- 9.Shtraizent N; Eliyahu E; Park JH; He X; Shalgi R; Schuchman EH Autoproteolytic cleavage and activation of human acid ceramidase. J. Biol. Chem 2008, 283(17), 11253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lucki N; Li D; Bandyopadhyay S; Wang E; Merrill A; Sewer MB Acid ceramidase (ASAH1) represses steroidogenic factor 1-dependent gene transcription in H295R human adrenocortical cells by binding to the receptor. Mol. Cell Biol, 2012, 32 (21), 4419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Realini N; Palese F; Pizzirani D; Pontis S; Basit A; Ganesan A; Piomelli D Acid ceramidase in Melanoma: expression, localization, and effects of pharmacological inhibition. J Biol. Chem, 2016, 291(5), 2422–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang WS; Moon HG; Kim HS; Choi EJ; YU MH; Noh DY; Lee C Proteomic approach reveals FKBP4 and S100A9 as potential predication markers of therapeutic response to neo-adjuvant chemotherapy in patients with breast cancer. J Proteome Res. 2012, 11(2), 1078–88. [DOI] [PubMed] [Google Scholar]
- 13.Mizutani N; Inoue M; Omori Y; Ito H; Tamiya-Koizumi K; Takagi A; Kojima T; Nakamura M; Iwaki S; Nakatochi M; Suzuki M; Nozawa Y; Murate T Increased acid ceramidase expression depends on up-regulation of androgen-dependent deubiquitinases, USP2, in a human prostate cancer cell line, LNCaP. J Biochem., 2015, 158(4), 309–19. [DOI] [PubMed] [Google Scholar]
- 14.Zeidan YH; Jenkins RW; Korman JB; Liu X; Obeid LM; Norris JS; Hannun YA Molecular targeting of acid ceramidase: implication to cancer therapy. Curr. Drug Targets, 2008, 9(8), 653–61. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan SF; Pearson JM; Feith DJ; Loughran TP Jr. The emergence of acid ceramidase as a therapeutic target for acute myeloid leukemia. Expert Opin Ther Targets. 2017, 21(6), 583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camacho L; Meca-Cortes O; Abad JL; Garcia S; Rubio N; Diaz A; Celia-Terrssa T; Cingolani F; Bermudo R; Fermandez PL; Blanco J; Delgado A; Casas J; Fabrias G; Thomson TM Acid ceramidase as a therapeutic target in metastatic prostate cancer. J Lipid Res. 2013, 54(5), 1207–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doan NB; Nguyen HS; Montoure A;Al-Glzawly MM; Mueller WM; Kurpad S; Rand SD; Connelly JM; Chitamber CR; Schmainda KM; Mirza SP Acid ceramidase is a novel drug target for pediatric brain tumors. Oncotarget. 2017, 8(15), 24753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugita M; Dulaney JT; Moser HW Ceramidase deficiency in Farber’s disease (lipogranulomatosis). Science, 1972, 178, 1100–2. [DOI] [PubMed] [Google Scholar]
- 19.Saad AF; Meacham WD; Bai A; Anellii V; Elojeimy S; Mahdy AE; Turner LS; Cheng J; Bielawska A; Bielawski J; Keane TE; Obeid LM; Hannun YA; Norris JS; Liu X The functional effects of acid ceramidase overexpression in prostate cancer progression and resistance to chemotherapy. Cancer Biol. Ther 2007, 6(9), 1455–60. [DOI] [PubMed] [Google Scholar]
- 20.Morales A; Paris R; Villanueva A; Llacuna L; Garcia-Ruiz C; Fernandez-Checa JC Pharmacological inhibition or small interfering RNA targeting acid ceramidase sensitizes hepatoma cells to chemotherapy and reduces tumor growth in vivo. Oncogene 2007, 26(6), 905–16. [DOI] [PubMed] [Google Scholar]
- 21.Mahdy AE; Cheng JC; Elojeimy S; Meacham WD; Turner LS; Bai A; Gault CR; MaPherson AS; Garcia N; Beckham TH; Saad A; Bielawska A; Bielawski J; Hannun YA; Keane TE; Taha MI; Hammouda HM; Norris JS; Liu X Acid ceramidase upregulation in prostate cancer cells confers resistance to radiation: AC inhibition, a potential radiosensitizer. Mol. Ther 2009, 17(3), 430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bielawska A; Linardic CM; Hannun YA Ceramide-mediated biology. Determination of structural and stereospecific requirements through the use of N-acylphenylaminoalcohol analogs. J. Biol. Chem 1992, 267 (26), 18493–7. [PubMed] [Google Scholar]
- 23.Bai A; Szulc ZM; Bielawski J; Mayroo N; Liu X; Norris J; Hannun YA; Bielawska A Synthesis and bioevaluation of omega-N-amino analogs of B13. Bioorg. Med. Chem 2009, 17(5), 1840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Selzner M; Bielawska A; Morse MA; Rudiger HA; Sindram D; Hannun YA; Clavien PA Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001, 61(3), 123–40. [PubMed] [Google Scholar]
- 25.Raisova M; Goltz G; Bektas M; Bielawska A; Riebeling C; Hossini AM; Eberle J; Hannun YA; Orfanos CE; Geilen CC Bcl-2 overexpression prevents apoptosis induced by ceramidase inhibitors in malignant melanoma and HaCaT keratinocytes. FEBS Lett. 2002, 516(1–3), 47–52. [DOI] [PubMed] [Google Scholar]
- 26.Holman DH; Turner LS; El-Zawahry A; Elojeimy S; Liu X; Bielawski J; Szulc ZM; Norris K; Zeidan Y; Hannun YA; Bielawska A; Norris JS Lysosomotropic acid ceramidase inhibitor induces apoptosis in prostate cancer cells. Cancer Chemother. Pharmacol 2008, 61(2), 231–42. [DOI] [PubMed] [Google Scholar]
- 27.Szulc ZM; Mayroo N; Bai A; Bielawski J; Norris JS; Hannun YA; Bielawska A Novel analogs of D-e-MAPP and B13. Part 1. Synthesis and evaluation as potential anticancer agents. Bioorg. Med. Chem 2008, 16(2), 1015–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai A; Szulc ZM; Bielawski J; Pierce JS; Rembisa B; Terzieva S; Mao C; Xu R; Wu B; Clark CJ; Newcomb B; Liu X; Norris J; Hannun YA; Bielawska A Targeting (cellular) lysosomal acid ceramidase by B13: design, synthesis and evaluation of novel DMG-B13 ester prodrugs. Bioorg. Med. Chem 2014. 22(24), 6933–44. 6083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng JC; Bai A; Beckham TH; Marrison T S; Yount, C.T.; Lyons, K.; Bartlett, A.M.; Wu, B.X.; Keane, B.J.; Armeson, K.E.; Marshall, D.T.; Keane, T.E.; Smith, M.T.; Jones, E.E.; Drake, R.R.; Bielawska, A.; Norris, J.S.; Liu, X. Radiation-induced acid ceramidase confers prostate cancer resistance and tumor relapse. J. Clin. Invest, 2013, 123(10), 4344–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bai A; Mao C; Jenkins RW; Szulc ZM; Bielawska A; Hannun YA Anticancer actions of lysosomally targeted inhibitor, LCL521, of acid ceramidase. PLoS One, 2017, 12(6), e0177805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camacho L; Meca-Cortes O; Abad JL; Garcia S; Rubio N; Diaz A; Cella-Terrassa T; Cingolani F; Bermudo R; Fernandez PL; Blanco J; Delgado A; Casas J; Fabrias G; Thomson TM Acid ceramidase as a therapeutic target in metastatic prostate cancer. J. Lipid Res 2013, 54(5), 1207–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyake Y; Kozutsumi Y; Nakamura S; Fujita T; Kawasaki T Serine palmitoyltransferase is the primary target of a sphingosine-like immunosuppressant, ISP-1/myriocin. Biochem. Biophys. Res. Commun 1995, 211(2), 396–403. [DOI] [PubMed] [Google Scholar]
- 33.Rahmaiyan M; Curley RW Jr; Obeid L.M.; Hannun Y.A.; Kraveka J.M. Identification of dihydroceramide desaturase as a direct in vitro target for fenretinide. J Biol Chem 2011, 286(28), 24754–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Colombo B; Felicetti L; Baglioni C Inhibition of protein synthesis by cycloheximide in rabbit reticulocytes. Biochem Biophys Res Commun. 1965, 18, 389–95. [DOI] [PubMed] [Google Scholar]
- 35.Liu F; Liu X; Lu C; Bai A; BIelawski J; Bielawska A; Marshall B; Schoenlein PV; Lebedyeva IO; Liu K Ceramide activates lysosomal cathepsin B and cathepsin D to attenuate autophagy and induces ER stress to suppress myeloid-derived suppressor cells. Oncotarget, 2016, 7(51), 83907–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bielawski J; Szulc ZM; Hannun YA; Bielawska A Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods, 2006, 39(2), 82–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.