Abstract
Phosphoglucomutase 1 (PGM1) deficiency is a recently defined disease characterized by glycogenosis and a congenital glycosylation disorder caused by recessive mutations in the PGM1 gene. We report a case of a 12-year-old boy with first-cousin parents who was diagnosed with a PGM1 deficiency due to significantly decreased PGM1 activity in his muscle. However, Sanger sequencing revealed no pathogenic mutation in the PGM1 gene in this patient. As this case presented with a cleft palate in addition to hypoglycemia and elevated transaminases and creatine kinase, karyotyping was performed and identified homozygous inv(1)(p31.1p32.3). Based on the chromosomal location of the PGM1 gene at 1p31, we analyzed the breakpoint of the inversion. Fluorescence in situ hybridization (FISH) combined with long PCR analysis revealed that the inversion disrupts the PGM1 gene within intron 1. Since the initiation codon in the PGM1 gene is located within exon 1, we speculated that this inversion inactivates the PGM1 gene and was therefore responsible for the patient’s phenotype. When standard molecular testing fails to reveal a mutation despite a positive clinical and biochemical diagnosis, the presence of a gross structural variant that requires karyotypic examination must be considered.
Keywords: Chromosomal inversion, Congenital disorders of glycosylation, Hypoglycemia, PGM1, Phosphoglucomutase 1 deficiency
Introduction
Phosphoglucomutase 1 (PGM1) deficiency is a recently defined disease, characterized by glycogenosis and a congenital disorder of glycosylation (CDG) (Tagtmeyer et al. 2014). Α PGM1 deficiency is rare with only 38 patients from 29 families with different ethnic backgrounds described in the literature so far (Perez et al. 2013; Ondruskova et al. 2014; Tagtmeyer et al. 2014; Loewenthal et al. 2015; Zeevaert et al. 2016; Wong et al. 2016; Preisler et al. 2017; Nolting et al. 2017; Voermans et al. 2017). PGM1 is an essential enzyme in carbohydrate biosynthesis and metabolism and functions both in glycogen synthesis and breakdown through a reversible conversion of glucose 1-phosphate to glucose 6-phosphate (Morava 2014). Since glucose 1-phosphate is a precursor of the nucleotide sugars used for glycan biosynthesis, PGM1 activity is also required for protein N-glycosylation (Beamer 2015). Hence, PGM1 deficiency has considerably diverse phenotypes. Most of the affected patients develop a congenital anomaly syndrome showing a bifid uvula, cleft palate, and Pierre Robin sequence as clinical manifestations from the time of birth. Hepatopathy, dilated cardiomyopathy (DCM), hypoglycemia, muscle weakness, exercise intolerance, growth retardation, and endocrine abnormalities emerge in these cases over time (Scott et al. 2014). Many of these manifestations can be linked to the role of PGM1 in glucose metabolism and glycosylation (Beamer 2015).
PGM1 deficiency is caused by homozygous or compound heterozygous nucleotide alterations in the PGM1 gene (Herbich et al. 1985). Several types of mutations have been reported to date including missense mutations, frameshifts, and splicing mutations (Tagtmeyer et al. 2014; Lee et al. 2014; Perez et al. 2013; Timal et al. 2012; Stojkovic et al. 2009; Ondruskova et al. 2014). In our current report, we describe a case of PGM1 deficiency caused by a homozygous chromosomal inversion that disrupts the PGM1 gene at chromosome 1p31.
Materials and Methods
Cytogenetic Analysis
Fluorescence in situ hybridization (FISH) analysis of the patient and his parents was performed using standard methods to detect the breakpoint region at the chromosome level. Briefly, phytohemagglutinin-stimulated lymphocytes or Epstein-Barr virus-transformed lymphoblastoid cell lines derived from the subjects were arrested by exposure to colcemid. Metaphase preparations were then obtained by hypotonic treatment with 0.075 M KCl followed by methanol/acetate fixation. A bacterial artificial clone (BAC) containing 1p31.1, RP4-534K7 (chr1:63,525,021-63,677,603), was used as the test probe, and a chromosome 1 centromere probe (CEN1 SpectrumOrange Probe; Abbott Laboratories, Abbott Park, IL) was used as a reference. The probes were labeled by nick translation with digoxigenin-11-dUTP. After hybridization, the probes were detected with DyLight 488 Anti-Digoxigenin/Digoxin. Chromosomes were visualized by counterstaining with 4,6-diamino-2-phenylindole.
Sequence Analysis
To isolate the breakpoint, long-range PCR with several sets of primers for the PGM1 gene was performed using LA Taq (TaKaRa, Shiga, Japan) (Fig. 3c). The PCR conditions were 35 cycles of 10 s at 98°C and 15 min at 60°C. PCR primers were designed using sequence data from the human genome database. PCR products were separated on 0.8% (w/v) agarose gels and visualized with ethidium bromide. The homology between the obtained sequence around the breakpoint within the PGM1 gene and the 1p32.3 sequence obtained from the database was examined using the BLAT in UCSC genome browser (http://genome-asia.ucsc.edu/human GRCh38/hg38).
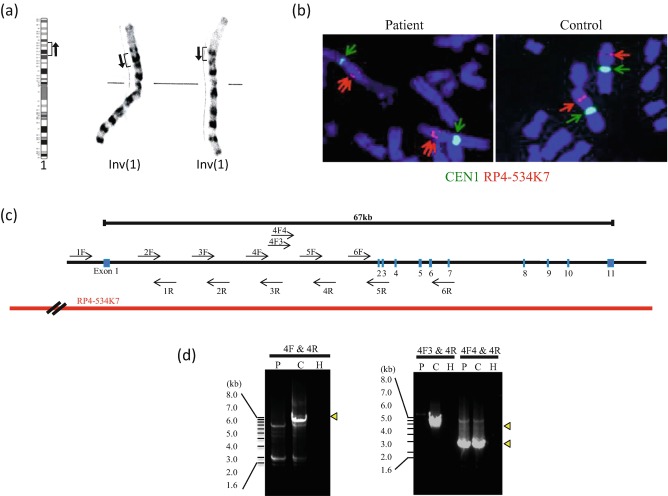
Fig. 3.
Disruption of the PGM1 gene in the study patient by a chromosomal inversion. (a) G-banding of the patient’s karyotype which was determined to be 46,XY,inv(1)(p31.1p32.3)x2, in which inv(1) was homozygous. (b) FISH signals for PGM1 (red arrow) are typically observed on the short arm of chromosome 1 in a normal karyotype. In contrast, the two distinctive signals were detected on the chromosome 1 arm in the study patient. (c) Schematic representation of the PGM1 gene structure. The blue boxes denote exons. The positions of the PCR primers are indicated by arrows. The position of the BAC probe is also indicated. (d) Agarose gel electrophoresis of long PCR products. 4F-4R and 4F3-4R primer pairs failed to amplify the PCR products in the study patient. P patient, C control, H H2O
Patient
The current study patient was a 12-year-old boy from consanguineous parents who are first cousins without a family history of congenital metabolic disease (Fig. 1). The patient’s height was 137 cm (z-score −2.3), and he had a normal body weight of 39 kg (z-score −0.6). He was born at term with a normal body weight and length. A cleft palate was noted at birth and closure surgery was performed at 12 months. Persistently elevated transaminases (AST 50–400 U/L [normal value <33 U/L] and ALT 40–300 U/L [normal value <30 U/L]) had been observed since that surgery. In addition, mild hypoglycemia after overnight fasting and an occasionally elevated serum creatine kinase (100–2,600 U/L [normal value <287 U/L]) were evident from 2 years of age. The echocardiogram and electrocardiogram readings showed no abnormalities, and his psychomotor development was normal. Oral administration of uncooked corn starch prior to bedtime was commenced to prevent morning hypoglycemia.
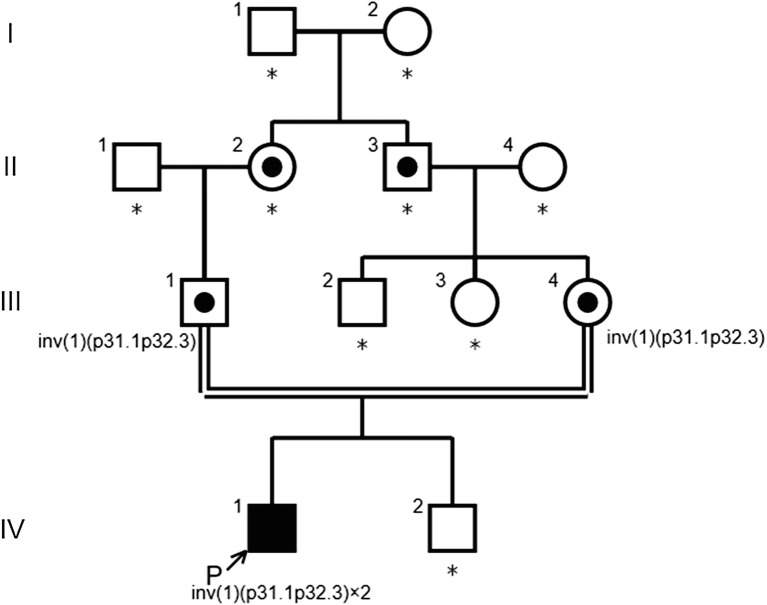
Fig. 1.
Pedigree of the family. Arrow indicates proband. Carriers are represented by a dot in the middle of circles or squares. Asterisks indicate the family members who have not been tested
At 2 years of age, the patient was referred to our department for further examination. Intravenous glucose loading at 2 g/kg led to an elevated lactate level (from 7 to 37 mg/dL at 120 min) with a normal lactate/pyruvate ratio. Intramuscular glucagon loading at 0.03 mg/kg caused no increase of blood sugar either during fasting or at 2 h after a meal, indicating a deficiency in the generation of hepatic glucose from glycogen. However, the activity of the debrancher enzyme responsible for glycogen storage disease (GSD) type III, phosphorylase involved in GSD type VI, and phosphorylase kinase enzyme associated with GSD type IX in the peripheral blood was normal. A forearm nonischemic exercise test was performed when the patient was 8 years old. No increase in venous lactate with a large elevation in his ammonia levels (297 μg/dL) was observed, suggesting inadequate glycogen utilization in the muscle. A muscle biopsy was therefore performed, and a significant decrease in PGM activity was identified (62.1 nmol/min/mg [controls 351.1 ± 81.1]). Isoelectric focusing (IEF) of serum transferrin was performed as previously described (Okanishi et al. 2008) and revealed a mixed type I and type II pattern, typical features of CDG-I and CDG-II (Fig. 2) (Tagtmeyer et al. 2014).
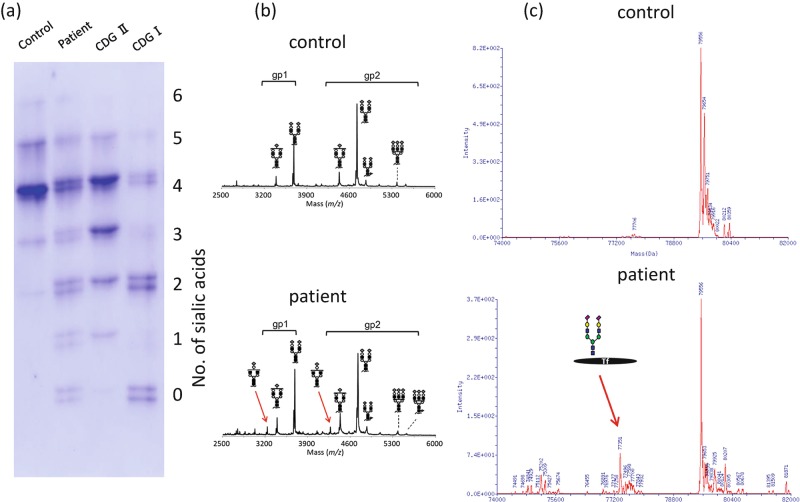
Fig. 2.
Serum transferrin isoelectric focusing (IEF) and mass spectrometry (MS) of serum glycoproteins. (a) IEF patterns of serum transferrin. The number of negatively charged sialic acids of transferrin is indicated on the right. Reduced glycosylation of transferrin including an unusual mixture of CDG-I and CDG-II patterns (increased tri-, di-, mono-, and asialotransferrin) is shown. (b) Matrix-assisted laser desorption/ionization (MALDI) mass spectrum of (glycol) tryptic peptides of transferrin. A biantennary glycan lacking galactose and sialic acid are observed in patient’s transferrin (arrows). (c) Electrospray ionization (ESI) mass spectrum of transferrin. An abnormal transferrin isoform having a single glycan is present in the patient (arrow)
Mass spectrometry to characterize the molecular abnormality of transferrin was performed as previously described (Wada 2016) and further revealed the presence of a variety of transferrin glycoforms, including forms lacking one or both glycans as well as forms with truncated glycan (Fig. 2). These findings were consistent with a PGM1 deficiency (Tagtmeyer et al. 2014), and genetic analysis was performed to confirm this. Sanger sequencing revealed only c.1258T>C, a common polymorphism in the database. The karyotype of the patient was determined to be 46,XY,inv(1)(p31.1p32.3)x2, of which inv(1) was homozygous (Fig. 3a). Since the PGM1 gene is localized at 1p31, we hypothesized that the inversion disrupts this gene in our patient, and we thus analyzed its distal breakpoint.
Results
FISH signals for the BAC RP4-534K7 probe that incorporates the entire PGM1 gene are observed on the short arm of chromosome 1 in an individual with a normal karyotype. In our current study patient however, two distinct signals were detected on the short arm of both chromosome 1 homologues (Fig. 3b). This result indicated that the inversion breakpoint in the patient had disrupted the PGM1 genomic region. Karyotype analysis of both parents showed 46,XY,inv(1)(p31.1p32.3). Both parents carried the inv(1) in a heterozygous state, suggesting that the two inv(1) homologues of the patient had been transmitted from each parent, respectively (data not shown).
Long PCR revealed that one of the PCR primer pairs (4F-4R) within intron 1 failed to amplify the products in the patient DNA, indicating that the breakpoint of the inversion was located in intron 1 (Fig. 3d). To analyze the breakpoint region in more detail, we performed additional long PCR. The 4F4-4R but not the 4F3-4R primer pair successfully yielded a PCR product. This indicated that the breakpoint was located between primer 4F3 and 4F4. We did not obtain the sequence of the other breakpoint region at 1p32.3. To ascertain the mechanism leading to the inversion, we obtained the sequence information of the 1p32.3 from the database and analyzed the homology with the 4F3-4F4 sequence. However, we did not find any sequence similarity between the 4F3-4F4 sequence and the genomic sequence at 1p32.3.
Discussion
PGM1 deficiency is a newly identified metabolic disorder which manifests features of both CDG and glycogenosis (Tagtmeyer et al. 2014). Our present case report describes a young male patient with PGM1 deficiency caused by a homozygous inv(1) inherited from his first-cousin parents that disrupts each of the two PGM1 alleles. To date, 38 PGM1 deficiency patients have been reported, and pathogenic mutations in the PGM1 gene were identified and genetically confirmed in most of these cases (Perez et al. 2013; Ondruskova et al. 2014; Tagtmeyer et al. 2014; Loewenthal et al. 2015; Zeevaert et al. 2016; Wong et al. 2016; Preisler et al. 2017; Nolting et al. 2017; Voermans et al. 2017). However, a small subset of patients exists without mutations in the PGM1 gene. In our present case, Sanger sequencing did not identify any pathogenic mutation in the PGM1 gene initially. However, subsequent chromosome karyotyping of our patient detected the presence of multiple congenital malformations and led to the identification of the aforementioned chromosomal inversion as the responsible mutation for his condition. Hence, when standard molecular testing does not reveal any abnormalities in patients who have been clinically and biochemically diagnosed with a known congenital disorder, chromosome testing may be a fruitful approach for identifying the responsible mutation in the candidate gene.
In mutational screening for single-gene disorders involving an autosomal recessive inheritance of a known causative gene, it is often the case that only one of the recessive mutations is identified. If standard PCR and Sanger methods fail to identify two pathogenic mutations within the exons or flanking intronic regions of the responsible gene, a subsequent approach can be MLPA (multiplex ligation-dependent probe amplification) analysis of structural variant copy number variations or repeat PCR/Sanger analysis to identify possible mutations in noncoding regions such as the promoter or enhancer. In addition to these methods, standard chromosomal karyotyping is important for identifying large-scale chromosomal abnormalities that may disrupt the causative gene.
A possible mechanism of inversion formation is interspersed repeat sequences that may induce chromosomal aberrations. Direct repeats can induce deletions or duplications via recombination between them, whereas inverted repeats sometimes cause pericentric or paracentric inversion (Lakich et al. 1993). In our present case, we didn’t find any specific segmental duplication sequences at the breakpoint region within the intron of the PGM1 gene. Likewise, there was no evidence of segmental duplication sequences that were common to the proximal and distal breakpoint regions. Our patient harbored a rare homozygous pericentric inversion of chromosome 1 inherited from first-cousin parents. We assume therefore that the inversion chromosome in this patient is rare in the general population and is not a recurrent type variation.
Since the initiation codon in the PGM1 gene is located within exon 1, the inversion in our patient that disrupts intron 1 produces a truncated protein containing only the amino acids encoded by exon 1 or no protein product at all due to nonsense-mediated mRNA decay. The crystal structure of human PGM1 has not been characterized, but the structure of the analogous PGM from rabbit has been described (Liu et al. 1997). Because of the high amino acid sequence identity (97%) between these two proteins, the rabbit PGM structure provides a highly accurate model for the human enzyme. PGM1 is a monomeric protein of 562 amino acids and 4 structural domains (Beamer 2015). The active site is located in a large, centrally located cleft and can be segregated into four highly conserved regions which are located behind exon 2. In our present case therefore, even if a truncated protein was produced, it would have no active site, and PGM1 deficiency would still arise. Further, we performed RT-PCR using the patient’s peripheral blood. The exon 1 transcript was found to be present, but we did not find any transcripts distal to the exon 2 (data not shown). Some residual enzymatic activity might be possibly due to other members of phosphoglucomutase family, PGM2 and PGM3, that could compensate the PGM1 activity (Maliekal et al. 2007; Wong et al. 2016).
In conclusion, we have identified and analyzed an inverted chromosome from a PGM1 deficiency patient. Our present report also emphasizes the potential benefits of karyotype analysis in congenital cases in which molecular genetic testing fails to identify the responsible mutations.
Acknowledgments
We thank the patient and his family for their participation in this study. We also thank past and present members of our laboratory. This research was partly supported by the intramural research grant (29-4) for Neurological and Psychiatric Disorders of NCNP (H. Sugie).
Synopsis Sentence
Karyotypic examination must be considered when standard molecular testing fails to reveal a mutation despite a positive clinical and biochemical diagnosis.
Conflict of Interest
Katsuyuki Yokoi, Yoko Nakajima, Ohye Tamae, Hidehito Inagaki, Yoshinao Wada, Tokiko Fukuda, Hideo Sugie, Isao Yuasa, Tetsuya Ito, and Hiroki Kurahashi declare that they have no conflict of interest.
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2005(5). Informed consent was obtained from all patients for inclusion in the study.
Author Contributions
Katsuyuki Yokoi retrieved the data and drafted and revised the manuscript.
Yoko Nakajima and Tetsuya Ito discovered the patients and provided many data.
Tamae Ohye did cytogenetic analysis and sequence analysis.
Hidehito Inagaki supported and supervised experiments.
Yoshinao Wada did mass spectrometry.
Tokiko Fukuda and Hideo Sugie estimated enzyme activity.
Isao Yuasa did IEF of serum transferrin.
Hiroki Kurahashi: conception and design, analysis and interpretation, and revising the article critically for important intellectual content.
All authors contributed to and reviewed the manuscript.
References
- Beamer LJ. Mutations in hereditary phosphoglucomutase 1 deficiency map to key regions of enzyme structure and function. J Inherit Metab Dis. 2015;38:243–256. doi: 10.1007/s10545-014-9757-9. [DOI] [PubMed] [Google Scholar]
- Herbich J, Szilvassy J, Schnedl W. Gene localisation of the PGM1 enzyme system and the Duffy blood groups on chromosome no. 1 by means of a new fragile site at 1p31. Hum Genet. 1985;70:178–180. doi: 10.1007/BF00273078. [DOI] [PubMed] [Google Scholar]
- Lakich D, Kazazian HH, Jr, Antonarakis SE, Gitschier J. Inversions disrupting the factor VII gene are a common cause of severe haemophilia A. Nat Genet. 1993;5:236–241. doi: 10.1038/ng1193-236. [DOI] [PubMed] [Google Scholar]
- Lee Y, Stiers KM, Kain BN, Beamer LJ. Compromised catalysis and potential folding defects in in vitro studies of missense mutants associated with hereditary phosphoglucomutase 1 deficiency. J Biol Chem. 2014;289:32010–32019. doi: 10.1074/jbc.M114.597914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ray W, Baranidharan S. Structure of rabbit muscle phosphoglucomutase refined at 2.4A resolution. Acta Crystallogr D. 1997;53:392–405. doi: 10.1107/S0907444997000875. [DOI] [PubMed] [Google Scholar]
- Loewenthal N, Haim A, Parvari R, Hershkovitz E. Phosphoglucomutase-1 deficiency: intrafamilial clinical variability and common secondary adrenal insufficiency. Am J Med Genet A. 2015;167A:3139–3143. doi: 10.1002/ajmg.a.37294. [DOI] [PubMed] [Google Scholar]
- Maliekal P, Sokolova T, Vertommen D, Veiga-da-Cunha M, Van Schaftingen E. Molecular identification of mammalian phosphopentomutase and glucose-1,6-bisphosphate synthase, two members of the alpha-D-phosphohexomutase family. J Biol Chem. 2007;282:31844–31851. doi: 10.1074/jbc.M706818200. [DOI] [PubMed] [Google Scholar]
- Morava E. Galactose supplementation in phosphoglucomutase-1 deficiency; review and outlook for a novel treatable CDG. Mol Genet Metab. 2014;112:275–279. doi: 10.1016/j.ymgme.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolting K, Park JH, Tegtmeyer LC, et al. Limitations of galactose therapy in phosphoglucomutase 1 deficiency. Mol Genet Metab Rep. 2017;13:33–40. doi: 10.1016/j.ymgmr.2017.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okanishi T, Saito Y, Yuasa I, et al. Cutis laxa with frontoparietal cortical malformation: a novel type of congenital disorder of glycosylation. Eur J Paediatr Neurol. 2008;12:262–265. doi: 10.1016/j.ejpn.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Ondruskova N, Honzik T, Vondrackova A, Tesarova M, Zeman J, Hansikova H. Glycogen storage disease-like phenotype with central nervous system involvement in a PGM1-CDG patient. Neuro Endocrinol Lett. 2014;35:137–141. [PubMed] [Google Scholar]
- Perez B, Medrano C, Ecay MJ, et al. A novel congenital disorder of glycosylation type without central nervous system involvement caused by mutations in the phosphoglucomutase 1 gene. J Inherit Metab Dis. 2013;36:535–542. doi: 10.1007/s10545-012-9525-7. [DOI] [PubMed] [Google Scholar]
- Preisler N, Cohen J, Vissing CR, et al. Impaired glycogen breakdown and synthesis in phosphoglucomutase 1 deficiency. Mol Genet Metab. 2017;122:117–121. doi: 10.1016/j.ymgme.2017.08.007. [DOI] [PubMed] [Google Scholar]
- Scott K, Gadomski T, Kozicz T, Morava E. Congenital disorders of glycosylation: new defects and still counting. J Inherit Metab Dis. 2014;37:609–617. doi: 10.1007/s10545-014-9720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojkovic T, Vissing J, Petit F, et al. Muscle glycogenosis due to phosphoglucomutase 1 deficiency. N Engl J Med. 2009;361:425–427. doi: 10.1056/NEJMc0901158. [DOI] [PubMed] [Google Scholar]
- Tagtmeyer LC, Rust S, van Scherpenzeel M, et al. Multiple phenotypes in phosphoglucomutase 1 deficiency. N Engl J Med. 2014;370:533–542. doi: 10.1056/NEJMoa1206605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timal S, Hoischen A, Lehle L, et al. Gene identification in the congenital disorders of glycosylation type I by whole-exome sequencing. Hum Mol Genet. 2012;21:4151–4161. doi: 10.1093/hmg/dds123. [DOI] [PubMed] [Google Scholar]
- Voermans NC, Preisler N, Madsen KL, et al. PGM1 deficiency: substrate use during exercise and effect of treatment with galactose. Neuromuscul Disord. 2017;27:370–376. doi: 10.1016/j.nmd.2017.01.014. [DOI] [PubMed] [Google Scholar]
- Wada Y. Mass spectrometry of transferrin and apolipoprotein C-III for diagnosis and screening of congenital disorder of glycosylation. Glycoconj J. 2016;33:297–307. doi: 10.1007/s10719-015-9636-0. [DOI] [PubMed] [Google Scholar]
- Wong SY, Beamer LJ, Gadomski T, et al. Defining the phenotype and assessing severity in phosphoglucomutase-1 deficiency. J Pediatr. 2016;175:130–136. doi: 10.1016/j.jpeds.2016.04.021. [DOI] [PubMed] [Google Scholar]
- Zeevaert R, Scalais E, Muino Mosquera L, et al. PGM1 deficiency diagnosed during an endocrine work-up of low IGF-1 mediated growth failure. Acta Clin Belg. 2016;71:435–437. doi: 10.1080/17843286.2016.1142043. [DOI] [PubMed] [Google Scholar]