Abstract
Background: Citrin (mitochondrial aspartate–glutamate transporter) deficiency causes the failures in both carbohydrate-energy metabolism and the urea cycle, and the alterations in the serum levels of several amino acids in the stages of newborn (NICCD) and adult (CTLN2). However, the clinical manifestations are resolved between the NICCD and CTLN2, but the reasons are still unclear. This study evaluated the serum amino acid profile in citrin-deficient children during the healthy stage.
Methods: Using HPLC-MS/MS analysis, serum amino acids were evaluated among 20 citrin-deficient children aged 5–13 years exhibiting normal liver function and 35 age-matched healthy controls.
Results: The alterations in serum amino acids characterized in the NICCD and CTLN2 stages were not observed in the citrin-deficient children. Amino acids involved in the urea cycle, including arginine, ornithine, citrulline, and aspartate, were comparable in the citrin-deficient children to the respective control levels, but serum urea was twofold higher, suggestive of a functional urea cycle. The blood sugar level was normal, but glucogenic amino acids and glutamine were significantly decreased in the citrin-deficient children compared to those in the controls. In addition, significant increases of ketogenic amino acids, branched-chain amino acids (BCAAs), a valine intermediate 3-hydroxyisobutyrate, and β-alanine were also found in the citrin-deficient children.
Conclusion: The profile of serum amino acids in the citrin-deficient children during the healthy stage showed different characteristics from the NICCD and CTLN2 stages, suggesting that the failures in both urea cycle function and energy metabolism might be compensated by amino acid metabolism.
Synopsis: In the citrin-deficient children during the healthy stage, the characteristics of serum amino acids, including decrease of glucogenic amino acids, and increase of ketogenic amino acids, BCAAs, valine intermediate, and β-alanine, were found by comparison to the age-matched healthy control children, and it suggested that the characteristic alteration of serum amino acids may be resulted from compensation for energy metabolism and ammonia detoxification.
Electronic supplementary material
The online version of this article (10.1007/8904_2018_99) contains supplementary material, which is available to authorized users.
Keywords: Age-matched control study, Amino acids, Energy metabolism, Gluconeogenesis, Mitochondria transporter, Urea cycle
Introduction
Citrin, a mitochondrial inner membrane liver-type aspartate–glutamate carrier-2, is a main component of the malate–aspartate (MA) NADH shuttle and is involved in urea synthesis (Palmieri et al. 2001). Citrin deficiency caused by a mutation in the SLC25A13 gene encoding citrin induces an increase in the NADH/NAD ratio in the cytoplasm leading to both an energy deficit with metabolic dysfunction in glycolysis and failure of the aspartate supply from the mitochondria to the cytoplasm for argininosuccinate synthesis leading to hypercitrullinemia and hyperammonemia (Kobayashi et al. 1993–2017; Saheki and Song 1993; Saheki and Kobayashi 2002).
Citrin deficiency in newborns presents with diverse clinical manifestations, including considerable liver dysfunction along with cholestasis, citrullinemia, mild hyperammonemia, galactosemia, and hypoglycemia, and is recognized as neonatal intrahepatic cholestasis caused by citrin deficiency (NICCD) (Saheki and Kobayashi 2002; Komatsu et al. 2008). However, the clinical features in the NICCD children resolve at 6 months to 1 year of age. Thereafter, the recurrence of clinical manifestations has a sudden onset in adults between 20 and 50 years of age (Yasuda et al. 2000; Chanprasert and Scaglia 2015), namely, adult-onset type II citrullinemia (CTLN2) (Kobayashi et al. 1993–2017).
During the period between the NICCD and CTLN2 stages, the citrin-deficient children are assumed to be apparently healthy (Kobayashi et al. 1993–2017). The resolution of clinical manifestations during the healthy period is probably due to either maturation of hepatocytes, metabolic adaptation, compensation by another mitochondrial malate–citrate (MC) NADH shuttle (Komatsu et al. 2008; Palmieri et al. 2015; Nagasaka et al. 2017), or lipid/protein-rich carbohydrate-restricted diets that citrin-deficient patients usually prefer or combinations of these processes (Saheki and Song 1993; Saheki et al. 2012). However, it is uncertain how and why these adaptations/compensations establish at about 1 year of age and then fail in the adult citrin-deficient patients.
In the NICCD and CTLN2 stages, an increase in ammonia level and characteristic alterations in blood amino acid levels are often observed, including increases in citrulline, arginine, methionine, phenylalanine, tyrosine, threonine, and threonine/serine ratio (Thr/Ser) (Kobayashi et al. 1993–2017; Tamamori et al. 2004). Many amino acids in the liver are involved in ammonia detoxification in the urea cycle and in energy production as glucogenic and ketogenic amino acids (Figs. 1a, 2, and 3a). Therefore, amino acid metabolism is likely to contribute to the resolution of clinical manifestations during the apparently healthy stage. Blood citrulline, arginine, and ammonia levels have been reported to be normal or slightly elevated in the healthy period between the NICCD and CTLN2 stages (Kobayashi et al. 1993–2017), the characteristics of blood amino acids involved in energy production are unknown in the healthy period.
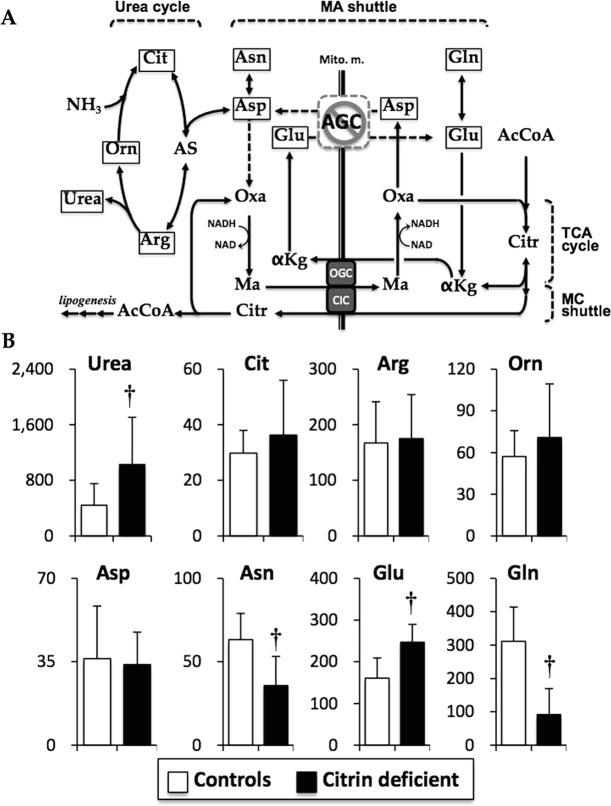
Fig. 1.
Metabolic pathways, including the urea cycle, TCA cycle, MA shuttle, and MC shuttle (a), and serum concentrations of amino acids related with the pathways (b). Asp that reacts with Cit to generate AS in the urea cycle is transported by AGC in exchange for Glu between the mitochondria and cytoplasm for the MA shuttle to maintain the NADH/NAD ratio in the cytoplasm. Asp is endogenously generated from Asn by asparaginase, and Glu is endogenously generated from Gln by glutaminase. In citrin deficiency, maintenance of the NADH/NAD ratio between the mitochondria and cytoplasm is compensated for by the MC shuttle, transporting MA by exchanging for Citr through CIC, and Citr is metabolized to Oxa in the cytoplasm. Data are presented as mean ± SD (μM). †P < 0.001 indicates a significant difference from the controls by the unpaired Student’s t-test. αKg α-ketoglutarate, AcCoA acetyl-CoA, AGC aspartate–glutamate carrier (citrin), Arg arginine, AS arginosuccinate, Asn asparagine, Asp asprartate, CIC citrate carrier, Cit citrulline, Citr citrate, Gln glutamine, Glu glutamate, MA shuttle malate–aspartate shuttle, Ma malate, MC shuttle malate–citrate shuttle, OGC oxoglutarate carrier, Orn ornithine, Oxa oxaloacetate
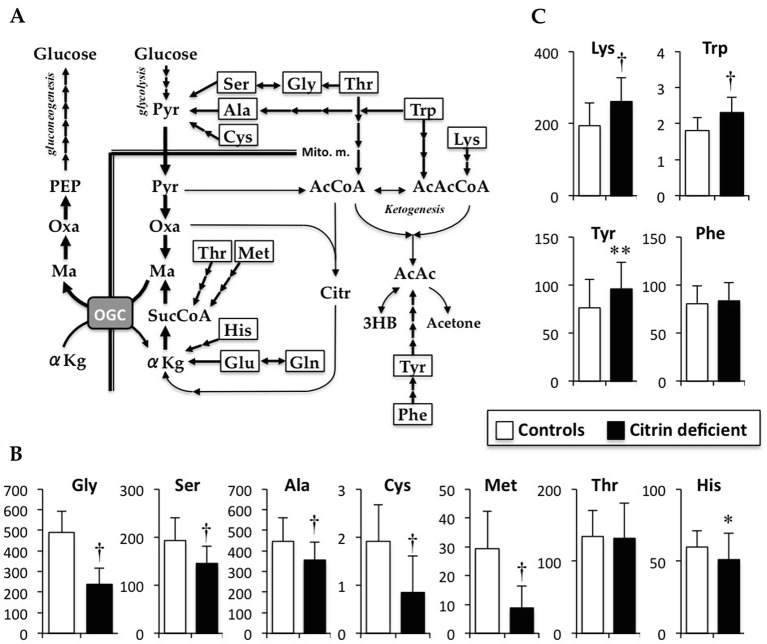
Fig. 2.
Metabolic pathways of gluconeogenesis and ketogenesis from amino acids (a), and serum concentrations of glucogenic amino acids (b) and ketogenic amino acids (c). In hepatocytes, some amino acids are metabolized for gluconeogenesis and for ketogenesis. In the cytoplasm, Ser, Gly, Thr, Ala, and Cys are directly or indirectly metabolized to Pyr. In gluconeogenesis, Pyr is directly converted to Oxa, but not to AcCoA, and metabolized to Ma in a reversal of the TCA cycle. Thr and Met are indirectly metabolized to SucCoA, and His, Glu, and Gln are either directly or indirectly metabolized to αKg. SucCoA and αKg are metabolized to Ma via the TCA cycle. Ma is transported to the cytoplasm, and reconverted to Oxa for gluconeogenesis. Thr is also metabolized to mitochondrial AcCoA. Trp and Lys are metabolized to AcAcCoA. A ketone body, AcAC, is converted from AcCoA and AcAcCoA, and metabolized from Tyr. Data are presented as mean ± SD (μM). *P < 0.05, **P < 0.01, and†P < 0.001 indicate a significant difference from the controls by the unpaired Student’s t-test. 3HB 3-hydroxyburyeate, αKg α-ketoglutarate, AcAc acetoacetate, AcAcCoA acetoacetyly-CoA, AcCoA acetyl-CoA, Ala alanine, Citr citrate, Cys cystine, Gln glutamine, Glu glutamate, Gluc glucose, Gly glycine, His histidine, Lys lysine, Ma malate, Mito.m. mitochondrial membrane, OGC oxoglutarate carrier, Oxa oxaloacetate, PEP phosphoenolpyruvate, Phe phenylalanine, Pyr pyruvate, Ser serine, Suc succinate, SucCoA succinyl-CoA, Thr threonine, Trp tryptophan, Tyr tyrosine
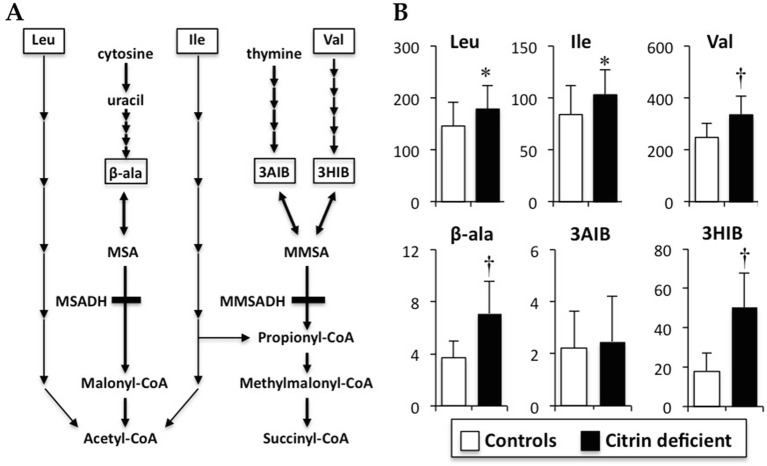
Fig. 3.
Metabolic pathways of the branched-chain amino acids (BCAAs) and pyrimidine (a), and serum concentrations of BCAAs, 3HIB, 3AIB, and β-alanine (b). Valine, leucine, and isoleucine are catabolized to succinyl-CoA and/or acetyl-CoA for energy generation mainly in the skeletal muscle. β-alanine is endogenously generated by the catabolism of cytosine and uracil. 3HIB is an intermediate of valine and is metabolized to MMSA, which is reversibly converted to 3AIB. 3AIB is also generated by thymine catabolism and is also reversibly converted to 3HIB via MMSA. Data are presented as mean ± SD (μM). †P < 0.001 indicates a significant difference from the controls by the unpaired Student’s t-test. 3AIB 3-aminoisobutyrate, 3HIB 3-hydroxyisobutyrate, β-ala β-alanine, Ile isoleucine, Leu leucine, MMSA methylmalonate semialdehyde, MMSADH MMSA dehydrogenase, MSA malonate semialdehyde, MSADH MSA dehydrogenase, n.s. no significance, Val valine
The goal of this study was to examine the blood amino acid profiles in citrin-deficient preschool and school children on lipid/protein-rich and carbohydrate-restricted diets exhibiting normal liver functions and compare the findings with that in healthy age-matched children.
Subjects and Methods
Subjects and Sample Collection
Twenty children aged from 5 years 5 months to 13 years 3 months (affected children) and 35 age-matched healthy children aged 5–13 years as healthy controls were enrolled for blood amino acid profile evaluation. The affected children were diagnosed by the SLC25A13 gene analysis (Yasuda et al. 2000) and considered as the patients in the apparently healthy period by the blood biochemical parameters including aspartate transaminase (AST), alanine transaminase (ALT), γ-glutamyl transpeptidase (γ-GTP), total bile acids (TBA), and total bilirubin. Total daily energy intake was similar between the affected and control children. The details of background in the affected children including plasma amino acids profile in the NICCD period were shown in the Supplementary Method 1 and Supplementary Table S1.
The methods and purpose of the study were explained to the parents, and their informed consent was obtained prior to enrollment. The study protocol was approved by the Ethics Committee of Tokyo Medical University Ibaraki Medical Center (#17-20) and accordance with the 1964 Helsinki declaration.
Serum Amino Acid Analyses
Serum concentrations of α-amino acids and other related molecules (β-alanine, GABA, taurine, 3-aminoisobutyrate [3AIB], and 3-hydroxyisobutyrate [3HIB]) were quantified by HPLC-ESI-MS/MS along with the previously reported methods (Shimbo et al. 2009; Nagasaka et al. 2017) (see the Supplementary Method 1 for the detail). The analyzed amino acid concentrations were used for calculation of the rate difference in the affected children to that in the controls per the SD value of the controls (Supplementary Fig. S1).
Statistical Analyses
Data are presented as mean ± SD. Statistical differences between the affected and control children were analyzed by the unpaired Student’s two-tailed t-test. The correlations were examined by Pearson’s correlation test. P < 0.05 was considered significant. Statistics were analyzed using JMP software (Version 9, SAS Institute, Cary, NC).
Results
Anthropometric and Basal Biochemical Data
Body weight, height, and weight SD scores in the affected children were significantly lower than those in the age-matched controls (Table 1). There were no significant differences in total protein, albumin, AST, ALT, γ-GPT, glucose, insulin, hemoglobin A1c, pyruvate, and ammonia between the affected and control children. TBA level in the affected children was within normal range (<10 μmol/L) (Matsuzaki 2008).
Table 1.
Background, and BCAAs and AAAs concentrations, and the amino acid ratios of citrin-deficient and control children
| Controls | Citrin-deficient | |
|---|---|---|
| Number (male/female) | 35 (18/17) | 20 (12/8) |
| Background | ||
| Ages (years) | 8.8 ± 1.4 | 9.2 ± 2.1 |
| Height (cm): SD score | 130.5 ± 9.3:0.06 ± 029 | 129.0 ± 9.5: −0.55 ± 0.49** |
| Body weight (kg): SD score | 27.8 ± 3.9: −0.02 ± 0.33 | 24.1 ± 3.6**: −1.01 ± 0.33† |
| Total protein (mg/dL) | 7.1 ± 0.2 | 6.9 ± 0.3 |
| Albumin (mg/dL) | 3.9 ± 0.2 | 3.8 ± 0.3 |
| Aspartate transaminase (IU/L) | 19 ± 3 | 20 ± 3 |
| Alanine transaminase (IU/L) | 21 ± 4 | 23 ± 3 |
| γ-Glutamyl transpeptitase (IU/L) | 15 ± 4 | 16 ± 3 |
| Total bile acids (μmol/L) | Not determined | 7 ± 3 |
| Fasting glucose (mg/dL) | 85 ± 5 | 83 ± 4 |
| Hemoglobin A1c (%) | 5.3 ± 0.2 | 5.1 ± 0.3 |
| Fasting insulin (mU/L) | 2.7 ± 2.2 | 2.5 ± 1.5 |
| Pyruvate (mg/dL) | 0.8 ± 0.2 | 0.8 ± 0.3 |
| Ammonia (mg/dL) | 35 ± 5 | 29 ± 5 |
| Serum BCAAs and AAAs concentrations and the amino acid ratios | ||
| BCAAs (μM) | 476.1 ± 125.7 | 615.4 ± 135.4† |
| AAAs (μM) | 157.6 ± 45.3 | 179.4 ± 40.1 |
| Fisher ratio | 3.09 ± 0.52 | 3.48 ± 0.63* |
| Thr/Ser | 0.71 ± 0.14 | 0.91 ± 0.25† |
| Cit/Ser | 0.16 ± 0.06 | 0.27 ± 0.16† |
| Cit/(Ile + Leu) | 0.14 ± 0.05 | 0.14 ± 0.11 |
| Cit × 100/TAAs | 1.00 ± 0.29 | 1.31 ± 0.71* |
Data are presented as mean ± SD. BCAAs, the sum of valine, leucine and isoleucine; AAAs, the sum of phenylalanine and tyrosine; Fisher ratio, BCAAs/AAAs; Thr/Ser, the ratio of threonine to serine; Cit/Ser, the ratio of citrulline to serine; Cit/Ser, the ratio of citrulline to the sum of isoleucine and leucine; Citr × 100/TAAs, the ratio of 100× citrulline to total amino acids (TAAs); TAAs, the sum of 20 amino acids including aspartate, glutamate, histidine, ornithine, lysine, arginine, citrulline, asparagine, serine, threonine, taurine, methionine, proline, glycine, alanine, valine, isoleucine, leucine, tyrocine, and phenylalanine. *P < 0.05, **P < 0.01, †p < 0.001 vs. the controls
Amino Acids Related to Citrin and the Urea Cycle
Serum citrulline concentration in the affected children (36.3 ± 19.7 μM; interquartile range [IQR], 26.6–36.4 μM) was decreased from the level in the NICCD period (130.2 ± 39.9 μM; Supplementary Table S1) to the comparable level of the controls (29.3 ± 8.1 μM; IQR, 24.5–34.9 μM) (Fig. 1b). Ornithine and arginine were also comparable in the affected children to those in the controls (Fig. 1b). Urea was significantly (greater than twofold) higher than in the affected children (P < 0.001). Aspartate was comparable, but asparagine was one-half and almost to −2SD in the affected children compared to that in the controls (Fig. 1b and Supplementary Fig. S1; P < 0.001). Glutamate was significantly increased and most to +2SD in the affected children compared to that in the controls (Fig. 1b and Supplementary Fig. S1; P < 0.001). On the other hand, glutamine was significantly decreased and less than -4SD in the affected children compared to that in the controls (Fig. 1b and Supplementary Fig. S1; P < 0.001).
Glucogenic and Ketogenic Amino Acids
Among glucogenic amino acids, glycine, serine, alanine, cysteine, histidine, and methionine levels were significantly lower in the affected children than those in the controls (Fig. 2b; P < 0.001 in all except for histidine [P < 0.01]). Among the ketogenic amino acids, lysine, tryptophan, and tyrosine levels were significantly increased in the affected children compared with those in the controls (Fig. 2c; P < 0.001 in lysine and tryptophan, P < 0.05 in tyrosine). There was no significant difference in phenylalanine between the affected and control children.
Branched-Chain Amino Acids, Valine Intermediates, and β-Alanine
Branched-chain amino acids (BCAAs) (valine, leucine, and isoleucine) and 3HIB, but not 3AIB, were significantly increased in the affected children compared to those in the controls (Fig. 3b; P < 0.05 in leucine and isoleucine, P < 0.001 in valine and 3HIB). β-alanine was significantly increased and greater than +2SD in the affected children compared to that in the controls (Fig. 3b and Supplementary Fig. S1; P < 0.001). There was a significant relationship between 3HIB and valine in both the affected children (r 2 = 0.27, P < 0.05) and controls (r 2 = 0.57, P < 0.0001), and between 3HIB and 3AIB in the affected children (r 2 = 0.23, P < 0.05).
Amino Acid Ratios
Table 1 shows Fischer ratio; BCAA/aromatic amino acids (AAA; tyrosine and phenylalanine), Thr/Ser, citrulline/serine (Cit/Ser), citrulline/(leucine + isoleucine) (Cit/[Ile + Leu]), and citrulline × 100/total amino acids; the sum of 20 amino acids (Cit × 100/TAAs) (Tamamori et al. 2004) as well as totals of BCAAs and AAAs. Fischer ratio was significantly higher in the affected children than that in the controls (P < 0.05), since total BCAA level was significantly increased (P < 0.001). Thr/Ser, Cit/Ser, and Cit × 100/TAAs ratios were significantly higher in the affected children compared with those in the controls (P < 0.001, P < 0.001, and P < 0.05, respectively), while there was no significant difference in Cit/(Ile + Leu) ratio.
Discussion
Present study evaluated serum amino acid concentrations in citrin-deficient children during the apparently healthy period between the NICCD and CTLN2 stages. In the NICCD period of the 20 affected children, higher levels of citrulline, threonine, and methionine in plasma that are the characteristics of both the NICCD children (Kobayashi et al. 1993–2017) and CTLN2 patients (Saheki et al. 1986) were observed compared to those in the affected and the age-matched healthy control children (Supplementary Table S1, Figs. 1 and 2), although the values in the NICCD could not be directly and statistically compared to those in the two groups because of differences in the analysis methods and blood sample types (i.e., plasma and serum). There was no significant difference in these amino acids as well as ammonia between the affected and control children. There was wide variation in citrulline of the affected children (Supplementary Fig. S1) because one value (108.5 μM) was over the healthy range; however, it was markedly lower than the values reported in the NICCD and CTLN2 (300 and 418 μM, respectively) (Kobayashi et al. 1993–2017). Therefore, the affected children were considered to be in the apparently healthy period by the serum amino acid levels.
In the citrin deficiency, ammonia detoxification is decreased because of declined argininosuccinate synthesis in the urea cycle (Fig. 1a) (Kobayashi et al. 1999, 2000; Palmieri et al. 2001, 2015). Indeed, higher citrulline and ornithine levels in plasma were found in the NICCD period indicating the reduced urea cycle activity (Supplementary Table S1). In the affected children, ammonia, arginine, ornithine, and citrulline levels were comparable to the respective control levels suggesting that the function of urea cycle was restored during this phase of the disease. However, urea was still significantly higher in the affected children than in the controls, although it was decreased from the level in the NICCD period. On the other hand, arginine that is the precursor of urea and ornithine in the urea cycle was lower in the NICCD than in the healthy period. Therefore, these results support that the declined argininosuccinate synthesis by decreased supplying of aspartate to the urea cycle occurred in the examined citrin-deficient children. The improvements in the affected children are likely due to sufficient aspartate for the urea cycle supplied through two possible ways. One possibility is that sufficient amounts of aspartate may be obtained from aspartate-rich foods, such as fish and soy that citrin-deficient patients prefer (Saheki et al. 2008; Saheki et al. 2012). Another source of aspartate may be the increase in endogenous levels through the metabolic conversion from asparagine by asparaginase in the cytoplasm (Fig. 1a) (Cooper et al. 2016). Sinasac et al. reported that asparagine administration together with NH4Cl during liver perfusion significantly increased urea production in the citrin (Slc25a13) knockout mice (Sinasac et al. 2004), supporting the concept that asparagine could serve as a source of cytoplasmic aspartate via asparaginase (Shiota et al. 1994). The significantly decreased asparagine level in serum of the affected children might be due to aspartate synthesis and a functioning urea cycle.
Although the citrin defect reduces glycolysis through elevation of the cytosolic NADH/NAD ratio (Kobayashi et al. 1999, 2000; Palmieri et al. 2001), blood glucose level and sugar-related parameters in the affected children were comparable to those in the controls (Table 1). The maintenance of blood glucose might be due to hepatic gluconeogenesis, because the low daily intake of carbohydrates was confirmed in the affected children. However, gluconeogenesis from lactate is impaired in the citrin deficiency (Sinasac et al. 2004), because the conversion of lactate to pyruvate yields cytoplasmic NADH that requires mitochondrial aspartate efflux. Therefore, gluconeogenesis in citrin deficiency might have to depend on glucogenic amino acids (Fig. 2a). Most glucogenic amino acids were significantly decreased in the affected children, supporting the enhancement of amino acid gluconeogenesis. Furthermore, glutamine was remarkably decreased in the affected children compared to that in both the NICCD and controls (Fig. 1b, Supplementary Table S1). Glutamine is utilized as the precursor of α-ketoglutamate and citrate in the TCA cycle for gluconeogenesis and lipogenesis, respectively, known as “glutaminolysis” in cancer cell biology (Reitzer et al. 1979; Dang 2010). Thus, mitochondrial metabolism might shift to a similar state of glutaminolysis (gluconeogenesis or lipogenesis) during the healthy period in this disease. In cases of gluconeogenesis and lipogenesis (MC shuttle), the malate transportation by the oxoglutarate carrier between the mitochondria and cytoplasm is in opposite direction (Figs. 1a and 2a). Although the upregulations of the MC shuttle involve hepatosteatosis in the CTLN2 patients (Komatsu et al. 2015), no distinct hepatic disorders were observed in the affected children. Therefore, gluconeogenesis is suggested to predominate in the healthy period. Further studies are needed to clarify these points.
Significant increases of the ketogenic amino acids were observed in the affected children. In the affected children, significantly increase of serum ketone body (3-hydroxybutyrate) level was previously observed, suggesting enhancement of hepatic fatty acid β-oxidation (Nagasaka et al. 2017). Therefore, the metabolisms of ketogenic amino acids might be inhibited due to the predominant production of ketone bodies from lipids in the affected children. On the other hand, hepatosteatosis along with the suppression of β-oxidation and the overproduction of fatty acids would be caused by compensatory upregulation of the MC shuttle in the CTLN2 patients (Komatsu et al. 2015). However, alterations in the ketogenic amino acid levels have not been observed in the stage. Therefore, the increased level of serum ketogenic amino acids is characteristic during the healthy period.
In the affected children, Thr/Ser was significantly higher than in the controls (Table 1), although the ratio was decreased to less than 1.0 from the higher ratio in the NICCD period (2.0 ± 0.2; Supplementary Table S1). The decrease of Thr/Ser from the NICCD period to the healthy period was due to the decrease of higher threonine level to the same level in the controls (Fig. 2b), and the characteristic was agreed with the previous report (Kobayashi et al. 1993–2017). However, serine in the affected children was significantly lower in the controls. The lower serine and higher threonine that derive the increased Thr/Ser are characteristics in the CTLN2 stage (Saheki et al. 1986). Therefore, Tamamori et al. observed significant increases in other parameters, such as the Cit/Ser, Cit/TAAs, and Cit/(Ile + Leu) that were accompanied by an increase of citrulline in the NICCD children (Tamamori et al. 2004). Cit/Ser and Cit/(Ile + Leu) in the NICCD period were higher than in the healthy period, because citrulline and Ile + Leu were higher and lower, respectively, in the NICCD (Table 1 and Supplementary Table S1). There was no significant difference in the Cit/(Ile + Leu) between the affected and control children, although Cit/Ser and Cit/TAAs were significantly higher in the affected children during healthy period than in the controls (Table 1). The Cit/(Ile + Leu) might be a useful parameter to distinguish the apparently healthy period of citrin-deficient children.
A significant increase in all of the BCAAs was observed in the affected children compared to those in both the NICCD period and the controls. This is likely due to the high protein diets, including BCAAs-rich foods, such as meats, fish, and beans. Otherwise, it is possible that metabolic dysfunction of BCAAs might be induced in the affected children. BCAAs are specifically metabolized mainly in the skeletal muscles for energy production (Shimomura et al. 2006). In the affected children, serum level of 3HIB, an intermediate of valine catabolism (Fig. 3a) (Avogaro and Bier 1989; Miyazaki et al. 2015), was significantly higher than in the controls. We also previously observed a significant decrease of serum acetylcarnitine level, which is an end product of fatty acid β-oxidation in the muscle, in the affected children (Nagasaka et al. 2017). Therefore, the preferred metabolic pathway in the skeletal muscle is likely predominantly BCAA catabolism rather than glycolysis and fatty acid oxidation in the healthy period. The significant increase of BCAA in the affected children was associated with the significant increase of Fischer ratio, but the ratio was over than 3 and comparable between the two groups (Table 1). Fischer ratio in the NICCD period (Supplementary Table S1) and in the CTLN2 (Saheki et al. 1986) was markedly decreased to less than 2 which is the level found in liver cirrhosis due to the significant decrease of BCAAs suggesting that muscular BCAA catabolism might further increase in the NICCD and CTLN2 stages. This point requires future study, because serum 3HIB level has been never investigated in the NICCD and CTLN2 stages.
In addition to 3HIB, the increase of β-alanine was significant in the affected children. β-alanine is endogenously generated during cytosine and uracil catabolism and is reversibly metabolized to malonic semialdehyde (MSA) that further metabolized to malonyl-CoA by its dehydrogenase (MSADH) (Fig. 3a). On the other hand, thymine is catabolized to 3AIB, and both 3HIB and 3AIB are reversibly convertible to each other via methylmalonic semialdehyde (MMSA) that metabolized to propionyl-CoA by its dehydrogenase (MMSADH). Because MSA and MMSA are unstable, simultaneous increases of β-alanine, 3HIB, and 3AIB in blood are elevated in cases of defects in these dehydrogenases (Pollitt et al. 1985; Marcadier et al. 2013). In the affected children, 3AIB was comparable to that in the controls, and 3HIB had a significant relationship with valine, but not β-alanine. It is unclear whether the significant increase of β-alanine is due to the pyrimidine catabolism or inactivated MSADH. However, the valine and thymine catabolism could be distinguished because 3HIB and 3AIB are generated as L- and D-forms in the respective catabolism (Marcadier et al. 2013). These points need to be clear in this diease in the future by separate measurement of the optical isomer.
Blood amino acid levels are influenced by both diet and metabolism, and therefore, it is difficult to ascertain which factor influences the blood level. Furthermore, amino acids are metabolized in either the cytoplasm or mitochondria, and blood amino acid levels may not necessarily reflect the levels in the cytoplasm and mitochondria. These points are a limitation of this study.
In conclusion, this study found that the characteristic phenotype of blood amino acids in the NICCD and CTLN2 patients, such as increased levels of citrulline, arginine, phenylalanine, threonine, and methionine, and the alterations of urea cycle-related amino acids were not found in citrin-deficient children during the apparently healthy period. Instead, decreased levels of glucogenic amino acids and increased levels of the ketogenic amino acids, BCAAs, and β-alanine were observed in the affected children. The different characteristics of blood amino acids in the healthy period to the NICCD and CTLN2 stages might contribute to the healthy clinical features.
Electronic Supplementary Material
∎∎∎ (JPEG 391 kb)
∎∎∎ (PDF 76 kb)
∎∎∎ (PDF 107 kb)
Acknowledgement
Details of the contributions of individual authors as (a) conception and design, (b) data analysis, (c) data interpretation, (d) drafting the article, (e) revising the article, and (f) clinical diagnosis/treatment and sample collection: Miyazaki T: (a, b, c, d); Nagasaka H: (a, b, c, d, f); Komatsu H: (c, f); Inui A: (c, f); Morioka I: (c, f); Tsukahara H: (c, f); SKaji S: (c, f); Hirayama S: (c, f); Miida T: (c, f); Kondou H: (c, f); Ihara K: (c, f); Yagi M: (c, f); Kizaki Z: (c, f); Bessho K: (c, f); Kodama T: (c, f); Iijima K: (c, f); Yorifuji T: (c, f); Matsuzaki Y: (e); and Honda A: (a, b, c, e).
The Name of the Corresponding Author
Teruo Miyazaki.
A Competing Interest Statement
All authors have no conflict of interest.
Details of Funding
The authors have no support and funding to this study.
Details of Ethics Approval
All procedures performed in the study were approved by the Ethics Committee of Tokyo Medical University Ibaraki Medical Center and accordance with the 1964 Helsinki declaration.
Patient Consent Statement
Informed consent was obtained from all patients of the participants prior to enrollment.
Documentation of Approval from the Institutional Committee for Care and Use of Laboratory Animals
Not available in this study.
References
- Avogaro A, Bier DM. Contribution of 3-hydroxyisobutyrate to the measurement of 3-hydroxybutyrate in human plasma: comparison of enzymatic and gas-liquid chromatography-mass spectrometry assays in normal and in diabetic subjects. J Lipid Res. 1989;30:1811–1817. [PubMed] [Google Scholar]
- Chanprasert S, Scaglia F. Adult liver disorders caused by inborn errors of metabolism: review and update. Mol Genet Metab. 2015;114:1–10. doi: 10.1016/j.ymgme.2014.10.011. [DOI] [PubMed] [Google Scholar]
- Cooper AJ, Shurubor YI, Dorai T, et al. Omega-Amidase: an underappreciated, but important enzyme in L-glutamine and L-asparagine metabolism; relevance to sulfur and nitrogen metabolism, tumor biology and hyperammonemic diseases. Amino Acids. 2016;48:1–20. doi: 10.1007/s00726-015-2061-7. [DOI] [PubMed] [Google Scholar]
- Dang CV. Glutaminolysis: supplying carbon or nitrogen or both for cancer cells? Cell Cycle. 2010;9:3884–3886. doi: 10.4161/cc.9.19.13302. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Saheki T, Song YZ, et al. Citrin deficiency. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. Citrin deficiency. Seattle, WA: University of Washington; 1993. [Google Scholar]
- Kobayashi K, Sinasac DS, Iijima M, et al. The gene mutated in adult-onset type II citrullinaemia encodes a putative mitochondrial carrier protein. Nat Genet. 1999;22:159–163. doi: 10.1038/9667. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Iijima M, Yasuda T, et al. Type II citrullinemia (citrin deficiency): a mysterious disease caused by a defect of calcium-binding mitochondrial carrier protein. Dordrecht, The Netherlands: Kluwer; 2000. [Google Scholar]
- Komatsu M, Yazaki M, Tanaka N, et al. Citrin deficiency as a cause of chronic liver disorder mimicking non-alcoholic fatty liver disease. J Hepatol. 2008;49:810–820. doi: 10.1016/j.jhep.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Kimura T, Yazaki M, et al. Steatogenesis in adult-onset type II citrullinemia is associated with down-regulation of PPARalpha. Biochim Biophys Acta. 2015;1852:473–481. doi: 10.1016/j.bbadis.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcadier JL, Smith AM, Pohl D, et al. Mutations in ALDH6A1 encoding methylmalonate semialdehyde dehydrogenase are associated with dysmyelination and transient methylmalonic aciduria. Orphanet J Rare Dis. 2013;8:98. doi: 10.1186/1750-1172-8-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki Y. Total bile acids. Tokyo: Ishiyaku Publishers, Inc.; 2008. [Google Scholar]
- Miyazaki T, Honda A, Ikegami T, et al. Simultaneous quantification of salivary 3-hydroxybutyrate, 3-hydroxyisobutyrate, 3-hydroxy-3-methylbutyrate, and 2-hydroxybutyrate as possible markers of amino acid and fatty acid catabolic pathways by LC-ESI-MS/MS. Springerplus. 2015;4:494. doi: 10.1186/s40064-015-1304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaka H, Komatsu H, Inui A, et al. Circulating tricarboxylic acid cycle metabolite levels in citrin-deficient children with metabolic adaptation, with and without sodium pyruvate treatment. Mol Genet Metab. 2017;120:207–212. doi: 10.1016/j.ymgme.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Palmieri L, Pardo B, Lasorsa FM, et al. Citrin and aralar1 are Ca(2+)-stimulated aspartate/glutamate transporters in mitochondria. EMBO J. 2001;20:5060–5069. doi: 10.1093/emboj/20.18.5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri EM, Spera I, Menga A, et al. Acetylation of human mitochondrial citrate carrier modulates mitochondrial citrate/malate exchange activity to sustain NADPH production during macrophage activation. Biochim Biophys Acta. 2015;1847:729–738. doi: 10.1016/j.bbabio.2015.04.009. [DOI] [PubMed] [Google Scholar]
- Pollitt RJ, Green A, Smith R. Excessive excretion of beta-alanine and of 3-hydroxypropionic, R- and S-3-aminoisobutyric, R- and S-3-hydroxyisobutyric and S-2-(hydroxymethyl)butyric acids probably due to a defect in the metabolism of the corresponding malonic semialdehydes. J Inherit Metab Dis. 1985;8:75–79. doi: 10.1007/BF01801669. [DOI] [PubMed] [Google Scholar]
- Reitzer LJ, Wice BM, Kennell D. Evidence that glutamine, not sugar, is the major energy source for cultured HeLa cells. J Biol Chem. 1979;254:2669–2676. [PubMed] [Google Scholar]
- Saheki T, Kobayashi K. Mitochondrial aspartate glutamate carrier (citrin) deficiency as the cause of adult-onset type II citrullinemia (CTLN2) and idiopathic neonatal hepatitis (NICCD) J Hum Genet. 2002;47:333–341. doi: 10.1007/s100380200046. [DOI] [PubMed] [Google Scholar]
- Saheki T, Song YZ (1993) Citrin deficiency. In: Adam MP, Ardinger HH, Pagon RA et al (eds) GeneReviews®, University of Washington, Seattle, WA
- Saheki T, Kobayashi K, Miura T, et al. Serum amino acid pattern of type II citrullinemic patients and effect of oral administration of citrulline. J Clin Biochem Nutr. 1986;1:129–142. doi: 10.3164/jcbn.1.129. [DOI] [Google Scholar]
- Saheki T, Kobayashi K, Terashi M, et al. Reduced carbohydrate intake in citrin-deficient subjects. J Inherit Metab Dis. 2008;31:386–394. doi: 10.1007/s10545-008-0752-x. [DOI] [PubMed] [Google Scholar]
- Saheki T, Inoue K, Ono H, et al. Effects of supplementation on food intake, body weight and hepatic metabolites in the citrin/mitochondrial glycerol-3-phosphate dehydrogenase double-knockout mouse model of human citrin deficiency. Mol Genet Metab. 2012;107:322–329. doi: 10.1016/j.ymgme.2012.07.021. [DOI] [PubMed] [Google Scholar]
- Shimbo K, Oonuki T, Yahashi A, Hirayama K, Miyano H. Precolumn derivatization reagents for high-speed analysis of amines and amino acids in biological fluid using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:1483–1492. doi: 10.1002/rcm.4026. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Honda T, Shiraki M, et al. Branched-chain amino acid catabolism in exercise and liver disease. J Nutr. 2006;136:250S–253S. doi: 10.1093/jn/136.1.250S. [DOI] [PubMed] [Google Scholar]
- Shiota M, Hiramatsu M, Fujimoto Y, et al. The capacity of the malate-aspartate shuttle differs between periportal and perivenous hepatocytes from rats. Arch Biochem Biophys. 1994;308:349–356. doi: 10.1006/abbi.1994.1050. [DOI] [PubMed] [Google Scholar]
- Sinasac DS, Moriyama M, Jalil MA, et al. Slc25a13-knockout mice harbor metabolic deficits but fail to display hallmarks of adult-onset type II citrullinemia. Mol Cell Biol. 2004;24:527–536. doi: 10.1128/MCB.24.2.527-536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamori A, Fujimoto A, Okano Y, et al. Effects of citrin deficiency in the perinatal period: feasibility of newborn mass screening for citrin deficiency. Pediatr Res. 2004;56:608–614. doi: 10.1203/01.PDR.0000139713.64264.BC. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Yamaguchi N, Kobayashi K, et al. Identification of two novel mutations in the SLC25A13 gene and detection of seven mutations in 102 patients with adult-onset type II citrullinemia. Hum Genet. 2000;107:537–545. doi: 10.1007/s004390000430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
∎∎∎ (JPEG 391 kb)
∎∎∎ (PDF 76 kb)
∎∎∎ (PDF 107 kb)