Abstract
Congenital disorders of glycosylation (CDG) are ultra-rare diseases showing a great phenotypic diversity ranging from mono- to multi-organ/multisystem involvement. Liver involvement, mostly nonprogressive, is often reported in CDG patients. The main objectives of this work were (1) to better understand liver involvement in CDG patients through a liver electronic questionnaire targeting CDG families (LeQCDG) and (2) to compare responses from LeQCDG participants with literature review regarding the prevalence of liver disease and the occurrence of liver symptoms in CDG patients. The network of patient advocacy groups, families and professionals (CDG & Allies – PPAIN) developed the LeQCDG by adapting validated published questionnaires. The LeQCDG was approved by an ethics committee, and the recruitment of patients and caregivers proceeded through social media platforms. Participants were asked to report past or present liver-related symptoms (e.g. hepatomegaly, liver fibrosis and cirrhosis) and laboratory results (e.g. biochemical and/or radiological). From 11 December 2016 to 22 January 2017, 155 questionnaires were completed. Liver disease was present in 29.9% of CDG patients. Main symptoms reported included hepatomegaly, increased levels of serum transaminases, fibrosis, steatosis and cirrhosis. The data obtained in this online survey confirm findings from a recent literature review of 25 years of published evidence (r = 0.927, P = 0.02). Our questionnaire collected large amounts of meaningful, clinical and patient-oriented data in a short period of time without geographic limitations. Internet-based approaches are especially relevant in the context of ultra-rare diseases such as CDG.
Electronic supplementary material
The online version of this chapter (10.1007/8904_2018_121) contains supplementary material, which is available to authorized users.
Keywords: Congenital disorder(s) of glycosylation (CDG), Literature review, Liver, PMM2-CDG, Questionnaire, Rare diseases
Introduction
Congenital Disorders of Glycosylation
Congenital disorders of glycosylation (CDG) are a group of mostly autosomal recessive disorders first described in 1980 by Jaak Jaeken (Jaeken et al. 1980). It is a rapidly growing family of very rare genetic diseases comprising more than 100 different subtypes (Jaeken and Péanne 2017). In glycosylation, glycans (‘sugar trees’) are assembled, processed and attached into proteins or lipids. It is the most important post-translational modification of proteins and a fundamental cellular process. Glycosylation defects are divided in several groups, namely, defects in N- and/or O-linked glycosylation, in lipid glycosylation and in GPI-anchor biosynthesis. N-glycosylation defects are the most common type, with almost 1,000 reported patients with the most frequent PMM2-CDG.
The majority of CDG patients present multisystem organ impairment with a vast clinical diversity ranging from mild to severe dysfunction (Monticelli et al. 2016; Marques-da-Silva et al. 2017a, b; Francisco et al. 2018). Liver involvement in CDG also ranges from mild to severe. Indeed, 22% of patients with CDG subtypes can present with liver involvement such as elevated serum transaminases, hepatomegaly, steatosis, fibrosis and cirrhosis (Marques-da-Silva et al. 2017a).
Data on the prevalence, severity and long-term evolution of liver disease in CDG is sparse. This hampers clinicians and families in recognizing symptoms and signs and in the prevention of liver disease-related complications, management and treatment. A recent literature review identified only 99 publications with information relevant to the subject (Marques-da-Silva et al. 2017a). Consequently, additional strategies/sources of medical information on this group of diseases are required.
Nowadays, there is a shift, especially in rare diseases, in the patient-physician relationship, and patients are often experts in their own rights obliged to become experts of their own medical conditions (Budych et al. 2012). Therefore, gathering data directly from patients and their caregivers is a unique approach with tremendous potential.
Quality of Life in Congenital Disorders of Glycosylation
Patient-reported outcomes (PROs) are assessed through validated questionnaires, which can evaluate quality of life (QoL) (Erhart et al. 2009; D’Ambrosi et al. 2017; Zeltner et al. 2016), symptoms (Malcolm et al. 2012; Neijenhuis et al. 2016), treatment and care satisfaction/effectiveness (D’Ambrosi et al. 2017) and even compliance to medication (Geissler et al. 2017). PROs are used clinically, as secondary endpoints in clinical trials and as tools employed in natural history studies and patient registries (Coons et al. 2009; Paulsen et al. 2010). Moreover, when patients are unable to reliably report for themselves, namely, in the case of children and/or due to the severity of their illness, impaired language ability speech or cognitive functioning, PROs may be completed by a proxy such as a parent (Matza et al. 2013).
Specifically for liver disease, there are already several validated tools, such as the Hepatitis Quality of Life Questionnaire (HQLQ), the Chronic Liver Disease Questionnaire (CLDQ), the Liver Disease Quality of Life questionnaire (LDQoL), the Liver Disease Symptom Index 2.0 (LDSI 2.0), the post-liver transplant quality of life (pLTQ) and the polycystic liver disease-specific symptom questionnaire (PLD-Q), which have been validated in target populations and have been translated to various languages (Younossi et al. 2001; Gutteling et al. 2007; Van Der Plas et al. 2007; Mucci et al. 2010; Saab et al. 2011; Neijenhuis et al. 2016). Indeed, in a recent study in which both CLDQ and a general HQoL questionnaire were taken from a heterogeneous population of patients with chronic liver disease, it was shown that combining PROs analysis with clinical objective scores might improve disease diagnosis, management as well as therapeutic effects (Obradovic et al. 2017).
Performing clinical research in rare diseases, like CDG, poses many challenges: there is complexity in collecting robust data in small-sized populations, that are globally dispersed, which is compounded by lack of funding (Augustine et al. 2013). Nevertheless, technology offers innovative approaches that help to overcome these hurdles. Electronic PROs (ePROs) have been gaining popularity and, a sudy comparing paper-based and ePROs revealed that both had the same reliability. The ePROs also presented advantages over the paper-based PROs, including the ease of data collection and processing while being more user-friendly (Coons et al. 2009). Additionally, social media has been emerging as a medical research recruitment platform, which is particularly helpful for geographically dispersed populations, like rare disease communities. Indeed, social media has repeatedly been used to conduct research surveys and questionnaires with high adherence and good results (Schumacher et al. 2014; Davies 2016; Topolovec-Vranic and Natarajan 2016; Burton-Chase et al. 2017). With 40% of the world population having access to the Internet, social media-based medical research is gaining support due to its accessibility, anonymity, simplicity, affordability, outreach and engagement capabilities (Fenner et al. 2012; Davies 2016; Burton-Chase et al. 2017).
Employing social media in clinical research, we implemented an electronic questionnaire aiming to:
Better understand liver involvement in CDG patients through a liver electronic questionnaire targeting CDG families (LeQCDG) where participants were asked to report past or present liver-related symptoms (e.g. hepatomegaly, liver fibrosis and cirrhosis) and the laboratory findings (e.g. biochemical and/or radiological).
Compare data reported by LeQCDG participants with previously published literature review regarding the reported prevalence of liver disease in CDG and the occurrence of liver symptoms in CDG patients.
The questions implemented in the LeQCDG were based on a recent literature review focusing on liver involvement in CDG and inspired us to adapt the existing PROs instruments to assess liver disease (Marques-da-Silva et al. 2017a).
Methods
Literature Search
To construct the liver electronic questionnaire for CDG (LeQCDG), we performed a literature search using Google Scholar and PubMed platforms. The keywords presented in Table S1 (Supplementary Material) were used to identify validated tools to evaluate liver-related symptoms and signs. This search was also performed to find validated tools to measure quality of life in liver disease patients. To maximize and refine the number and quality of the retrieved tools, an additional search was performed as explained in Table S2 (Supplementary Material). The identified instruments were characterized according to the following parameters: generic/specific disease questionnaire, main domains, specific domains, mode of administration, number of items, score system, completion time, available translations (cross-cultural studies) and validated tools.
Building and Testing the LeQCDG
Based on the information collected from the above-mentioned literature search, a liver questionnaire was constructed. Since we specifically wanted to collect data from CDG patients, the tool was then tailored to this patient group. For this, we used information available from our recent published work (Marques-da-Silva et al. 2017a) that describes the main liver symptoms reported in CDG patients. The questionnaire integrated the following sections: ‘Participants Data’, ‘Liver Signs and Symptoms’, ‘Liver Symptoms’, ‘Impact of Liver Involvement’, ‘Liver Transplantation’ and ‘Awareness and Information About Liver Involvement in CDG’.
The online platform used to implement the questionnaire was SurveyMonkey (http://www.surveymonkey.net – Copyright©1999–2018 SurveyMonkey) which allowed construction of the questionnaire with different question types such as multiple choice, text insertion or classification scale (Fig. S1 in Supplementary Material). The SurveyMonkey platform also allows automatic capture of responses. The questionnaire was an open survey and directed to CDG patients and caregivers, henceforth referred to as ‘participants’.
Using the SurveyMonkey platform, the LeQCDG was pilot tested by relevant partners: two researchers, two physicians and six CDG caregivers. The pilot testing included face validity concerning the relevance of the items as they appear to participants and possible effects of literacy on reading comprehension. Suggestions derived from the pilot allowed us to refine the questionnaire to record not only the views of patients with liver disease but also patients that had clinical liver-related symptoms, thus expanding the scope of participants. The sample was derived from participants reporting on CDG patients with liver disease, who answered to specific questions regarding liver symptoms (e.g. incidence of fibrosis, cirrhosis and portal hypertension).
Questions related to the impact of liver disease on the wellbeing of the CDG patients were also suggested by our CDG partners during the piloting phase. A glossary, explaining different liver disease symptoms, was made available to participants (Fig. S2 in Supplementary Material).
LeQCDG Recruitment, Dissemination and Analysis
The LeQCDG was launched online on 11 December 2016 and was available for participation for 42 days, until 22 January 2017. The survey was available in different languages (English, French, Spanish and Portuguese) and took between 15 and 20 min to complete. To assure the participants’ anonymity, the IP identification number of respondents was not recorded. Multiple entries from the same individual were avoided choosing the SurveyMonkey option ‘the questionnaire cannot be answered several times from the same device’. The respondents could review and change their answers (in this case, through a ‘Prev.’ button).
The LeQCDG participants were recruited from clinics and from the CDG community. Due to geographic limitations, social networks like Facebook, LinkedIn, Twitter and the Rareconnect platform (https://www.rareconnect.org/en/community/cdg) were used to disseminate the LeQCDG to the CDG community. In December 2016 at the LeQCDG launching time, the CDG community included 720 participants of the ‘CDG Global Alliance’, a closed group on Facebook, and 1,100 followers of the ‘Sindrome CDG’ Facebook page. The Facebook page was started in 2000, and the members have been responsive to activity calls. An example of patient engagement in research activities is the constant participation of the CDG community in conferences and workshops.
An example of the survey announcement is shown in Fig. S3 (Supplementary Material). The participants identified themselves as CDG caregivers or as CDG patients and as having liver disease or being on a transplant list. Medical records and disease confirmation were not required for participation, as we considered ‘random’ participation of non-CDG patients/caregivers very unlikely.
Epidemiological data suggest that at least 20–30% of CDG patients may present liver disease symptoms (Marques-da-Silva et al. 2017a). Therefore, the sample size should contain, with high probability (e.g. 95%), sufficient numbers of positive and negative cases of liver disease. To achieve these conditions and the desired significance level of P < 0.05, a sample size of minimum 138 participants was calculated to be necessary, in order to achieve enough power to allow differentiation of the major liver symptoms in CDG patients (Lachin 1981).
Data Analysis
LeQCDG responses were exported from SurveyMonkey to excel. All questionnaires were analysed, except for participants reporting unknown CDG types or NGLY1 deficiency since NGLY-1 is a congenital disorders of deglycosylation (CDDG) which is different from CDG. These exclusion criteria were based on the fact that these patients do not qualify as ‘diagnosed CDG patient’. Descriptive statistics were used to analyse and report the data. In addition, correlation between the survey and bibliographic data was evaluated with Pearson’s linear correlation coefficient and correspondent P value (Prob>F) using OriginPro 8.5 software (OriginLab Corp., Northampton, USA).
Ethical approval for this study was obtained from the Ethics Committee at the Faculty of Psychology, Lisbon University (20/07/2016), and an informed consent was obtained from all participants. The online survey was conducted in accordance with the CHERRIES checklist (Eysenbach 2004).
Results
During 42 days, 203 participants accessed the LeQCDG. Four participants did not give informed consent and were excluded from further participation. According to the CHERRIES checklist (Eysenbach 2004), the completion rate was 77.9% (a total of 155 out of 199 participants who agreed to participate).
Participants
All but 1 participant were caregivers (1 was a CDG patient); 90% (162 out of 180) of participants knew that CDG patients can be affected by liver disease. Patients of both sexes were represented in a very similar percentage, 52.8% (95 out of 180) female patients and 47.2% (85 out of 180) male patients (Table S3 in Supplementary Material). The majority of CDG patients they represented were 10 years or younger (61.2% – 109 out of 178) (Table S4 in Supplementary Material). The major CDG types represented were PMM2-CDG (76.1% – 137 out of 180) and ALG6-CDG (4.4% – 8 out of 180) (Fig. S4 in Supplementary Material). These results were expected since PMM2-CDG is the most prevalent CDG followed by ALG6-CDG as the second most prevalent one.
Liver Disease in CDG
Among the CDG patients represented by the participants, 29.9% (53 out of 177) had liver disease, 41.8% (74 out of 177) did not have liver disease, and in 28.3% (50 out of 177) of patients, the participant did not know whether the CDG patient suffered from liver disease or not. However, in only 63.6% (105 out of 165) of all CDG patients reported, biochemical and/or radiological examinations were used to diagnose liver disease. For 96.1% (49 out of 51) of CDG patients with liver disease, the diagnosis of liver disease was made before or at the age of 10 years, and in 49% (25 out of 51) of the cases, liver problems were diagnosed before 12 months of age.
Monitoring rate of liver disease is detailed in Table 1. Hepatomegaly was described in 29% (47 out of 162) of CDG patients; additional reported symptoms and pathology findings were steatosis, ascites, splenomegaly and oesophageal varices (Fig. 1).
Table 1.
Monitoring rate of liver function in all CDG patients
| Characteristic | Value |
|---|---|
| Monitoring rate of liver function | |
| Twice a year | 19.9% (32) |
| Once a year | 37.3% (60) |
| Once in 2 years | 9.9% (16) |
| Other | 30.4% (49) |
| Don’t know/specify | 2.5% (4) |
‘Other’ includes every 2 weeks, 6–12 times per year, 3–4 times per year, once in every 3 years, once and never/no needed. The results are expressed as percentage and absolute number; the total number of participants answering this question was 161
Fig. 1.
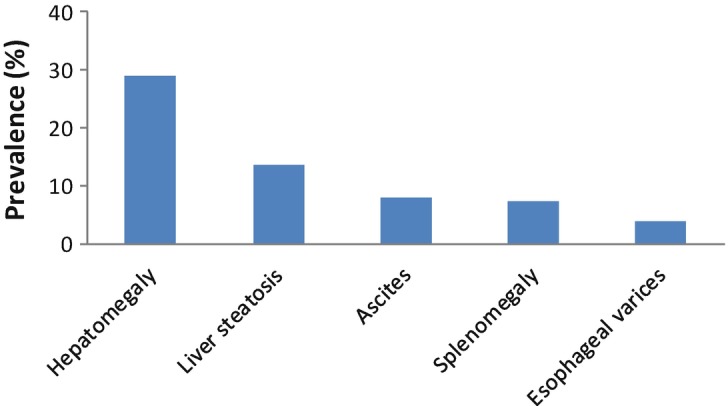
Prevalence of liver symptoms in all CDG patients represented in the LeQCDG
Liver Symptoms in CDG
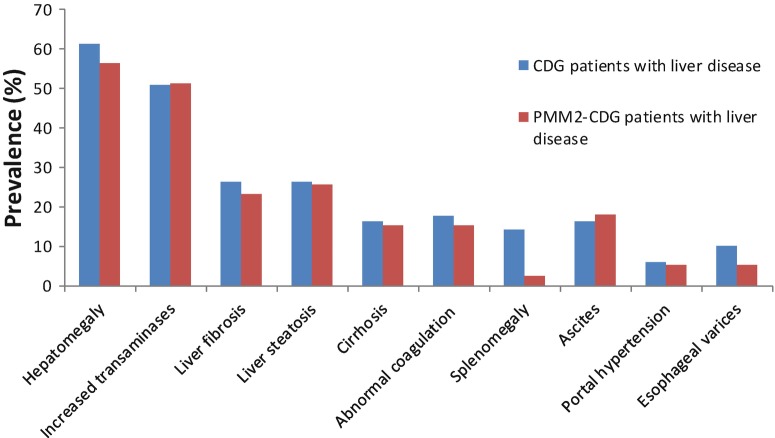
Within the population of CDG patients with known liver disease (n = 53), 17.6% (9 out of 51) answered that there was an alternative cause for liver disease put forward besides CDG; 27.5% (14 out of 51) noticed hepatic disturbances concomitant with an infectious syndrome. 10.2% (5 out of 49) were taking medication specifically for their liver condition at the time of the questionnaire. Liver symptoms were reported as shown in Fig. 2, with a high incidence of hepatomegaly (61.2% – 30 out of 49) and increased levels of serum transaminases (51% – 26 out of 51). Within this group of patients, liver transplantation was proposed to 5 patients (10.4% – 5 out of 48), 2 of whom had MPI-CDG and 3 had PMM2-CDG.
Fig. 2.
Prevalence of liver symptoms obtained in the LeQCDG for CDG patients with PMM2-CDG (red) and with other CDG (blue)
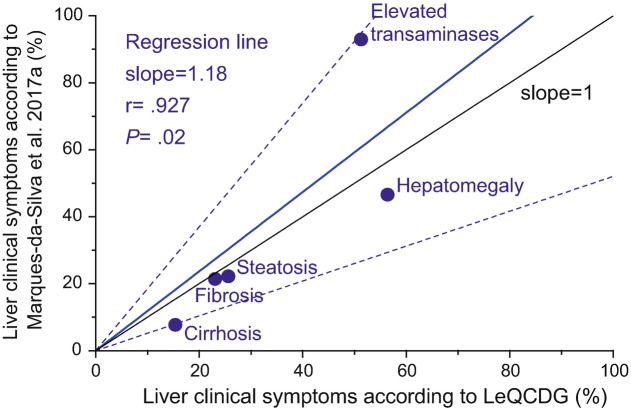
In the LeQCDG, 30.2% (39 out of 129) PMM2-CDG patients reported liver disease, and a specific subgroup analysis on these patients was performed. The main symptoms were also hepatomegaly (56.4% – 22 out of 39) and increased levels of serum transaminases (51.3% – 20 out of 39) (Fig. 2). In fact, for PMM2-CDG, we observed a significant correlation between the results obtained through the LeQCDG and the descriptions retrieved from the medical literature (reviewed in Marques-da-Silva et al. 2017a) (Fig. 3).
Fig. 3.
Scatterplot and regression line (in blue) for the relation between the prevalence of liver symptoms in PMM2-CDG patients obtained from LeQCDG results and from literature data (Marques-da-Silva et al. 2017a). The slope of the regression line was found 1.18 ± 0.24. The 95% confidence interval is plotted as confidence bands (dashed lines) around the regression line. Pearson’s correlation coefficient (r) and observed significance of the test (P) are indicated. The line with slope = 1 corresponds to perfect agreement between both methods
Within this subgroup of 39 patients with PMM2-CDG, liver transplantation was suggested to 3 patients; however only 1 patient reported severe liver disease in the LeQCDG supporting the suggestion of liver transplantation with symptoms that include hepatomegaly, increased levels of transaminases, liver fibrosis, steatosis and cirrhosis. The other two participants reported only increased levels of transaminases and in one case also the occurrence of ascites.
Impact of Liver Involvement
Liver disease-associated symptoms in CDG patients can be found in Table 2; most symptoms were not present. In line with this, the participants reported that the liver disease has no or only minor impact on the patient’s wellbeing and functioning (Table S5 in Supplementary Material) or emotional impact (Table S6 in Supplementary Material). Besides the low impact of liver disease on the physical and emotional health of CDG patients, most respondents (all but one being caregivers) reported their constant concerns regarding physical health and emotional wellbeing of the patient (Table S7 in Supplementary Material).
Table 2.
Associated liver symptoms in CDG patients with liver disease
| Classification rate | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Abdominal distension | 53% (27) | 31% (16) | 8% (4) | 8% (4) |
| Feeling sleepy or drowsy during the day (particularly after eating) | 55% (28) | 24% (12) | 14% (7) | 8% (4) |
| Having difficulties sleeping during the night | 55% (28) | 20% (10) | 16% (8) | 10% (5) |
| Nausea | 53% (27) | 24% (12) | 8% (4) | 16% (8) |
| Body temperature fluctuations (too low temperature or fever) | 61% (31) | 12% (6) | 20% (10) | 8% (4) |
| Jaundice (yellow discoloration of the whites of your eyes) | 88% (45) | 4% (2) | 2% (1) | 6% (3) |
| Itching | 80% (41) | 12% (6) | 6% (3) | 2% (1) |
| Bad breath or body odour | 69% (35) | 20% (10) | 4% (2) | 8% (4) |
| Loss of appetite | 55% (28) | 18% (9) | 14% (7) | 14% (7) |
| Pain or discomfort | 59% (30) | 20% (10) | 10% (5) | 12% (6) |
| Fatigue or low levels of energy | 31% (16) | 33% (17) | 16% (8) | 20% (10) |
The results are expressed as percentage and absolute number; the total number of participants answering this question was 51. Scoring system used: 0, symptom not present; 1, mild/rare; 2, moderate/sometimes; 3, severe/often
Awareness and Information About Liver Involvement in CDG: All Participants
We found that 50% of the participants that did not know if the CDG patient they reported on had liver disease also reported that the possibility of having liver disease has never been mentioned to the CDG patient. This is at least in part due to a lack of awareness in the medical community regarding CDG and related signs and symptoms. The participants classify the CDG patient’s doctor’s knowledge about CDG as represented in Table 3. Most participants think that the medical community should be trained better about CDG signs and symptoms (42.6% – 66 out of 155) and/or refer a patient to a more experienced colleague, in order to help in diagnosis and management (40% – 62 out of 155).
Table 3.
Opinion of participants about CDG patient’s doctor’s knowledge about CDG
| Question | Value |
|---|---|
| How would you rate the CDG patient’s doctor’s knowledge about CDG? | |
| Excellent | 14.2% (22) |
| Good | 26.5% (41) |
| Neutral | 20.6% (32) |
| Fair | 16.1% (25) |
| Poor | 22.6% (35) |
The results are expressed as percentage and absolute number; the total number of participants answering this question was 155
The treating physicians were reported to have recommended the patient to visit a hepatologist in 23.2% (36 out of 155) of cases. Approximately 62.6% (97 out of 155) of the participants feel they should know more about liver involvement in CDG because this allows them to communicate and share information with the doctor responsible for the CDG patient (43.8% – 67 out of 153). The majority of LeQCDG participants (i.e. 60.6%) gathered information about liver involvement in CDG themselves. The main information sources reported were ‘to talk with CDG treating physician’, ‘information from a patient group website’ and ‘information from a practical CDG guide’. However, only 7.7% (12 out of 155) of participants have an informative document regarding liver disease in CDG. From all participants, 57.4% (89 out of 155) of the participants plan to try to learn more about liver involvement in CDG on the next scheduled medical check-up.
Discussion
Principal Results
The results show that 29.9% of the reported CDG patients have liver disease; in 49% of these patients, the liver problems were diagnosed before 12 months of age. We analysed the entire cohort but also analysed the subgroup of PMM2-CDG patients, as this subtype made up 76.1% of our cohort of participants.
Limitations of This Work
The number of known CDG patients worldwide is unknown but estimated to be between 1,500 and 2,000. The number of CDG subtypes that are actually known has reached a count of 125 and is steadily increasing (Jaeken and Péanne 2017). These facts make it difficult to estimate the representative participation of CDG patients overall and of all CDG subtypes to this questionnaire. One hundred and fifty-five participants completed the questionnaire, representing 21 CDG types, and each CDG (apart from PMM2-CDG) was represented by only a few participants. This urged us to focus on a sub-analysis of PMM2-CDG, the CDG with the highest number of participants (76.1%). To increase the total number of participants and of CDG subtypes in future work, the recruitment strategy could be refined, and additional languages could be used.
This is a patient-oriented questionnaire focused on the patient/caregiver perspective. Due to the lack of a structured platform to keep files confidentially and in a secure way, medical records and pathology confirmation were not reviewed for confirmation of the reported data.
Comparison Between LeQCDG and Literature Data
The major liver disease symptoms identified, within all CDG or within the subgroup of PMM2-CDG patients, were hepatomegaly, increased transaminases, liver fibrosis, steatosis and cirrhosis and changes in coagulation factors. These are the same symptoms as we found in our recent literature review (Marques-da-Silva et al. 2017a). The good correlation between both methods (Fig. 3) even for symptoms with lower prevalence (up to 30% in cirrhosis, fibrosis and steatosis) indicates that the population sample in the query was at least as adequate as that in the medical literature, to allow differentiation of patients with less common symptoms.
The data from the LeQCDG show prevalence to be slightly higher to those from the literature review, except for increased transaminases. This is probably because not all CDG patients with liver disease are reported, leading to fewer reports for hepatomegaly, liver fibrosis, steatosis and cirrhosis in the literature than actually found in the population. Regarding serum transaminases, the higher incidence of increased levels obtained in literature may be due, at least in some cases, to non-hepatic conditions. Liver transplantation was suggested for PMM2-CDG and MPI-CDG patients, indicating that liver disease in CDG can become life-threatening. Inversely, liver disease seems to have no or minimal impact on the patient’s wellbeing and functioning in the large majority of patients which can be related to the low severity of liver disease-associated symptoms for CDG patients with liver disease described in this questionnaire. This is in accordance with previous studies that correlated quality of life and liver disease severity (Younossi et al. 2001; Saffari et al. 2016; Neijenhuis et al. 2016). This validates the experience of LeQCDG participants to evaluate CDG patients’ quality of life.
The fact that 90% of the LeQCDG participants knew that CDG may affect the liver and more than half of the participants will try to learn more about liver disease in CDG in the next medical check-up of the CDG patient is important in the context of patient empowerment. In fact, in an open question requesting additional data from blood analysis, some participants added quantitative values for specific parameters, e.g. transaminases. This detailed information means we can have confidence in the respondents’ knowledge about the CDG patient they are reporting on.
CDG Electronic Questionnaires as an Additional Source of Patient Information
The Internet, as a fast source of information and with the possibility to share health information, represents a potent tool for collecting data from families of children affected by rare diseases (Tozzi et al. 2013), such as CDG. Internet-based electronic questionnaires are increasingly being used among the rare diseases community to better understand, for example, the disease impact in terms of health (Molster et al. 2016; Price et al. 2016) and economy (Chevreul et al. 2016). In this electronic questionnaire, besides the information about clinical symptoms related to liver disease and its impact on the wellbeing of patients, relevant data regarding CDG awareness and information related to the disease was also gathered. In doing so, we learned that CDG awareness and information are lacking within this community meaning that there is a need to educate physicians and create public awareness on CDG. However, we observed that regarding clinical information in the LeQCDG, the families presented additional information that is not described in the literature. This adds value to available medical CDG information.
Conclusions
The results obtained with this online questionnaire confirmed the data obtained in the previous literature review (Marques-da-Silva et al. 2017a) in terms of prevalence, severity and phenotype of liver disease, with a high incidence of hepatomegaly and increased levels of serum transaminases. In addition, the consequences of liver disease on the patient’s wellbeing and the emotional impact are considered minor, although physical health and emotional wellbeing of the CDG patient are both important concerns.
The information was collected in 42 days and confirmed data representing 25 years of scientific publications dedicated to CDG patients. It should be noted that all the information was given by the participants that were mostly family caregivers. This work demonstrated that the participants are empowered and capable of completing validated tools about CDG, clinical symptoms and their impact on the patient’s quality of life.
We believe that the recent vision on patient-centered health will move patient associations to embrace patient/caregiver-driven research within their community. Our study shows that geographic limitations can be overcome and that large amounts of meaningful, representative clinical and patient-oriented data can be collected, in a short period of time, when patient advocacy groups, families and professionals (CDG & Allies – PPAIN) work closely together. These findings and the model of this questionnaire can be especially relevant for other ultra-rare diseases.
Note
This study is a result of a collaborative study between patient advocacy groups, families and professionals (CDG Professionals and Patient Associations International Network; CDG & Allies – PPAIN).
Electronic Supplementary Material
■■■ (DOCX 1092 kb)
Acknowledgements
Dorinda Marques-da-Silva acknowledges the support from the Rare Disease Foundation’s microgrant and ‘Liliana Scientific Scholarship’; Rita Francisco acknowledges Fundação para a Ciência e Tecnologia for the grant SFRH/BD/124326/2016 awarded to her.
We also thank the CDG & Allies – Professionals and Patient Associations International Network (CDG & Allies PPAIN), whose network expertise greatly helped in the creation of this manuscript.
Abbreviations
- CDG & Allies
PPAIN – CDG Professionals and Patient Associations International Network
- CDG
Congenital disorder(s) of glycosylation
- CLDQ
Chronic liver disease questionnaire
- ePROs
Electronic patient-reported outcomes
- HQLQ
Hepatitis quality-of-life questionnaire
- LDQoL
Liver disease quality-of-life questionnaire
- LDSI 2.0
Liver disease symptom index 2.0
- LeQCDG
Liver electronic questionnaire for CDG
- PLD-Q
Polycystic liver disease-specific symptom questionnaire
- pLTQ
Post-liver transplant quality of life
- PROs
Patient-reported outcomes
- QoL
Quality of life
Synopsis
Liver involvement in CDG was accessed through a CDG family-targeted online questionnaire based on a recent literature review. Main symptoms reported included hepatomegaly, increased levels of serum transaminases, fibrosis, steatosis and cirrhosis. Electronic questionnaires can boost knowledge on rare diseases, improving possible treatments and management. This is also useful with regard to future clinical trials.
Compliance with Ethics Guidelines
Conflict of Interests
Vanessa dos Reis Ferreira is the president and founder of the Portuguese Association for CDG and other Rare Metabolic Diseases (APCDG-DMR). All other authors declare no competing financial interests.
Details of the Contributions of Individual Authors
Dorinda Marques-da-Silva participated in planning, conducted the work, analysed data, conceived the article outline, participated in the conception and design, drafted all the manuscript including figures and tables, obtained final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Rita Francisco participated in conducting the work; conceiving the article outline, conception and design; and drafting the manuscript.
Vanessa dos Reis Ferreira participated in planning and conducting the work; conceiving the article outline, conception and design; and critically revising it for important intellectual content.
Liz Forbat participated in conception and design and in critically revising it for important intellectual content.
Ricardo Lagoa helped in conception and design, data analysis and drafting the tables and participated in critically revising the manuscript for important intellectual content.
Paula A. Videira participated in planning the work and the conception, design and revision of the literature and in critically revising it for important intellectual content.
Peter Witters participated in conception and design and in critically revising the manuscript for important intellectual content.
Jaak Jaeken conceived in planning the work and the article outline; participated in the conception, design and analysis of the article; and was involved in drafting the manuscript and critically revising it for important intellectual content.
David Cassiman participated in planning the work and conceiving the article outline and the conception, design and analysis of the article and was involved in drafting the manuscript and in critically revising it for important intellectual content. He is the guarantor of the article, accepts full responsibility for the work submitted and controlled the decision to publish.
All authors gave final approval of the version to be published.
Ethical Guidelines, Human and Animal Rights and Consents
This work has been carried out in accordance with The Code of Ethics of the World Medical Association. Ethical approval for this study was obtained from the Ethics Committee at the Faculty of Psychology, Lisbon University (20/07/2016). Besides, the informed consent was obtained from participants, and the privacy rights of human subjects were ensured.
Funding
Dorinda Marques-da-Silva acknowledges the support from the Rare Disease Foundation’s microgrant and ‘Liliana Scientific Scholarship’; Rita Francisco acknowledges Fundação para a Ciência e Tecnologia for the grant SFRH/BD/124326/2016 awarded to her.
The authors confirmed independence from the sponsors; the content of the article has not been influenced by sponsors.
References
- Augustine EF, Adams HR, Mink JW. Clinical trials in rare disease: challenges and opportunities. J Child Neurol. 2013;28:1142–1150. doi: 10.1177/0883073813495959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budych K, Helms TM, Schultz C. How do patients with rare diseases experience the medical encounter? Exploring role behavior and its impact on patient-physician interaction. Health Policy. 2012;105:154–164. doi: 10.1016/j.healthpol.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Burton-Chase AM, Parker WM, Hennig K, et al. The use of social media to recruit participants with rare conditions: lynch syndrome as an example. JMIR Res Protoc. 2017;6:e12. doi: 10.2196/resprot.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevreul K, Gandré C, Brigham KB, et al. Social/economic costs and health-related quality of life in patients with fragile X syndrome in Europe. Eur J Health Econ. 2016;17(Suppl 1):43–52. doi: 10.1007/s10198-016-0784-3. [DOI] [PubMed] [Google Scholar]
- Coons SJ, Gwaltney CJ, Hays RD, et al. Recommendations on evidence needed to support measurement equivalence between electronic and paper-based patient-reported outcome (PRO) measures: ISPOR ePRO good research practices task force report. Value Health. 2009;12:419–429. doi: 10.1111/j.1524-4733.2008.00470.x. [DOI] [PubMed] [Google Scholar]
- D’Ambrosi R, Ragone V, Caldarini C, et al. The impact of hereditary multiple exostoses on quality of life, satisfaction, global health status, and pain. Arch Orthop Trauma Surg. 2017;137:209–215. doi: 10.1007/s00402-016-2608-4. [DOI] [PubMed] [Google Scholar]
- Davies W. Insights into rare diseases from social media surveys. Orphanet J Rare Dis. 2016;11:1–5. doi: 10.1186/s13023-016-0532-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhart M, Ellert U, Kurth BM, Ravens-Sieberer U. Measuring adolescents’ HRQoL via self reports and parent proxy reports: an evaluation of the psychometric properties of both versions of the KINDL-R instrument. Health Qual Life Outcomes. 2009;7:77. doi: 10.1186/1477-7525-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysenbach G. Improving the quality of web surveys: the checklist for reporting results of Internet E-surveys (CHERRIES) J Med Internet Res. 2004;6(3):e34. doi: 10.2196/jmir.6.3.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner Y, Garland SM, Moore EE, et al. Web-based recruiting for health research using a social networking site: an exploratory study. J Med Internet Res. 2012;14:e20. doi: 10.2196/jmir.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco R, Pascoal C, Marques-da-Silva D, Morava E, Gole GA, Coman DJJ (2018) Keeping an eye on congenital disorders of O-glycosylation. A systematic literature review. J Inherit Metab Dis. 10.1007/s10545-017-0119-2 [DOI] [PubMed]
- Geissler J, Sharf G, Bombaci F, et al. Factors influencing adherence in CML and ways to improvement: results of a patient-driven survey of 2546 patients in 63 countries. J Cancer Res Clin Oncol. 2017;143:1167–1176. doi: 10.1007/s00432-017-2372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteling JJ, de Man RA, Busschbach JJ, Darlington AS. Overview of research on health-related quality of life in patients with chronic liver disease. Neth J Med. 2007;65:227–234. [PubMed] [Google Scholar]
- Jaeken J, Péanne R. What is new in CDG? J Inherit Metab Dis. 2017;40(4):569–586. doi: 10.1007/s10545-017-0050-6. [DOI] [PubMed] [Google Scholar]
- Jaeken J, Vanderschueren-Lodeweyckx M, Casaer P, et al. Familial psychomotor retardation with markedly fluctuating serum prolactin, FSH and GH levels, partial TBG deficiency, increased serum arylsulfatase A and increased CSF protein: a new syndrome. Pediatr Res. 1980;14:179. doi: 10.1203/00006450-198002000-00117. [DOI] [Google Scholar]
- Lachin JM. Introduction to sample size determination and power analysis for clinical trials. Control Clin Trials. 1981;2:93–113. doi: 10.1016/0197-2456(81)90001-5. [DOI] [PubMed] [Google Scholar]
- Malcolm C, Hain R, Gibson F, et al. Challenging symptoms in children with rare life-limiting conditions: findings from a prospective diary and interview study with families. Acta Paediatr Int J Paediatr. 2012;101:985–992. doi: 10.1111/j.1651-2227.2012.02680.x. [DOI] [PubMed] [Google Scholar]
- Marques-da-Silva D, dos Reis Ferreira V, Monticelli M, et al. Liver involvement in congenital disorders of glycosylation (CDG). A systematic review of the literature. J Inherit Metab Dis. 2017;40:195. doi: 10.1007/s10545-016-0012-4. [DOI] [PubMed] [Google Scholar]
- Marques-da-Silva D, Francisco R, Webster D, et al. Cardiac complications of congenital disorders of glycosylation (CDG): a systematic review of the literature. J Inherit Metab Dis. 2017;40:657. doi: 10.1007/s10545-017-0066-y. [DOI] [PubMed] [Google Scholar]
- Matza LS, Patrick DL, Riley AW, et al. Pediatric patient-reported outcome instruments for research to support medical product labeling: report of the ISPOR PRO good research practices for the assessment of children and adolescents task force. Value Health. 2013;16:461–479. doi: 10.1016/j.jval.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Molster C, Urwin D, Di Pietro L, et al. Survey of healthcare experiences of Australian adults living with rare diseases. Orphanet J Rare Dis. 2016;11:30. doi: 10.1186/s13023-016-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli M, Ferro T, Jaeken J, et al. Immunological aspects of congenital disorders of glycosylation (CDG): a review. J Inherit Metab Dis. 2016;39:765–780. doi: 10.1007/s10545-016-9954-9. [DOI] [PubMed] [Google Scholar]
- Mucci S, Citero VA, Gonzalez AM, et al. Cross-cultural adaptation of the Chronic Liver Disease Questionnaire (CLDQ) to the Brazilian population. Cad Saude Publica. 2010;26:199–205. doi: 10.1590/S0102-311X2010000100021. [DOI] [PubMed] [Google Scholar]
- Neijenhuis MK, Gevers TJG, Hogan MC, et al. Development and validation of a disease-specific questionnaire to assess patient-reported symptoms in polycystic liver disease. Hepatology. 2016;64:151–160. doi: 10.1002/hep.28545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradovic M, Gluvic Z, Petrovic N, et al. A quality of life assessment and the correlation between generic and disease-specific questionnaires scores in outpatients with chronic liver disease-pilot study. Rom J Intern Med. 2017;55:129–137. doi: 10.1515/rjim-2017-0014. [DOI] [PubMed] [Google Scholar]
- Paulsen A, Pedersen AB, Overgaard S, Roos E. Feasibility of four patient reported outcome measures in the Danish Hip Arthroplasty Registry. A cross-sectional study of 6000 patients. Hip Int. 2010;20:354. [Google Scholar]
- Price MA, Barghout V, Benveniste O, et al. Mortality and causes of death in patients with sporadic inclusion body myositis: survey study based on the clinical experience of specialists in Australia, Europe and the USA. J Neuromuscul Dis. 2016;3:67–75. doi: 10.3233/JND-150138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saab S, Ng V, Landaverde C, et al. Development of a disease-specific questionnaire to measure health-related quality of life in liver transplant recipients. Liver Transplant. 2011;17:567–579. doi: 10.1002/lt.22267. [DOI] [PubMed] [Google Scholar]
- Saffari M, Alavian SM, Naderi MK, et al. Cross-cultural adaptation and psychometric assessment of the liver disease symptom index 2.0 to measure health-related quality of life among Iranian patients with chronic hepatitis B. J Transcult Nurs. 2016;27:496–508. doi: 10.1177/1043659615577698. [DOI] [PubMed] [Google Scholar]
- Schumacher KR, Stringer KA, Donohue JE, et al. Social media methods for studying rare diseases. Pediatrics. 2014;133:e1345–e1353. doi: 10.1542/peds.2013-2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topolovec-Vranic J, Natarajan K. The use of social media in recruitment for medical research studies: a scoping review. J Med Internet Res. 2016;18:e286. doi: 10.2196/jmir.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi AE, Mingarelli R, Agricola E, et al. The Internet user profile of Italian families of patients with rare diseases: a web survey. Orphanet J Rare Dis. 2013;8:76. doi: 10.1186/1750-1172-8-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Plas SM, Hansen BE, De Boer JB, et al. Generic and disease-specific health related quality of life of liver patients with various aetiologies: a survey. Qual Life Res. 2007;16:375–388. doi: 10.1007/s11136-006-9131-y. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Boparai N, Price LL, et al. Health-related quality of life in chronic liver disease: the impact of type and severity of disease. Am J Gastroenterol. 2001;96:2199–2205. doi: 10.1111/j.1572-0241.2001.03956.x. [DOI] [PubMed] [Google Scholar]
- Zeltner NA, et al. Development and validation of a quality of life questionnaire for paediatric patients with intoxication-type inborn errors of metabolism. J Inherit Metab Dis. 2016;39:S133. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
■■■ (DOCX 1092 kb)