Abstract
Background: Mutations in SLC25A4 (syn. ANT1, Adenine nucleotide translocase, type 1) are known to cause either autosomal dominant progressive external ophthalmoplegia (adPEO) or recessive mitochondrial myopathy, hypertrophic cardiomyopathy, and lactic acidosis.
Methods and Results: Whole exome sequencing in a young man with myopathy, subsarcolemmal mitochondrial aggregations, cardiomyopathy, lactic acidosis, and L-2-hydroxyglutaric aciduria (L-2-HGA) revealed a new homozygous mutation in SLC25A4 [c.653A>C, NM_001151], leading to the replacement of a highly conserved glutamine by proline [p.(Q218P); NP_001142] that most likely affects the folding of the ANT1 protein. No pathogenic mutation was found in L2HGDH, which is associated with “classic” L-2-HGA. Furthermore, L-2-HGDH enzymatic activity in the patient fibroblasts was normal. Long-range PCR and Southern blot confirmed absence of mtDNA-deletions in blood and muscle.
Conclusion: The disturbed ADP/ATP transport across the inner mitochondrial membrane may lead to an accumulation of different TCA-cycle intermediates such as 2-ketoglutarate (2-KG) in our patient. As L-2-HG is generated from 2-KG we hypothesize that the L-2-HG increase is a secondary effect of 2-KG accumulation. Hence, our report expands the spectrum of laboratory findings in ANT1-related diseases and hints towards a connection with organic acidurias.
Electronic supplementary material
The online version of this article (10.1007/8904_2018_93) contains supplementary material, which is available to authorized users.
Keywords: Cardiomyopathy, Giant mitochondria, L-2-hydroxyglutaric aciduria, Mitochondrial disease, Myopathy, Ragged-red-fibers
Introduction
ANT (Adenine nucleotide translocase) is a solute carrier which is embedded in the inner mitochondrial membrane and exchanges matrix ATP for cytosolic ADP, thereby channeling mitochondrial energy to the cytosol. Dysfunction of this transporter is expected to dramatically reduce the mitochondrial energy production. Human ANT occurs in four tissue specific isoforms (ANT1–4), which are encoded by four closely related nuclear genes (Stepien et al. 1992; Dolce et al. 2005). ANT1 (SLC25A4) is mainly expressed in heart and skeletal muscle.
Mutations in SLC25A4 can lead to three different clinical phenotypes. Heterozygous mutations can cause adult-onset autosomal dominant progressive external ophthalmoplegia (adPEO, OMIM #609283) (Napoli et al. 2001; Komaki et al. 2002; Deschauer et al. 2005; Thompson et al. 2016) or severe early-onset mitochondrial disease (OMIM #617184) (Thompson et al. 2016), whereas autosomal recessively inherited SLC25A4 mutations cause childhood-onset mitochondrial myopathy with hypertrophic cardiomyopathy (OMIM #615418) (Palmieri et al. 2005; Echaniz-Laguna et al. 2012; Strauss et al. 2013; Körver-Keularts et al. 2015). Dominant adPEO SLC25A4 mutations have been shown to affect protein folding with subsequent protein aggregation and augmented proteostatic stress leading to reduced cell viability in the yeast model (Liu et al. 2015), while biallelic SLC25A4 mutations predominantly act via the loss of ATP-transport activity (Palmieri et al. 2005).
Patients with autosomal recessive and dominant SLC25A4 mutations show a time-dependent accumulation of multiple mitochondrial DNA deletions, which have been documented in muscle of man and mouse (Esposito et al. 1999; Palmieri et al. 2005; Echaniz-Laguna et al. 2012). This mtDNA instability, which has been associated with excessive reactive oxygen species production (Esposito et al. 1999) and nucleotide imbalance (Kaukonen et al. 2000) might contribute to a more progressive disease course.
Until now, compound heterozygous p.(Q39Lfs*14)|p.(R236P), homozygous p.(A123D) and homozygous c.111+1G>A SCL25A4 mutations have been described in single patients (Palmieri et al. 2005; Echaniz-Laguna et al. 2012; Körver-Keularts et al. 2015) and the homozygous p.(Q175Rfs*38) mutation 10 affected members of a large Mennonite family. Interestingly, in the latter family faster progression of the heart disease was associated with the mtDNA haplogroup U. In contrast, more mildly affected members carried the haplogroup H (Strauss et al. 2013).
Here we describe a new missense mutation in SCL25A4 in a patient with hypertrophic cardiomyopathy, myopathy, and lactic acidosis, who additionally exhibited a mild L-2-hydroxyglutaric-aciduria.
Case History
The now 24-year-old male patient of Lebanese origin is the second child of healthy consanguineous parents who are first degree cousins. Two sisters and a brother are healthy. He was born at term after normal pregnancy with normal length and birth weight. His motor development was retarded and he only achieved independent walking by the age of 2.5 years. He complained of easy fatigability and muscle pain since early childhood.
By the age of 16 years, he was unable to walk more than 500 m; climbing the stairs to his third floor apartment was progressively difficult. Physical examination revealed generalized muscle wasting and symmetrical weakness. His body-length was 3 cm and his weight 13 kg below the third percentile. Deep tendon reflexes were weak and Gower’s sign or contractures were absent. Puberty was delayed (G3) and testosterone values were pre-pubertal (0.6 ng/mL, normal range 2.5–9.0). Neuropsychological testing revealed a total IQ of 81. Serum lactate and pyruvate levels were elevated (lactate 31–96 mg/dL, normal range <19; pyruvate 1.9–2.9 mg/dL, normal range <0.9) but serum creatine phosphokinase (CPK) levels were normal. The acylcarnitine profile was normal. Repeated urine examinations at the age of 14, 16, and 20 years revealed elevated L-2-hydroxyglutaric acid (L-2-HG) excretion (66–98 mmol/mol creatinine, normal range 1.2–18.9), but L-2-hydroxyglutarate dehydrogenase (L-2-HGDH) enzyme activity in fibroblasts was normal. Urine excretion of D-2-hydroxyglutaric acid (D-2-HG) was below the normal range and 2-ketoglutaric acid (2-KG) excretion was elevated. Urine excretion of lactate, pyruvate, acetoacetate, and alanine was massively increased.
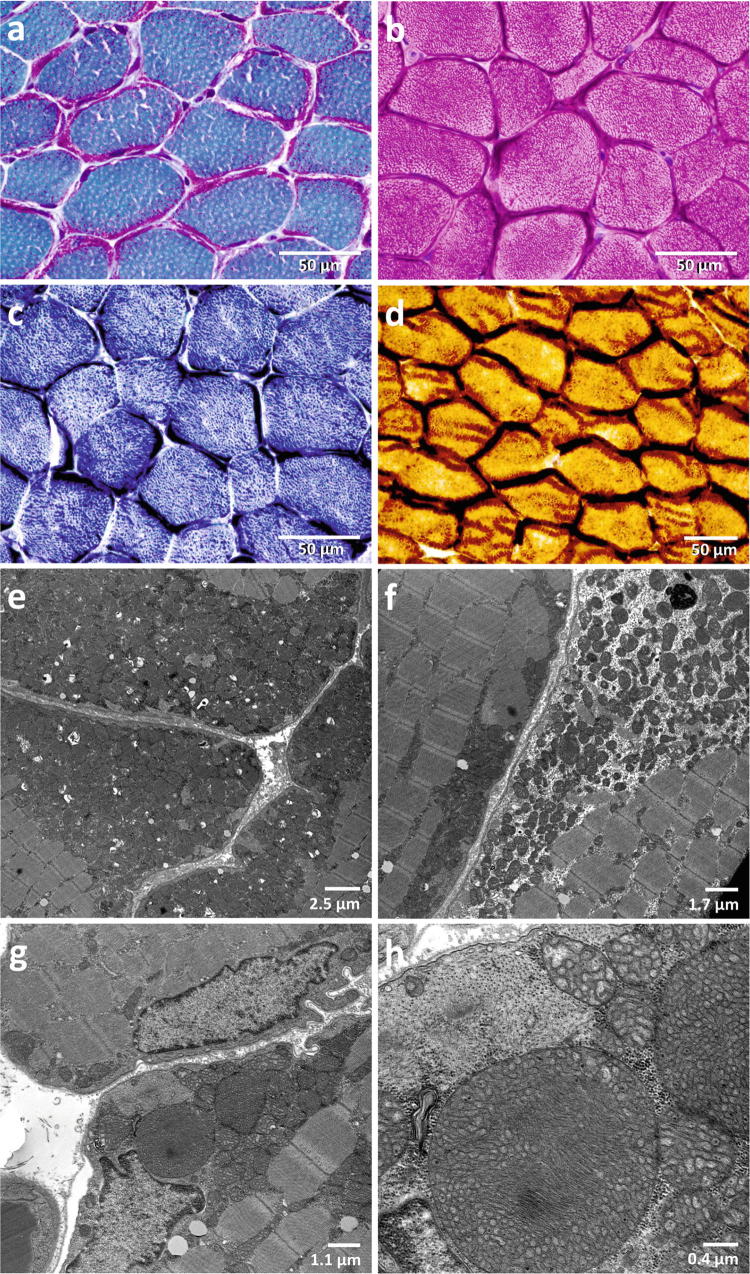
Regular follow-up visits revealed non-progressive, non-obstructive left ventricular hypertrophy with a left ventricular shortening fraction of 40–53% and a restrictive respiratory disorder with a forced vital capacity of 56%. Sensory and motor nerve conduction studies and the cranial MRI were normal. Muscle biopsy at the age of 5 and 16 years showed massive subsarcolemmal accumulations of mitochondria (Fig. 1), and the presence of giant mitochondria on electron microscopy (Fig. 1g, h). Activities of the isolated mitochondrial respiratory chain complexes I and IV in muscle tissue were normal, activity of the citrate synthase was increased (354 mU/mg protein; normal range 45–187), which could be an indirect marker for mitochondrial proliferation. No deletions could be detected in the mtDNA of muscle and blood, neither by long-range PCR nor by Southern blot, and no mtDNA mutations were present in the NGS dataset.
Fig. 1.
Light and electron microscopy of the patient muscle. The muscle biopsy specimen was obtained at the age of 16 years. The muscle shows a massive presence of ragged-red fibers, which are lined by subsarcolemmal mitochondrial accumulations, which impose purple in the Gömöri trichrome stain (a), dark red in the haematoxylin eosin (HE) stain (b), dark blue in the succinate dehydrogenase (SDH) stain (c), and dark brown in the cytochrome c oxidase (COX) stain (d). Electron microscopic images show massive accumulations of mitochondria below the sarcolemma (e) and between the myofibrils (f); presence of giant mitochondria in the muscle measuring up to 3 μm in diameter (g, h)
Material and Methods
Autozygosity Mapping and Whole Exome Sequencing
DNA was extracted from peripheral blood leukocytes according to standard procedures. Exonic sequences were enriched using the Agilent SureSelect V4 Human All Exon 51 Mb Kit (Agilent Technologies). Sequencing was performed on a HiSeq2000 machine (Illumina), which produced 55.5 million 100 bp paired-end reads. The combined paired-end FASTQ files were aligned to the human GRCh37.p11 (hg19/Ensembl 72) genomic sequence using the BWA-MEM V.0.7.1 aligner. The mean coverage was 127.24x, 99.7% of the enriched bases were covered >3x and 97.6% >20x. The raw alignments were fine adjusted and called for deviations from the human reference sequence (GRCh37.p11) in all exonic ±50 bp flanking regions using the Genome Analysis Toolkit (GATK v3.8) software package. The resulting variant (VCF) file comprised 90,903 variants and was sent to the MutationTaster Query Engine software (http://www.mutationtaster.org/StartQueryEngine.html) for assessment of potential pathogenicity. The filtering options were used as described (von Renesse et al. 2014). All relevant variants were inspected visually using the Integrative Genomics Viewer (IGV, http://www.broadinstitute.org/igv/). The c.653A>C mutation in SLC25A4 was verified by Sanger sequencing using the BigDye v3.1 chemistry with the oligonucleotide primers FOR 5′-TGGTCATATGTGAAGCACCTG-3′, REV 5′-TCCAGTAAGGAAGACTGAAAGGA-3′.
Due to consanguinity of the parents we performed autozygosity mapping with the resulting VCF file from WES in order to further restrict the genomic region in which the mutation would be located using our HomozygosityMapper2012 software (Seelow and Schuelke 2012) at http://www.homozygositymapper.org. All potential pathogenic variants within the autozygous regions are discussed in Supplementary Table 1.
MtDNA Deletion Screening
Southern blot and long-range PCR screening for mtDNA-deletions in muscle and blood of the patient along with samples from patients with Kearns-Sayre syndrome and normal controls was performed as described (Tang et al. 2000; Lorenz et al. 2017). Briefly, for Southern blot DNA was incubated with the restriction endonuclease Pvu II, separated by 1% agarose gel electrophoresis and blotted on a Hybond® membrane. MtDNA-fragments were detected by 32P-labeled single stranded DNA probes that had been transcribed from human mtDNA using the Klenow fragment. For long-range PCR, two overlapping long-range PCR fragments were generated from whole DNA extracts of muscle and blood using a proof-reading long-range polymerase (Roche) and two oligonucleotide primer sets FOR#1: 5′-CCCTCTCTCCTACTCCTG-3′, REV#1: 5′-CAGGTGGTCAAGTATTTATGG-3′ (PCR-product size 9,932 bp), and FOR#2: 5′-CTTTATCTGCCTCTTCCTACACATCG-3′, REV#2: 5′-GTATGTAGGAGTTGAAGATTAGTCCGCC-3′ (PCR-product size 9,506 bp), which were separated on an 0.8% agarose gel along with control samples and a high molecular size standard. Two different thermocycler protocols were used, one with an elongation time of 10 min and one with a shorter elongation time of 4 min, favoring shorter deleted mtDNA molecules, if present (Fig. 2).
Fig. 2.
Screening for mtDNA-deletions in DNA extracts from muscle and blood of the patient. (a) Two overlapping long-range PCR fragments were generated that span the entire mtDNA. The shorter elongation time of 4 min amplifies preferentially the shorter fragments as seen in the samples of two patients with Kearns-Sayre Syndrome (KSS). (b) Position of the primers and the mtDNA coverage of the long-range PCR products. (c) Southern blot autoradiography of Pvu II digested DNA labeled with a radioactive mtDNA probe. *Other diagnostic samples not related to the study
Biochemistry
The D- and L-enantiomers of 2-hydroxyglutarate were determined in urine as published previously (Struys et al. 2004).
Results
Mutation Analysis
In our patient we identified a new homozygous missense mutation in exon 3 of the SLC25A4 gene [chr4:g.186,066,967A>C, c.653A>C, NM_001151], which leads to the substitution of a glutamine at position 218 for a proline [p.(Q218P), NP_001142]. This variant was neither listed in the 1000 Genomes Project (The 1000 Genomes Project Consortium 2015) nor the exomes of 60,706 unrelated individuals of the Exome Aggregation Consortium (ExAC) or in the exomes/genomes of in 138,000 individuals from the Genome Aggregation Database (gnomAD) (Lek et al. 2016). The glutamine at position 218 is highly conserved among different species including Xenopus tropicalis (Fig. 3c). Unfortunately we were unable to confirm the genotype-phenotype segregation in the family as no blood samples of parents and healthy siblings were available. Long-range PCR and Southern blot excluded single or multiple mtDNA-deletions in the patient’s muscle and blood.
Fig. 3.
Molecular and biochemical findings in the patient. (a) Results of the autozygosity mapping of the patient, a child of consanguineous parents. The red bars depict the autozygous genomic blocks of a total of 55.6 Mbp, which likely contain the disease mutation. Potentially pathogenic homozygous variants in the autozygous blocks are discussed in Supplementary Table 1. Mitochondria associated genes within the autozygous blocks (n = 17 from a total of 1,130 “mitochondrial” genes) are also listed on Supplementary Table 1. (b) Sequence electropherograms of the patient and of a wildtype control depicting the localization of the homozygous mutation. (c) High evolutionary conservation of the mutated amino acid down to Caenorhabditis elegans. (d) Biochemical flow scheme of the tricarboxylic acid cycle (TCA) and enzymes that act downstream of 2-ketoglutarate (2-KG), which is an intermediate of the TCA. GHB γ-hydroxybutyrate, SSA succinic semialdehyde, HOT hydroxyacid-oxoacid transhydrogenase, NAD nicotinamide adenine dinucleotide, L-malDH L-malate dehydrogenase, FAD flavin adenine dinucleotide, D-2-HGDH D-2-hydroxyglutarate dehydrogenase, L-2-HGDH L-2-hydroxyglutarate dehydrogenase
Interestingly, no disease causing gene variant was found in the L-2-hydroxyglutarate dehydrogenase gene (L2HGDH), which confirms the result of normal enzymatic activity of the L-2-HGDH in fibroblasts.
mtDNA Haplotype Analysis
The mtDNA haplotype was determined by uploading the VCF file to the HaploGrep2 (v.2.1.1) software on the internet (https://haplogrep.uibk.ac.at) (Weissensteiner et al. 2016). The mtDNA variants of the patient are listed on Supplementary Table 2. This mtDNA haplotype was K1, which is a subgroup of mtDNA haplogroup U8b, with m.4640C>T, m.9647T>C, and m.11017T>C as private variants. All positions were visually inspected on the BAM file to exclude sequencing or alignment errors.
Enzyme Diagnostics
Due to the constantly elevated L-2-HG levels we determined the activity of the L-2-HGDH in the patient fibroblasts according to published protocols (Kranendijk et al. 2009), which yielded results within the reference range of normal controls.
Discussion
ANT is one of the most abundant mitochondrial proteins and humans have four tissue specific isoforms, encoded by four different genes: SLC25A4 (ANT1) is mainly expressed in heart and skeletal muscle, SLC25A6 (ANT3) is expressed in all tissues, at levels depending on the state of oxidative metabolism, SLC25A5 (ANT2) is expressed in kidney, liver, and spleen, but also in cancer cells (Stepien et al. 1992), and SLC25A31 (ANT4) is mainly expressed in testis and male germ cells (Dolce et al. 2005). Until now, 13 patients with autosomal recessive mutations in SLC25A4 have been described (Palmieri et al. 2005; Echaniz-Laguna et al. 2012; Strauss et al. 2013; Körver-Keularts et al. 2015), all suffering from mitochondrial myopathy, cardiomyopathy, and exercise intolerance.
We report here on a patient with a similar spectrum of clinical symptoms and a novel mutation in SLC25A4 (c.653A>C), which leads to a proline for glutamine substitution. The glutamine at position 218 of the amino acid chain is highly evolutionary conserved down to Caenorhabditis elegans (Fig. 3c) and it is located in the third of five transmembrane helices of the protein. 218Pro most probably affects folding of a highly conserved α-helical transmembrane domain through steric hindrance. According to the ACMG guidelines (Richards et al. 2015) the novel mutation can be classified as “likely pathogenic” with “moderate to strong evidence for pathogenicity” (evidence levels: 1x strong PS3, 1x moderate PM2, 2x supporting PP3 and PP4).
In contrast to previously described patients with recessive SLC25A4 mutations, we did not find any mtDNA-deletions in muscle tissue and blood by the age of 16 years. However, following the evidence of Strauss et al. (2013), the mtDNA haplotype U of our patient would be a risk factor for more severe a disease progression.
Repeated urine-testing revealed elevated L-2-HG levels in our patient. Interestingly, these metabolic abnormalities did not match with those typically seen in classic L-2-hydroxyglutaric aciduria (L-2-HGA, OMIM#236792), which is an autosomal-recessive encephalopathy manifesting with developmental delay, epilepsy, and cerebellar ataxia with abnormalities of the subcortical white matter and basal ganglia on cMRI. L-2-HGA is caused by mutations in L2HGDH.
The reason for the absence of classic L-2-HGA related symptoms in our patient is probably due to the only mild increase of L-2-HG levels. His urine L-2-HG excretion was only increased fourfold, whereas L-2-HGDH deficient patients generally exhibit a larger, 10- to 300-fold increase.
L-2-HG is generated from 2-ketoglutarate (2-KG) in a side-reaction by the L-malate dehydrogenase (L-malDH), an enzyme of the TCA-cycle, which at the same time catalyzes the conversion of L-malate to oxaloacetate. L-2-HGDH reconverts L-2-HG to 2-KG to maintain carbon balance and prevent potential toxic effects (Fig. 3d). In the search for an explanation of the potentially reduced L-2-HGDH activity, we measured the enzyme activity in the patient’s fibroblasts but obtained normal results, which was in line with the absence of mutations in the coding sequence and flanking intronic regions of L2HGDH.
Other diseases with elevated 2-HG levels are often secondary to a primary accumulation of 2-KG (James et al. 2007). Increased levels of other Krebs cycle intermediates such as fumarate, malate, succinate, and citrate have been reported in ANT1-deficient mice and humans (Graham et al. 1997; Echaniz-Laguna et al. 2012): as the electron transport chain at the inner mitochondrial membrane (IMM) is directly coupled to the ATP-synthase (Complex V), a defect of the ADP/ATP-transport across the IMM leads to a net reduction of the electron transport through the respiratory chain thereby resulting in an NADH accumulation. The increased NADH/NAD+ ratio in turn slows down the Krebs cycle, building-up different Krebs cycle intermediates in the process.
Urine testing in our patient indeed revealed elevated 2-KG urine excretion. We now hypothesize that the increase of L-2-HGA in our patient might be a secondary effect of 2-KG accumulation due to ANT1-deficiency. Intriguingly, D-2-hydroxyglutarate (D-2-HG) showed levels below the normal range, whereas in the normal state one would expect to find combined D/L-2-hydroxyglutaric aciduria, because D-2-HG is equally formed from 2-KG, albeit by a different enzyme (hydroxyacid-oxoacid transhydrogenase, HOT). A possible explanation for our findings would be that L-malDH, which forms L-2-HG from 2-KG, uses NADH as a cofactor, while the reduction of 2-KG to D-2-HG is cofactor-independent (Kaufman et al. 1988a, b; Struys et al. 2005; Rzem et al. 2007). As a result of the described accumulation of NADH, the ratio of L-2-HG and D-2-HG might shift in favor of L-2-HG.
In summary, we report on a patient with a new homozygous missense mutation in SLC25A4 in a transmembrane domain of the protein. While the phenotype with hypertrophic cardiomyopathy, lactic acidosis, and exercise intolerance corresponds to those of other patients, we additionally found elevated L-2-HG urine levels despite normal L-2-HGDH activity, but no mtDNA-deletions. We speculated that increased L-2-HG excretion might be secondary to NADH accumulation in the mitochondrial matrix. More patients with SLC25A4 mutations have to be examined to see whether increased L-2-HG would be a constant feature of the disease.
Electronic Supplementary Material
■■■ (PDF 126 kb)
■■■ (PDF 60 kb)
Acknowledgments
The authors thank the patient for participation in the study and Angelika Zwirner for excellent technical assistance.
Synopsis
Mutations in SLC25A4, the gene encoding Adenine nucleotide translocase type 1 may be associated with increased urinary excretion of L-2-hydroxyglutaric acid and massive subsarcolemmal aggregations of mitochondria.
Compliance with Ethics Guidelines
Ethics Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (IRB of the Charité, EA2/107/14) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from the patient for being included in the study.
Conflict of Interest
All authors declare that they have no conflict of interest.
Funding
The project was funded by the Deutsche Forschungsgemeinschaft (SFB 665 TP C4), and the NeuroCure Center of Excellence (Exc 257).
Authors’ Contributions
Anja von Renesse analyzed and clinically verified the NGS data, interpreted the data, together with MS wrote the first draft of the manuscript,
Susanne Morales-Gonzalez performed mtDNA deletion screening, isolated DNA from patients muscle and blood,
Esther Gill performed DNA Sanger sequencing for confirmation of NGS results,
Gajja S. Salomons did the L-2- and D-2-HG measurements in the urine and the L-2-HGDH enzyme activity measurements in the patient fibroblasts,
Werner Stenzel performed the histological and electron microscopic analyses of the patient’s muscle,
Markus Schuelke conception of the study, investigated the patient, performed the bioinformatic analyses, did the genetic counseling of the family, together with AvR wrote the first draft of the manuscript.
All authors read the final version of the manuscript for intellectual content and consented to its publication.
Contributor Information
Anja von Renesse, Email: anja.von-renesse@charite.de.
Susanne Morales-Gonzalez, Email: susanne.morales-gonzalez@charite.de.
Esther Gill, Email: esther.gill@charite.de.
Gajja S. Salomons, Email: g.salomons@vumc.nl
Werner Stenzel, Email: werner.stenzel@charite.de.
Markus Schuelke, Email: markus.schuelke@charite.de.
References
- Deschauer M, Hudson G, Müller T, et al. A novel ANT1 gene mutation with probable germline mosaicism in autosomal dominant progressive external ophthalmoplegia. Neuromuscul Disord. 2005;15:311–315. doi: 10.1016/j.nmd.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Dolce V, Scarcia P, Iacopetta D, Palmieri F. A fourth ADP/ATP carrier isoform in man: identification, bacterial expression, functional characterization and tissue distribution. FEBS Lett. 2005;579:633–637. doi: 10.1016/j.febslet.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Echaniz-Laguna A, Chassagne M, Ceresuela J, et al. Complete loss of expression of the ANT1 gene causing cardiomyopathy and myopathy. J Med Genet. 2012;49:146–150. doi: 10.1136/jmedgenet-2011-100504. [DOI] [PubMed] [Google Scholar]
- Esposito LA, Melov S, Panov A, et al. Mitochondrial disease in mouse results in increased oxidative stress. Proc Natl Acad Sci U S A. 1999;96:4820–4825. doi: 10.1073/pnas.96.9.4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BH, Waymire KG, Cottrell B, et al. A mouse model for mitochondrial myopathy and cardiomyopathy resulting from a deficiency in the heart/muscle isoform of the adenine nucleotide translocator. Nat Genet. 1997;16:226–234. doi: 10.1038/ng0797-226. [DOI] [PubMed] [Google Scholar]
- James AW, Miranda SG, Culver K, et al. DOOR syndrome: clinical report, literature review and discussion of natural history. Am J Med Genet A. 2007;143A:2821–2831. doi: 10.1002/ajmg.a.32054. [DOI] [PubMed] [Google Scholar]
- Kaufman EE, Nelson T, Fales HM, Levin DM. Isolation and characterization of a hydroxyacid-oxoacid transhydrogenase from rat kidney mitochondria. J Biol Chem. 1988;263:16872–16879. [PubMed] [Google Scholar]
- Kaufman EE, Nelson T, Miller D, Stadlan N. Oxidation of γ-Hydroxybutyrate to succinic semialdehyde by a mitochondrial pyridine nucleotide-independent enzyme. J Neurochem. 1988;51:1079–1084. doi: 10.1111/j.1471-4159.1988.tb03071.x. [DOI] [PubMed] [Google Scholar]
- Kaukonen J, Juselius JK, Tiranti V, et al. Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science. 2000;289:782–785. doi: 10.1126/science.289.5480.782. [DOI] [PubMed] [Google Scholar]
- Komaki H, Fukazawa T, Houzen H, et al. A novel D104G mutation in the adenine nucleotide translocator 1 gene in autosomal dominant progressive external ophthalmoplegia patients with mitochondrial DNA with multiple deletions. Ann Neurol. 2002;51:645–648. doi: 10.1002/ana.10172. [DOI] [PubMed] [Google Scholar]
- Körver-Keularts IMLW, de Visser M, Bakker HD, et al. Two novel mutations in the SLC25A4 gene in a patient with mitochondrial myopathy. JIMD Rep. 2015;22:39–45. doi: 10.1007/8904_2015_409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranendijk M, Salomons GS, Gibson KM, et al. Development and implementation of a novel assay for l-2-hydroxyglutarate dehydrogenase (l-2-HGDH) in cell lysates: l-2-HGDH deficiency in 15 patients with l-2-hydroxyglutaric aciduria. J Inherit Metab Dis. 2009;32:713. doi: 10.1007/s10545-009-1282-x. [DOI] [PubMed] [Google Scholar]
- Lek M, Karczewski KJ, Minikel EV, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–291. doi: 10.1038/nature19057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang X, Chen XJ. Misfolding of mutant adenine nucleotide translocase in yeast supports a novel mechanism of Ant1-induced muscle diseases. Mol Biol Cell. 2015;26:1985–1994. doi: 10.1091/mbc.E15-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz C, Lesimple P, Bukowiecki R, et al. Human iPSC-derived neural progenitors are an effective drug discovery model for neurological mtDNA disorders. Cell Stem Cell. 2017;20:659–674.e9. doi: 10.1016/j.stem.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Napoli L, Bordoni A, Zeviani M, et al. A novel missense adenine nucleotide translocator-1 gene mutation in a Greek adPEO family. Neurology. 2001;57:2295–2298. doi: 10.1212/WNL.57.12.2295. [DOI] [PubMed] [Google Scholar]
- Palmieri L, Alberio S, Pisano I, et al. Complete loss-of-function of the heart/muscle-specific adenine nucleotide translocator is associated with mitochondrial myopathy and cardiomyopathy. Hum Mol Genet. 2005;14:3079–3088. doi: 10.1093/hmg/ddi341. [DOI] [PubMed] [Google Scholar]
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzem R, Vincent M-F, Schaftingen EV, Veiga-da-Cunha M. L-2-hydroxyglutaric aciduria, a defect of metabolite repair. J Inherit Metab Dis. 2007;30:681. doi: 10.1007/s10545-007-0487-0. [DOI] [PubMed] [Google Scholar]
- Seelow D, Schuelke M. HomozygosityMapper2012 – bridging the gap between homozygosity mapping and deep sequencing. Nucleic Acids Res. 2012;40:W516–W520. doi: 10.1093/nar/gks487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien G, Torroni A, Chung AB, et al. Differential expression of adenine nucleotide translocator isoforms in mammalian tissues and during muscle cell differentiation. J Biol Chem. 1992;267:14592–14597. [PubMed] [Google Scholar]
- Strauss KA, DuBiner L, Simon M, et al. Severity of cardiomyopathy associated with adenine nucleotide translocator-1 deficiency correlates with mtDNA haplogroup. Proc Natl Acad Sci. 2013;110:3453–3458. doi: 10.1073/pnas.1300690110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struys EA, Jansen EEW, Verhoeven NM, Jakobs C. Measurement of urinary D- and L-2-hydroxyglutarate enantiomers by stable-isotope-dilution liquid chromatography–tandem mass spectrometry after derivatization with diacetyl-L-tartaric anhydride. Clin Chem. 2004;50:1391–1395. doi: 10.1373/clinchem.2004.033399. [DOI] [PubMed] [Google Scholar]
- Struys EA, Verhoeven NM, Brink HJT, et al. Kinetic characterization of human hydroxyacid–oxoacid transhydrogenase: relevance toD-2-hydroxyglutaric and γ-hydroxybutyric acidurias. J Inherit Metab Dis. 2005;28:921–930. doi: 10.1007/s10545-005-0114-x. [DOI] [PubMed] [Google Scholar]
- Tang Y, Schon EA, Wilichowski E, et al. Rearrangements of human mitochondrial DNA (mtDNA): new insights into the regulation of mtDNA copy number and gene expression. Mol Biol Cell. 2000;11:1471–1485. doi: 10.1091/mbc.11.4.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The 1000 Genomes Project Consortium A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K, Majd H, Dallabona C, et al. Recurrent de novo dominant mutations in SLC25A4 cause severe early-onset mitochondrial disease and loss of mitochondrial DNA copy number. Am J Hum Genet. 2016;99:860–876. doi: 10.1016/j.ajhg.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Renesse A, Petkova MV, Lützkendorf S, et al. POMK mutation in a family with congenital muscular dystrophy with merosin deficiency, hypomyelination, mild hearing deficit and intellectual disability. J Med Genet. 2014;51:275–282. doi: 10.1136/jmedgenet-2013-102236. [DOI] [PubMed] [Google Scholar]
- Weissensteiner H, Pacher D, Kloss-Brandstätter A, et al. HaploGrep 2: mitochondrial haplogroup classification in the era of high-throughput sequencing. Nucleic Acids Res. 2016;44:W58–W63. doi: 10.1093/nar/gkw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
■■■ (PDF 126 kb)
■■■ (PDF 60 kb)