Abstract
Gamma-aminobutyric acid transaminase (GABA-T) deficiency is a rare, autosomal recessive disorder characterized by severe psychomotor retardation, early-onset epileptic encephalopathy, intractable seizures, hypotonia, and hyperreflexia. The disease is caused by mutation in the 4-aminobutyrate aminotransferase (ABAT) gene, which encodes an enzyme involved in GABA catabolism. In this chapter, a 10-year follow-up of GABA-T deficiency in a rare case of a long-term survivor patient is discussed. The patient showed a progression of clinical phases with increasing age. In infancy, the patient developed psychomotor retardation and recurrent encephalopathic episodes associated with febrile illness. In early childhood, the patient presented with refractory involuntary and hyperkinetic movements and dystonic hypertonicity. In childhood, the patient gradually progressed into the chronic stable phase of the condition. Magnetic resonance imaging demonstrated high signal intensity on diffusion-weighted images involving the internal and external capsules and cerebral white matter in infancy which disappeared gradually by the age of 3 years, and showed subsequently diffuse brain atrophy in childhood. Using proton magnetic resonance spectroscopy, GABA levels in the basal ganglia were shown to be markedly elevated at the age of 1–2 years, and subsequently decreased with increasing age (toward 5 years). These findings suggest that the encephalopathic episodes in infancy and clinical severity of involuntary and hyperkinetic movements may be correlated with levels of GABA in the basal ganglia. The high levels of GABA in the cerebrospinal fluid remained unaltered, whereas levels of GABA in the serum decreased during childhood. Further investigation of long-term clinical surveillance may improve the understanding of GABA-T deficiency.
Keywords: 4-Aminobutyrate aminotransferase, Basal ganglia, Gamma-aminobutyric acid, Gamma-aminobutyric acid transaminase, Magnetic resonance spectroscopy
Introduction
Gamma-aminobutyric acid transaminase (GABA-T) deficiency is a rare, autosomal recessive disorder characterized by severe psychomotor retardation, early-onset epileptic encephalopathy, intractable seizures, hypotonia, and hyperreflexia (Jaeken et al. 1984). GABA is metabolized by a combination of GABA-T and succinic semialdehyde dehydrogenase (SSADH) (Parviz et al. 2014). The disease is caused by a mutation in the 4-aminobutyrate aminotransferase (ABAT) gene (OMIM 13715), which encodes an enzyme involved in GABA catabolism and salvage of mitochondrial nucleotides. In GABA-T deficiency, GABA is unable to convert to succinic semialdehyde resulting in a build-up of GABA, beta-alanine, homocarnosine, GABA ketone, and 2-pyrrolidinone.
To date, only ten patients have been diagnosed with GABA-T deficiency worldwide (Louro et al. 2016; Koenig et al. 2017). Of those, five patients have survived for more than 3 years, in contrast to the previously reported mortality expected within the first 2 years of life. Currently, there are no studies investigating in detail the clinical information of long-term survivor cases.
Proton magnetic resonance spectroscopy (1H-MRS) is a noninvasive technique for the investigation of brain metabolites and may also be useful for estimating the levels of in vivo neurotransmitters (Novotny et al. 2003). 1H-MRS combined with the LCModel method may facilitate metabolite separation and be applied for the measurement of GABA levels. However, quantifying GABA levels is challenging due to its low concentration masked by the larger peaks of the glutamine–glutamate complex (Provencher 2001).
This report describes the clinical course of a patient with GABA-T deficiency, during a 10-year follow-up, focusing particularly on changes in GABA levels in the brain, serum, and cerebrospinal fluid (CSF).
Case Report
The patient was a Japanese female aged 10 years who was followed up since infancy. The patient was born without complications, and developed severe psychomotor retardation, with hypotonia and recurrent encephalopathic episodes with intractable seizures during infancy. She was diagnosed with GABA-T deficiency via 1H-MRS, based on elevated levels of GABA in the brain. Subsequently, enzyme and molecular studies at 16 months of age confirmed the initial diagnosis. The patient demonstrated compound heterozygosity for a deletion of one exon and a missense mutation 275G>A in the ABAT gene, as well as decreased enzymatic activity of GABA-T in the lymphoblasts. A detailed description of clinical information until the age of 28 months has been reported previously (Tsuji et al. 2010).
The patient developed lethargy associated with febrile illness at the age of 8, 10, and 12 months. These encephalopathic episodes were consistently associated with neurological deterioration and led to opisthotonic posturing with generalized dystonia and segmental myoclonic jerks during wakefulness. The patient showed intense involuntary movements such as choreoathetosis, myoclonic jerks, and dystonic opisthotonic posturing in early childhood. These symptoms were refractory to multiple medications such as phenobarbital, clonazepam, diazepam, baclofen, dantrolene, and haloperidol. However, these symptoms gradually improved during childhood.
The patient revealed an accelerated increase in height reaching +3.0 standard deviations from the normal height during late infancy, and remained at the upper limit of normal height in childhood. At the age of 10 years, the patient was non-verbal, non-ambulatory with spastic quadriparesis and dystonia, and dependent on gastrostomy tube feedings. No further deterioration was reported in childhood.
Radiological Findings
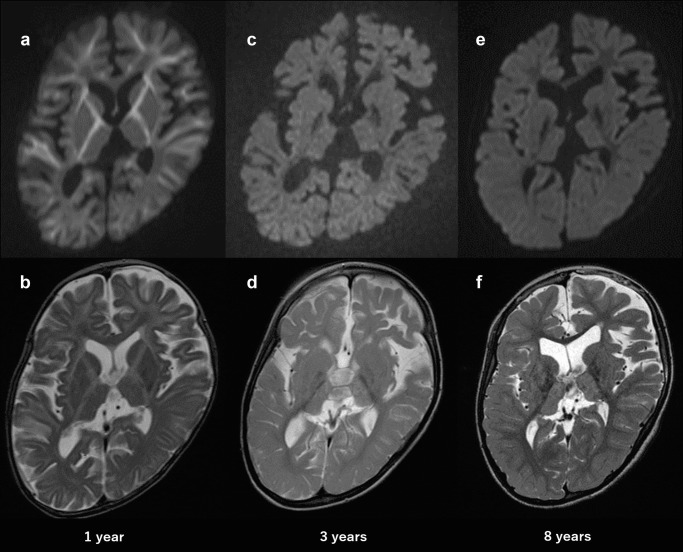
Magnetic resonance imaging (MRI, Siemens 1.5T) at the age of 12 months demonstrated high signal intensity on diffusion-weighted images involving the internal and external capsules and the cerebral white matter with reduced apparent diffusion coefficiency. These high-signal intense lesions disappeared gradually by the age of 3 years. MRI (3T, Siemens) at 8 years of age showed diffuse atrophy of the cerebrum and cerebellum and abnormal high-signal intensity in the bilateral thalamus (Fig. 1). The loss of signal of the bilateral globus pallidus on susceptibility-weighted images (SWI) appeared from the age of 2 years and became more apparent at the age of 8 years.
Fig. 1.
Axial MRI at 1, 3, and 8 years of age (top: diffusion-weighted images, bottom: T2-weighted images). MRI at 1 year of age showing high signal intensity involving the internal and external capsules and cerebral white matter (a, b). MRI at 3 years of age showing the disappearance of high signal intensity in a diffusion-weighted image (c, d). MRI at 8 years of age showing diffuse brain atrophy and symmetrical high signal in the bilateral thalamus (e, f)
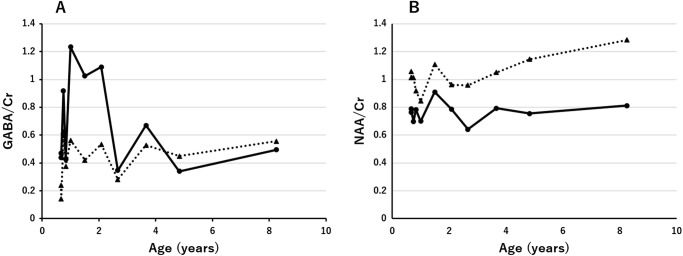
Serial 1H-MRS data (echo time/repetition time = 30/5,000 ms) were obtained from the basal ganglia (gray matter region) and the semioval center (white matter region). Concentrations of GABA and N-acetylaspartate (NAA) were quantified via the LCModel method software, using the internal water reference approach (water concentration of 43.30 and 35.88 mol/kg for the basal ganglia and semioval center, respectively). Concentrations of GABA in the basal ganglia at 1, 3, and 8 years of age were 6.35, 5.07, and 3.96 mmol/kg (GABA/Cr were 1.23, 0.67, and 0.50), respectively. Concentrations of GABA in the semioval center at the same ages were 2.03, 2.32, and 2.79 mmol/kg (GABA/Cr were 0.56, 0.53, and 0.56), respectively (Fig. 2a) (normal volunteers n = 17, 1–9 years: GABA concentrations 1.43 ± 0.36 in the basal ganglia; 0.82 ± 0.38 in the semioval center). NAA concentrations in the basal ganglia at 1, 3, and 8 years of age were 3.60, 5.99, and 6.50 mmol/kg (NAA/Cr were 0.70, 0.79, and 0.81), respectively. Concentrations of NAA in the semioval center were 3.05, 4.61, and 6.45 mmol/kg (NAA/Cr were 0.84, 1.05, and 1.28), respectively (Fig. 2b).
Fig. 2.
Changes in the level ratio of GABA to Cr and NAA to Cr with increasing age (years) quantified by the LCModel method. Basal ganglia (solid line); semioval center (dotted line)
GABA in the Serum and Cerebrospinal Fluid
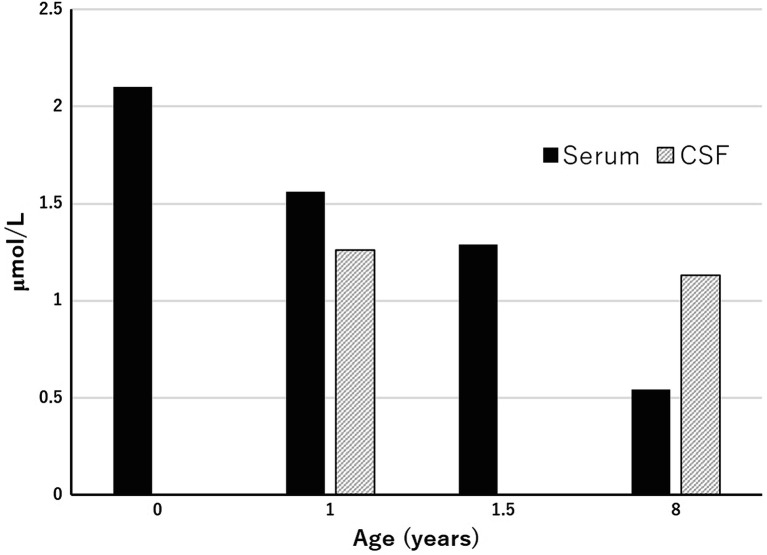
Free GABA levels in the serum at 1 and 8 years of age were 2.10 and 0.54 μmol/L (reference range 0.12–0.50 μmol/L), respectively. Free GABA levels in the CSF at 1 and 8 years of age were 1.261 and 1.133 μmol/L (reference range <2 years; 0.017–0.067 μmol/L, >2 years; 0.032–0.167 μmol/L), respectively (Fig. 3) (Van Hove and Thomas 2014).
Fig. 3.
Changes in GABA levels in the serum and cerebrospinal fluid
Electroencephalography (EEG)
EEG revealed a diffuse slow spike and wave discharges with 1–2 s suppression during encephalopathic episodes in infancy. This examination at 3 years of age showed diffuse occipital dominant 2.5–3 Hz slow activities on background, occasionally containing paroxysmal sharp waves and diffuse polyspike and slow waves. EEG at 8 years of age revealed 13–14 Hz occipital dominant rhythmic activities on background having 4–5 Hz slow waves on the centro-occipital area, and demonstrated sporadic focal spikes. In contrast to the patient’s infancy, childhood was characterized by infrequent seizures such as myoclonic or complex partial seizures.
Discussion
The patient showed a progression of clinical phases with increasing age. In infancy, the patient developed psychomotor retardation and recurrent encephalopathic episodes associated with febrile illness. In early childhood, she presented refractory involuntary and hyperkinetic movements and dystonic hypertonicity through encephalopathic episodes. In childhood, the patient gradually progressed into the chronic stable phase of the condition.
Using 1H-MRS, GABA/Cr levels in the basal ganglia were shown to be markedly elevated at 1–2 years of age, and subsequently decreased with increasing age (toward 5 years). Levels of GABA/Cr in the semioval center remained slightly elevated over time. These findings suggest that the levels of GABA in the basal ganglia were highest during the encephalopathic phase in infancy. Moreover, it is suggested that the clinical severity of involuntary and hyperkinetic movements in early childhood may be correlated with the levels of GABA in the basal ganglia. Levels of NAA/Cr in the basal ganglia remained low over time, whereas those in the semioval center increased slightly from the age of 3 years onwards. This finding suggests that neuronal loss of the cerebrum, particularly in the semioval center, may not progress during childhood.
High levels of GABA in the CSF remained unaltered, whereas GABA levels in the serum decreased in childhood. GABA levels in the CSF may reflect those of the semioval center. The pathophysiology of the decrease in the serum levels of GABA could not be disclosed in this study.
In the developing brain, elevated GABA levels have been associated with an excitatory effect. However, in the mature brain, GABA exerts an inhibitory effect (Kilb 2012). Moreover, it has been suggested that supraphysiological levels of GABA result in a neurotoxic effect. Inhibition of GABA-T by the irreversible inhibitor vigabatrin has been shown to induce intramyelinic edema via elevation of GABA levels in dogs (Peyster et al. 1995). In the case described herein, the paradoxical excitatory effect of GABA was manifested prominently until 5 years of age particularly in the basal ganglia, in terms of the correlation between GABA levels and the severity of involuntary movement. The disappearance of the restricted diffusion as shown through MRI also suggested withdrawal of the neurotoxic effect of GABA. The relief of hyperkinetic movements with increasing age may be due to a decrease in the levels of GABA in the basal ganglia or GABAergic postsynaptic changes resulting in the resolution of excitatory/neurotoxic effects. Regional differences in GABA metabolism in the brain coupled with age-dependent susceptibility of the developing brain may also be responsible for movement disorder.
Although loss of signal of the globus pallidus was shown on SWI, its pathophysiology could not be disclosed in this study. This finding may be correlated with the abnormal elevation in GABA levels and involuntary movement such as SSADH deficiency and vigabatrin-associated brain abnormality. SSADH deficiency is the most prevalent among GABA degeneration disorders leading to accumulation of γ-hydroxybutyric acid and GABA, and reveals an increased hyperintense signal of the globus pallidus as shown on MRI (Ziyeh et al. 2002). Furthermore, vigabatrin may induce reversible MRI signal changes and restricted diffusion in the globus pallidus, thalamus, brainstem, and dentate nucleus, with an increase in GABA levels in the brain during treatment of infantile spasms (Dracopoulos et al. 2010).
A limitation of this study was that the control individuals of GABA concentration were not completely age-matched between the three ages that the MRS scans obtained from the patient. In addition, the natural history of GABA concentrations in the brain with age has remained to be elucidated in GABA-T deficiency. This study included only a patient with GABA-T deficiency. Therefore, the meaning of the lowering GABA is unclear and is uncertain as to whether unique to GABA-T deficiency. Decrease of GABA levels in the plasma and urine was also reported even in SSADH deficiency (Jansen et al. 2016).
In this report, a 10-year follow-up of GABA-T deficiency in a rare case of a long-term survivor patient is discussed. Encephalopathic episodes as well as hyperkinetic and involuntary movements seemed to correlate with the levels of GABA in the basal ganglia. 1H-MRS combined with the LCModel method may be clinically applicable to measure region-specific GABA levels. The pathophysiology of GABA-T deficiency remains to be elucidated. Investigation of additional patients with long-term follow-up data may improve the understanding of GABA-T deficiency. The findings discussed in this chapter may provide important insights into the pathogenesis of GABA-T deficiency and the role of the GABAergic system in human brain development.
Acknowledgement
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 26461843.
Authors’ Contributions
K.I. wrote the manuscript and provided medical care for the patient; Y.T. and M.T. provided medical care for the patient; M.T. performed the analysis of magnetic resonance spectroscopy and interpreted the results; N.A. interpreted magnetic resonance images; T.G. approved the final manuscript. All authors read and approved the final version of the manuscript.
Corresponding Author
Kazushi Ichikawa.
Conflict of Interest
Kazushi Ichikawa, Megumi Tsuji, Yu Tsuyusaki, Moyoko Tomiyasu, Noriko Aida, and Tomohide Goto declare that they have no conflict of interest.
Funding
Japan Society for the Promotion of Science (JSPS) KAKENHI (Grant Number 26461843) [Aida, Goto, Tsuyusaki].
Compliance with Ethics Guideline
Informed Consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from the patient’s parents for being included in the study.
References
- Dracopoulos A, Widjaja E, Raybaud C, Westall CA, Snead OC., 3rd Vigabatrin-associated reversible MRI signal changes in patients with infantile spasms. Epilepsia. 2010;51:1297–1304. doi: 10.1111/j.1528-1167.2010.02564.x. [DOI] [PubMed] [Google Scholar]
- Jaeken J, Casaer P, de Cock P, et al. Gamma-aminobutyric acid-transaminase deficiency: a newly recognized inborn error of neurotransmitter metabolism. Neuropediatrics. 1984;15:165–169. doi: 10.1055/s-2008-1052362. [DOI] [PubMed] [Google Scholar]
- Jansen EE, Vogel KR, Salomons GS, et al. Correlation of blood biomarkers with age informs pathomechanisms in succinic semialdehyde dehydrogenase deficiency (SSADHD), a disorder of GABA metabolism. J Inherit Metab Dis. 2016;39:795–800. doi: 10.1007/s10545-016-9980-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilb W. Development of the GABAergic system from birth to adolescence. Neuroscientist. 2012;18:613–630. doi: 10.1177/1073858411422114. [DOI] [PubMed] [Google Scholar]
- Koenig MK, Hodgeman R, Riviello JJ, et al. Phenotype of GABA-transaminase deficiency. Neurology. 2017;88:1919–1924. doi: 10.1212/WNL.0000000000003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louro P, Ramos L, Robalo C, et al. Phenotyping GABA transaminase deficiency: a case description and literature review. J Inherit Metab Dis. 2016;39:743–747. doi: 10.1007/s10545-016-9951-z. [DOI] [PubMed] [Google Scholar]
- Novotny EJ, Jr, Fulbright RK, Pearl PL, Gibson KM, Rothman DL. Magnetic resonance spectroscopy of neurotransmitters in human brain. Ann Neurol. 2003;54(Suppl 6):S25–S31. doi: 10.1002/ana.10697. [DOI] [PubMed] [Google Scholar]
- Parviz M, Vogel K, Gibson KM, Pearl PL. Disorders of GABA metabolism: SSADH and GABA-transaminase deficiencies. J Pediatr Epilepsy. 2014;3:217–227. doi: 10.3233/PEP-14097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyster RG, Sussman NM, Hershey BL, et al. Use of ex vivo magnetic resonance imaging to detect onset of vigabatrin-induced intramyelinic edema in canine brain. Epilepsia. 1995;36:93–100. doi: 10.1111/j.1528-1157.1995.tb01672.x. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Aida N, Obata T, et al. A new case of GABA transaminase deficiency facilitated by proton MR spectroscopy. J Inherit Metab Dis. 2010;33:85–90. doi: 10.1007/s10545-009-9022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hove JLK, Thomas JA. Disorders of glycine, serine, GABA, and proline metabolism. In: Blau N, Duran M, Gibson KM, Dionisi-Vici C, editors. Physician’s guide to the diagnosis, treatment, and follow-up of inherited metabolic diseases. Berlin, Heidelberg: Springer; 2014. pp. 63–83. [Google Scholar]
- Ziyeh S, Berlis A, Korinthenberg R, Spreer J, Schumacher M. Selective involvement of the globus pallidus and dentate nucleus in succinic semialdehyde dehydrogenase deficiency. Pediatr Radiol. 2002;32:598–600. doi: 10.1007/s00247-002-0717-4. [DOI] [PubMed] [Google Scholar]