Abstract
Mutations in the ABCD1 gene that encodes peroxisomal ABCD1 protein cause X-linked adrenoleukodystrophy (X-ALD), a rare neurodegenerative disorder. More than 70% of the patient fibroblasts with this missense mutation display either a lack or reduction of the ABCD1 protein because of posttranslational degradation. In this study, we analyzed the stability of the missense mutant ABCD1 proteins (p.A616T, p.R617H, and p.R660W) in X-ALD fibroblasts and found that the mutant ABCD1 protein p.A616T has the capacity to recover its function by incubating at low temperature. In the case of such a mutation, chemical compounds that stabilize mutant ABCD1 proteins could be therapeutic candidates. Here, we prepared CHO cell lines stably expressing ABCD1 proteins with a missense mutation in fusion with green fluorescent protein (GFP) at the C-terminal. The stability of each mutant ABCD1-GFP in CHO cells was similar to the corresponding mutant ABCD1 protein in X-ALD fibroblasts. Furthermore, it is of interest that the GFP at the C-terminal was degraded together with the mutant ABCD1 protein. These findings prompted us to use CHO cells expressing mutant ABCD1-GFP for a screening of chemical compounds that can stabilize the mutant ABCD1 protein. We established a fluorescence-based assay method for the screening of chemical libraries in an effort to find compounds that stabilize mutant ABCD1 proteins. The work presented here provides a novel approach to finding therapeutic compounds for X-ALD patients with missense mutations.
Electronic supplementary material
The online version of this chapter (10.1007/8904_2018_118) contains supplementary material, which is available to authorized users.
Keywords: ABCD1, Bortezomib, Green fluorescent protein, Missense mutation, X-linked adrenoleukodystrophy
Introduction
X-linked adrenoleukodystrophy (X-ALD) (OMIM 300100) (Bezman et al. 2001) is a human genetic disorder caused by mutation of the ABCD1 gene (Mosser et al. 1993) that encodes the peroxisomal membrane protein ABCD1, which consists of 745 amino acids. To date, more than 340 nonrecurrent missense mutations of the ABCD1 gene have been identified (http://www.x-ald.nl). In these missense mutations, approximately 70% of the ABCD1 proteins are either not detected or reduced in amount in X-ALD fibroblasts. We previously reported that the mutant ABCD1 protein is degraded via the proteasome pathway (Takahashi et al. 2007), at least in part, because the degradation of the missense mutant ABCD1 was partially inhibited by treatment with MG132. Zhang et al. have reported that X-ALD fibroblasts with certain temperature missense mutation recovered their function by incubating them at a low temperature (Zhang et al. 2011). These results indicate that a part of the mutant ABCD1 protein, which is incorrectly folded and becomes degraded in the cytosol, might have a capacity to recover its function by stabilization. Thus, a chemical compound with the capacity to stabilize the mutant ABCD1 protein is a potential therapeutic agent for X-ALD.
Certain strategies and candidate drugs for X-ALD treatment have been reported (Morita et al. 2011). To date, however, no effective therapeutic drugs for X-ALD have been developed. Thus, development of a high-throughput system for screening of therapeutic compounds based on direct stabilization and stimulation of functional activity of mutant ABCD1 is of paramount importance for this condition. In this study, we prepared CHO cells expressing missense mutant ABCD1 fused with GFP and found that GFP at the C-terminal was posttranslationally degraded together with the mutant ABCD1 protein. Using these model cells, we demonstrate a novel approach to finding therapeutic compounds for X-ALD by stabilizing the ABCD1 protein having a missense mutation.
Materials and Methods
Materials
pEGFPN-1 was purchased from Clontech Laboratories (Mountain View, CA). The KOD-plus mutagenesis kit was from Toyobo (Osaka, Japan). The MTT assay kit was from Roche Applied Science. Bortezomib was purchased from Cell Signaling Technology (Danvers, MA). [1-14C]lignoceric acid (53 mCi/mmol) was purchased from Moravek Biochemicals (Brea, CA). ECL Plus, a Western blotting detection system, and Fluorolink Cy3-labeled goat anti-rabbit IgG were purchased from GE Healthcare (Buckinghamshire, England). The mouse anti-human ALDP/ABCD1 monoclonal antibody (MAB2162) and the rabbit anti-catalase antibody were purchased from Millipore (Billerica, MA) and Immunochemicals (Gilbertsville, PA), respectively. The rabbit anti-PMP70/ABCD3 antibody was raised against the C-terminal 15 amino acids of rat PMP70/ABCD3 (Imanaka et al. 2000). The chemical library of existing drugs was a gift provided by the Drug Discovery Initiative at the University of Tokyo.
Plasmid Construction
pEGFP vectors, each harboring a missense ABCD1 mutant, were constructed with a KOD-plus mutagenesis kit using pEGFP/ABCD1 as the template (Takahashi et al. 2007). The oligonucleotide primer sets were designed on the basis of their sequences (Suppl. Table 1). The mutation in the constructs was confirmed by the dye-terminator cycle sequencing method using an ABI PRISM310 DNA sequencer (Life Technologies Corporation, Carlsbad, CA).
Cell Culture and Stable Transfection
CHO-K1 cells were cultured in F12 medium (Nissui, Tokyo, Japan) with 10% FCS containing streptomycin and penicillin at 37°C and 5% CO2. CHO cells expressing wild or mutant ABCD1-GFP were prepared by the transfection of pEGFP/wild ABCD1 or pEGFP/mutant ABCD1 into CHO cells. The transfection procedures were performed using Effectene Transfection Reagent (Qiagen, Valencia, CA) according to the manufacturer’s instructions. At 48-h post-transfection, the medium was replaced with the same medium containing G418 (500 μg/mL). In the G418-resistant cells, we selected several clones expressing wild ABCD1-GFP or each mutant ABCD1-GFP as determined by means of fluorescence imaging. The cell lines expressing mutant ABCD1-GFP were identified by the observation of GFP fluorescence under a condition in which the CHO cells were incubated in the presence of MG132 (20 μM) for 20 h.
Human skin fibroblasts from a healthy individual and X-ALD patients with a missense mutation (R617H, A616T, and R660W) were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Grand Island, NY) containing 10% FCS.
Fluorescence-Based Assay Method for the Screening of Chemical Libraries
The CHO cells stably expressing mutant ABCD1-GFP (referred to as CHO/mutABCD1-GFP) were seeded in a 96 well plate (Greiner, blk/clr bottom) with 100 μL of DMEM/F12 (phenol red-free) containing 10% FCS (4 × 104 cells/well). Chemical compounds were added to each well on the next day. One μL of stock solution (2 mM in DMSO) was mixed with the culture medium to give a final yield of 20 μM. CHO/mutABCD1-GFP was cultured in the wells from columns 1 to 11, and CHO/wildABCD1-GFP was in cultured column 12 as a positive control. DMSO was added to each well in columns 1 and 12, and each compound was added to 80 wells from columns 2 to 11. After 48-h incubation, culture medium was discarded, and cells were washed with 170 μL of 1 × Hank’s balanced salt solution (HBSS). After the addition of 100 μL of 1 × HBSS, the fluorescence intensity was measured at an excitation wavelength of 485 nm and an emission wavelength of 535 nm with Filter Max F5 (Molecular Devices, Sunnyvale, CA).
Immunofluorescence Analysis
Immunofluorescence analysis was performed as described previously (Morita et al. 2013). The primary antibodies were rabbit antibodies against catalase (1:200) and mouse antibodies against ABCD1 (1:200). Alexa 488-conjugated goat anti-rabbit (1:500) was utilized to label the anti-catalase antibodies. Cy3-conjugated goat anti-mouse was used to label the anti-ABCD1 antibodies. The cells were mounted in Vectarshield with DAPI (Vector Laboratories, Burlingame, CA) to allow examination under confocal microscopy (confocal microscope LSM780, Carl Zeiss Microscopy, Tokyo, Japan).
Other Methods
Fatty acid β-oxidation was measured essentially as described by Watkins et al. (Morita et al. 2013; Watkins et al. 1991). Immunoblotting was performed as described previously using ECL Plus Western blotting detection reagent (Kurisu et al. 2003). The protein concentration was determined by the Lowry method (Lowry et al. 1951) using bovine serum albumin as the standard.
Results
Stability of Missense Mutant ABCD1 Proteins in X-ALD Fibroblasts
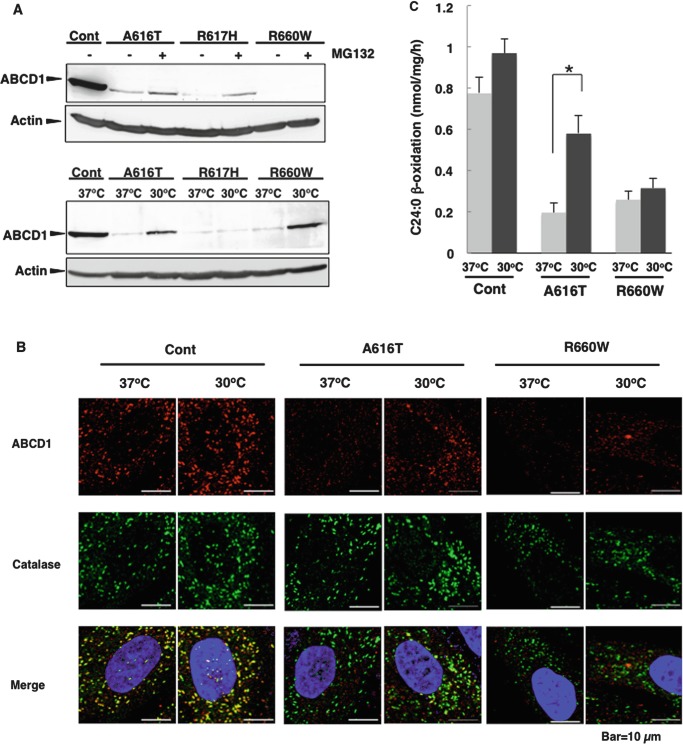
We analyzed the stability of mutant ABCD1 proteins in X-ALD fibroblasts with a missense mutation of A616T, R617H, and R660W (Suppl. Fig. 1). We selected these mutant ABCD1 proteins because they had been demonstrated to be temperature-sensitive mutations and culturing X-ALD cells harboring these mutations at lower temperature partially restored their function (Zhang et al. 2011). Indeed, when these X-ALD fibroblasts were treated with MG132, a proteasome inhibitor, the mutant ABCD1 proteins A616T and R617H partially recovered (Fig. 1a), indicating that these mutant ABCD1 proteins are unstable due to incorrect folding and are partly degraded by proteasomes. In contrast, the mutant ABCD1 protein R660W did not recovered, indicating that p.R660W is degraded by other proteases. When these X-ALD fibroblasts were incubated at 30°C for 7 days, the mutant ABCD1 proteins A616T and R660W recovered (Fig. 1a). The recovered mutant ABCD1 protein A616T was partially localized to peroxisomes, but p.R660W was not (Fig. 1b). The VLCFA β-oxidation activity of X-ALD fibroblasts (A616T) cultured at 30°C was approximately 75% of that in the control fibroblasts (Fig. 1c), even though the expression level of the mutant ABCD1 protein was approximately 30% compared with normal fibroblasts (Fig. 1a). We previously reported that the C26:0/C22:0 ratio became near normal level by culturing at 30°C for 3 weeks (Zhang et al. 2011). These results suggest that the mutant ABCD1 protein A616T has the capacity to recover its function and can be rescued by chemical compounds that stabilize it. In contrast, the mutant ABCD1 protein R660W did not show any recovery of VLCFA β-oxidation, probably because of mislocalization (Fig. 1b). In the case of the X-ALD fibroblasts (R617H), the mutant ABCD1 protein was not recovered by incubation at a low temperature.
Fig. 1.
Recovery of mutant ABCD1 proteins in X-ALD fibroblasts. (a) X-ALD fibroblasts (A616T, R617H, and R660W) were incubated at 37°C in the absence or presence of MG132 (20 μM) for 20 h or incubated at 30°C for 7 days. After the incubation, the expression of ABCD1 was analyzed by immunoblotting. Total cellular protein (100 μg protein/sample) were separated by SDS-PAGE and transferred to nitrocellulose membranes. ABCD1 and actin were stained with an anti-ABCD1 antibody and anti-β-actin antibody, respectively. (b) Control (Cont) and X-ALD fibroblasts (A616T and R660W) were incubated at 37°C or 30°C for 7 days and subjected to immunofluorescence analysis. ABCD1 was stained with an anti-ABCD1 antibody followed by a Cy3-labeled secondary antibody. Catalase was stained with an anti-catalase antibody followed by an Alexa 488-labeled secondary antibody. Catalase appears as green dots and ABCD1 as red dots. The figure displays a merged image. (c) VLCFA β-oxidation activities in control (Cont) and X-ALD fibroblasts (A616T and R660W) were measured using [1-14C]C24:0 as the substrate. Control and X-ALD fibroblasts (A616T and R660W) were incubated at 37°C or 30°C. After 7-day incubation, cells were harvested and incubated with a reaction buffer containing dissolved α-cyclodextrin [1-14C]C24:0. The reaction was stopped by the addition of 1M KOH for alkaline hydrolysis. After neutralization and Folch extraction, the radioactivity in the water-soluble fraction was measured by scintillation counting. Results are the means ± S.D.; n = 3. Statistical analysis of the data was performed with Student’s t-test (*, p < 0.02)
Stability of the Mutant ABCD1 Proteins Expressed in CHO Cells
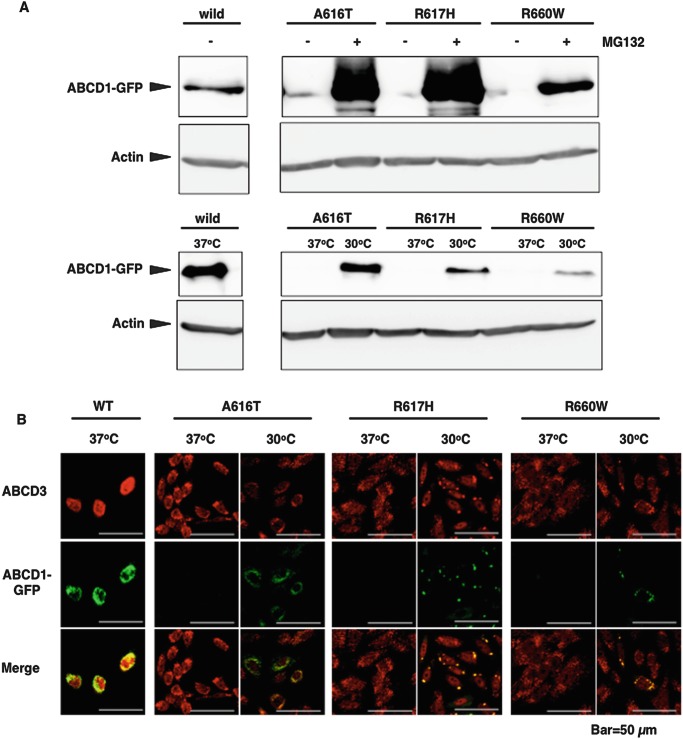
To evaluate the stabilization of each mutant ABCD1 protein more easily, we expressed missense mutant ABCD1 proteins fused with GFP at the C-terminal (p.A616T, p.R617H, and p.R660W) in CHO cells. When CHO cells stably expressing each mutant ABCD1-GFP (CHO/mutABCD1-GFP) were cultured at 37°C, the expression of the mutant ABCD1-GFP was beneath the limit of detection (Fig. 2a and Suppl. Fig. 2). However, the mutant ABCD1-GFPs A616T, R617H, and R660W were largely recovered by treatment with MG132, suggesting that these mutant ABCD1-GFPs were posttranslationally degraded by proteasomes. However, the recovery of p.R660W was less than that of p.A616T and p.R617H, which is consistent with the findings on mutant ABCD1 proteins in X-ALD fibroblasts (Fig. 1a). In this experiment, it should be noted that the GFP at the C-terminal was degraded together with the mutant ABCD1 protein. When these cells were cultured at 30°C for 3 days, the mutant ABCD1-GFPs became detectable and co-localized with the ABCD3 protein, a peroxisomal membrane protein (Fig. 2a, b). However, peroxisomes expressing mutant ABCD1-GFPs R617H and R660W became large as if a subset of peroxisomes aggregate. The recovery of the mutant ABCD1-GFP A616T was time-dependent and reached a maximum level at 3 days (Suppl. Fig. 3). When CHO/mutABCD1-GFP (A616T) were incubated for 5 days at 30°C followed by incubation at 37°C, the mutant ABCD1-GFP was still detectable for at least up to 24 h (Suppl. Fig. 4), indicating that the mutant ABCD1 protein is relatively stable once it localizes to peroxisomes.
Fig. 2.
Missense mutant ABCD1-GFPs recovered by treatment with MG132 or incubation at a low temperature. CHO cells expressing wild or mutant ABCD1-GFPs (A616T, R617H, and R660W) were cultured in the presence of MG132 (20 μM) for 20 h or at 30°C for 5 days. After the incubation, the expression of ABCD1-GFP was analyzed by immunoblotting (a) or immunofluorescence (b). ABCD1-GFP and actin were detected by using an anti-GFP antibody or anti-β-actin antibody, respectively. Peroxisomes were stained with an anti-ABCD3 antibody followed by a Cy3-labeled secondary antibody. ABCD1-GFP appears as green dots and ABCD3 as red dots. The antibody used in this experiment did not react with endogenous ABCD1 in CHO cells
The stability of each mutant ABCD1 protein in X-ALD fibroblasts was similar to that of mutant ABCD1-GFP in CHO cells, suggesting that CHO/mutABCD1-GFP can be used as a model cells. Additionally, the fluorescent intensity in CHO/mutABCD1-GFP is a good indicator of the recovery of the mutant ABCD1 protein. Therefore, these cells are very useful for the screening of chemical compounds that are able to stabilize temperature-sensitive mutant ABCD1 protein.
Screening Method Using CHO/mutABCD1-GFP
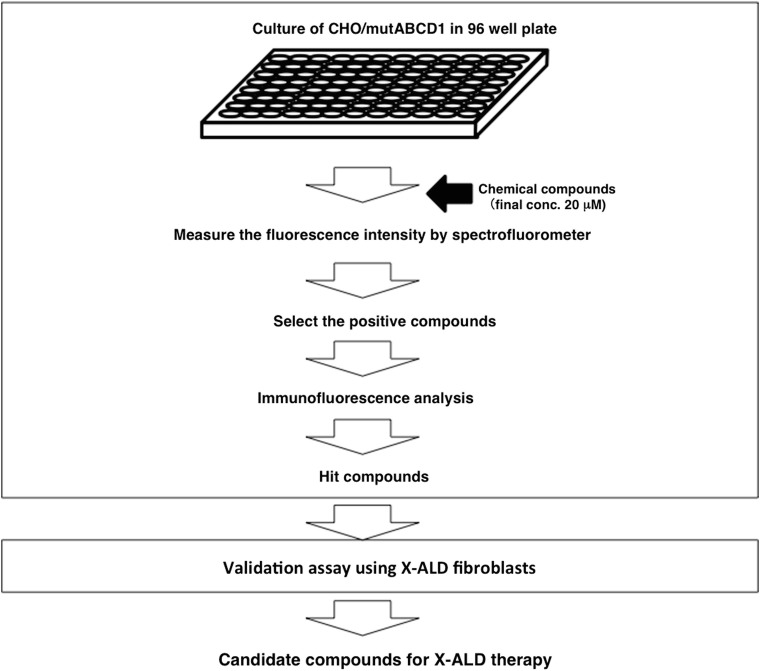
We used the CHO/mutABCD1-GFP (A616T) cells to develop a screening method to identify chemical compounds that can restore temperature-sensitive ABCD1 mutants (Fig. 3). The GFP fluorescent was detected by culturing at a low temperature or with MG132 (Suppl. Fig. 5). The average Z-factor for the fluorescence intensity was calculated to be more than 0.5 in the CHO/wildABCD1-GFP and CHO/mutABCD1-GFP (A616T). The compounds were first screened by measuring the fluorescence intensity with a spectrofluorometer. In this screening, we selected the chemical compounds that exhibited a greater than 30% of the fluorescence intensity of CHO/wildABCD1-GFP. Next, the positive compounds were analyzed for their capacity to recover the mutant ABCD1-GFP by immunofluorescence or by immunoblot analysis to exclude the false-positive compounds. Finally, the ability of the positive compounds to recover mutant ABCD1 protein was analyzed in X-ALD fibroblasts (A616T).
Fig. 3.
Flowchart for the screening of chemical compounds. CHO/wildABCD1-GFP and CHO/mutABCD1-GFP (A616T) cells cultured in 96 well plates (4 × 104 cells/well) were treated with chemical compounds (final conc. 20 μM) and incubated for 2 days. After washing with 1 × HBSS, their fluorescent intensity was directly measured with a spectrofluorometer. Next, the effect of positive compounds on the localization of mutant ABCD1-GFP was determined by immunofluorescence analysis. Hit compounds from the fluorescence-based assay were finally validated on ALD fibroblasts
Screening of Existing Drugs
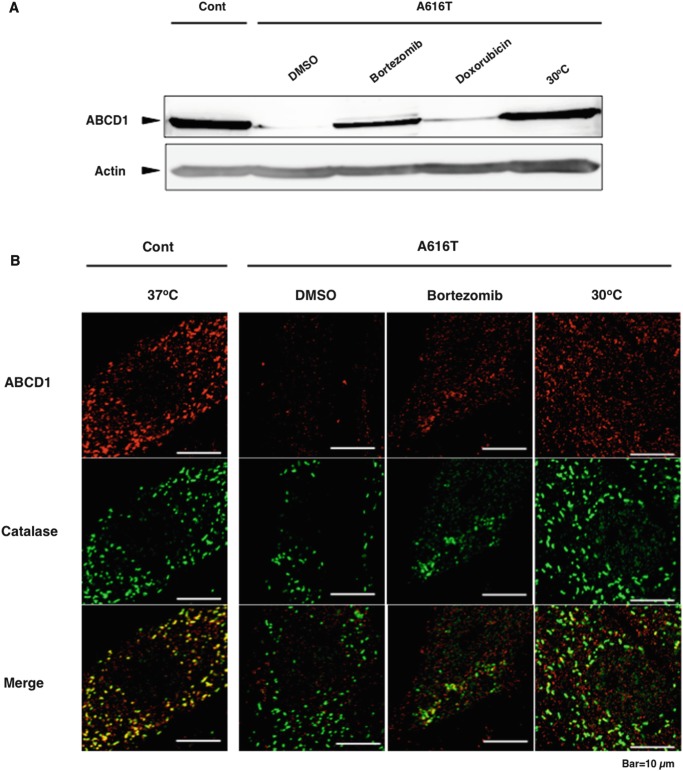
In the present study, we screened 1,948 compounds from the Drug Discovery Initiative library of the University of Tokyo and found 19 compounds that exhibited the increase in the fluorescent intensity. These 19 drugs were used to study their effect on the peroxisomal localization of mutant ABCD1 proteins in X-ALD patient fibroblasts. In this experiment, the concentration of drugs was decreased to 5 μM because higher doses altered the cell morphology. Four drugs, including the anthracycline anticancer agents (doxorubicin, idarubicin, and aclarubicin) and bortezomib, induced the recovery of mutant ABCD1-GFP in peroxisomes (Suppl. Fig. 6). The other 15 drugs did not elicit any fluorescence in peroxisomes (data not shown). When X-ALD fibroblasts (A616T) were incubated with bortezomib and doxorubicin, only bortezomib induced the recovery of mutant ABCD1 proteins (Fig. 4a). Other anthracycline antibiotics such as idarubicin and aclarubicin did not bring about any recovery (data not shown). This discrepancy between X-ALD fibroblasts and CHO/mutABCD1-GFP is due to the promoter induction by anthracycline antibiotics (Kurisu et al. 2003). We found that the transcription of the mutant ABCD1-GFP gene as well as GFP-SKL gene was stimulated by the treatment of doxorubicin (Suppl. Fig. 7). This result suggests that anthracycline antibiotics such as doxorubicin, idarubicin, and aclarubicin activate the CMV promoter derived from the pEGFP vector, resulting in the induction of a large amount of mutant ABCD1-GFP. Therefore, we believe anthracycline antibiotics do not have the capacity to stabilize the mutant ABCD1 protein. In contrast, bortezomib exhibited a dose- and time-dependent recovery of mutant ABCD1 proteins in X-ALD fibroblasts (Suppl. Fig. 8a, b) without significant effects on cell viability up to 500 nM for 2 days (Suppl. Fig. 9). When ALD fibroblasts (A616T) were incubated with bortezomib at 50 nM for 2 days, the mutant ABCD1 protein A616T partially localized to peroxisomes, although the expression of catalase was relatively decreased (Fig. 4b).
Fig. 4.
Recovery of mutant ABCD1 (A616T) by the treatment of bortezomib in X-ALD fibroblasts. (a) X-ALD fibroblasts (A616T) were incubated in the presence or absence of bortezomib (50 nM) or doxorubicin (5 μM) for 2 days or incubated at 30°C for 7 days. After the incubation, the cells were analyzed as indicated in Fig. 1a. (b) X-ALD fibroblasts (A616T) were incubated in the presence or absence of bortezomib at 50 nM for 2 days or incubated at 30°C for 7 days. After the incubation, they were subjected to immunofluorescence analysis as indicated in Fig. 1b
Discussion
Recently, small molecules with chaperone activity have garnered attention for clinical importance in protein folding diseases with missense mutations (Loo and Clarke 2007). In lysosomal storage diseases, pharmacological chaperone therapy is currently being investigated as a potential therapeutic approach (Sawkar et al. 2006; Fan 2008). Furthermore, several missense mutant ABC proteins, including ABCG2 (BCRP), ABCB4 (MDR3), ABCC7 (CFTR), ABCC1 (MRP1), and ABCC8 (SUR1), can be recovered by chemical chaperones (Loo et al. 2005, 2011; Sampson et al. 2011; Yu et al. 2011; Zhang et al. 2012).
In the missense mutations in X-ALD, approximately 70% of the mutant ABCD1 proteins were posttranslationally degraded. However, some mutant proteins still have the capacity for functional recovery by stabilization. Indeed, in cases in which mutant ABCD1 proteins could be rescued by culturing X-ALD fibroblasts at lower temperature, they showed residual biological activity (Zhang et al. 2011). This indicates that stabilization of the mutant protein is an attractive therapeutic approach. In the present study, we characterized three missense mutant ABCD1 proteins: R617H is located in the Walker B motif, A616T in the ABC signature motif, and R660W in the C-terminal downstream from the Walker B motif (Suppl. Fig. 1). Among them, the mutant ABCD1 protein A616T was functionally recovered by incubation at a low temperature. These data are in line with an earlier study that reported that the function of p.A616T could be restored when X-ALD fibroblasts were cultured at 30°C (Zhang et al. 2011). These data suggest that pharmacological chaperone therapy is adaptable to X-ALD patients with a temperature-sensitive missense mutation.
The expression of GFP fused with a desired protein in living cells is useful for monitoring the fate of the protein. Although it should be noted that the CMV promoter that is genetically transfected in CHO/mutABCD1-GFP can be stimulated by chemical compounds such as anthracycline antibiotics (Kinoshita et al. 2008), CHO/mutABCD1-GFP allows monitoring of the stabilization of mutant ABCD1 proteins. In these cells, GFP at the C-terminal is degraded along with the mutant ABCD1 protein, indicating that GFP fluorescence is detected only when they are stabilized. Based on these properties, we established a screening method and found bortezomib, a potent proteasome inhibitor, which is commonly used as a drug for multiple myeloma patients (Dou and Goldfarb 2002). Recently, bortezomib has been reported to improve the function of mutant lysosomal α-glucosidase in fibroblasts from Pompe disease patients (Shimada et al. 2011) and also to stabilize the deltaF508-cystic fibrosis transmembrane conductance regulator (CFTR) (Wilke et al. 2012). In the present study, the mutant ABCD1 proteins were recovered and partially localized in peroxisomes by treatment with bortezomib (Fig. 4b). Unfortunately, bortezomib did not show significant recovery of peroxisomal fatty acid β-oxidation in X-ALD fibroblasts (A616T) (data not shown). The failure to increase the peroxisomal fatty acid β-oxidation might be due to cellular damage caused by the treatment of bortezomib. In the CFTR, pharmacological chaperones, together with proteostasis regulators, that bind directly to the mutant CFTR and indirectly affect the cellular pathways, are being investigated as a novel pharmacological strategy (Hanrahan et al. 2013). At present, bortezomib seems to be not applicable for therapeutic drugs for X-ALD patients due to the significant side effects. Instead, bortezomib provides a piece of supporting evidence that small molecule interventions that stabilize a subset of ABCD1 mutant proteins could have therapeutic value for X-ALD in the future. Testing in animal model is required for verifying the possibility of therapeutic use.
The discovery of bortezomib confirms that the fluorescence-based screening protocol presented here is useful for the screening of a large number of chemical compounds for drug candidates that can stabilize the missense mutant protein. It should be considered that our assay method is only effective to a part of mutant ABCD1 proteins with missense mutation. Nevertheless, the present study provides a novel approach to finding therapeutic compounds for X-ALD.
Electronic Supplementary Material
ABCD1-GFP and missense mutations in X-ALD patients. The mutated positions in the ABCD1 protein in fusion with GFP are used in this study (TIFF 1521 kb)
Recovery of mutant ABCD1-GFP by the treatment of MG132. CHO/mutABCD1-GFP (A616T, R617H and R660W) cells were cultured in the presence of MG132 (20 μM) for 20 h. After the incubation, cells were harvested and subjected to immunoblot analysis as in Fig. 2 (TIFF 1521 kb)
Recovery of mutant ABCD1-GFP (A616T) by incubating low temperature. CHO/mutABCD1-GFP (A616T) cells were cultured by incubating at 30°C for up to 5 days. After the incubation, cells were harvested and subjected to immunoblot analysis as in Fig. 2 (TIFF 1521 kb)
Stability of mutant ABCD1-GFP (A616T) in peroxisomes. CHO/mutABCD1-GFP (A616T) cells were incubated at 30°C for 5 days and incubated at 37°C for further 0, 12, 24, and 48 h. After fixation, cells were stained as in Fig. 2b (TIFF 1521 kb)
Measurement of fluorescence intensity with a spectrofluorometer. CHO/mutABCD1-GFP (A616T) cells were cultured on 96 well plate and incubated at 30°C for 5 days or with MG132 (20 μM) for 20 h. After the incubation, the cells were washed with 1 × HBSS and directly analyzed the fluorescence intensity. The fluorescence images of CHO/wildABCD1-GFP cells and CHO/mutABCD1-GFP (A616T) cells were also shown in the figure (TIFF 1521 kb)
Recovery of mutant ABCD1-GFP by the treatment of positive compounds. CHO/mutABCD1-GFP(A616T) cells were incubated in the medium containing each 19 positive compounds (5 μM) that were selected by measuring fluorescent intensity. After the 2-day incubation, cells were prepared for both immunofluorescence and immunoblot analysis as in Fig. 2. Among the 19 positive compounds, only 4 drugs (doxorubicin, idarubicin, aclarubicin, or bortezomib) showed the recovery of mutant ABCD1-GFPs (TIFF 1521 kb)
Effect of doxorubicin on the gene expression in CHO/mutABCD1-GFP and CHO/GFP-SKL. CHO/mutABCD1-GFP (A616T) cells and CHO/GFP-SKL cells were incubated in the presence or absence of doxorubicin (5 μM) for 48 h. After the incubation, total RNA was extracted and reverse transcribed into cDNA. The cDNA was used as template for real-time PCR using specific primer. The result shows the arbitral unit based on 18SrRNA (TIFF 1521 kb)
Effect of bortezomib on the recovery of mutant ABCD1 protein in X-ALD fibroblasts. X-ALD fibroblasts (A616T) were treated with bortezomib at the concentration of up to 100 nM for 2 days (a) or with bortezomib at 50 nM for 0, 3, 6, 12, 24, or 48 h (b). After the incubation, the total cellular protein (150 μg protein/lane) was separated by SDS-PAGE followed by immunoblotting using anti-ABCD1 or anti-β-actin antibodies (TIFF 1521 kb)
Cell viability of X-ALD fibroblasts in the presence of bortezomib. X-ALD fibroblasts (A616T) were cultured in the medium containing bortezomib (0, 5, 10, 20, 50, 100, 200, and 500 nM) for 2 days (closed bar) or 7 days (gray bar). After the incubation, cells were subjected to MTT assay. The values in the figure were indicated as percentage of the fibroblasts in the absence of bortezomib. Results are the means ± S.D.; n = 3 (TIFF 1521 kb)
Primer for mutation (DOCX 44 kb)
Acknowledgments
This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology (16K09961), by the Platform Project for Supporting in Drug Discovery and Life Science Research from Japan Agency for Medical Research and Development (AMED), and by JSPS Core-to-Core Program, B. Asia-Africa Science Platforms. D.G.K. acknowledges the Matsumae International Foundation, Takeda Science Foundation, and Goho Life Sciences International Fund for fellowships. Pacific Edit reviewed the manuscript prior to submission.
Take-Home Message
A novel approach to finding therapeutic compounds for X-ALD patients.
Conflict of Interest
Masashi Morita, Shun Matsumoto, Airi Sato, Kengo Inoue, Dzmitry G. Kostsin, Kozue Yamazaki, Kosuke Kawaguchi, Nobuyuki Shimozawa, Stephan Kemp, Ronald J. Wanders, Hirotatsu Kojima, Takayoshi Okabe, and Tsuneo Imanaka declare that they have no conflict of interest.
Informed Consent
All procedures were in accordance with the ethical standards of the responsible committee on human studies (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. Written informed consent was obtained from all patients for being included in the study.
Animal Rights
The article does not contain animal subjects.
Author Contributions
TI conceived and supervised the study; TI, MM, AH, HK, TO, NS, SK, and RJW designed the experiments; MM, SM, AS, KI, DGK, and KY performed the experiments; and MM, SM, and DGK wrote the manuscript, which was discussed by all authors.
References
- Bezman L, Moser AB, Raymond GV, et al. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol. 2001;49:512–517. doi: 10.1002/ana.101. [DOI] [PubMed] [Google Scholar]
- Dou QP, Goldfarb RH. Bortezomib (millennium pharmaceuticals) IDrugs. 2002;5:828–834. [PubMed] [Google Scholar]
- Fan JQ. A counterintuitive approach to treat enzyme deficiencies: use of enzyme inhibitors for restoring mutant enzyme activity. Biol Chem. 2008;389:1–11. doi: 10.1515/BC.2008.009. [DOI] [PubMed] [Google Scholar]
- Hanrahan JW, Sampson HM, Thomas DY. Novel pharmacological strategies to treat cystic fibrosis. Trends Pharmacol Sci. 2013;34:119–125. doi: 10.1016/j.tips.2012.11.006. [DOI] [PubMed] [Google Scholar]
- Imanaka T, Aihara K, Suzuki Y, et al. The 70-kDa peroxisomal membrane protein (PMP70), an ATP-binding cassette transporter. Cell Biochem Biophys. 2000;32:131–138. doi: 10.1385/CBB:32:1-3:131. [DOI] [PubMed] [Google Scholar]
- Kinoshita A, Kobayashi D, Hibino Y, et al. Regulation of CMV promoter-driven exogenous gene expression with doxorubicin in genetically modified cells. J Pharm Pharmacol. 2008;60:1659–1665. doi: 10.1211/jpp.60.12.0012. [DOI] [PubMed] [Google Scholar]
- Kurisu M, Morita M, Kashiwayama Y, et al. Existence of catalase-less peroxisomes in Sf21 insect cells. Biochem Biophys Res Commun. 2003;306:169–176. doi: 10.1016/S0006-291X(03)00913-6. [DOI] [PubMed] [Google Scholar]
- Loo TW, Clarke DM. Chemical and pharmacological chaperones as new therapeutic agents. Expert Rev Mol Med. 2007;9:1–18. doi: 10.1017/S1462399407000361. [DOI] [PubMed] [Google Scholar]
- Loo TW, Bartlett MC, Clarke DM. Rescue of folding defects in ABC transporters using pharmacological chaperones. J Bioenerg Biomembr. 2005;37:501–507. doi: 10.1007/s10863-005-9499-3. [DOI] [PubMed] [Google Scholar]
- Loo TW, Bartlett MC, Clarke DM. Benzbromarone stabilizes DeltaF508 CFTR at the cell surface. Biochemistry. 2011;50:4393–4395. doi: 10.1021/bi2004813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Morita M, Shimozawa N, Kashiwayama Y, et al. ABC subfamily D proteins and very long chain fatty acid metabolism as novel targets in adrenoleukodystrophy. Curr Drug Targets. 2011;12:694–706. doi: 10.2174/138945011795378577. [DOI] [PubMed] [Google Scholar]
- Morita M, Kobayashi J, Yamazaki K, et al. A novel double mutation in the ABCD1 gene in a patient with X-linked adrenoleukodystrophy: analysis of the stability and function of the mutant ABCD1 protein. JIMD Rep. 2013;10:95–102. doi: 10.1007/8904_2012_209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser J, Douar AM, Sarde CO, et al. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 1993;361:726–730. doi: 10.1038/361726a0. [DOI] [PubMed] [Google Scholar]
- Sampson HM, Robert R, Liao J, et al. Identification of a NBD1-binding pharmacological chaperone that corrects the trafficking defect of F508del-CFTR. Chem Biol. 2011;18:231–242. doi: 10.1016/j.chembiol.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Sawkar AR, Schmitz M, Zimmer KP, et al. Chemical chaperones and permissive temperatures alter localization of Gaucher disease associated glucocerebrosidase variants. ACS Chem Biol. 2006;1:235–251. doi: 10.1021/cb600187q. [DOI] [PubMed] [Google Scholar]
- Shimada Y, Kobayashi H, Kawagoe S, et al. Endoplasmic reticulum stress induces autophagy through activation of p38 MAPK in fibroblasts from Pompe disease patients carrying c.546G>T mutation. Mol Genet Metab. 2011;104:566–573. doi: 10.1016/j.ymgme.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Morita M, Maeda T, et al. Adrenoleukodystrophy: subcellular localization and degradation of adrenoleukodystrophy protein (ALDP/ABCD1) with naturally occurring missense mutations. J Neurochem. 2007;101:1632–1643. doi: 10.1111/j.1471-4159.2007.04457.x. [DOI] [PubMed] [Google Scholar]
- Watkins PA, Ferrell EV, Jr, Pedersen JI, et al. Peroxisomal fatty acid beta-oxidation in HepG2 cells. Arch Biochem Biophys. 1991;289:329–336. doi: 10.1016/0003-9861(91)90419-J. [DOI] [PubMed] [Google Scholar]
- Wilke M, Bot A, Jorna H, et al. Rescue of murine F508del CFTR activity in native intestine by low temperature and proteasome inhibitors. PLoS One. 2012;7:e52070. doi: 10.1371/journal.pone.0052070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Kim Chiaw P, Bear CE. Probing conformational rescue induced by a chemical corrector of F508del-cystic fibrosis transmembrane conductance regulator (CFTR) mutant. J Biol Chem. 2011;286:24714–24725. doi: 10.1074/jbc.M111.239699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, De Marcos Lousa C, Schutte-Lensink N. Conservation of targeting but divergence in function and quality control of peroxisomal ABC transporters: an analysis using cross-kingdom expression. Biochem J. 2011;436:547–557. doi: 10.1042/BJ20110249. [DOI] [PubMed] [Google Scholar]
- Zhang D, Ciciriello F, Anjos SM, et al. Ouabain mimics low temperature rescue of F508del-CFTR in cystic fibrosis epithelial cells. Front Pharmacol. 2012;3:176. doi: 10.3389/fphar.2012.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ABCD1-GFP and missense mutations in X-ALD patients. The mutated positions in the ABCD1 protein in fusion with GFP are used in this study (TIFF 1521 kb)
Recovery of mutant ABCD1-GFP by the treatment of MG132. CHO/mutABCD1-GFP (A616T, R617H and R660W) cells were cultured in the presence of MG132 (20 μM) for 20 h. After the incubation, cells were harvested and subjected to immunoblot analysis as in Fig. 2 (TIFF 1521 kb)
Recovery of mutant ABCD1-GFP (A616T) by incubating low temperature. CHO/mutABCD1-GFP (A616T) cells were cultured by incubating at 30°C for up to 5 days. After the incubation, cells were harvested and subjected to immunoblot analysis as in Fig. 2 (TIFF 1521 kb)
Stability of mutant ABCD1-GFP (A616T) in peroxisomes. CHO/mutABCD1-GFP (A616T) cells were incubated at 30°C for 5 days and incubated at 37°C for further 0, 12, 24, and 48 h. After fixation, cells were stained as in Fig. 2b (TIFF 1521 kb)
Measurement of fluorescence intensity with a spectrofluorometer. CHO/mutABCD1-GFP (A616T) cells were cultured on 96 well plate and incubated at 30°C for 5 days or with MG132 (20 μM) for 20 h. After the incubation, the cells were washed with 1 × HBSS and directly analyzed the fluorescence intensity. The fluorescence images of CHO/wildABCD1-GFP cells and CHO/mutABCD1-GFP (A616T) cells were also shown in the figure (TIFF 1521 kb)
Recovery of mutant ABCD1-GFP by the treatment of positive compounds. CHO/mutABCD1-GFP(A616T) cells were incubated in the medium containing each 19 positive compounds (5 μM) that were selected by measuring fluorescent intensity. After the 2-day incubation, cells were prepared for both immunofluorescence and immunoblot analysis as in Fig. 2. Among the 19 positive compounds, only 4 drugs (doxorubicin, idarubicin, aclarubicin, or bortezomib) showed the recovery of mutant ABCD1-GFPs (TIFF 1521 kb)
Effect of doxorubicin on the gene expression in CHO/mutABCD1-GFP and CHO/GFP-SKL. CHO/mutABCD1-GFP (A616T) cells and CHO/GFP-SKL cells were incubated in the presence or absence of doxorubicin (5 μM) for 48 h. After the incubation, total RNA was extracted and reverse transcribed into cDNA. The cDNA was used as template for real-time PCR using specific primer. The result shows the arbitral unit based on 18SrRNA (TIFF 1521 kb)
Effect of bortezomib on the recovery of mutant ABCD1 protein in X-ALD fibroblasts. X-ALD fibroblasts (A616T) were treated with bortezomib at the concentration of up to 100 nM for 2 days (a) or with bortezomib at 50 nM for 0, 3, 6, 12, 24, or 48 h (b). After the incubation, the total cellular protein (150 μg protein/lane) was separated by SDS-PAGE followed by immunoblotting using anti-ABCD1 or anti-β-actin antibodies (TIFF 1521 kb)
Cell viability of X-ALD fibroblasts in the presence of bortezomib. X-ALD fibroblasts (A616T) were cultured in the medium containing bortezomib (0, 5, 10, 20, 50, 100, 200, and 500 nM) for 2 days (closed bar) or 7 days (gray bar). After the incubation, cells were subjected to MTT assay. The values in the figure were indicated as percentage of the fibroblasts in the absence of bortezomib. Results are the means ± S.D.; n = 3 (TIFF 1521 kb)
Primer for mutation (DOCX 44 kb)