Abstract Abstract
An annotated checklist of the psyllids of the Cook Islands is presented. The presence of Syntomozatahuata (Klyver, 1932) and Triozaalifumosa Klyver, 1932 in the archipelago, based on new material collected, is reported for the first time. This is the first record from these islands of the genus Syntomoza and the family Liviidae. An identification key to the psyllid species known from the Cook Islands is provided, and their origin and provenance are discussed in relation to their biogeographic implications.
Keywords: Jumping plant lice, Pacific Islands, Polynesia, Rarotonga, Sternorrhyncha
Introduction
The superfamily Psylloidea (Hemiptera: Sternorrhyncha) is composed of almost 4000 described species worldwide (Ouvrard 2018). These include taxa used for biological control, such as Arytainillaspartiophila (Förster, 1848) released in New Zealand to control Scotch broom, Cytisusscoparius (L.) Link (Fabaceae) (Syrett et al. 2007), and also a number of species listed as pests (EPPO/CABI 1997). Among these, a few taxa are known to vector plant pathogenic bacteria (e.g. Munyaneza 2007, 2014). Such a broad range of ecological functions ensures that psyllids’ movement between countries is of interest. For example, a recent study implemented modelling analyses to assess the risk and predict the spread of the pest species Russellianasolanicola Tuthill, 1959, the South American potato psyllid, to several countries where it is not yet present (Syfert et al. 2017). Similarly, the recent establishment of the tomato/potato psyllid Bactericeracockerelli (Šulc), vector of Candidatus Liberibacter solanacearum and agent of the zebra chips disease, has caused great economic losses in New Zealand (Vereijssen et al. 2018). Therefore, understanding psyllid distributions is fundamental to assess the risk associated with new invasions. In recent years, research on psyllid biodiversity has been conducted in a number of regions and islands of the Austro-Pacific. These include the description of taxa in Australia (Taylor et al. 2016, Taylor 2018), the reclassification of Pariaconus Enderlein, 1926 and Swezeyana Caldwell, 1940 in the Hawaiian Islands (Percy 2017, 2018) and reports of the arrival of alien species in Australia (Taylor and Kent 2013), New Zealand (Thorpe 2013, Martoni et al. 2016, Martoni et al. 2018) and French Polynesia (Claridge et al. 2014). However, the psyllid fauna of most other Pacific Islands has not been updated for many years (Ouvrard 2018).
The first report on the psyllid fauna of the Cook Islands appears in Hodkinson’s checklist of the Austro-Oriental and Pacific area that listed three species: Mesohomotomahibisci (Froggatt, 1901); Leptynopterasulfurea Crawford, 1919; and Triozavitiensis Kirkaldy, 1907 (Hodkinson 1983). An additional species, Heteropsyllacubana Crawford, 1914, was reported a few years later (Muddiman et al. 1992). The most recent addition was a Trioza species similar to T.zimmermani Tuthill, 1942, identified by P. Dale and recorded in the online Cook Island Biodiversity and Natural Heritage database (McCormack 2007).
The geographical location of the Cook Islands puts them in a central position between French Polynesia and other countries such as Samoa, Tonga, Fiji, and New Zealand. This makes this small archipelago important for evaluating biogeographic hypotheses and testing theories of biological dispersal within the Pacific. Additionally, due to the high movement of people and produce between the Cook Islands, New Zealand and Australia, understanding the biodiversity of the Cook Islands allows evaluation of potential biosecurity risks for New Zealand or Australian agriculture.
For these reasons, recent field collections from the Cook Islands presented in this work have contributed to updating our knowledge of the psyllid biodiversity of the Islands, with the discovery of two additional taxa: Syntomozatahuata (Klyver, 1932), and Triozaalifumosa Klyver, 1932, both originally described from French Polynesia (Marquesas) (Klyver 1932).
Materials and methods
Specimens were collected by SDJB on the island of Rarotonga, Cook Islands, in March and April 2017. Collections were made by beating host foliage over a beating tray. Insects were stored in propylene glycol until morphological identification was performed. Photographs were taken using a Nikon DS-Ri2 camera connected to a Nikon SMZ25 microscope. Pictures presented in the plates are the result of stacking photographs using the software Nikon NIS-Elements D v4.5 resulting in a single image with an extended depth of field. Plates were prepared using GIMP version 2.8.14. For closer examination, two specimens of each species (male and female) were mounted on microscope slides following the protocol of Taylor et al. (2016). Morphological terms follow Taylor et al. (2011) and wing venation follows Hodkinson and White (1979) and Hollis (1984). Psyllid specimens from the recent field collection have been deposited in the New Zealand Arthropod Collection (NZAC, Manaaki Whenua Landcare Research, Tamaki, Auckland), and the Lincoln University Entomology Collection (LUNZ, Canterbury). Plants were identified by SDJB using Sykes (2016). Specimens of the host plants collected at the same time as insect specimens were deposited in the Allan Herbarium (Landcare Research, Lincoln, New Zealand), with catalogue numbers CHR644589 (Homaliumacuminatum), CHR644590 (Weinmanniasamoensis), and CHR644584 and CHR644585 (Metrosideroscollina). Paratype specimens of T.alifumosa and T.alipellucida Klyver, 1932 were examined in the Bernice Pauahi Bishop Museum (BPBM, Honolulu, Hawaii).
Identification of the newly reported species
Syntomoza tahuata
(Klyver, 1932)
Figures 1–10.
Syntomozatahuata. 1 lateral habitus of female 2 lateral habitus of male 3 dorsal habitus of female 4 dorsal habitus of male 5 head of female, dorsal view 6 head of male, dorsal view 7 wing of male 8 mesotibia of male 9 terminalia of female, lateral view of left side 10 terminalia of male, lateral view of left side. Abbreviation: par = paramere. Scale bars: 1 mm (1–7); 0.5 mm (8); 0.25 mm (9, 10).
Figures 22–29.
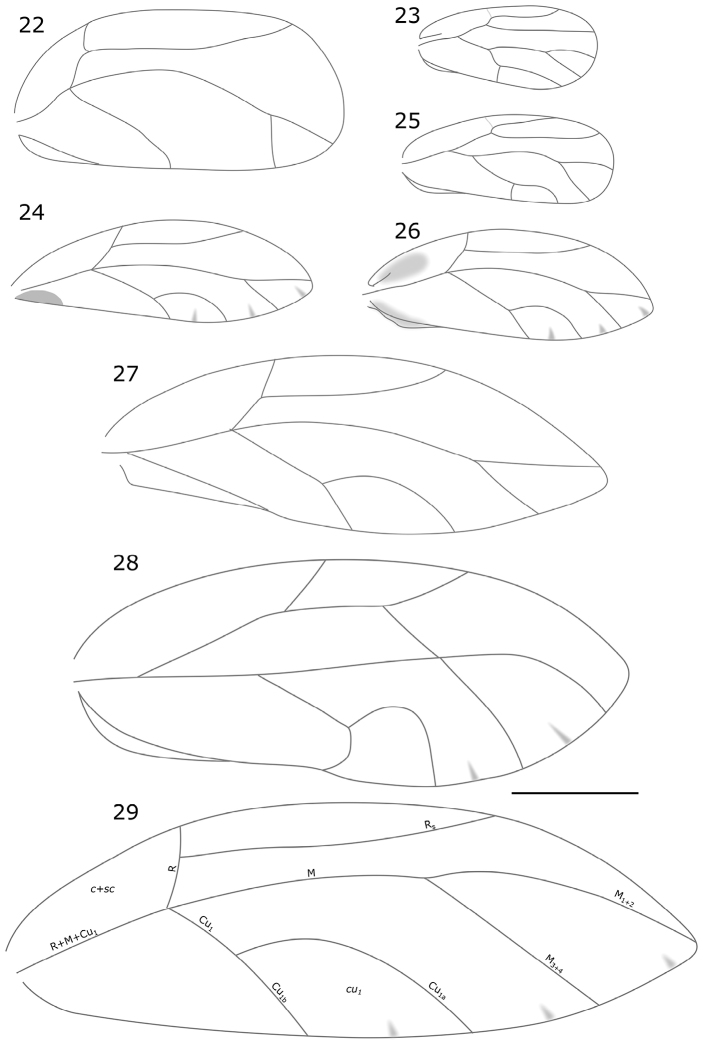
Wings, schematic. 22Leptynopterasulfurea (after Crawford 1919) 23Syntomozatahuata (from slide-mounted Rarotonga specimen) 24Triozacf.zimmermani (from photograph of Rarotonga specimen by G. McCormack) 25Heteropsyllacubana (after Tuthill 1959) 26Triozaalifumosa (from slide-mounted Rarotonga specimen) 27Triozazimmermani (after Tuthill 1942) 28Mesohomotomahibisci (after Froggatt 1901) 29Triozavitiensis (after Klyver 1932). Scale bar: 1 mm.
Material examined.
4 females, 10 males. This species was collected on two separate occasions on Rarotonga: on 15 April 2017 on Te Manga at elevations between 540 m and 560 m, collected from two host plants: from Weinmanniasamoensis A.Gray (Cunionaceae) (five specimens) and from Freycinetiawilderi Martelli ex Wilder (Pandanaceae, plant specimens not collected) (two specimens), and on 17 April 2017 in the Avana Valley around 70 m elevation, from the foliage of a fallen Homaliumacuminatum Cheeseman (Salicaceae) (seven specimens collected, with several more observed). Three additional specimens collected around Avatiu in November 1979 by NLH Krauss were located in the Bishop Museum.
Measurements.
Measurements are in mm (n = 3 ♂, 2 ♀ unless reported differently in brackets). Length of body (vertex to terminalia) ♂ 0.98–1.30 (n = 2), ♀ 1.17–1.53; length of body (vertex to apex of folded wings) ♂ 1.72–1.88 (n = 2), ♀ 2.21–2.22; width of head (HW) ♂ 0.53–0.60 (n = 2), ♀ 0.63–0.65; length of genal processes (GCL) ♂ 0.10 (n=1), ♀ 0.12; length of vertex (VL) ♂ 0.12–0.18 (n = 2), ♀ 0.18–0.19; width of vertex (VW) ♂ 0.30–0.35 (n = 2), ♀ 0.34–0.38; length of antenna (AL) ♂ 0.40–0.49 (n = 2), ♀ 0.44–0.57; length of fore wing ♂ 1.40–1.49, ♀ 1.71–1.77; width of fore wing ♂ 0.63–0.68, ♀ 0.75–0.85; length of vein Rs ♂ 0.82–0.87, ♀ 1.02–1.04; length of vein M (M) ♂ 0.44–0.46, ♀ 0.52–0.53; length of vein M1+2 (M1) ♂ 0.36–0.40, ♀ 0.48–0.51; marginal width of cell m1 ♂ 0.18–0.20, ♀ 0.26–0.27; marginal width of cell cu1 ♂ 0.50–0.54, ♀ 0.62–0.63; length of vein Cu1b ♂ 0.11–0.14, ♀ 0.13–0.16; length (height) of proctiger (PL) ♂ 0.21–0.24 (n = 2); length of paramere ♂ 0.17–0.19 (n = 2); length of proximal aedeagal segment ♂ 0.19 (n = 1); length of distal aedeagal segment ♂ 0.09 (n = 1); length of proctiger (PL) ♀ 0.44–0.52; length of circum-anal ring (CL) ♀ 0.16–0.20; length of subgenital plate (SL) ♀ 0.35–0.46.
Description.
The stout body shape, and the distinct dorsal patterning of orange stripes on a black background makes this psyllid readily recognised within the Cook Island fauna. This psyllid was identified using the original description (Klyver 1932) and the subsequent reclassification that attributed this species to the genus Syntomoza Enderlein, 1921 (Burckhardt and Mifsud 2003). Other features that allow it to be placed in S.tahuata include the greatly modified tergites and the secondary groups of small teeth at the apex of the posterior tibiae in both sexes (Figure 8), which are characteristic of this genus, together with the strongly inclined head (at about 90° to the longitudinal body axis; Figures 1, 2). Furthermore, the female terminalia which are pronouncedly down-turned at about 45° (Figure 1), the shape of the male parameres (Figure 10), and wing shape and venation (Figure 7) allowed identification of this species as per the description and figures presented by Klyver (1932).
Trioza alifumosa
Klyver, 1932
Figures 11–20.
Triozaalifumosa. 11 lateral habitus of female 12 lateral habitus of male 13 dorsal habitus of male 14 dorsal habitus of female 15 head of female, dorsal view 16 head of male, dorsal view 17 wing of male 18 mesotibia of male 19 terminalia of female, lateral view of left side 20 terminalia of male, lateral view of left side. Abbreviations: aed = aedeagus, par = paramere, ptg = proctiger, sgp = subgenital plate. Scale bars: 1 mm (11–17); 0.5 mm (18); 0.25 mm (19, 20).
Material examined.
11 females, 8 males. A single population of this species was collected on Rarotonga, on the summit of Raemaru at an elevation of 380 m. On 16 March 2017 all 19 individuals were collected from a single plant of Metrosideroscollina (J.R.Forst. and G.Forst.) A.Gray.
Measurements.
Measurements are in mm (n = 2 ♂, 3 ♀ unless reported differently in brackets). Length of body (vertex to terminalia) ♂ 1.30–1.45, ♀ 1.60–1.78; length of body (vertex to apex of folded wings) ♂ 2.57–2.81, ♀ 2.86–3.10; width of head (HW) ♂ 0.50–0.53, ♀ 0.52–0.57 (n = 2); length of genal processes (GCL) ♂ 0.09–0.14 ♀ 0.10–0.13 (n = 2); length of vertex (VL) ♂ 0.21, ♀ 0.20–0.25 (n = 2); width of vertex (VW) ♂ 0.31–0.32, ♀ 0.32–0.33 (n = 2); length of antenna (AL) ♂ 0.78–0.79, ♀ 0.81–0.85 (n = 2); length of fore wing ♂ 2.27–2.28, ♀ 2.38–2.57 (n = 2); width of fore wing ♂ 0.83–0.86, ♀ 0.85–0.96 (n = 2); length of vein Rs ♂ 0.91–0.99, ♀ 1.00–1.08 (n = 2); length of vein M (M) ♂ 1.11–1.12, ♀ 1.15–1.24 (n = 2); length of vein M1+2 (M1) ♂ 0.44–0.48, ♀ 0.54–0.56 (n = 2); marginal width of cell m1 ♂ 0.28–0.32, ♀ 0.38 (n = 2); marginal width of cell cu1 ♂ 0.40–0.42, ♀ 0.42–0.44 (n = 2); length of vein Cu1b ♂ 0.23–0.25, ♀ 0.21–0.25 (n = 2); length (height) of proctiger (PL) ♂ 0.15–0.20; length of paramere ♂ 0.11–0.13; length of proximal aedeagal segment ♂ 0.17 (n = 1); length of distal aedeagal segment ♂ 0.16 (n = 1); length of proctiger (PL) ♀ 0.30–0.51; length of circum-anal ring (CL) ♀ 0.10–0.13; length of subgenital plate (SL) ♀ 0.29–0.34.
Description.
This psyllid can be identified by the following combination of characters: habitus as in Figures 11–14, with a dark brown colour, fore wings with an infuscate spot in the apical costal cell as in Figures 13, 17, female proctiger short and bearing setae on the subgenital plate (Figure 19); male parameres elongate, slightly back-turned apically and bearing setae (Figure 20). Both this species and T.alipellucida Klyver, 1932, were described from material collected on Metrosideroscollina. The evenly dark colouration of the dorsal surface and head, the presence of an infuscate spot in the apical costal cell (c+sc), the rounded but elongated shape of the aedeagus, the elongated shape of the male proctiger and the slightly back-turned parameres lead us to place it in T.alifumosa. Triozaalipellucida differs from T.alifumosa by most specimens having a wide lighter brown stripe on the pronotum, not having an infuscate spot at the base of the forewing, and for a shorter male proctiger associated with parameres that are not as back-turned. The morphological distinction between T.alifumosa and T.zimmermani appears more immediate, with the latter presenting light stripes dorsally on a dark brown abdomen and having hyaline wings without any dark spot in the cell c+sc (Tuthill 1942).
Checklist of the Cook Islands psyllids
The following checklist includes all species known to be present in the Cook Islands. Information on their taxonomy is reported together with their worldwide distribution and host plant associations. For species of socio-economic interest, such as pests, basic information on their biology is summarised.
Family Carsidaridae
Mesohomotoma hibisci
(Froggatt, 1901)
Figure 21.
Mesohomotomahibisci nymphs and adult on Hibiscustiliaceus on Rarotonga, showing white waxy exudates formed by the nymphs.
Tyora hibisci Froggatt, 1901: 287.
Udamostigma hibisci (Froggatt); Enderlein 1910: 138.
Mesohomotoma hibisci (Froggatt); Crawford 1925: 32.
Distribution.
Reported on the Cook Islands by Hodkinson (1983). Known from Rarotonga and Mangaia. Other locations include: Australia (Hollis 2004), Africa [Cameroon, Democratic Republic of the Congo, Kenya, Madagascar, Seychelles, South Africa, Tanzania, Uganda and Zimbabwe (Yana et al. 2015; Burckhardt and Van Harten 2006)], Asia [Chagos archipelago, China, India, Japan, Malaya, Malaysia, Mauritius, Philippines, Ryukyu Islands, Singapore, Yemen (Hodkinson 1983, Hodkinson 1986, Burckhardt and Van Harten 2006, Percy 2017)], Pacific Islands [Bismarck Archipelago, Caroline Islands, Fiji, French Polynesia (Australs, Societies, Marquesas), Gilbert Islands, New Caledonia, Palau, Tonga, Solomon Islands, Vanuatu (Hodkinson 1983)].
Host plant.
Hibiscus species, especially H.tiliaceus L. (Malvaceae).
Common name.
Hibiscus (woolly) psyllid (David Hockings 2013).
Remarks.
the genus Mesohomotoma Kuwayama was reviewed by Hollis (1987). The species included in the genus have a lot of variation between populations, and subtle differences between species. Although Hollis (1987) suspected all nominal taxa may represent a single species, he did not formally synonymise them, recommending that further research into their biology and hostplants be undertaken to further investigate species boundaries in the genus. This species breeds in the tips of Hibiscustiliaceus branches. The nymphs produce filamentous exudates, which forms a woolly coating on the leaves and stem of the plant (Figure 21). Mesohomotomahibisci is considered a pest (David Hockings 2013).
Family Liviidae
Syntomoza tahuata
(Klyver, 1932)
Anomoterga tahuata Klyver, 1932: 94.
Syntomoza tahuata (Klyver); Burckhardt and Mifsud 2003: 17.
Distribution.
Reported on the Cook Islands in the present study. Known only from Rarotonga. Other locations include: French Polynesia (Marquesas) (Klyver 1932).
Host plant.
No host plants have been previously proposed (Burckhardt and Mifsud 2003; Ouvrard 2018). In June 2002, however, Percy (pers. comm.) collected a high number of adult specimens (> 30) from Weinmanniaparviflora in French Polynesia (Marquesas) with no specimens found on surrounding plants.
Family Psyllidae
Heteropsylla cubana
Crawford, 1914
Heteropsylla cubana Crawford, 1914.
Distribution.
Reported on the Cook Islands by Hodkinson (1983). Known only from Rarotonga. Other locations include: Australia (Muddiman et al. 1992), America [Bahamas, Bermuda, Brazil , Central America, Chile, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, El Salvador, Guatemala, Jamaica, Mexico, Nicaragua, Panama, Peru, Suriname, Trinidad and Tobago, USA (Brown and Hodkinson 1988, Burckhardt and Queiroz 2012, Hodkinson and White 1981, Hodkinson 1988, Hodkinson and Muddiman 1993, Muddiman et al. 1992, Olivares and Burckhardt 2002, Percy et al. 2012)], Africa [Burundi, Cameroon, Kenya, KwaZulu-Natal, Mauritius, Mpumalanga, Reunion, Tanzania, Uganda, Zimbabwe (FAO 1994, Dzokou et al. 2009, Matimati et al. 2009, Muddiman et al. 1992, Olckers 2011)], Asia [Bangladesh, Cambodia, China, Christmas Islands, India, Indonesia, Japan, Malaysia, Mariana Islands, Nepal, Ryukyu Islands, Philippines, Sri Lanka, Taiwan, Thailand, Vietnam (Muddiman et al. 1992, Martin and Lau 2011, Inoue and Miyatake 2001, Geiger and Gutierrez 2000)], Pacific Islands [Fiji, French Polynesia (Australs), Guam, Haiti, Hawaiian Islands, New Caledonia, Niue, Papua New Guinea, Samoa, Solomon Islands, Tonga (Beardsley and Uchida 1990, Claridge et al. 2014, Muddiman et al. 1992, FAO 1994)], Europe [Ireland (Muddiman et al. 1992)].
Host plant.
Leucaenaleucocephala (Lam.) de Wit (Fabaceae).
Common name.
Leucaena psyllid (Asadi et al. 2011).
Remarks.
Heterpsyllacubana is considered an agricultural pest both in the Asia-Pacific area and in Africa (FAO 1994). The biological control agent that has been used most and with better results is the parasitoid Psyllaephagusyaseeni Noyes, 1990 (Encyrtidae), but Curinuscoeruleus Mulsant, 1850 (Coccinellidae) and Tamarixialeucaenae Boucek, 1988 (Eulophidae) have been used as well (Geiger and Gutierrez 2000).
Biology.
The biology and life cycle of H.cubana is reported here with the intent of summarising information (mostly from Showler and Melcher 1995 and CABI 1990) that may be relevant for a better understanding of this pest species. The incubation period for eggs is generally 2–5 days. Immature stages grow from the egg through five instars to adulthood in 10–20 days. Nymphs feed at first gregariously near the oviposition site and then, more and more solitarily, they colonise and feed on other parts of stems, branches, and petioles of young leaves. Generations are overlapping, and longevity of adults is on average 14.5 days for females and 9.7 days for males. Mating can occur more than once for both males and females (Rauf et al. 1990) and eggs are laid in groups on very young shoots, often covering the whole leaflet. Each female can produce 300–500 eggs throughout a lifetime and can lay as many as 60 eggs in one day. Heteropsyllacubana is diurnal, and flight of adults can occur in the morning and afternoon.
Family Triozidae
Leptynoptera sulfurea
Crawford, 1919
Leptynoptera sulfurea Crawford, 1919: 147.
Distribution.
Reported on the Cook Islands by Hodkinson (1983). Known only from Rarotonga. Other locations include: Australia (Hollis 2004), Asia [China, Chagos Islands, Cocos Islands, India, Indonesia, Japan, Malaysia, Philippines, Ryukyu Islands, Singapore, Sulawesi, Taiwan, Thailand (Martin and Hollis 1992, Hodkinson 1983, 1986, Neville et al. 2015)], Pacific Islands [Caroline Islands, Fiji, French Polynesia (Australs), Guam, Hawaiian Islands, Mariana Islands, New Caledonia, Palau, Papua New Guinea, Tonga (Hodkinson 1983, Martin and Hollis 1992, Percy 2017].
Host plant.
Calophylluminophyllum L. (Calophyllaceae).
Remarks.
Leptynopterasulfurea forms galls along the leaf margins of Calophylluminophyllum (Neville et al. 2015), a tree of particular significance for Cook Islanders in that the trunks were preferentially used for building canoes (Hiroa 1927).
Trioza alifumosa
Klyver, 1932
Trioza alifumosa Klyver, 1932: 96.
Distribution.
Reported on the Cook Islands in the present study. Known only from Rarotonga. Other locations include: French Polynesia (Marquesas, Fatu Hiva) (Klyver 1932).
Host plant.
Metrosideroscollina (J.R. Forst. & G. Forst.) A. Gray (Myrtaceae).
Trioza vitiensis
Kirkaldy, 1907
Trioza vitiensis Kirkaldy, 1907: 103.
Megatrioza vitiensis (Kirkaldy); Crawford 1919: 195.
Phyllopecta vitiensis (Kirkaldy); Klyver 1932: 99.
Trioza vitiensis Kirkaldy, 1907 combinatio revivisco according to Mathur (1975): 348.
Distribution.
Reported on the Cook Islands by Hodkinson (1983). Known only from Rarotonga. Other locations include: Asia [China, India, Indonesia, Malaya, Malaysia, Philippines, Singapore, Sri Lanka (Hodkinson 1983, 1986)], Pacific Islands [Caroline Islands, Fiji, French Polynesia (Societies, Marquesas), Samoa (Kirkaldy 1907, Hodkinson 1983)].
Host plant.
Syzygiummalaccense (L.) Merr. & L.M.Perry, 1938 (Myrtaceae).
Trioza cf. zimmermani
Tuthill, 1942
Distribution.
Reported on the Cook Islands by P.J. Dale (McCormack 2007). Known only from Rarotonga.
Host plant.
Metrosideroscollina (J.R. Forst. & G. Forst.) A. Gray (Myrtaceae).
Remarks.
no specimens of this psyllid were collected by the authors. Photographs provided by G McCormack were consistent with the morphology of T.zimmermani, with the greatest difference shown in the wings (Figures 24, 27), with the Rarotongan specimens being shorter and with a less acute apex (Figure 24), than those from Raivaevae drawn by Tuthill (1942, Figure 27). However, since no specimens could be examined in person, this taxon is reported here based on the identification made by Dale. The distribution of T.zimmermani includes French Polynesia (Australs) (Tuthill 1942, Percy 2017).
Key to the Cook Islands psyllids
| 1 | Forewing with vein R+M+Cu1 bi-furcating to form R and M+Cu1 (Figures 23, 25, 28) | 2 |
| – | Forewing with vein R+M+Cu1 tri-furcating to form R, M and Cu1 (Figures 24, 29) | 4 |
| 2 | Forewing with veins R and M+Cu1 equally long or M+Cu1 slightly longer than R (Figures 7, 23). Body colour black with orange stripes on the dorsum | Syntomozatahuata (Klyver, 1932) |
| – | Forewing with vein R longer than M+Cu1 (Figures 25, 28). Body colour light green | 3 |
| 3 | Forewing with vein Rs very short (♂ 0.91, ♀ 1.14), strongly bent towards margin at apex, with a transverse vein crossing from centre of Rs to the bi-furcation between M1+2 and M3+4 (Figure 28) | Mesohomotomahibisci (Froggatt, 1901) |
| – | Forewing with vein Rs not turning upward and no transverse vein crossing the wing (Figure 25) | Heteropsyllacubana Crawford, 1914 |
| 4 | Forewing with vein Cu1 not bi-furcating and therefore not forming cell cu1 (Figure 22). Body colour light brown | Leptynopterasulfurea Crawford, 1919 |
| – | Cell cu1 present (Figures 24, 29). Body colour darker brown/black | 5 |
| 5 | Forewing with dark spot on cell c+sc (Figures 17, 26). Body colour black with subtle brown patterning | Triozaalifumosa Klyver, 1932 |
| – | Forewing with no spots (Figures 24, 29). Body colour brown with tan pattern or black with pale stripe on the abdomen | 6 |
| 6 | Male genitalia with parameres pointing forward at apex and proctiger bearing long setae on the apical part facing the parameres. Female genitalia extremely short, approximately ¼ of abdomen. Length of psyllid to tip of folded wings between 5 mm and 6 mm. Body colour brown, with tan patterning | Triozavitiensis Kirkaldy, 1907 |
| – | Male parameres pointing backward at apex, proctiger bearing short setae uniformly, female terminalia longer (half of the rest of abdomen). Length of the psyllid to tip of folded wings only 3.5mm. Body colour black with pale stripe at base of abdomen | Triozacf.zimmermani Tuthill, 1942 |
Discussion
Based on the similarity of the samples analysed with the description and the drawings provided by the literature, the presence of the psyllids Syntomozatahuata and Triozaalifumosa is reported on the Cook Islands for the first time. Host plants for these two species in the Cook Islands are hypothesised to be Weinmanniasamoensis or Homaliumacuminatum and Metrosideroscollina respectively, based on collection data. Percy’s collection of a large number of individuals of S.tahuata from Weinmanniaparviflora suggests this genus could be a true host plant (Percy, personal communication). However, we consider that H.acuminatum should remain under consideration as a possible host. No specimens of W.samoensis were seen near the Avana Valley site where S.tahuata was collected from H.acuminatum, and the elevation of the site is well below the lower elevational limit of W.samoensis (Sykes 2016). The number of S.tahuata observed during this collecting event was much greater than were captured, and were much more abundant than on the occasions when S.tahuata was beaten from W.samoensis. A search for immature stages of S.tahuata on both H.acuminatum and W.samoensis should be undertaken to differentiate between these host plant hypotheses or confirm whether S.tahuata is a generalist (Burckhardt et al. 2014).
We consider these two species to be indigenous to the Cook Islands, despite their not having been recorded here previously. The Cook Islands are underexplored entomologically, with relatively little collecting having been done in indigenous vegetation in particular. Moreover, these species were found in areas of relatively intact vegetation, with little human modification, which tend to be more resistant to invasive species (Brockerhoff et al. 2010). We hypothesise that further investigation of the psyllid fauna in other islands of Eastern Polynesia will locate these species there also, in areas where Metrosideroscollina and Homalium species may be found. However, this in itself would not provide sufficient evidence to distinguish between hypotheses of recent or distant arrival in the Cook Islands. In the absence of past collections, analysis of rapidly evolving DNA regions would be necessary to provide further data to infer the arrival of these species in the Cook Islands.
The psyllid fauna of the Cook Islands now includes seven psyllid taxa from five genera and four families. The addition of S.tahuata is not only the first report for the genus in the Cook Islands, but also for the family Liviidae.
Compared with the psyllid fauna of other nearby archipelagos, the Cook Islands appear to have a very similar psyllid biodiversity. In fact, the single taxon present in Niue (H.cubana) and three of the four taxa present in Tonga (H.cubana, M.hibisci, and L.sulfurea) are also present in the Cook Islands (Ouvrard 2018). Similarly, the psyllid fauna of French Polynesia lists eight species, four of which are in common with the Cook Islands: M.hibisci, T.zimmermani, T.alifumosa, and S.tahuata (Ouvrard 2018). On the other hand, the Cook Islands do not share any of the three taxa present in American Samoa (Ouvrard 2018). A recent review indicates that the biota of the Society islands in many cases show close sister-taxon relationships with the Cook, Austral, and Marquesas Islands (Hembry and Balukjian 2016). They also found that many taxa showed patterns of multiple colonisation of the islands, indicating high species turnover in the Eastern Polynesian region (Hembry and Balukjian 2016). We believe that the records of the two psyllid species reported here for the first time from the Cook Islands provides further evidence of the recognition of a distinctive Eastern Polynesian fauna.
Supplementary Material
Acknowledgements
The authors would like to thank Gerald McCormack (Natural Heritage Trust, Cook Islands) for assistance, advice, and support in the Cook Islands. A special thank you to Dr Diana Percy, for reviewing the manuscript and providing important information regarding the host plant of S.tahuata. Ines Schoenberger and Mary Korver (Allan Herbarium, Manaaki Whenua Landcare Research, Lincoln) facilitated plant specimen collections and identification. Jim Boone and Neal Evenhuis (Bishop Museum, Honolulu, Hawaii) provided access to the type specimens of the psyllids of interest. Fieldwork in the Cook Islands was carried out under research permit 10/17, dated 21 April 2017, issued by Bredina Drollet through the Office of the Prime Minister. Thanks to John Marris and the Bio-Protection Research Centre (Lincoln University) for providing working space and equipment for sorting and imaging specimens. This research was supported by a Winston Churchill Memorial Fellowship awarded to SDJB, and Plant and Food Research Strategic Science Investment Funding (SSIF).
Citation
Martoni F, Brown SDJ (2018) An annotated checklist of the Cook Islands psyllids with keys to the species and two new records (Hemiptera, Psylloidea). ZooKeys 811: 91–108. https://doi.org/10.3897/zookeys.811.28829
References
- Asadi R, Talebi AA, Khalghani J, Fathipour Y, Moharramipour S, Burckhardt D. (2011) Comparative development and demographic parameters of Euphyllurapakistanica on four olive cultivars. Bulletin of Insectology 64(2): 159–165. [Google Scholar]
- Beardsley JW, Uchida GK. (1990) Parasites associated with the Leucaena psyllid, Heteropsyllacubana Crawford, in Hawaii. Journal of the Hawaii Institute of Tropical Agriculture and Human Resources 30: 155–156. [Google Scholar]
- Brockerhoff EG, Barratt BIP, Beggs JR, Fagan LL, Kay MK, Phillips CB, Vink CJ. (2010) Impacts of exotic invertebrates on New Zealand’s indigenous species and ecosystems. New Zealand Journal of Ecology 34(1): 158–174. [Google Scholar]
- Brown RG, Hodkinson ID. (1988) Taxonomy and ecology of the jumping plant-lice of Panama (Homoptera: Psylloidea). Entomonograph 9: 1–304. [Google Scholar]
- Burckhardt D, Mifsud D. (2003) Jumping plant-lice of the Paurocephalinae (Insecta, Hemiptera, Psylloidea): systematics and phylogeny. Contributions to Natural History, Bern 2: 3–34. [Google Scholar]
- Burckhardt D, Van Harten A. (2006) Jumping plant-lice (Insecta: Hemiptera: Psylloidea) of Yemen. Fauna of Arabia 21: 189–216. [Google Scholar]
- Burckhardt D, Queiroz DL. (2012) Checklist and comments on the jumping plant-lice (Hemiptera: Psylloidea) from Brazil. Zootaxa 3571: 26–48. [DOI] [PubMed] [Google Scholar]
- Burckhardt D, Ouvrard D, Queiroz D, Percy D. (2014) Psyllid host-plants (Hemiptera: Psylloidea): resolving a semantic problem. Florida Entomologist 97: 242–246. [Google Scholar]
- CABI (1990) Economic review of psyllid damage on Leucaena in southeast Asia and Australia. Australian International Development Assistance Bureau, Canberra, 30 pp. [Google Scholar]
- Claridge EM, Garb JE, Gillespie RG, Percy DM. (2014) Insects and spiders. In: Meyer JY, Claridge EM. (Eds) Terrestrial Biodiversity of the Austral Islands, French Polynesia.Muséum national d’Histoire naturelle, Paris, 92–101.
- Crawford DL. (1914) A monograph of the jumping plant-lice or Psyllidae of the new world. Government Printing Office, Washington, 186 pp. [Google Scholar]
- Crawford DL. (1919) The jumping plant lice of the Palaeotropics and the South Pacific Islands – Family Psyllidae, or Chermidae, Homoptera. Philippine Journal of Science 15: 139–207. 10.5962/bhl.part.11765 [DOI] [Google Scholar]
- David Hockings F. (2013) Pests, Diseases and Beneficials: Friends and Foes of Australian Gardens. CSIRO Publishing, 280 pp.
- Dzokou VJ, Tamesse JL, Burckhardt D. (2009) Jumping plant-lice of the family Psyllidae (Hemiptera: Psylloidea) from west-Cameroon: biodiversity and host plants. Journal of Entomology 6(1): 1–17. 10.3923/je.2009.1.17 [DOI] [Google Scholar]
- EPPO/CABI (1997) Quarantine Pests for Europe. 2nd edition. CABI, Wallingford, UK, 1425 pp. [Google Scholar]
- FAO (1994) Leucaena psyllid in the Asia-Pacific region: implications for its management in Africa. RAPA publication: 1994/13, RAPA/FAO, Bangkok, 27 pp. [Google Scholar]
- Froggatt WW. (1901) Australian Psyllidae. Part II. Proceedings of the Linnean Society of New South Wales 26: 242–298. [Google Scholar]
- Geiger CA, Gutierrez AP. (2000) Ecology of Heteropsyllacubana (Homoptera: Psyllidae): Psyllid Damage, Tree Phenology, Thermal Relations, and Parasitism in the Field. Environmental Entomology 29(1): 76–86. 10.1603/0046-225X-29.1.76 [DOI] [Google Scholar]
- Hembry DH, Balukjian B. (2016) Molecular phylogeography of the Society Islands (Tahiti; South Pacific) reveals departures from hotspot archipelago models. Journal of Biogeography 43: 1372–1387. 10.1111/jbi.12723 [DOI] [Google Scholar]
- Hiroa TR. (1927) The material Culture of the Cook Islands (Aitutaki). Thomas Avery and Sons Ltd, New Plymouth, 384 pp. [Google Scholar]
- Hodkinson ID. (1983) The psyllids (Homoptera: Psylloidea) of the Austro-Oriental, Pacific and Hawaiian zoogeographical realms: an annotated check list. Journal of Natural History 17: 341–377. 10.1080/00222938300770251 [DOI] [Google Scholar]
- Hodkinson ID. (1986) The psyllids (Homoptera: Psylloidea) of the Oriental Zoogeographical Region: an annotated check-list. Journal of Natural History 20: 299–357. 10.1080/00222938600770251 [DOI] [Google Scholar]
- Hodkinson ID. (1988) The Nearctic Psylloidea (Insecta: Homoptera): an annotated check list. Journal of Natural History 22(5): 1179–1243. 10.1080/00222938800770751 [DOI] [Google Scholar]
- Hodkinson ID, Muddiman SB. (1993) A new species of Heteropsylla Crawford from Ecuador with new host-plant and distribution records for the genus (Homoptera, Psylloidea). Beiträge zur Entomologie 43(2): 441–443. [Google Scholar]
- Hodkinson ID, White IM. (1979) Handbooks for the Identification of British Insects. Volume II, Part 5(a). Homoptera, Psylloidea. Royal Entomological Society of London, London, 98 pp. [Google Scholar]
- Hodkinson ID, White IM. (1981) The Neotropical Psylloidea (Homoptera: Insecta): an annotated checklist. Journal of Natural History 15(3): 491–523. 10.1080/00222938100770361 [DOI] [Google Scholar]
- Hollis D. (1984) Afrotropical jumping plant lice of the family Triozidae (Homoptera: Psylloidea). Bulletin of the British Museum (Natural History), Entomology Series 49: 1–102. [Google Scholar]
- Hollis D. (1987) A review of the Malvales-feeding psyllid family Carsidaridae (Homoptera). Bulletin of the British Museum (Natural History), Entomology Series 56: 87–127. [Google Scholar]
- Hollis D. (2004) Australian Psylloidea. Jumping plantlice and lerp insects. Australian Biological Resources Study, Canberra (Australia), 216 pp. [Google Scholar]
- Inoue H, Miyatake Y. (2001) Taxonomic study of the superfamily Psylloidea (Homoptera: Sternorrhyncha) on the Ogasawara (Bonin) Islands. Part 1, Aphalaridae, Psyllidae and Carsidaridae, with descriptions of four new species. Entomological Science 4: 459–475. [Google Scholar]
- Kirkaldy GW. (1907) On two new Vitian Chermidae (Hem.). Proceedings of the Hawaiian Entomological Society 1: 103–104. [Google Scholar]
- Klyver FD. (1932) Anomotergatahuata, new genus and new species, and other Chermidae from the Marquesas. Bulletin of the B.H.P. Bishop Museum 98: 93–101. [Google Scholar]
- Martin JH, Hollis D. (1992) The Calophyllum-feeding triozid genus Leptynoptera (Hemiptera: Psylloidea). Journal of Natural History 26: 555–585. 10.1080/00222939200770351 [DOI] [Google Scholar]
- Martin JH, Lau CSK. (2011) The Hemiptera-Sternorrhyncha (Insecta) of Hong Kong, China – an annotated inventory citing voucher specimens and published records. Zootaxa 2847: 1–122. [Google Scholar]
- Martoni F, Burckhardt D, Armstrong K. (2016) An annotated checklist of the psyllids of New Zealand (Hemiptera: Psylloidea). Zootaxa 4144: 556–574. 10.11646/zootaxa.4144.4.6 [DOI] [PubMed] [Google Scholar]
- Martoni F, Bulman S, Pitman A, Taylor G, Armstrong K. (2018) DNA barcoding highlights cryptic diversity in the New Zealand Psylloidea (Hemiptera: Sternorrhyncha). Diversity 10, 50, 18 pp 10.3390/d10030050 [DOI] [Google Scholar]
- Mathur RN. (1975) Psyllidae of the Indian Subcontinent. Indian Council of Agricultural Research, New Delhi, 429 pp. [Google Scholar]
- Matimati I, Maasdorp BV, Hove L. (2009) On-farm productivity of Acaciaangustissima, Calliandracalothyrsus and Leucaenaleucocephala in a subhumid area in Zimbabwe. African Journal of Range & Forage Science 26(2): 75–80. 10.2989/AJRFS.2009.26.2.4.847 [DOI] [Google Scholar]
- McCormack G. (2007) Cook Islands Biodiversity Database, Version 2007.2. Available from: http://cookislands.bishopmuseum.org [accessed 2 June 2018]
- Muddiman SB, Hodkinson ID, Hollis D. (1992) Legume-feeding psyllids of the genus Heteropsylla (Homoptera: Psylloidea). Bulletin of Entomological Research 82: 73–117. 10.1017/S0007485300051518 [DOI] [Google Scholar]
- Munyaneza JE, Crosslin JM, Upton JE. (2007) Association of Bactericeracockerelli (Homoptera: Psyllidae) with “zebra chip,” a new potato disease in southwestern United States and Mexico. Journal of Economic Entomology 100: 656–663. 10.1093/jee/100.3.656 [DOI] [PubMed] [Google Scholar]
- Munyaneza JE, Sengoda VG, Sundheim L, Meadow R. (2014) Survey of “Candidatus Liberibacter solanacearum” in carrot crops affected by the psyllid Triozaapicalis (Hemiptera: Triozidae) in Norway. Journal of Plant Pathology 96: 397–402. [Google Scholar]
- Neville PJ, Burckhardt D, Yen AL. (2015) First record of the psyllid Leptynopterasulfurea Crawford (Hemiptera: Psylloidea: Triozidae) from the Cocos (Keeling) Islands. Journal of Asia-Pacific Entomology 18: 497–499. 10.1016/j.aspen.2015.06.008 [DOI] [Google Scholar]
- Olivares TS, Burckhardt D. (2002) Presencia de Heteropsyllacubana Crawford en Chile (Hemiptera: Psyllidae: Ciriacreminae) [Presence of Heteropsyllacubana Crawford in Chile (Hemiptera: Psyllidae: Ciriacreminae)]. Gayana 66(1): 81–82. 10.4067/S0717-65382002000100012 [DOI] [Google Scholar]
- Olckers T. (2011) Biological control of Leucaenaleucocephala (Lam.) de Wit (Fabaceae) in South Africa: a tale of opportunism, seed feeders and unanswered questions. African Entomology 19(2): 356–365. 10.4001/003.019.0219 [DOI] [Google Scholar]
- Ouvrard D. (2018) Psyl’list – The World Psylloidea Database. Available from: http://www.hemiptera-databases.com/psyllist [accessed 30 October 2018]
- Percy DM, Rung A, Hoddle MS. (2012) An annotated checklist of the psyllids of California (Hemiptera: Psylloidea). Zootaxa 3193: 1–27. [Google Scholar]
- Percy DM. (2017) Making the most of your host: the Metrosideros-feeding psyllids (Hemiptera: Psylloidea) of the Hawaiian Islands. ZooKeys 649: 1–163. 10.3897/zookeys.649.10213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy DM. (2018) Revision of the Hawaiian psyllid genus Swezeyana, with descriptions of seven new species (Hemiptera, Psylloidea, Triozidae). ZooKeys 758: 75–113. 10.3897/zookeys.758.23019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf A, Hidayat P, Maryana N, Winasa IW. (1990) Biology and demography of Heteropsyllacubana Crawford (Homoptera: Psyllidae). In: Napompeth B, MacDicken KG. (Eds) Leucaena Psyllid: Problems and Management.Funny Publishing Limited Partnership, Bangkok, 114–118.
- Showler AT, Melcher J. (1995) Environmental assessment for implementation of biological control for the leucaena psyllid in Asia and Africa. U.S. Agency for International Development, Washington DC, 18 pp. [Google Scholar]
- Syfert MM, Serbina L, Burckhardt D, Knapp S, Percy DM. (2017) Emerging New Crop Pests: Ecological Modelling and Analysis of the South American Potato Psyllid Russellianasolanicola (Hemiptera: Psylloidea) and Its Wild Relatives. PLoS ONE 12(1): e0167764. 10.1371/journal.pone.0167764 [DOI] [PMC free article] [PubMed]
- Syrett P, Fowler SV, Harman HM, Hayes LM, Memmott J, Sheat JJ. (2007) Establishment of Arytainillaspartiophila Förster (Hemiptera: Psyllidae), a new biological control agent for broom, Cytisusscoparius, in New Zealand. New Zealand Entomologist 30: 53–62. 10.1080/00779962.2007.9722151 [DOI] [Google Scholar]
- Sykes WR. (2016) Flora of the Cook Islands. National Tropical Botanical Garden, Kalaheo, 973 pp. [Google Scholar]
- Taylor GS. (2018) New species of Acizzia (Hemiptera: Psyllidae) from an Australian endemic Solanum (Solanaceae). Austral Entomology 57: 297–302. 10.1111/aen.12278 [DOI] [Google Scholar]
- Taylor GS, Jennings JT, Purcell MF, Austin AD. (2011) A new genus and ten new species of jumping plant-lice (Hemiptera: Triozidae) from Allocasuarina (Casuarinaceae) in Australia. Zootaxa 3009: 1–45. [Google Scholar]
- Taylor GS, Kent DS. (2013) Potential economic pests of solanaceous crops: a new species of Solanum-feeding psyllid from Australia and first record from New Zealand of Acizziasolanicola (Hemiptera: Psyllidae). Zootaxa 3613: 257–273. 10.11646/zootaxa.3613.3.4 [DOI] [PubMed] [Google Scholar]
- Taylor GS, Fagan-Jeffries EP, Austin AD. (2016) A new genus and twenty new species of Australian jumping plant-lice (Psylloidea: Triozidae) from Eremophila and Myoporum (Scrophulariaceae: Myoporeae). Zootaxa 4073: 1–84. 10.11646/zootaxa.4073.1.1 [DOI] [PubMed] [Google Scholar]
- Thorpe SE. (2013) Casuarinicolaaustralis Taylor, 2010 (Hemiptera: Triozidae), newly recorded from New Zealand. Biodiversity Data Journal 1: 1–3. 10.3897/BDJ.1.e953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuthill LD. (1942) Psyllidae from Rapa, the Caroline, Society, and Austral Islands (Homoptera). Occasional Papers of Bernice P. Bishop Museum, Honolulu, Hawaii 17(6): 71–78. [Google Scholar]
- Tuthill LD. (1956) Psyllidae of Pacific Entomological Survey. Occasional Papers of Bernice P. Bishop Museum, Honolulu, Hawaii 22: 1–5. [Google Scholar]
- Vereijssen J, Smith GR, Weintraub PG. (2018) Bactericeracockerelli (Hemiptera: Triozidae) and Candidatus Liberibacter solanacearum in Potatoes in New Zealand: Biology, Transmission, and Implications for Management. Journal of Integrated Pest Management 9(1): 13: 1–21. 10.1093/jipm/pmy007 [DOI]
- Yana W, Mveyo Ndankeu YP, Dzokou VJ, Tamesse JL. (2015) Jumping plant lice of the family Carsidaridae (Hemiptera: Psylloidea) from Cameroon: taxonomic, faunistic, phenology and host plants. Journal of Biodiversity and Environmental Sciences 6(6): 1–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.