Abstract
The growing process of industrialization was a milestone for world economic evolution. Since the 1940s, social movements have revolutionized green chemistry and provided shifts in industrial positions and sustainable processes with advances in environmental impact and awareness of companies and population. Paul Anastas and John Warner, in the 1990s, postulated the 12 principles of Green Chemistry, which are based on the minimization or non-use of toxic solvents in chemical processes and analyzes, as well as, the non-generation of residues from these processes. One of the most active areas of Research and Development in Green Chemistry is the development of analytical methodologies, giving rise to the so-called Green Analytical Chemistry. The impacts of green chemistry on pharmaceutical analyzes, environmental, population, analyst and company are described in this review and they are multidimensional. Every choice and analytical attitude has consequences both in the final product and in everything that surrounds it. The future of green chemistry as well as our future and the environment is also contemplated in this work.
Keywords: Green chemistry, Green analytical chemistry, Multidimensional impacts, Future
1. Introduction
Since the 1940s, environmental issues began to emerge in relation to the growth of industrial activities. In the face of environmental problems and concerns, companies have changed their position on conventional production and product development habits through conferences, political agreements and advances in chemical research and ecological engineering adopting sustainable processes to the present.
In the 1990s, Paul Anastas and John Warner postulated the 12 principles of Green Chemistry, still in use today, that rely on the minimization or non-use of toxic solvents in chemical processes and analyzes, as well as the non-generation of wastes from these processes. These principles propose environmentally favorable actions from the planning of the product to its synthesis, processing, analysis and its destination after use (Anastas, 1999). The main objective is to minimize the environmental and occupational hazards inherent in industrial activities (Anastas, 1999, Lenardão et al., 2003, Prado, 2003).
Later, Paul Anastas discussed the importance of using these 12 principles in the development of new methods and analytical techniques, with the purpose of reducing their environmental impacts (Anastas, 1999). Thus, one of the most active areas of Research and Development in Green Chemistry is the development of analytical methodologies. New methods and techniques that are able to reduce the use and generation of hazardous substances in all stages of chemical analysis are the main goals of the so-called Green Analytical Chemistry (Anastas, 1999, Sanseverino, 2000, Nolasco et al., 2006, Guardia and Armenta, 2012). In this context, Galuszka, Migaszewski and Namienski, in the year 2013, adapted the 12 principles of Green Chemistry, to better fit the Green Analytical Chemistry.
The impacts of green chemistry are multidimensional. Each analytical choice has consequences both in the final product and in everything that surrounds it, from the environment, population, analyst and even the company.
Thus, this work shows a critical review of the (i) history and (ii) evolution of green chemistry, as well as its (iii) impact on pharmaceutical analyzes, environment, population, analyst, company and our future.
2. History
The growing process of industrialization was a milestone for the world economic evolution. Despite the contribution to the increase in quality of life, the global government policies remained far from the environmental impact that the growth of industrial activities could cause in our planet (Tobiszewski et al., 2009).
The rapid increase in population resulted in increased food production with excessive industrialization, which led to increased pollution and resource depletion. In this way, natural resources began to be used as if there were no consequences on environmental problem (Tobiszewski et al., 2009).
Although the first concerns about the environment occurred since 1949 at United Nations Scientific Conference on the Conservation and Use of Resources (UNSCCUR) in the USA, environmental issues came into focus in 1968 from the Intergovernmental Conference of Experts on the Scientific Bases for Rational Use and Conservation of Biosphere Resources, known as the Biosphere Conference (Farias and Fávaro, 2011).
In the 1960s the publication of the book “Silent Spring” stimulated a contemporary environmental movement. The historical book has raised awareness about ecological perception and has provided major government initiatives marked by concern about the risks associated with over-exploitation of natural resources. Robert Downs, listed the book as “the book that changed America” and John Kenneth Galbraith cited it as one of the most important books in Western literature (Lutts, 1985).
The Stockholm Conference occurred in Sweden in 1972, and it was attended by representatives from a number of countries, including members of the United Nations (UN) and non-governmental organizations, where environmental law was also considered in the legal field (Pereira, 2009). From this conference, the world began to be alerted on the environmental damages that the depletion of the ecosystem could cause to humanity (Jungstedt, 2002).
The 1980s were marked by numerous world conferences on the Environment. After an evaluation of the 10 years of the proposed actions at Stockholm Conference, the UN created the World Commission on Environment and Development in 1983 to produce a report on world development and environment. This commission was established at a time of unprecedented pressure on the global environment and a growing recognition that much of the development was not sustainable (Brundtland, 1985).
The report known as the “Brundtland Report” reconciled environmental and social issues and was published in 1987, which for the first time defined the concept of sustainable development as development that meets the needs of the current generation without compromising the future generation. The report also emphasized the dangers of ozone depletion and the effects on global warming, stating that scientists' ability to evaluate and propose solutions were lower than the speed of climate change (Marcondes, 2005).
In 1985, during a meeting of the Environment Ministers of the countries of the Organization for Economic Co-operation and Development (OECD), several decisions were made on three main themes: Economic Development and Environment, Pollution Prevention and Control and Environmental Information and National Reviews, these decisions persisted until the year 1990. Interventions based on these main themes were central to issues of chemical product risk reduction and pollution prevention and control (Linthorst, 2009).
The US Environmental Protection Agency (EPA) launched the “Alternative Synthetic Routes for Pollution Prevention” program in 1991 that reported a new philosophy and policy on controlling the risks of toxic chemical products to prevent problems with these substances, emphasizing that the correct would be the non-production of these products in the first instance (Woodhouse and Breyman, 2005).
Since 1992, the inclusion of other topics as environmentally friendly solvents and safer chemical compounds has been the expansion and rename of this program, which since then officially adopted the name of green chemistry (Farias and Fávaro, 2011).
The 1990s were marked by a worldwide consensus on environmental preservation. In Brazil there was a United Nations International Conference on Environment and Development in 1992 called (ECO-92). The participation of heads of state resulted in the elaboration of a document entitled “Agenda 21”, which had the commitment of countries to value sustainable development by moving environmental issues, economic policies and decision-making (Strong, 1991).
Although the advances in the environment had been awakened worldwide, the environmental awareness of the companies was very insecure. The companies were submitted to controls established by the government when they were pressured by the media and civil society, taking this environmental dimension as a necessary evil (Almeida, 2002).
In order to transform the business sector, a program called “Responsible Care”, was developed in 1984 in Canada and until the present day it is practiced in 68 economies around the world, improvements in the behavior of industries in relation to the environment, the health and safety of workers (Responsible Care, 2017).
With this program, human activities began to be performed in pursuit of progress, replacing harmful activities with activities that emphasized quality of life and a safe environment such as: investments in infrastructure security; improvements in energy efficiency; employee safety records; voluntary follow-up of process incidents and reduction of hazardous emissions to air, earth and water (Responsible Care, 2017, Baird, 2002).
Although environmental issues have had major impacts on industrial and economic sectors, a survey of the European Chemical Industry Council (CEFIC) in 1994 showed that the population's views on the chemical industry were not favorable. In general, the population was more attentive to the pharmaceutical and plastics sectors because of the benefits associated with their needs (Pandey, 2015).
Most interviewees did not believe that the chemical industries were concerned about the development of sustainable actions. Opinions generated dislikes about the transportation, safety and waste of these industries, making opinions more favorable to the oil, gas, electricity, wood and paper industries (Clark, 1999).
The US Government in 1995 announced the Presidential Green Chemistry Challenge (PGCC) program. It contemplates the technological innovations that were constituted in the chemical industries to reduce the production of waste in several sectors of production. The works are awarded annually in five different categories: Academic; Small Business; Alternative Synthetic Routes; Reactive Alternative Conditions and Safer Chemical Designs (Cann, 1999).
In 1997 the Green Chemistry Institute (GCI) was created as a non-profit corporation to promote through the knowledge, experience and capacity, moves of the chemical company toward sustainability, which advanced in the applications of green chemistry (ACS Chemistry, 2017).
The GCI joined the American Chemical Society (ACS) in 2001 to address global issues in the meeting of chemistry and environment. Through research, work has integrated green chemistry in every aspect such as industries, business, education, planning conferences as well as organizing efforts with international networks (ACS Chemistry, 2017).
The groundbreaking book Green Chemistry: Theory and Practice, presenting Paul Anastas and John C. Warner as co-authors in 1998, was another important development for green chemistry. In the book, the 12 Principles of Green Chemistry are clearly outlined with a philosophy that has always encouraged academic scientists and industries to pursue environmentally correct actions (ACS Chemistry, 2017).
In 2002, after 30 years of the Stockholm Conference, an event called Rio + 10 or the World Summit on Sustainable Development took place in the city of Johannesburg, South Africa, attended by thousands of people (Sequinel, 2002).
Governmental and non-governmental organizations, large companies, sectoral associations, delegations and journalists attended this meeting to assign a single objective: to discuss the solutions proposed in “Agenda 21”, so that not only the government can apply them, but the general population, in addition to implementing what had been discussed in ECO-92 (Marcondes, 2005, Sequinel, 2002).
The ACS's Green Chemistry Institute (GCI) and the global pharmaceutical corporations established a panel discussion in 2005 to enable and encourage green chemistry and green engineering in the pharmaceutical industries. The panel discussion defined “continuous processing” as the key to the implementation to advance “the green” (Poechlauer et al., 2012, Constable et al., 2007).
The International Union of Pure and Applied Chemistry (IUPAC), together with ACS and GCI, held four conferences on Green Chemistry between 1997 and 2011. The conferences included topics such as green products and processes to the environment, production energy, renewable sources of chemical waste in addition to adopting green policies and education in green chemistry (Lenardão et al., 2003).
Although advances in chemistry and ecological engineering research have adopted sustainable processes over the years, continuing to invest in industrial techniques and policies will be extremely important in the process of implementing environmental improvements (Jenck et al., 2004).
3. Green chemistry
Cathcart (Cathcart, 1990), who presented a discussion on the growth of the Irish chemical industry, probably used the term “Green Chemistry” for the first time in a paper title in the year 1990. However, only in 1996, the first publication, by Anastas and Williamson, approached Green Chemistry with the philosophy adopted today (Cathcart, 1990, Anastas and Williamson, 1996).
The main concept of Green Chemistry is the use of chemical skills and knowledge to reduce or eliminate the use or generation of hazardous substances during the planning, manufacturing and application of chemicals in order to minimize threats to the health of operators and the environment (Anastas, 1999). Thus, the concern to eliminate or minimize the generation of toxic waste has become greater than treating the waste already generated.
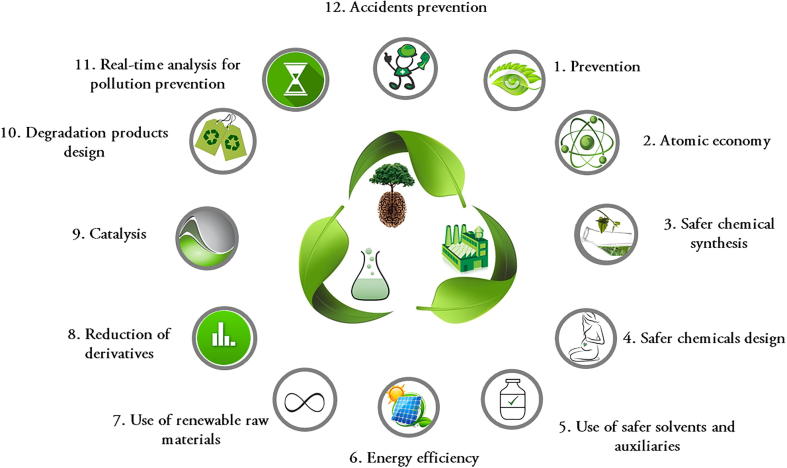
In 1998, Paul Anastas and John Warner published the first manual of Green Chemistry, in which they proposed 12 principles for the theme, which have been described in Table 1 and Fig. 1 (Anastas and Warner, 1998).
Table 1.
12 Principles of Green Chemistry proposed by Anastas and Warner (Anastas and Warner, 1998).
| Number | Principle | Description of principle |
|---|---|---|
| 1 | Prevention | It concerns the prevention of waste generation. It is better to avoid generating waste than to treat it after its generation |
| 2 | Atomic economy | Synthetic methods should be planned so that the final product incorporates as much of the reagents used during the process as possible. Thus, waste generation will be minimized |
| 3 | Safer chemical synthesis | Synthetic methods should be designed to use and generate substances with low or no occupational and environmental toxicity. Thus, replacement of toxic solvents with low or no toxicity solvents is highly recommended |
| 4 | Safer chemicals design | Great importance should be given to the toxicity of the designed chemicals. They should obviously fulfill their functions, but should also present the lowest possible toxicity |
| 5 | Use of safer solvents and auxiliaries | The use of solvents and other reagents should be avoided where possible. When it is not possible, these substances should be innocuous |
| 7 | Use of renewable raw materials | Whenever it is economically and technically feasible, renewable raw materials should be used instead of non-renewable |
| 8 | Reduction of derivatives | Unnecessary derivatization processes should be avoided or minimized, as they require the additional use of reagents and, therefore, generate waste |
| 9 | Catalysis | The use of catalytic reagents (as selective as possible) is better than the use of stoichiometric reagents |
| 10 | Degradation products design | Chemicals should be designed so that at the end of their function they decompose into harmless degradation products and do not persist in the environment |
| 11 | Real-time analysis for pollution prevention | Analytical methods should be monitored in real time to avoid the formation of hazardous substances |
| 12 | Accidents prevention | Both the substances and the way they are used in a chemical process should be chosen considering the minimization of potential accidents, such as leaks, explosions and fires, aiming at greater occupational and environmental safety |
Fig. 1.
12 Principles of Green Chemistry proposed by Anastas and Warner (Anastas and Warner, 1998).
In summary, the 12 principles of Green Chemistry are based on the minimization or non-use of toxic solvents in the chemical processes and analyzes, as well as on the non-generation of residues resulting from these processes. For this, the atomic and energy economies occupy prominent places, as well as the use of renewable and innocuous raw materials. In addition, the acceleration of chemical reactions through catalysis can help, for example, in energy savings and less waste generation. One of the principles is also concerned with the conscious development of chemicals, so that after their useful life they must decompose and become degradation products harmless to the environment, also avoiding bioaccumulation. Thus, it is observed that these principles are concerned with the planning of the product, through its synthesis, processing, analysis and its destination after the use. The main objective is to minimize the environmental and occupational risks inherent in industrial activities (Anastas, 1999, Lenardão et al., 2003, Prado, 2003).
At the same time, it is possible to predict some of the economic benefits generated by the adoption of Green Chemistry in industrial chemical processes, such as the lesser need for investments in effluent storage and treatment, as well as the payment of indemnities for environmental damages (Prado, 2003). This is an important aspect, since it is clear that if Green Chemistry does not bring economic benefits to the market, it will not be viable. However, if the market ignores the needs of the environment, it will not prosper (Tundo et al., 2000).
To illustrate, we can mention the worst environmental disaster ever recorded in Brazil in 2015, after the collapse of the waste dam of Samarco mining company, in the city of Mariana, state of Minas Gerais. In this tragedy (Mariana Catastrophe), according to the Brazilian magazine Exame (2017), more than 34 million cubic meters of mud were flowed on down in the Rio Doce, the “sweet river”, damaging the life of communities for six hundred kilometers, affecting water supplies and threatening the livelihoods before spilling into the Atlantic Ocean. Almost 1500 ha of vegetation were compromised and dozens of species of aquatic and terrestrial fauna were affected (Exame Magazine, 2017). After this episode, the company accorded with the Brazilian government a socioeconomic and environmental recovery work in the amount of 20 billion reais.
However, two years after the Mariana Catastrophe, Samarco, the mining company is still trying to evade its responsibilities by co-opting the state. Thus, it is possible to understand the extent of environmental, financial and reputational damage that a tragedy of this level can cause (Exame Magazine, 2017, Guardian, 2017).
The efforts that emerged in the 1990s regarding the manufacture of chemicals with the minimum possible use of toxic reagents and solvents also raised a factor used to assess how green an industrial process is. This factor, named “E factor” (efficiency factor), was proposed by Roger Sheldon (Sheldon, 1997). Its calculation is based on the division of the mass of the waste generated (kg) by the mass of the finished product (kg). The meaning of “waste” in this context is everything that was formed during the process, with the exception of the final product. Thus, all processes must aim for the lowest possible E factor. For truly green processes, the calculation of this factor should generate a value equal to zero.
It is possible to observe that the higher the production of the segment, the smaller the E factor. The oil refinery, for example, which produces from 1 to 100,000,000 tons per year, presents an E factor of 0.1, while the pharmaceutical industry, which produces from 10 to 1000 tons per year, presents an E factor of 25–100. Thus, through the calculation of E factor, it can be noted that large-scale manufacturing processes, although perceived to be worse for the environment compared to small-scale operations, are in fact not (Lenardão et al., 2003, Sheldon, 1997, Sheldon, 1996, Poliakoff and Licence, 2007). The low performance of the pharmaceutical industry in this regard, which also occurs in the fine chemical industry, comes from the fact that its industrial plants were designed to employ classical stoichiometric reactions, which generate large amounts of inorganic salts as waste (Lenardão et al., 2003).
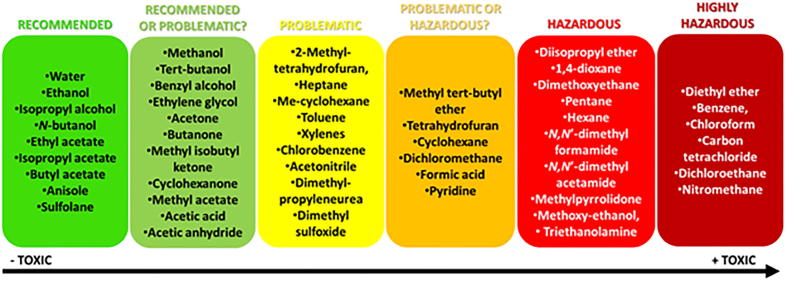
In relation to the toxicity of solvents, there are several guides in this regard, carried out by many institutions and companies. However, Prat and coworkers, in an paper published in 2014, made an interesting comparison of several of these guides and elaborated a table with the compilation of them, in which they divided the solvents into six categories: “recommended”, “recommended or problematic?”, “problematic”, “problematic or hazardous?”, “hazardous” and “highly hazardous” (Prat et al., 2014). Fig. 2 illustrates this classification.
Fig. 2.
Solvent classification published by Prat et al. (Prat et al., 2014).
Most solvents are highly volatile, easily causing air pollution. In addition, many of them are flammable and toxic. Thus, wherever possible, organic solvents should be replaced with water. However, this is often not possible, since most organic compounds are not soluble in this solvent. Another alternative to organic solvents is the use of supercritical CO2, which is non-toxic and does not contribute to climate change, since it is a byproduct of other processes. Ionic liquids are also a good choice, since they suffer very little evaporation and therefore are not lost to the atmosphere (Poliakoff and Licence, 2007).
4. Green analytical chemistry
In 1999, Paul Anastas published a paper in which he discussed the importance of using the 12 principles of Green Chemistry, postulated by him and John Warner in the previous year (1998), in the development of new methods and analytical techniques, in order to reduce their environmental impacts (Anastas, 1999). One of the most active areas of Research and Development in Green Chemistry is the development of analytical methodologies. New methods and techniques that are able to reduce and eliminate the use and generation of hazardous substances in all stages of chemical analysis are the main targets of the so-called Green Analytical Chemistry (Anastas, 1999, Sanseverino, 2000, Nolasco et al., 2006, Guardia and Armenta, 2012).
Galuszka, Migaszewski and Namiénski, in 2013, adapted the 12 principles of Green Chemistry, to better fit the Green Analytical Chemistry (Galuszka et al., 2013). Thus, the 12 principles of Green Analytical Chemistry are described in Table 2.
Table 2.
12 Principles of Green Analytical Chemistry, proposed by Galuszka, Migaszewski and Namiénski (2013).
| Number | Principle and description |
|---|---|
| 1 | Direct analytical techniques should be applied to avoid the sample treatment step |
| 2 | The size and quantity of samples should be as small as possible |
| 3 | In situ analyzes should be carried out |
| 4 | Integration of analytical processes and operations must be performed, as it promotes energy savings and reduces the use of reagents |
| 5 | Automated and miniaturized methods must be selected |
| 6 | Derivatizations should be avoided as they require the use of additional reagents and, therefore, generate waste |
| 7 | Generation of large volumes of analytical waste must be avoided and the correct handling of this waste must be provided |
| 8 | Multi-analyte methods should be preferred over methods that analyze one analyte at a time |
| 9 | The use of electric energy should be minimized |
| 10 | Reagents obtained from renewable sources should be preferred |
| 11 | Hazardous reagents should be discarded |
| 12 | Safety of operators should be increased |
The principles suggested by Galuszka, Migaszewski and Namiénski (2013) are based mainly on the elimination or minimization of the use of chemical substances, on the minimization of the consumption of electricity, on the correct handling of the generated analytical residues and on the greater safety of the operators (Galuszka et al., 2013).
5. Impacts of green chemistry
5.1. Pharmaceutical analysis
Currently the chemical-pharmaceutical industries and laboratories must contemplate green chemistry through, and not only, their analysis.
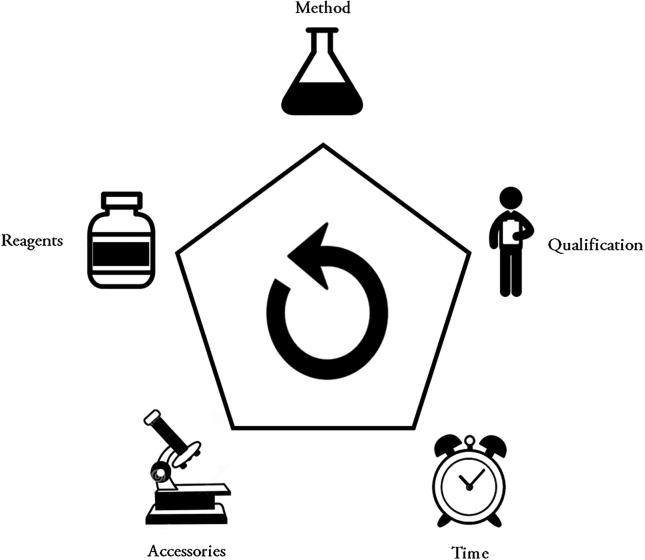
The chosen method, reagents, accessories, personnel qualification, time to evaluate the quality of a product are part of the ecologically correct thinking, shown in Fig. 3 (Kogawa and Salgado, 2016).
Fig. 3.
The pentagon of ecologically correct thinking.
The method of choice for the determination of active pharmaceutical ingredients as well as the investigation of impurities and degradation products is high performance liquid chromatography (HPLC). Most of these methods use as organic solvents, acetonitrile and/or methanol. Many also opt for buffer solutions. This is indisputable. However, most of them have never even attempted to use another organic solvent in addition to the acetonitrile/methanol combination or do not use buffer solutions in the mobile phase. Why? Lack of knowledge, negligence of consequences, ease and/or accommodation? (Tótoli and Salgado, 2014, Pedroso et al., 2016, Spagnol et al., 2016, Rodrigues and Salgado, 2016, Figueiredo et al., 2017, Kogawa et al., 2017, Marco and Salgado, 2017).
Buffer solutions, in addition to requiring a certain amount of time to prepare, have a low shelf life which requires a new preparation and thus a longer dispensing time. The use of it also requires an extensive cleaning process of both the column and the entire chromatographic system (Kogawa and Salgado, 2012).
Toxic organic solvents, such as acetonitrile and methanol, in addition to damaging the health of the operator who is exposed daily to these solvents also requires proper waste management for the disposal of this contaminant. This has a cost that will certainly be included in the final product (Pedroso et al., 2016).
Even the accessories used in the methods of analysis can contemplate green thinking. Chromatographic pre-columns are often not needed, but are used by lack of knowledge of the analyst who understands that it must be present. Steps, that are not necessary but, which are carried out by lack of knowledge of the analyst who understands that if he does not do it the method will be incorrect and will lead to a result out of specification. Devices that can be reused but that are not because the company always buys more and so it is more convenient to throw away and wait for the new one to arrive (Anastas, 1999, Breslow, 1996, Dichiarante et al., 2010, Kogawa and Salgado, 2015, McElroy et al., 2015, Ravikiran et al., 2015).
Often qualified personnel are assigned to develop banal tasks, repetition of tasks such as a robot, overprocessing products and processes rather than develop, create, and evolve within their work area. This is a waste of intellect, one of the eight wastes we have today. It is a sophisticated and qualified workforce hired to perform mediocre services (Kogawa et al., 2017).
Is the time for each process or analysis measured? It must be. It is part of green chemistry. The longer an activity takes, the longer the analyst will need to be dependent on it and fewer activities he will develop and therefore there will be less production and the final product will be more expensive. Time, an item that starts an entire process or service, has direct consequences on the final product (Kogawa et al., 2017).
Therefore, faster and cheaper methods with personnel adequately qualified for the service, using materials and accessories worthy of analysis and with ecologically correct reagents are currently required.
In the literature there are many physical-chemical and microbiological methods for the evaluation of drugs and pharmaceuticals which contemplate green analytical chemistry items such as HPLC methods using only ethanol and water in the mobile phase (Tótoli and Salgado, 2014, Pedroso et al., 2016, Spagnol et al., 2016, Rodrigues and Salgado, 2016, Figueiredo et al., 2017, Kogawa et al., 2017, Marco and Salgado, 2017), spectrophotometry in the ultraviolet region (UV) using aqueous solution as diluent (Kogawa and Salgado, 2013, Alessio et al., 2017, Kogawa and Salgado, 2016), spectrophotometry in the visible region (Vis) using aqueous solution as diluent (Brbaklic et al., 2017, Rechelo et al., 2017), spectrophotometry in the infrared region (IR) using only potassium bromide as reagent (Moreno and Salgado, 2012, Tótoli and Salgado, 2012, Vieira et al., 2012, Kogawa and Salgado, 2013, Piantavini et al., 2014, Mallah et al., 2015, Marco and Salgado, 2016, Trindade and Salgado, 2017), capillary electrophoresis (CE) with migration time less than 5 min (Kogawa et al., 2014, Tótoli et al., 2015, Chierentin et al., 2016) and microbiological methods with results in 4 h (Kogawa et al., 2012, Tótoli and Salgado, 2013, Cazedey and Salgado, 2013, Vieira et al., 2014, Pedroso and Salgado, 2014, Chierentin and Salgado, 2015, Silva and Salgado, 2015, Tótoli and Salgado, 2015, Curbete and Salgado, 2016, Kogawa and Salgado, 2016).
5.2. Environment
The residues generated in the chemical-pharmaceutical analyzes must be pre-treated before being returned to the environment. However, this process requires a cost that is more expensive depending on the toxicity and hazardousness of the solvent.
Acetonitrile, for example, is incinerated and this process generates waste that contributes to acid rain. Even using a process to neutralize the toxicity of the solvent, it negatively affects us otherwise (World Health Organization, 1993). Acid rain damages cars, buildings, monuments, vegetation, rivers, lakes and so on.
The vegetation can contemplate plantations that feed thousands of people. The waters can be affected with a lower pH and change the habitat previously favorable to certain organisms that lived there. An effect like this will never be isolated! This is when waste is treated, but when are not? When industrial wastes are dumped directly into the waters ecological disasters can occur. Fish and vegetation die, contaminated water changes its characteristics and eutrophication occurs (World Health Organization, 1997). In some cases this water would be used for the irrigation of plantations, which in this case would also be impaired, as shown in Fig. 4.
Fig. 4.
The consequences of analytical choices on the environment.
5.3. Population
The population is impacted by current chemistry in different ways and on different fronts. Patients who frequently get their medication from pharmacies or health centers are affected by the choice of methods of analysis and reagents used by analysts or chemical-pharmaceutical operators. An expensive method generates an expensive product on the market. An expensive method with accessories (not always necessary) generates a more expensive product on the market. An expensive method with accessories and several steps (not always necessary) generates an even more expensive product on the market (Kogawa and Salgado, 2015).
A time-consuming method that releases results within 24 h or more, such as microbiological results for antibiotics, will make products expensive or, if released without this analysis, perhaps inefficient which can promote the overload of the health system and contribute to microbial resistance (Kogawa et al., 2012, Tótoli and Salgado, 2013, Cazedey and Salgado, 2013, Vieira et al., 2014, Pedroso and Salgado, 2014, Chierentin and Salgado, 2015, Silva and Salgado, 2015, Tótoli and Salgado, 2015, Curbete and Salgado, 2016, Kogawa and Salgado, 2016).
The patient is undoubtedly affected by the analytical decision in the analysis of a pharmaceutical, in the evaluation of the quality of a raw material and in the development of an industrial or laboratory process (Taylor, 2016).
All the steps of a process have a cost that is passed on to the final product that the patient pays and is also affected by its effects, good or bad.
5.4. Analyst
The physical-chemical analyst has direct and daily contact with the pharmaceutical analyzes. He is the first affected by the entire analytic chain.
Toxic solvents such as acetonitrile are absorbed quickly by the body and its metabolism generates cyanide that impairs respiration (World Health Organization, 1997). Another example, which is also lovely by pharmaceutical analyzes, is methanol. In addition to being excreted more slowly than ethanol the products of its metabolism generates formaldehyde and mainly formic acid which are responsible for severe intoxication (Kogawa et al., 2017).
The analyst can also suffer from the execution of time-consuming and non-reproducible analytical methods or that require specific accessories or that have several stages or that depend on other professionals, anyway, the analyst, in addition to all exposure to toxic solvents and reagents, can also suffer emotionally (Kogawa and Salgado, 2017).
A time consuming method discourages the analyst and generates waste of intellect and time. A valuable time coming from a qualified professional who could be developing another activity. According toWilliam Edwards Deming Fig. 5), the methods that do not reproduce bring a feeling that the professional is not qualified, and 85% of the time the problem is not the analyst but the method that needs to be improved (Kogawa and Salgado, 2017).
Fig. 5.
William Edwards Deming, one of the quality gurus.
Specific accessories will result in expensive products. Is it possible to change the accessory or system that does not use it? They will not always be delivered promptly. What will happen if the accessory production is discontinued?
The fact of depending on a person to perform an activity is emotionally exhausting. How many times did someone wait for another to be able to perform certain activity? At this time surely the qualification of this waiting person was wasted. Waiting and intellect are wastes that must be eliminated for the success of a company.
Then, in addition to physical health, the analyst can also suffer from emotional health. Discouragement, feeling of incapacity and fear are examples of factors that affect the analyst's emotional. Additionally, this can also affect physical health.
Quality tools can be used in process improvement, interaction between people and decrease of the iceberg of the quality cost (Kogawa and Salgado, 2017).
5.5. Company
Chemical-pharmaceutical companies must increasingly contemplate the principles of green chemistry and/or green analytical chemistry, since switching off the light until choosing the reagent to be used in the evaluation of a pharmaceutical, since the interaction with the collaborator until the provision of training for a team.
Green chemistry must be seen as a sustainable idea since a better world until a better company, people and conviviality. A company that values this kind of attitude, modern and current, will certainly succeed. In it there will be no employees but collaborators. In it there will be no chief but leaders. In it there will be no the vision only in the final product but in the whole chain, in order to be sustainable, green and clean (Marco and Salgado, 2017, Marco et al., 2017, Trindade et al., 2017). Thus, automatically, the company grows. The vision of the company is also benefited, because it becomes a model and reference of the ecologically correct, clean and sustainable, besides being competitive in the market. Companies like Coca-Cola™, Google™, Apple™ are examples of this concept (Coca-Cola Company, 2017, Google, 2017, Apple environment, 2017).
5.6. Future
World leaders have already begun this theoretical process through the United Nations Conference on the Human Environment in Stockholm in 1972, Conference of Nairobi in 1982, United Nations Conference on Environment and Development in Rio de Janeiro in 1992, World Summit on Sustainable Development in Johannesburg in 2002, United Nations Conference on Sustainable Development in Rio de Janeiro in 2012 and the Paris Agreement in 2015 (United Nations, 2017).
In the academic-professional context there is the “Green & Sustainable Chemistry Conference” that brings together academic and business representatives to exhibit work and exchange ideas and learning (Green & Sustainable Chemistry Conference, 2017).
These initiatives show that many work in favor of green chemistry, sustainable, clean and ecologically correct. One way to achieve the impossible is to design the possible. So we just have to do our part. If each one plays a part, it does not matter if it is small, when we join all the parts they will be significant.
Finally, we must have positive perspectives for the future of green chemistry because it encompasses the future of our world. Green chemistry is not restricted to a chemical analysis in which a less toxic solvent is used. This is not green chemistry. Green chemistry is a set of actions and attitudes, it is multidimensional (Kogawa and Salgado, 2015). It is thinking about the whole process and minimizes reagents, steps, costs and energy. In this scenario, the protagonist must also be considered. The physical and emotional health of the collaborator is the differential of the companies, because they know that a man alone will never add the skills of an effective team.
6. Conclusion
Research advances have enabled sustainable processes over the years with investments in environmentally correct analytical and policy techniques in line with world conferences since 1968. Despite these efforts, industries need to visualize the economic viability of applying green chemistry to their processes, which prevents us from leveraging the use of this ideology. Investments and dissemination on the importance of green chemistry and how they affect directly from the start of pharmaceutical analyzes, employees and patient health until to the environmental sustainability are extremely important for the process of future improvements.
Conflict of interest
The authors report no declarations of interest.
Acknowledgments
The authors acknowledge CNPq (Brasília, Brazil), FAPESP (São Paulo, Brazil) and CAPES (São Paulo, Brazil)
Footnotes
Peer review under responsibility of King Saud University.
References
- ACS Chemistry for Life. <https://www.acs.org/content/acs/en/greenchemistry/what-is-green-chemistry/history-of-green-chemistry.html> (accessed October 2017).
- ACS Chemistry for Life. <https://www.acs.org/content/acs/en/greenchemistry/about/history.html> (accessed October 2017).
- Alessio P.V., Kogawa A.C., Salgado H.R.N. J. Anal. Methods Chem. 2017:1–4. doi: 10.1155/2017/7530242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, F. 2002. The Good Business of Sustainability, New Frontier.
- Anastas P.T. Crit. Rev. Anal. Chem. 1999;29:167–175. [Google Scholar]
- Anastas, P.T., Williamson, TC.1996. Green Chemistry: Designing Chemistry for the Environment. In: ACS Symposium Series, Washington, v. 626.
- Anastas P.T., Warner J.C. Oxford University Press; New York: 1998. Green Chemistry: Theory and Practice. [Google Scholar]
- Apple environment. <https://www.apple.com/br/environment/> (access September 2017).
- Baird C. Bookman; 2002. Environmental Chemistry. [Google Scholar]
- Brbaklic V., Kogawa A.C., Salgado H.R.N. Curr. Pharm. Anal. 2017;13:532–537. [Google Scholar]
- Breslow R. The Greening of Chemistry. Chem. Eng. News. 1996 [Google Scholar]
- Brundtland G.H. Environ. Policy Law. 1985;14:26–30. [Google Scholar]
- Cann C.M. J. Chem. Educ. 1999;76:1639. [Google Scholar]
- Cathcart C. Chem. Ind. (London) 1990;5:684–687. [Google Scholar]
- Cazedey E.C.L., Salgado H.R.N. J. Pharm. Anal. 2013;3:382–386. doi: 10.1016/j.jpha.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chierentin L., Barth T., Bonato P.S., Salgado H.R.N. Int. J. Basic Life Sci. 2016;4:21–32. [Google Scholar]
- Chierentin L., Salgado H.R.N. Brazil. J. Pharm. Sci. 2015;51:629–635. [Google Scholar]
- Clark J.H. Green Chem. 1999;1:1–8. [Google Scholar]
- Coca-Cola Company. <http://www.coca-colacompany.com/sustainability, http://www.coca-colacompany.com/stories/environmental-initiatives> (access September 2017).
- Constable D.J.C., Peter D.J., Hayler J.D., Humphrey G.R., Leazer J.L., Linderman R.J., Lorenz K., Manley J., Pearlman B.A., Wells A., Zaks A., Zhang Y. Green Chem. 2007;9:411–420. [Google Scholar]
- Curbete M.M., Salgado H.R.N. Talanta. 2016;153:51–56. doi: 10.1016/j.talanta.2016.01.069. [DOI] [PubMed] [Google Scholar]
- Dichiarante V., Ravelli D., Albini A. Green Chem. Lett. Rev. 2010;3:105–113. [Google Scholar]
- Exame Magazine. <https://exame.abril.com.br/brasil/mariana-o-arrastado-processo-de-indenizacao-das-familias/> (accessed October 2017).
- Farias L.A., Fávaro D.I.T. New Chem. 2011;34:1089–1093. [Google Scholar]
- Figueiredo A.L., Kogawa A.C., Salgado H.R.N. Built Environ. 2017;1:16–23. [Google Scholar]
- Galuszka A., Migaszewski Z., Namiésnik J. Trends Anal. Chem. 2013;50:78–84. [Google Scholar]
- Google. <https://environment.google/> (access September 2017).
- Green & Sustainable Chemistry Conference. <https://www.elsevier.com/events/conferences/green-and-sustainable-chemistry-conference> (access September 2017).
- Guardia M.D., Armenta S. Anal. Bioanal. Chem. 2012;404:625–626. doi: 10.1007/s00216-012-6208-z. [DOI] [PubMed] [Google Scholar]
- The Guardian. <https://www.theguardian.com/sustainable-business/2016/oct/15/samarco-dam-collapse-brazil-worst-environmental-disaster-bhp-billiton-vale-mining> (accessed October 2017).
- Jenck J.F., Agterberg F., Droescher J.M. Green Chem. 2004;6:544–556. [Google Scholar]
- Jungstedt, L.O.C., 2002. Administrative law: legislation. Thex.
- Kogawa A.C., Salgado H.R.N. World Res. J. Pharm. Res. 2013;1:21–24. [Google Scholar]
- Kogawa A.C., Salgado H.R.N. Scientific. 2016:1–9. [Google Scholar]
- Kogawa A.C., Tomita L.K., Salgado H.R.N. Int. J. Microbiol. Res. 2012;4:316–321. [Google Scholar]
- Kogawa A.C., Salgado H.R.N. Phys. Chem. 2013;3:1–6. [Google Scholar]
- Kogawa A.C., Aguiar F.A., Gaitani C.M., Salgado H.R.N. World J. Pharm. Pharm. Sci. 2014;3:283–297. [Google Scholar]
- Kogawa A.C., Salgado H.R.N. J. Chromatogr. Sci. 2012;50:1–7. [Google Scholar]
- Kogawa A.C., Salgado H.R.N. J. Int. Res. Med. Pharm. Sci. 2015;2:99–105. [Google Scholar]
- Kogawa A.C., Salgado H.R.N. Scholars Acad. J. Pharm. 2016;5:240–244. [Google Scholar]
- Kogawa A.C., Mendonça J.N., Lopes N.P., Salgado H.R.N. Curr. Pharm. Anal. 2017;13:520–524. [Google Scholar]
- Kogawa A.C., Salgado H.R.N. Pharm. Anal. Acta. 2016;7:1–7. [Google Scholar]
- Kogawa A.C., Salgado H.R.N. Int. J. Business Process Integr. Manage. 2017;8:153–159. [Google Scholar]
- Kogawa A.C., Cernic B.G., Couto L.G.D., Salgado H.R.N. Saudi Pharm. J. 2017;25:934–938. doi: 10.1016/j.jsps.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardão J.E., Freitag R.A., Dabdoub M.J., Batista A.C.F., Silveira C.C. New Chem. 2003;26:123–129. [Google Scholar]
- Linthorst J.A. Found. Chem. 2009;12:55–68. [Google Scholar]
- Lutts R.H. Environ. Rev. 1985;9:211–225. [PubMed] [Google Scholar]
- Mallah M.A., Sherazi S.T.H., Bhanger M.I., Mahesar S.A., Bajeer M.A.A. Spectrochim. Acta Part A. 2015;141:64–70. doi: 10.1016/j.saa.2015.01.036. [DOI] [PubMed] [Google Scholar]
- Marco B.A., Salgado H.R.N. Phys. Chem. 2016;6:67–74. [Google Scholar]
- Marco B.A., Natori J.S.H., Fanelli S., Tótoli E.G., Salgado H.R.N. Crit. Rev. Anal. Chem. 2017;47:267–277. doi: 10.1080/10408347.2017.1281097. [DOI] [PubMed] [Google Scholar]
- Marco B.A., Salgado H.R.N. World J. Pharm. Pharm. Sci. 2017;6:2074–2091. [Google Scholar]
- Marco B.A., Salgado H.R.N. Crit. Rev. Anal. Chem. 2017;47:93–98. doi: 10.1080/10408347.2016.1219649. [DOI] [PubMed] [Google Scholar]
- Marcondes, S. 2005. Brazil, Love at First Sight! Environmental Travel in Brazil from the 16th to the 21st Century, Peirópolis.
- McElroy C.R., Constantinou A., Jones L.C., Summerton L., Clark J.H. Green Chem. 2015;17:3111–3121. [Google Scholar]
- Moreno A.H., Salgado H.R.N. Phys. Chem. 2012;2:6–11. [Google Scholar]
- Nolasco F.R., Tavares G.A., Bendassolli J.A. Sanitary Environ. Eng. 2006;11:118–124. [Google Scholar]
- Pandey G. Int. J. Adv. Res. Innovation. 2015;1:53–59. [Google Scholar]
- Pedroso T.M., Medeiros A.C.D., Salgado H.R.N. Talanta. 2016;160:745–753. doi: 10.1016/j.talanta.2016.08.016. [DOI] [PubMed] [Google Scholar]
- Pedroso T.M., Salgado H.R.N. Anal. Methods. 2014;6:1391–1396. [Google Scholar]
- Pereira J.V.I. Global Economy Manage. 2009;14:115–126. [Google Scholar]
- Piantavini M.S., Pontes F.L.D., Uber C.P., Stremel D.P., Sena M.M., Pontarolo R. Spectrochim. Acta Part A. 2014;125:396–403. doi: 10.1016/j.saa.2014.01.078. [DOI] [PubMed] [Google Scholar]
- Poechlauer P., Manley J., Broxterman R., Gregertsen B., Ridemark M. Org. Process Res. Dev. 2012;16:1586–1590. [Google Scholar]
- Poliakoff M., Licence P. Nature. 2007;450:810–812. doi: 10.1038/450810a. [DOI] [PubMed] [Google Scholar]
- Prado A.G.S. New Chem. 2003;26:738–744. [Google Scholar]
- Prat D., Hayler J., Wells A. Green Chem. 2014;16:4546–4551. [Google Scholar]
- Ravikiran T.N., Prasad Y.R., Anoop K. World J. Pharm. Pharm. Sci. 2015;4:353–367. [Google Scholar]
- Rechelo B.S., Fernandes F.H.A., Kogawa A.C., Salgado H.R.N. Euro. Chem. Bull. 2017;6:238–245. [Google Scholar]
- Responsible Care. <https://responsiblecare.americanchemistry.com/> (accessed October 2017).
- Rodrigues D.F., Salgado H.R.N. Curr. Pharm. Anal. 2016;12:306–314. [Google Scholar]
- Sanseverino A.M. New Chem. 2000;23:102–107. [Google Scholar]
- Sequinel M.C.M. Conjunctural Anal. 2002;24:12–15. [Google Scholar]
- Sheldon R.A. J. Mol. Catal. A: Chem. 1996;107:75–83. [Google Scholar]
- Sheldon R.A. J. Chem. Technol. Biotechnol. 1997;68:381–388. [Google Scholar]
- Silva L.M., Salgado H.R.N. J. Microbiol. Methods. 2015;110:49–53. doi: 10.1016/j.mimet.2015.01.017. [DOI] [PubMed] [Google Scholar]
- Spagnol C.M., Isaac V.L., Corrêa M.A., Salgado H.R.N. J. Chromatogr. Sci. 2016;54:305–311. doi: 10.1093/chromsci/bmv142. [DOI] [PubMed] [Google Scholar]
- Strong M.F. J. Int. Affairs. 1991;2:287–300. [Google Scholar]
- Taylor D. Pharma. Environ. 2016;41:1–33. [Google Scholar]
- Tobiszewski M., Mechlińska A., Zygmunt B., Namieśnik J. TrAC Trends Anal. Chem. 2009;28:943–951. [Google Scholar]
- Tótoli E.G., Salgado H.R.N. Phys. Chem. 2012;2:103–108. [Google Scholar]
- Tótoli E.G., Garg S., Salgado H.R.N. Ther. Drug Monit. 2015;37:699–710. doi: 10.1097/FTD.0000000000000222. [DOI] [PubMed] [Google Scholar]
- Tótoli E.G., Salgado H.R.N. Anal. Methods. 2013;5:5923–5928. [Google Scholar]
- Tótoli E.G., Salgado H.R.N. World J. Pharm. Pharm. Sci. 2014;3:1928–1943. [Google Scholar]
- Tótoli E.G., Salgado H.R.N. Pharmaceutics. 2015;7:106–121. doi: 10.3390/pharmaceutics7030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trindade M.T., Salgado H.R.N. Phys. Chem. 2017;7:55–62. [Google Scholar]
- Trindade M.T., Kogawa A.C., Salgado H.R.N. Crit. Rev. Anal. Chem. 2017 [Google Scholar]
- Tundo P., Anastas P.T., Black D.S., Breen J., Collins T., Memoli S., Miyamoto J., Polyakoff M., Tumas W. Pure Appl. Chem. 2000;72:1207–1228. [Google Scholar]
- United Nations. <http://www.un.org/> (access September 2017).
- Vieira D., Fiuza T., Salgado H.R.N. Pathogens. 2014;3:656–666. doi: 10.3390/pathogens3030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira D.C.M., Ricarte P.C., Salgado H.R.N. Adv. Anal. Chem. 2012;2:80–87. [Google Scholar]
- Woodhouse E.J., Breyman S. Sci. Technol. Human Values. 2005;30:199–222. [Google Scholar]
- World Health Organization. 1993. <http://www.inchem.org/documents/ehc/ehc/ehc154.htm> (access September 2017).
- World Health Organization. 1997. <http://www.inchem.org/documents/ehc/ehc/ehc196.htm> (access September 2017).