Abstract
This paper reports comparative extraction efficiencies and enhancement methods for natural herbicidal (growth inhibitors) compounds, momilactone A and B, respectively from the dried husks of Oryza sativa using different extraction techniques and different solvent systems. Four different extraction techniques viz. percolation, agitation with heat, sonication and soxhlet using five solvent systems as ethyl acetate, acetone, acetonitrile, methanol and methanol:water (8:2) were evaluated. In these studies, it was observed that maximum extract yield was obtained using in methanol and methanol/water mixture as extracting solvent by soxhlet technique although the content of total momilactones A and B was higher in the methanol/water mixture in comparison to other extractions. The successive and simple isolation enrichment technique for momilactones A and B were achieved by solid-matrix partitioning after the treatment of methanolic extract with charcoal and using ethyl acetate as extracting solvent for momilactones A and B. The quantitative analysis of the extraction and enrichment development protocol was validated by a simple, accurate, reproducible RP-HPLC-UV–VIS method using a binary gradient elution comprising of acetonitrile and water (70:30). The separation was achieved on a waters Spherisorb S10 ODS 2 column (250 × 4.6 mm, I.D., 10 µm) that achieved a greater degree of linearity within an overall concentration of extracts and momilactones A and B, 1 mg mL−1 and higher degree of correlation (0.9928 ≤ r2 ≤ 0.9936) for momilactones A and B. So far, comparative extraction of momilactones A and B and HPLC of these compounds has not been reported. Standards of momilactones A (1) and B (2) were isolated along with other two compounds as orizaterpenoid (3) and 7-ketostigmaterol (4) from ethyl acetate extract of rice hulls of O. sativa and checked purity by HPLC-PDA-MS and identification of these isolated compounds (1–4) by complete spectroscopic techniques as IR, 1H NMR, 13C NMR, 2D NMR and HR-MS. The qualitative analysis of momilactone A and B separation technique by thin layer chromatography was also developed.
Keywords: Oryza sativa L. Gramineae, Rice husks, Momilactones A and B isolation, Soxhlet extraction, RP-HPLC quantification, Solid-matrix partitioning, High resolution MS
1. Introduction
Rice (Oryza sativa L.) is the main cereal food in Asia and the majority of the population in the world. It generally occurs as two types, with white and coloured husks, although the white husks variety is more common (85%). The germination of rice seed is of great agricultural importance, and it has long been known to be influenced by compounds present in the seed coat (husks) (Dutta, 1973). The compounds momilactone A and B from rice husks cause germination and growth inhibition in the roots of rice (Kato et al., 1973, Kato et al., 1977, Takahashi et al., 1976). They were later found in rice leaves and straw as phytoalexins (Cartwright et al., 1981, Kodama et al., 1988) and putative growth inhibitor was isolated from rice root exudates and identified as momilactone B (Kato-Noguchi et al., 2002). Several isolation methods of momilactones with cold percolation technique have been reported (Kato et al., 1973, Kato et al., 1977, Chung et al., 2005a). Cytotoxic and antitumor activity of momilactone B from rice husks of O. sativa were evaluated for against human colon cancer cells (Kim et al., 2007). Among these, still the classical organic solvent extraction methods primarily using solvents methanol and methanol/water mixture in the ratio (8:2). Newer extraction techniques such as, heat with agitation, sonication and soxhlet extraction have not been used earlier for rice husks extraction, as these techniques were reported in literature (Nagar et al., 2015, Chatterjee et al., 2014, Tandon, 2010). Several analytical methods for the identification of momilactones A and B have also been reported, few like reverse phase-high performance liquid chromatography (RP-HPLC) (Kim et al., 2007), TLC/FID analytical technique used for the analysis of momilactones A and B (Saha et al., 1981), momilactones A and B were identified at different growth stages by HPLC-MS-MS (Lee et al., 1999) and also in rice husks by GC–MS analysis (Chung et al., 2006).
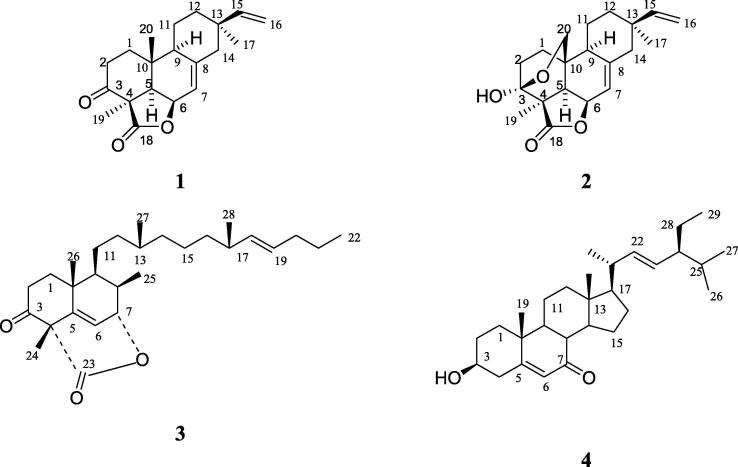
As per newer techniques (Nagar et al., 2015, Chatterjee et al., 2014, Tandon, 2010), the rice husks of O. sativa used for the extraction for enrichment and quantification of momilactones. As per literature, such type of extraction, enrichment and quantification techniques have not been reported by HPLC and by others methods of momilactones A and B. We have extracted and quantified of momilactones by HPLC in different extracts with different extraction techniques. The standards of momilactone A (1) and B (2) were isolated along with other two compounds orizaterpenoid (3) and 7-ketostigmaterol (4) from methanol extract of rice husks of O. sativa and identified of these isolated compounds (1–4; Fig. 1) by complete spectroscopic techniques as 1H NMR, 13C NMR, 2D NMR (COSY, HMBC & HSQC), FAB-MS and high resolution mass. Therefore, we report a detailed study of solvent extraction techniques employing different solvents to study the extraction kinetics with respect to different solvents, different extraction techniques, variability in extraction methods and there by developing enhancement protocol validated by an improved, rapid and RP-HPLC and simple isolation method by column chromatography of momilactones from O. sativa (Fig. 2). The qualitative analysis of momilactones A and B separation technique by thin layer chromatography was also developed different as reported earlier (Cartwright et al., 1981).
Fig. 1.
Structures of momilactones A (1) and momilactone B (2), other isolated compounds orizaterpenoid (3), 7-ketostigmasterol (4).
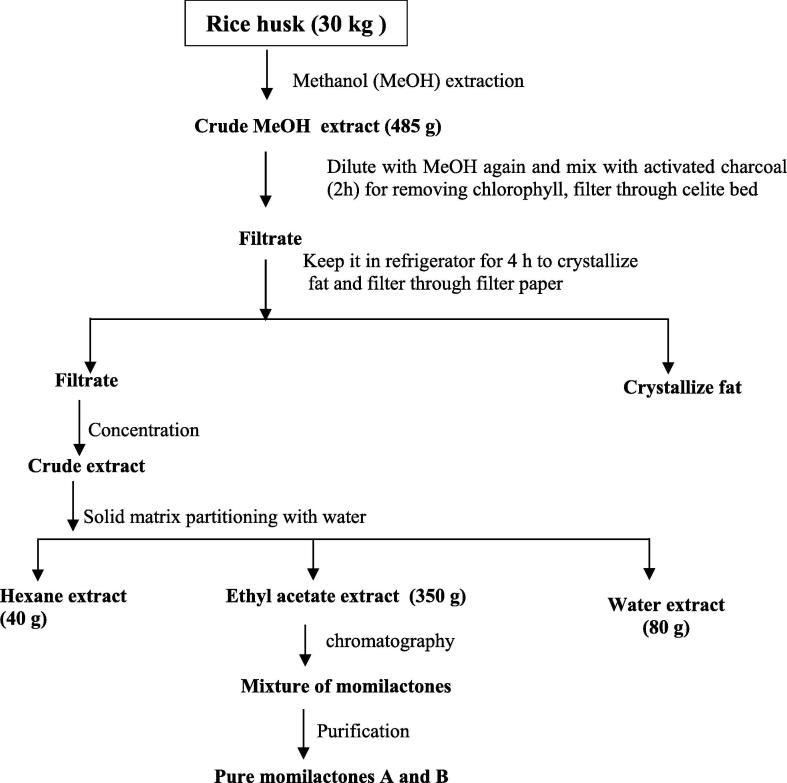
Fig. 2.
Enrichment and simple isolation of momilactone A and B in different partitioned extracts through solid-matrix partitioning of methanolic extract.
2. Materials and methods
2.1. Chemicals and standards
The solvents used for extraction of the rice husks were of L.R. grade and was procured from Junsei Chemical Co., Ltd., Japan. The solvents used for HPLC analysis of the extracts as well as standards were of HPLC grade and procured from Merck, Mumbai, India. The HPLC standards of momilactones A and B were isolated our self and purity of 98%, 97% by HPLC. The solvents were filtered through a 0.45 µm Millipore membrane (Millipore, Billerica, MA) before injecting into the HPLC stream. Digital melting point apparatus from Sonar India was used to determine melting points, whereas Rudolf autopol model polarimeter for measurement of the optical rotations. Pre-coated TLC plates of thickness 0.25 mm and silica gel 60–120 mesh ASTM for column chromatography were procured from Merck. Visualization of the TLC spots was performed using 5% H2SO4 in ethanol with vanillin spray reagent. Both 1H and 13C NMR spectra were obtained on a Brucker DRX-500 model spectrometer operating at 500 and 125 MHz, respectively. NMR spectra were obtained in deuterated chloroform using tetramethylsilane (TMS) as an internal standard, FAB/MS data was recorded on a JEOL SX-102 spectrometer and Electrospray ionization mass (ESI) in direct mass analysis of HPLC-PDA-MS spectrometer and HR/MS was measured on Agilent technology 6545Q-TOF LC/MS. Infrared spectroscopy was recorded on an FT-IR spectrophotometer Shimadzu 8201 PC (4000–400 cm−1).
2.2. Plant material and preparation of extract
The husks of O. sativa (var. Koshihikari) were collected after harvesting and drying from rice mill of Higashi-Hiroshima, Japan in September/October 2016. The voucher specimen (HUJ SO 17) has been dried and deposited in the herbarium of our department. Dried husks of O. sativa (30 kg) were immersed in methanol (MeOH) for 2 week at room temperature and concentrated under vacuum to produce an extract (485 g).
2.3. Extraction and isolation of momilactones A and B
The MeOH extract (485 g) after processing as per (Fig. 2) was subjected to normal-phase column chromatography over silica gel column (60–120 mesh, 1.8 kg, 5.0 cm × 160 cm) to yield 60 fractions (each fr. 1 L) with the following eluants: fraction 1–10 in hexane, fractions 11–20 in hexane:EtOAc (9.5:0.5), fractions 21–30 in hexane:EtOAc (9:1), fractions 31–50 in hexane:EtOAc (8:2), fractions 51–60 in hexane:EtOAc (7:3). All fractions were examined by thin layer chromatography (TLC). Fractions 41–50, were showing same on TLC and further column chromatography over silica gel with chloroform and chloroform:methanol, (100% and 99:1) yielded mixture of compounds momilactone A and momilactone B. These mixture was further purified by column chromatography over silica gel with chloroform:methanol (99.8:0.2; 99.60.4; 99.4:0.6; 99.2:0.2; 99:1) and obtained pure compounds: momilactone A (1, 350 mg) and momilactone B (2, 200 mg) along with two more known compounds orizaterpenoid (3) and 7-ketostigmasterol (4, Fig. 1) and identified with the help of spectroscopic techniques.
2.4. Separation techniques by thin layer chromatography (TLC)
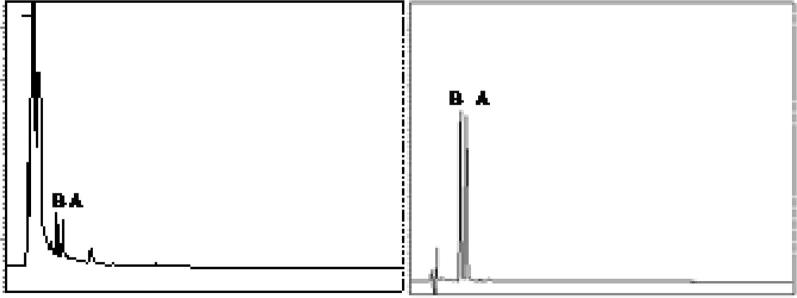
The momilactones A and B mixture was not separated in solvent system hexane/ethyl acetate (7:3) and showing a single spot in TLC (Fig. 3). The same mixture was well separated in chloroform/methanol (9.5:0.5) into two distinct spots of momilactone A and B (Fig. 3). This type of qualitative analysis of momilactones A and B separation technique by thin layer chromatography has not been reported earlier.
Fig. 3.
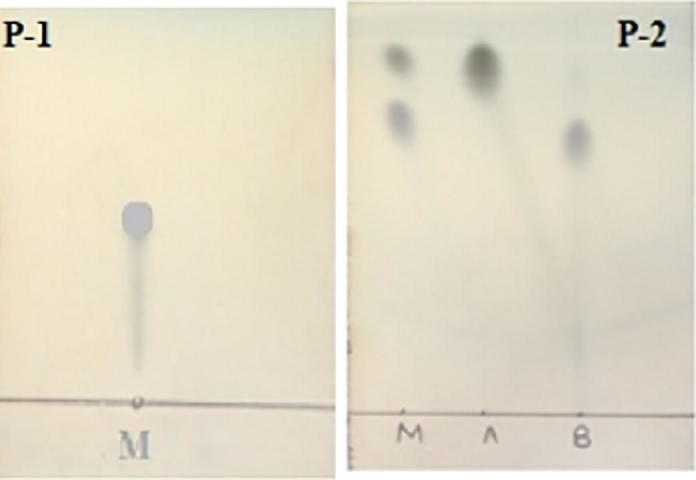
P-1, TLC shows mixture (M) of momilactone A and B not separating in solvent system (Hexane-EtOAc; 7:3) and P-2, shows mixture (M) of well separation of momilactones A and B in solvent system (CHCl3:MeOH; 9.5:0.5), along with isolated momilactone A and B.
2.5. NMR data of momilactones A and B, and other isolated compounds
Momilactone A (1). Colourless crystalline compound; Rf 0.48 (CHCl3:MeOH; 9.5:0.5); m.p. 234–236 °C; IR νmax: 2936, 1766, 1698, 1637, 1390, 1188, 990, 908 cm−1; 1H NMR (CDCl3; 500 MHz): δ 1.90 (m, H2-1α), 2.59–2.63 (m, H-2), 2.31 (d, J = 5.0, H-5), 4.84 (t, J = 5.0, H-6), 5.70 (d, J = 5.0, H-7), 1.74–1.80 (m, H-9, H-11α), 1.32 (m, H2-11β), 1.56–1.62 (m, complex, H2-1β, H2-12), 2.20, 2.19 (d, J = 12.5, H2-14), 5.84 (d d, J = 17.0, 11.0, H-15), 4.97, 4.93 (d d, J = 17.0 & 1; 10.0 & 1, H-16), 0.88 (s, H-17), 1.52 (s, H-18), 0.98 (s, H-20). 13C NMR (CDCl3; 125 MHz): δ 34.89 (C-1), 31.21 (C-2), 205.20 (C-3), 53.57 (C-4),46.46 (C-5), 73.17 (C-6), 114.03 (C-7), 148.96 (C-8), 50.18 (C-9), 32.46 (C-10), 23.99 (C-11), 37.24 (C-12), 40.13 (C-13), 47.53 (C-14), 148.03 (C-15), 110.17 (C-16), 21.80 (C-17), 21.47(C-18), 174.32 (C-19), 21.96 (C-20); HPLC-PDA-MS ESI+: 315 [M+H]+(C20H27O3); ESI−: 313 [M−H]−(C20H25O3); HRESIMS: 315.1959 [M+H]+ (calc for C20H27O3, 315.1960). (Compare NMR data with previous literature Kato et al., 1973, Kato et al., 1977, Cartwright et al., 1981, Kim et al., 2007, Chung et al., 2005a).
Momilactone B (2). Colourless crystalline compound; Rf 0.42 (CHCl3:MeOH; 9.5:0.5); m.p. 240 °C; IR νmax: 2920, 1737, 1662, 1637, 1461, 1296, 992, 916 cm−1; 1H NMR (CDCl3; 500 MHz): δ 1.99 (m, H-1α), 2.13–2.06 (m, complex H-2, H-14), 2.20 (dd, J = 6.5, 2.0, H-5), 4.97 (t, J = 4.5, H-6), 5.68 (d, J = 5.0, H-7), 1.72–1.64 (m, H-9, H-11α), 1.30 (m, H-11β), 1.56 –1.51 (m, complex, H-1β, H-12), 5.82 (dd, J = 17.0, 11.0, H-15), 4.93 (d d, J = 10.0 & 1, H-16), 0.87 (s, H-17), 1.43 (s, H-18), 3.58, 4.07 (dd, 9.0, 3.1 7 9.0, 3.5). 13C NMR (CDCl3; 125 MHz): δ 28.81 (C-1), 26.44 (C-2), 96.60 (C-3), 50.35 (C-4), 42.97 (C-5), 73.76 (C-6), 114.00 (C-7), 146.70 (C-8), 44.68 (C-9), 30.74 (C-10), 24.79 (C-11), 37.22 (C-12), 39.99 (C-13), 47.42 (C-14), 148.83 (C-15), 110.23 (C-16), 21.86 (C-17), 18.99 (C-18), 180.48 (C-19), 72.72 (C-20); HPLC-PDA-MS-ESI+: 331 [M+H]+(C20H27O4); ESI−: 329 [M−H]− (C20H25O4); HRESIMS 330.1905 [M+H]+ (calc for C20H27O4, 315.1909). (Compare NMR data with previous literature Kato et al., 1973, Kato et al., 1977, Cartwright et al., 1981, Kim et al., 2007, Chung et al., 2005a).
4, 8β, 10β-trimethyl-9-(13,17-dimethyldodec-18-enyl)decalin-5-en-3-one-23,7α-olide (3). Rf 0.32 (Hex–EtOAc; 8:2); mp 211–215 °C, [α]D26 + 24.0° (c, 0.1 MeOH); IR spectrum (KBr, ν, cm−1): 2948, 2855, 1725, 1698, 1620, 1462, 1405, 1219, 960, 810, 757 cm−1; 1H NMR (CDCl3); δ 1.01 (d, J = 6.5 Hz, H-1α), 1.15 (m H-1β), 2.49, 2.53 (dd, J = 3.1 Hz, H-2), 5.81 (br s, H-6), 4.34 (s, H-7), 2.02 (m, H-8), 1.44 (m, H-9), 1.26, 1.23, (m, H-11, 12), 1.86 (m, H-13), 1.13–1.50 (H-14, 15, 16), 1.17, 1.46 (m, H-16), 2.36 (m, H-17), 5.15 (dd, J = 8.6, 8.6 Hz, H-18), 5.05 (dd, J = 8.6, 8.6 Hz, H-19), 1.50, 2.02 (m, H-20), 1.14, 1.17 (m, H-21), 0.84 (t, J = 5.4, H-22), 1.37 (br s, H-24), 0.93 (d, J = 6.4 Hz, H-25), 0.73 (br s, H-26), 0.83 (d, J = 4.4 Hz, H-27), 0.80 (d, J = 7.9 Hz, H-28); 13C NMR (CDCl3): δ 38.53(C-1), 39.58 (C-2), 199.74 (C-3), 56.03 (C-1), 138.31(C-5), 129.26 (C-6), 71.81 (C-7), 42.30 (C-8), 56.85 (C-9), 51.22 (C-10), 21.08 (C-11), 24.18 (C-12), 45.81 (C-13), 26.04 (C-14), 28.38 (C-15), 29.35 (C-16), 53.85(C-17), 126.46 (C-18), 126.25 (C-19), 39.81(C-20), 21.38 (C-21), 12.17 (C-22),168.91(C-23), 20.01(C-24), 19.69 (C-25), 20.71 (C-26), 19.23 (C-27), 18.93 (C-28); EI MS m/z (rel. int.) 428 [M]+ (C28H44O3 (1 0 0); HRESIMS m/z 429.3721 [M+H]+; (calculated for C28H45O3, 429.3368); (Compare NMR data with previous literature Chung et al., 2005b).
7-Ketostigmasterol (4): Colourless solid; IR spectrum (KBr, ν, cm−1): 3427, 2956, 2870, 1671, 1645, 1462, 1381, 1297, 1180, 1060, 967 cm−1; 1H NMR (CDCl3): δ 5.71 1H, s, H-6), 5.17 (1H, m, H-22), 5.07 (1H, m, H-23), 3.69 (1H, m, w1/2 = 16.5 Hz, H-3α), 1.21 (3H, s, Me-19), 0.94 (3H, d, J = 6.5 Hz, Me-21), 0.86 (3H, d, J = 6.3 Hz, Me-26), 0.84 (3H, d, J = 6.6 Hz, Me-27), 0.81 (3H, t, J = 6.2 Hz, Me-29), 0.70 (3H, s, Me-18), 2.52–1.26 (23H, m, 8 × CH2, 7 × CH); 35.83 (C-1), 32.41 (C-2), 68.70 (C-3), 42.46 (C-4), 138.08 (C-5), 119.66 (C-6), 202.35 (C-7), 34.16 (C-8), 49.97 (C-9), 36.37 (C-10), 21.23 (C-11), 38.29 (C-12), 43.11 (C-13), 55.57 (C-14), 24.18 (C-15), 28.56 (C-16), 54.72 (C-17), 11.94 (C-18), 19.05 (C-19), 36.09 (C-20), 17.62 (C-21), 129.59 (C-22), 126.11 (C-23), 45.42 (C-24), 26.41 (C-25), 18.93 (C-26), 18.70 (C-27), 28.56 (C-28), 29.54 (C-29); FABMS m/z (rel. int.): 426 [M]+. (C29H46O2) (46.3), 408 (21.2), 287 (78.7), 269 (51.4), 254 (18.6), 107 (78.9), 55 (100); HRESIMS m/z 427.3580 [M+H]+; (calculated for C29H47O2, 427.3576); (Compare NMR data with previous literature Laparra et al., 2015).
3. Comparative experimental extraction techniques
Five different experimental treatments were designed which were the combination of four extraction techniques viz. percolation, soxhlet, agitation with heat and sonication with five solvent systems as ethyl acetate, acetone, acetonitrile, methanol and methanol:water (8:2) having varying degree of polarity. The each treatment was done in three times.
3.1. Percolation
Plant material (rice husks, 10 g) was extracted separately in a conical flask (250 mL) with ethyl acetate, acetone, acetonitrile, methanol and methanol:water (8:2). Three washes with 100 mL of fresh solvent at ambient conditions with a hold time of 24 h was carried out. The extracts thus obtained was further concentrated under controlled conditions using rotary evaporator. The dried extracts were stored under cold conditions prior to analysis.
3.2. Sonication
The sonication (ultrasound assisted extraction) was carried out by ultrasonic vibrations using Sonics vibra cell (Model VCX-750) (Sonics, USA). Plant material (10 g) was taken in a conical flask and sonicated with each solvent as described earlier. Three washes with 100 mL of fresh solvent at 30 °C with an extraction time of 2 h were carried out. The extracts were filtered and concentrated under vacuum and low temperatures.
3.3. Agitation with heat
Plant material (10 g) was extracted in a conical flask using magnetic stirrer with heating facility. Three washes with100 mL of the solvents with a hold time of four hour each at a constant temperature of 40 °C was carried out. The washes were pooled, filtered and concentrated under low temperatures and vacuum in rotary evaporator.
3.4. Soxhlet extraction
Plant material (10 g) was exhaustively extracted using soxhlet apparatus with 100 mL of each solvent in a thermostatically controlled water bath for 8 h. The extracts were concentrated using rotary vacuum evaporator under controlled conditions. Prior to analysis the samples were stored under cold conditions. The hot percolation process was performed to check the variation in the content of momilactones with the conventional percolation techniques and also the effect of temperature.
3.5. Standards stock solutions and sample preparation
The stock solutions of 1 mg mL−1, i.e. 1000 ppm standards of momilactone A and B were prepared after dissolving in methanol. The prepared solution was sonicated for 5 min. Sample solutions of these extracts obtained by the different extraction techniques and solvents at a concentration of 10 mg ml−1 were prepared in methanol and analyzed. All the samples were filtered through 0.45 µm Millipore membrane prior injection for HPLC analysis.
3.6. Chromatographic instrumentation and separation conditions (RP-HPLC)
The gradient liquid chromatographic system (model LC-10A series; Shimadzu, Tokyo, Japan) consisted of two LC-10AD pumps controlled by a CMB-10A interface module, a model 7725i manual injector valve (Rheodyne) equipped with a 20 mL sample loop, and a multi-dimensional UV–VIS detector (model SPD-10A). Data were collected and analyzed using a class LC-10 solution software. Work station equipped with an HP-Desk Jet printer. The method involves the use of a Waters Spherisorb S10 ODS2 column (250 × 4.6 mm, I.D., 10 µm) and binary gradient mobile phase profile. The various other aspects of analysis viz. extraction efficiency, peak purity and similarity were validated using a photo diode array detector, and a mobile phase consisting of 0.1% TFA in acetonitrile:water (70:30, v/v). The mobile phase was filtered through 0.45 μm Millipore filter and degassed by sonication for 30 min. The flow rate was adjusted to 4 mL−1 with run time of 50 min. Injection volume was adjusted to 10 μL and detection was made at 210 nm. Linearity was observed in the range of 10–250 μg mL−1 with correlation coefficient of momilactone-A 0.9936 and B-0.9928. Detection limit of A was 0.9552, ng mL−1 and quantitation limit was 3.1840, ng mL−1. Detection limit of B was 1.008, ng mL−1 and quantitation limit was 3.3617, ng mL−1.
3.7. Data analysis
For comparing the extraction efficiency of different extraction techniques with the various solvents data analysis was performed. Statistical treatment of data (least square regression, RSD%) were performed using MS-Office.
4. Results and discussions
The dried rice husks of O. sativa was procured from rice mill Higashi-Hiroshima city, Hiroshima, Japan in the month of September/Octpober 2016. The MeOH extract from the rice husks of O. sativa after defatting with methanol and treatment with charcoal and solid matrix partitioning then prepared ethyl acetate extract for isolation of momilactones as shown in Fig. 2. The standards of momilactone A (1) and momilactone B (2) were isolated from the ethyl acetate extract was subjected to column chromatography over silica gel normal-phase to give four pure compounds as above two (1, 2), and orizaterpenoid (3), 7-ketostigmasterol (4, Fig. 1). The Compounds 1 and 2 (Kato et al., 1973, Kato et al., 1977) and (3, Chung et al., 2005a, Chung et al., 2005b) already reported from rice husks of O. sativa and 7-ketostigmasterol (4) has been found for the first time in this plant. Quantification of momilactones A and B by HPLC has not been reported by different extraction techniques earlier. All isolated compounds were identified by complete spectroscopic techniques as 1H NMR, 13C NMR, 2D NMR (COSY, HMBC & HSQC), FAB-MS and after that finally confirmed the all compounds (1–4) by high resolution mass (HR-MS).
4.1. Rapid gradient for fast and accurate determination of momilactones A and B
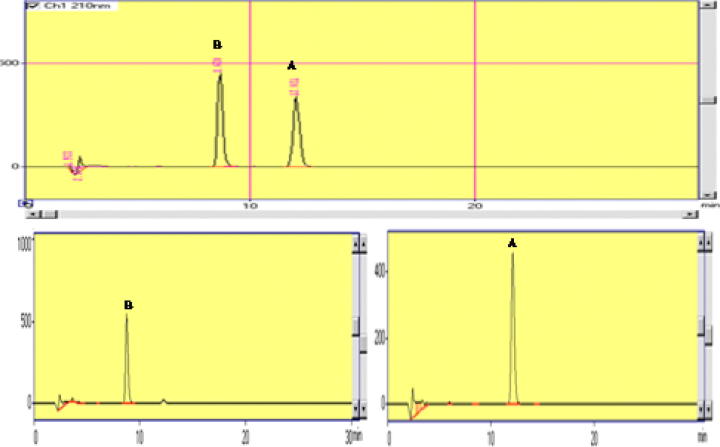
Considering the solubility of momilactones A and B and reviewing different literatures on analysis by GC, GC–MS and HPLC (Cartwright et al., 1981, Saha et al., 1981, Lee et al., 1999, Chung et al., 2006, Kim et al., 2007), the chromatographic method was developed by using solvent mixture comprising of acetonitrile and water with a rapid binary gradient of 30 min analysis time ensuring sufficient column wash and eluting any adhering non-polar impurities of sample matrix. Several stationary phases were tried like symmetry C8 (250 mm × 4.6 mm, 5 µm) and X Terra (250 mm × 4.6 mm, 5 µm) from Waters (Waters, Milford, MA, USA) and Spherisorb-5, RP-8, Brownlee (100 mm × 2.1 mm, 5 µm) and Spherisorb-5 RP-18, Brownlee (100 mm × 2.1 mm, 5 µm) from Perkin-Elmer (Perkin-Elmer, USA). As per above literature, there is no proper reports of HPLC of momilactones A and B percentage or quantification in rice husks. It can be seen from the chromatogram (Fig. 5, Fig. 6) that the best separation was showed on Waters Spherisorb-10 ODS-2 column (100 mm × 2.1 mm, 5 µm) and binary gradient mobile phase profile, where the standards containing momilactones mixture A and B resolved well with relatively high sensitivities at mean retention times of momilactone A and B, respectively following an improvement in the gradient programming to that of earlier method (Lee et al., 1999). Identification and authentication of the targeted standards of momilactones A and B has been analyzed by the confirmation of spectroscopic techniques 1H, 13C NMR, IR, HR-MS spectra in this paper.
Fig. 5.
HPLC chromatograms of momilactone mixture A, B in extracts and isolated mixture of momilactone A and B.
Fig. 6.
HPLC chromatograms of mixture of momilactone A, B (upper) and pure A and B (lower).
4.2. Method validation
The validation was performed in compliance with International Conference on Harmonization (%, RSD, least square regression and residual analysis) (ICH, 2005).
4.3. Linearity and sensitivities (detection limits)
The linearity of the detector response was determined based on calibration curves. Ten test solutions ranging from 5 µg mL to 100 µg mL−1were prepared by dilution of the original stock solution of 1 mg mL−1. Linear regression curves were obtained by plotting the UV detector response in terms of peak area of individual momilactones A and B at each level (y-axis) against the concentration (x-axis) of each injection for each momilactones mixture and separately A and B. A good correlation (0.9936 ≤ r2 ≤ 0.9928) was found on computation for the respective momilactone A and B. Limits of detection (LOD) were determined of momilactones A and B 0.9552, 1.008, limits of quantitation (LOQ) were 3.18409, 3.3617, respectively.
4.4. Extraction recovery study of momilactones A and B through different extraction techniques
The HPLC analysis indicated that in case of soxhlet, agitation with heat and sonication techniques more than 95% of the momilactones are obtained in first wash and remaining in second wash whereas in case of percolation only 90% of momilactones is obtained in first wash, additional 5% second wash and remaining in third wash. The third wash obtained during soxhlet, agitation with heat and sonication did not yield any momilactones. This implies that soxhlet (hot continuous extraction), agitation with heat and sonication all involves the assistance of energy that augments the continuous extraction process which facilitates efficient mass transfer and thereby completely leaches out the analyte in two successive elutions. Whereas percolation itself implies static extraction and hence the recovery occur by a favourable distribution coefficient in consecutive stages. The details of extraction techniques, extractive yields and quantification of momilactones of rice husks are given in Table 1 and Fig. 4.
Table 1.
Extractive yield of extracts (%) of different rice husks of O. sativa obtained by different extraction techniques.
| S. no. | Three extraction each technique | Solvent used | Weight of pl material (g) | Weight of extract (g) | Extract yield (%) |
|---|---|---|---|---|---|
| 1 | Percolation | Ethyl acetate | 10 | 0.10 | 1.00 |
| 2 | Agitation with heat | Ethyl acetate | 10 | 0.12 | 1.20 |
| 3 | Sonication | Ethyl acetate | 10 | 0.11 | 1.10 |
| 4 | Soxhlet | Ethyl acetate | 10 | 0.12 | 1.20 |
| 5 | Percolation | Acetone | 10 | 0.11 | 1.10 |
| 6 | Agitation with heat | Acetone | 1 0 | 0.13 | 1.30 |
| 7 | Sonication | Acetone | 10 | 0.12 | 1.20 |
| 8 | Soxhlet | Acetone | 10 | 0.12 | 1.20 |
| 9 | Percolation | Acetonitrile | 10 | 0.11 | 1.10 |
| 10 | Agitation with heat | Acetonitrile | 10 | 0.12 | 1.20 |
| 11 | Sonication | Acetonitrile | 10 | 0.12 | 1.10 |
| 12 | Soxhlet | Acetonitrile | 10 | 0.13 | 1.10 |
| 13 | Percolation | Methanol | 10 | 0.13 | 1.20 |
| 14 | Agitation with heat | Methanol | 10 | 0.13 | 1.30 |
| 15 | Sonication | Methanol | 10 | 0.14 | 1.40 |
| 16 | Soxhlet | Methanol | 10 | 0.13 | 1.30 |
| 17 | Percolation | Methanol:water (8:2) | 10 | 0.13 | 1.30 |
| 18 | Agitation with heat | Methanol:water (8:2) | 10 | 0.14 | 1.40 |
| 19 | Sonication | Methanol:water (8:2) | 10 | 0.15 | 1.50 |
| 20 | Soxhlet | Methanol:water (8:2) | 10 | 0.15 | 1.50 |
Fig. 4.
Percent of momilactone A and B content determined in different extraction techniques.
4.5. The influence of the extraction techniques and solvents on the momilactones A and B content
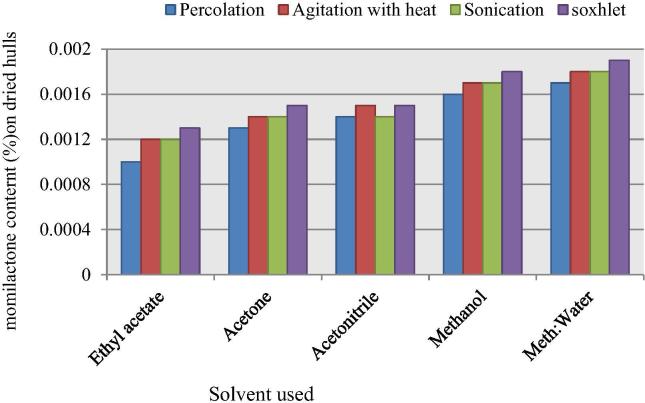
The extraction efficiency based on the momilactones content for these treatments was observed on the basis of HPLC analysis and total momilactones percentage as shown in Table 2. Methanol/water mixture were used with soxhlet gave the best results, while ethyl acetate used with percolation gave the least momilactones content as also depicted in Table 2 and Fig. 4. In our present studies it was observed that extraction employing soxhlet technique using methanol and methanol/water mixture as solvent gave the best yield and comparable content. Although energy assisted extraction techniques like agitation and sonication enhanced the extractive yield by little in comparison to percolation 10–20% with most solvents in comparison to percolation but the best results were obtained using soxhlet method wherein continuous recycling of solvents at slightly elevated temperatures was applied. The percentage details of momilactones A and B of rice husks by different extraction techniques are given in Table 2.
Table 2.
HPLC analysis of total momilactones content in each extract (%) obtained by different extraction techniques with various solvents.
| Extraction techniques | Solvents |
||||
|---|---|---|---|---|---|
| Ethyl acetate | Acetone | Acetonitrile | Methanol | Methanol:Water (8:2) | |
| Percolation | 0.0010 | 0.0013 | 0.0014 | 0.0016 | 0.0017 |
| Agitation with heat | 0.0012 | 0.0014 | 0.0015 | 0.0017 | 0.0018 |
| Sonication | 0.0012 | 0.0014 | 0.0014 | 0.0017 | 0.0018 |
| Soxhlet | 0.0013 | 0.0015 | 0.0015 | 0.0018 | 0.0019 |
4.6. Extraction yield and selection of extract for further downstream processing (solid matrix partitioning)
The extractive yield turns out to be an important factor in designing an extraction process technology for further recovery of analyte in better yields. Thus as seen from Table 1, that of all the solvents tried at the percolation and heat level with ethyl acetate, acetone, acetonitrile methanol and methanol/water mixture represents a congruency and the methanol and methanol/water mixture extract showed a better extractive yield in comparison to others extractions. Again, in the previous extraction methodology of momilactones (Kato et al., 1973, Kato et al., 1977), it is clear that methanol favours extraction and in the current experiments do also follow the same pattern. But the extractive yield varies substantially with that of solvent selectivity. Thus, in the current experiments and also observed in the Table 1, it follows that the highest extract yield is resultant from methanol, methanol water mixture and the lowest in ethyl acetate. Hence for up gradation at pilot scale the process was designed initially to get the extract in substantial yield and then follow the partition and enrichment process which will also result into high yield of enriched extracts of momilactones (Fig. 2).
4.7. Enrichment of momilactones
For a large scale extraction technology, process development is a critical procedure. The process should comprise of minimum unit operations, with good extraction efficiency and better clarification of the extracts in simple processing steps. In preparation of rice husks extract it is generally desired to chromatographic steps which would make the process simple, less tedious, efficient and economical. One such method previously experimented by the authors in the development of process technology is the application of solid-matrix partitioning technique that uses celite as the filtration aid (Tandon, 2010, Chatterjee et al., 2014) in downstream processing of extracts to effectively reduce the high costs of solvent exhausted in liquid-liquid partitioning. As shown in Fig. 2 different solvents such as hexane, ethyl acetate and water were used to check the enriching efficiency by weight of extracts and increasing percentage of momilactones checked by HPLC. Simple isolation techniques of momilactones by solid matrix partitioning technique using ethyl acetate extract as shown in Fig. 2. It was observed that partitioning of the extract by using water and extracted with hexane, ethyl acetate afforded the best enriched fraction of ethyl acetate having up to 95% of total momilactones. The pure momilactone A and B separation was achieved by column chromatography as shown experimental part.
5. Conclusion
During the development of any extraction process the main objectives includes high extraction efficiency, eco friendly solvent systems and less cumbersome unit operations and downstream processing steps. In this work, the extraction permeability and efficiency has been reviewed by studying four extraction techniques (percolation, agitation with heat, sonication, and soxhlet). Secondly, the enrichment process has also been simplified by selective solvent partitioning and employing simple chromatographic enrichment techniques (Fig. 2) which enhanced the assay by almost many fold. In enrichment process is usually carried out using methanol for initial extraction for momilactones, and if extraction done by other polar solvents (methanol: water mixture) the extract enriched with unwanted chemicals like high molecular weight compounds, tannins, sugar etc. However, the other process which comprises of ethyl acetate, acetone, and acetonitrile as extracting solvent for momilactones of lesser yields. Thus, this research study signifies an economic scale-up process technique which can be further exploited commercially for obtaining momilactones rich fraction in a greater relative purity and strength and simple chromatographic process to save time and solvents.
Acknowledgments
The author (AA) are grateful to Graduate School for International Development and Cooperation, Hiroshima University, Higashi-Hiroshima City, Hiroshima, Japan for inviting as a Visiting Associate Professor to Dr. Ateeque Ahmad for three months October 2016 to December 2016 for providing the necessary facilities and infrastructure for carrying out the work. The authors thank and grateful to Do Tan Khang, Phung Thi Tuyen, Hasan Shamim Mandal for his help in plant material collection and isolation of compounds in this work.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jsps.2018.07.014.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Cartwright D.W., Langcake P., Pryce R.J., Leworthy D.P., Ride J.P. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry. 1981;20:535–537. [Google Scholar]
- Chatterjee A., Tandon S., Ahmad A. Comparative extraction and downstream processing techniques for quantitative analysis of rosmarinic acid in Rosmarinus officinalis. Asian J. Chem. 2014;26(14):4313–4318. [Google Scholar]
- Chung, I.M., Hahn, S.J., Ahmad, A., 2005a. Confirmation of potential herbicidal agents in hullsof rice, Oryza sativa. 31, 1339–1352. [DOI] [PubMed]
- Chung I.M., Ali M., Hahn S.J., Siddiqui N.A., Lim Y.H., Ahmad A. Chemical constituents from the the hulls of Oryza sativa with cytotoxic activity. Chem. Nat. Compds. 2005;41:182–189. [Google Scholar]
- Chung I.M., Kim J.T., Kim S.H. Evaluation of allelopathgic potential and quantification of momilactonesA, B from rull extracts and assessment of inhibitory bioactivity on paddy field weeds. J. Agric. Food Chem. 2006;54:2527–2536. doi: 10.1021/jf052796x. [DOI] [PubMed] [Google Scholar]
- Dutta A.K. Germination and growth-inhibitors in relation to non-viability of rice seeds. Ind. J. Agric. Res. 1973;42:894–900. [Google Scholar]
- ICH (Q2), 2005. Proceedings of the International Conference on Harmonization. IFPMA, Geneva.
- Kato T., Kabuto C., Sasaki N., Tunagawa M., Aizawa H., Fujita K., Kato Y., Kitahara Y. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahederon Lett. 1973;39:3861–3864. [Google Scholar]
- Kato T., Tunakawa M., Sasaki N., Aizawa H., Fujita K., Kitahara Y., Takahashi N. Growth and germination inhibitors in rice husks. Phytochemistry. 1977;16:45–48. [Google Scholar]
- Kato-Noguchi H., Ino T., Sata N., Yamamura S. Isolation and identification of a potent allelopathic substance in rice root exudates. Physiol. Plant. 2002;115:401–405. doi: 10.1034/j.1399-3054.2002.1150310.x. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Park H.R., Park E., Lee S.C. Cytotoxic and antitumor activity of momilactone B from rice hulls. J. Agric. Food Chem. 2007;55:1702–1706. doi: 10.1021/jf062020b. [DOI] [PubMed] [Google Scholar]
- Kodama O., Suzuki T., Miyakawa J., Akatsuka T. Ultraviolet-induced accumulation of phytoalexin in rice leaves. Agric. Biol. Chem. 1988;52:2469–2473. [Google Scholar]
- Laparra J.M., Alfonso-Garcia A., Alegria A., Barbera R., Cilla A. 7-keto-stigmasterol and 7-keto-cholesterol induce differential proteome changes to intestinal epitelial (Caco-2) cells. Food Chem. Toxi. 2015;84:29–36. doi: 10.1016/j.fct.2015.06.021. [DOI] [PubMed] [Google Scholar]
- Lee C.W., Yoneyama K., Taekeuch Y., Konnai M., Tamogami S., Kodama O. Momilactones A and B in rice straw harvested at different growth stages. Biosci. Biotechnol. Biochem. 1999;63:1318–1320. doi: 10.1271/bbb.63.1318. [DOI] [PubMed] [Google Scholar]
- Nagar A., Chatterjee A., Rehman L., Ahmad A., Tandon S. Comparative extraction and enrichment techgniques for pyrethrins from flowers of Chrysanthemum cinerarriaefolium. Ind. Crops Prod. 2015;76:955–960. [Google Scholar]
- Saha P.K., Hatakeda K., Kato T. A convenient method for analysis of momilactones. Jpn. J. Crop Sci. 1981;50:382–387. [Google Scholar]
- Takahashi N., Kato T., Tunagawa M., Sasaki N., Kitahara Y. Mechanism of dormancy in rice seeds. II. New growth inhibitors, momilacxtones A and B isolated from the hulls of rice seeds. Jpn. J. Plant Breed. 1976;26:91–98. [Google Scholar]
- Tandon S. Pilot scale processing technology for extraction of Cliv-92: a combination of three coumari-nolignoids cleomiscosins A, B and C from Cleome viscosa. Ind. Crops Prod. 2010;31:335–343. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.