Abstract
Background
A severe mismatch between the supply and demand of oxygen is the common feature of all types of shock. We present a newly developed, clinically oriented classification of the various types of shock and their therapeutic implications.
Methods
This review is based on pertinent publications (1990–2018) retrieved by a selective search in PubMed, and on the relevant guidelines and meta-analyses.
Results
There are only four major categories of shock, each of which is mainly related to one of four organ systems. Hypovolemic shock relates to the blood and fluids compartment while distributive shock relates to the vascular system; cardiogenic shock arises from primary cardiac dysfunction; and obstructive shock arises from a blockage of the circulation. Hypovolemic shock is due to intravascular volume loss and is treated by fluid replacement with balanced crystalloids. Distributive shock, on the other hand, is a state of relative hypovolemia resulting from pathological redistribution of the absolute intravascular volume and is treated with a combination of vasoconstrictors and fluid replacement. Cardiogenic shock is due to inadequate function of the heart, which shall be treated, depending on the situation, with drugs, surgery, or other interventional procedures. In obstructive shock, hypoperfusion due to elevated resistance shall be treated with an immediate life-saving intervention.
Conclusion
The new classification is intended to facilitate the goal-driven treatment of shock in both the pre-hospital and the inpatient setting. A uniform treatment strategy should be established for each of the four types of shock.
In the first descriptions of shock the focus was exclusively on traumatic hemorrhagic shock, but later this changed and five different types of shock came to be distinguished (1). Although it is true that all types of shock can lead to the same final stage of multiorgan failure as a result of the imbalance between oxygen demand and supply, the differences in their pathogenesis and pathophysiology make it desirable to change their classification, partly for teaching purposes, but also, especially, because different therapeutic measures are needed for the different types of shock. The new classification makes no claim to be binding, and the therapeutic effects are as a rule limited primarily to restoration of vital functions, in particular cardiovascular function consistent with survival.
For the reasons given above, the new classification comprises just four main categories:
Hypovolemic shock
Distributive shock
Cardiogenic shock
Obstructive shock.
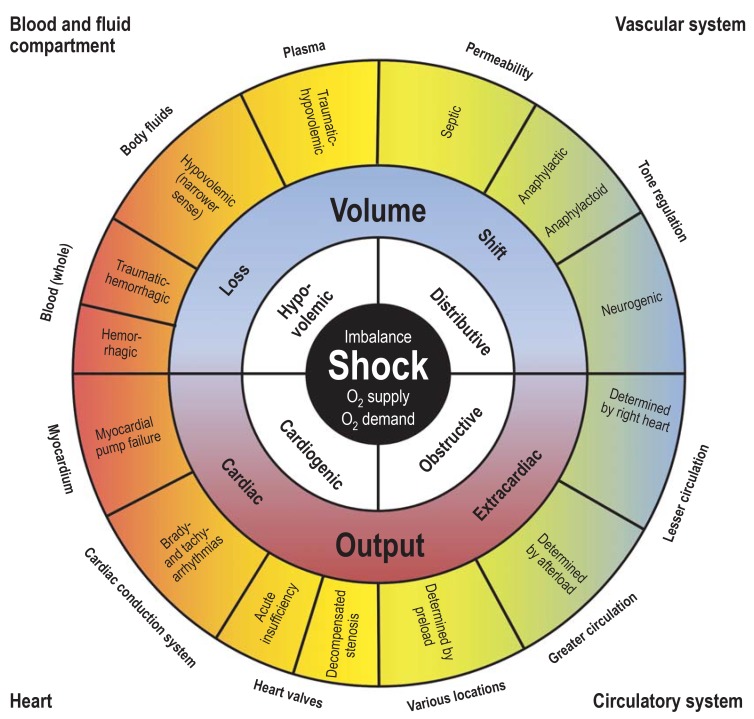
Of these, hypovolemic shock is divided into four subcategories and distributive shock into three. Obstructive shock has been given a category of its own. Although this nomenclature and classification is schematic and there is some overlapping between the main groups, these four main groups can be basically assigned to four organ systems (figure 1) that, owing to differences in their pathogenesis and pathophysiology, require group-specific—or, in other words, organ-specific—treatment (figure 2):
Figure 1.
Synoptic view of the four types of shock (inner, white field) with the organ systems primarily associated with them (outer corners), sites and mechanisms of manifestation (outside the circle), and pathogenetic and pathophysiologic features (outer and middle sectors of the circle). To maintain clarity, mixed types of shock are not depicted.
Blood and fluids compartment
Vascular system
Heart
Circulatory system.
Classification of types of shock.
Hypovolemic shock
Distributive shock
Cardiogenic shock
Obstructive shock
Because of the difficulty of carrying out prospective randomized studies in shock patients, the recommendations for treatment are based largely on guidelines and registry studies. If available, the recommendation grade (RG) from the guidelines is given. Where no recommendation grade is available, the recommendation is that of the present authors (etable 1). The effects of the interventions presented on survival and disability-free survival are in some cases not strong.
eTable 1. Definition of recommendation grades.
| Recommendation grade | Description | In words | Symbol |
| A | Strong recommendation | Should/should not | ↑↑ |
| B | Recommendation | Should/should not (weaker) |
↑ |
| O | No recommendation | May be considered/ rejected |
↔ |
Learning goals
After reading this article, the reader should:
Be familiar with the new classification of types of shock
Understand the different pathogenesis and pathophysiology of the four main categories of shock
Know the different therapeutic approaches to the various types of shock.
Hypovolemic shock
Hypovolemic shock is a condition of inadequate organ perfusion caused by loss of intravascular volume, usually acute. The result is a drop in cardiac preload to a critical level and reduced macro- and microcirculation, with negative consequences for tissue metabolism and the triggering of an inflammatory reaction.
Hypovolemic shock is divided into four subtypes (2):
Hemorrhagic shock, resulting from acute hemorrhage without major soft tissue injury
Traumatic hemorrhagic shock, resulting from acute hemorrhage with soft tissue injury and, in addition, release of immune system activators
Hypovolemic shock in the narrower sense, resulting from a critical reduction in circulating plasma volume without acute hemorrhage
Traumatic hypovolemic shock, resulting from a critical reduction in circulating plasma volume without acute hemorrhage, due to soft tissue injury and the release of immune system mediators.
Pathogenesis and pathophysiology
Hypovolemic shock.
Hypovolemic shock is a condition of inadequate organ perfusion caused by loss of intravascular volume, usually acute.
The characteristic feature of both, hemorrhagic and traumatic hemorrhagic shock is bleeding. However, differences exist between the two subcategories in terms of the extent of soft tissue damage. Clinically the most significant cause of hemorrhagic shock is acute bleeding from an isolated injury to a large blood vessel, gastrointestinal bleeding, nontraumatic vascular rupture (e.g., aortic aneurysm), obstetric hemorrhage (e.g., uterine atony), and hemorrhage in the region of the ear, nose, and throat (vascular erosion). The shock is triggered by the critical drop in circulating blood volume; massive loss of red blood cells intensifies the tissue hypoxia.
Traumatic hemorrhagic shock is distinguished from hemorrhagic shock by the additional presence of major soft tissue injury which aggravates the shock. A typical example of this type of shock is polytrauma, most usually caused by road traffic accidents and falls from a great height. Diffuse bleeding, hypothermia (especially = 34 °C), and acidosis lead to life-threatening coagulopathy (3, 4). The soft tissue injury leads to postacute inflammation, further reinforcing this process. At the microcirculatory level, leukocyte–endothelium interactions (5) and destruction of endothelial membrane-bound proteoglycans and glycosaminoglycans cause microvascular dysfunction with capillary leak syndrome. At the intracellular level a metabolic imbalance arises (6) with possible mitochondrial damage (7) and a negative influence on the vasomotor system (8).
Hypovolemic shock in the narrower sense and traumatic hypovolemic shock show significant fluid loss without hemorrhage.
Hypovolemic shock in the narrower sense arises from external or internal fluid loss coupled with inadequate fluid intake. It can be caused by hyperthermia, persistent vomiting and diarrhea (e.g., cholera), or uncompensated renal losses (e.g., diabetes insipidus, hyperosmolar diabetic coma). Sequestration of large quantities of fluid in the abdomen, e.g., in ileus or liver cirrhosis, also leads to a reduction of circulating plasma volume. The pathologically raised hematocrit as well as the increased leukocyte and platelet interactions additionally impair the rheologic properties of the blood and can lead to persistent organ damage even after the patient has been treated for shock (“no-reflow phenomenon”).
Typical causes of traumatic hypovolemic shock are large surface burns, chemical burns, and deep skin lesions. The trauma also activates the coagulation cascade and the immune system, potentiating the impairment of the macro- and microcirculation. The inflammatory reaction results in damage to the endothelium, increases capillary leak syndrome, and causes severe coagulopathy (9, 10).
Physiology of hypovolemic shock.
The result is a drop in cardiac preload to a critical level and reduced macro- and microcirculation, with negative consequences for tissue metabolism and the triggering of an inflammatory reaction.
It may be possible to draw some cautious conclusions about the incidence of traumatic hypovolemic and traumatic hemorrhagic shock from the Trauma Registry of the German Trauma Society (Deutsche Gesellschaft für Unfallchirurgie). In the 2017 annual report, out of 40 836 patients, 27 147 (66%) had a maximum severity of injury of AIS 3 (Abbreviated Injury Score) or more, and 10 639 (26%) had life-threatening injuries (ISS, Injury Severity Score = 11), on the basis of which the number of patients can be calculated to be around 30 000 per year. The incidence of gastrointestinal hemorrhage in Germany is around 100 000 patients per year, of whom roughly 10 000 suffer hypovolemic shock. These figures, together with those for the remaining subtypes of hypovolemic shock, lead to a total of about 50 000 patients per year (table 1).
Table 1. Relative incidences of the various types of shock.
| Type of shock |
Relative incidence (authors’ own calculations) |
Relative incidence (representative published figures [25]) |
| Hypovolemic | 27% | 16% |
| Distributive | 59% | 66% |
| Made up of: septic 55%, anaphylactic and neurogenic 4% |
Made up of: septic 62%, anaphylactic and neurogenic 4% |
|
| Cardiogenic | 13% | 16% |
| Obstructive | 1% | 2% |
Hypovolemic shock in the narrower sense and traumatic hypovolemic shock.
Hypovolemic shock in the narrower sense and traumatic hypovolemic shock show significant fluid loss without hemorrhage.
Treatment
Causes.
Typical causes of traumatic hypovolemic shock are large surface burns, chemical burns, and deep skin lesions.
The preclinical and clinical treatment of hypovolemic shock consists of immediate intravascular volume replacement (fluid resuscitation) with balanced crystalloids (recommendation grade: B) using wide-bore peripheral venous access and, in a patient who is hemorrhaging, rapid bleeding control (table 2). To prevent or alleviate hypoxia, endotracheal intubation with normoventilation usually follows (recommendation grade: A). The extent of blood loss can be roughly estimated using the ATLS (Advanced Trauma Life Support) score (11). Trauma patients with shock should be transferred directly to a trauma center (recommendation grade: B).
Table 2. Typical drugs for treatment of the various types of shock.
| Drug | Indication | Main effect | Important adverse effects | Dosage |
| Blood and coagulation products | ||||
| Red cell concentrates (RCC) | Hemorrhagic shock, traumatic hemorrhagic shock, all other types of shock in patients with signs of anemic hypoxia | Replace lost red blood cells, increase blood oxygen concentration, increase blood coagulability | Hyperkalemia (check length of storage of RCC), acute transfusion reaction, sensitization in case of non-identical subgroup infection (cytomegaly, HIV, hepatitis A, B, C, E) | According to effect, need, and transfusion trigger in the individual case, 1 RCC raises Hb value by approx. 1 g/dL. In patients with massive hemorrhage: RCC:FFP:PC = 4:4:1 |
| Fresh frozen plasma (FFP) | Hemorrhagic shock, traumatic hemorrhagic shock, all other types of shock in patients with acquired coagulopathy and bleeding | Replaces coagulation factors and volume | Anaphylaxis, acute transfusion reaction, sensitization in case of non-identical subgroup infection, volume overload, TRALI, infection (cytomegaly, HIV, hepatitis A, B, C, E) | Initially 20 mL/kg, then according to effect and individual need. 1 mL/kg raises the coagulation factor(s) concerned by approx. 1%.. In patients with massive hemorrhage: RCC:FFP:PC = 4:4:1 |
| Coagulation factors (fibrinogen, PPSB = F II, VII, IX and X) | Hemorrhagic shock, traumatic hemorrhagic shock, all other types of shock in patients with acquired coagulopathy and bleeding | Selectively replace individual factors after loss/use of vitamin K inhibitor and NOAC-induced hemorrhage | Risk of thromboembolism, contraindication: HIT2 | 1 IU/kg causes the relevant factor to rise by approx. 0.5–1% |
| Platelet concentrates (PC) | Trauma and hemorrhage-induced coagulopathy with thrombocytopenia | Replaces platelets | Acute transfusion reaction, sensitization in case of non-identical subgroup infection, anaphylaxis | 1 apheresis PC raises the platelet count by approx. 20 G/dL. In patients with massive hemorrhage: RCC:FFP:PC = 4:4:1 |
| Tranexamic acid | Hemorrhagic shock, traumatic hemorrhagic shock, peripartum hemorrhage | Inhibits plasmin activation, reduces hyperfibrinolysis | Diarrhea, vomiting, nausea, allergic dermatitis; administration later than 3 h after trauma may be harmful | Early (<3 h) in patients with hemorrhage, especially when peripartum or due to trauma: 1–2 g i. v. |
| Solutions for infusion | ||||
| Isotonic balanced full electrolyte solutions | All types of shock, when cardiac preload is concomitantly reduced due to intravascular volume depletion or obstruction | Replaces fluids lost due to electrolyte imbalance or volume shift, increases stroke volume by raising cardiac preload | Volume overload, pulmonary edema, peripheral edema | Initially 10–20 mL/kg i. v. repeatedly according to effect and volume response |
| Vasoconstrictors, positive inotropic agents, and vasodilators | ||||
| Epinephrine*1,* 2 | All types of shock, when use of other catecholamines fails to achieve adequate vasoconstriction and increased inotropy: cardiopulmonary resuscitation, anaphylactic shock | α1-Receptor-mediated vasoconstrictionβ1-Receptor-mediated positive inotropia β2-Receptor-mediated bronchodilation | Myocardial ischemia, stress cardiomyopathy, tachyarythmias, oliguria/anuria | 0.3–0.6 mg i.m. (autoinjector in anaphylaxis cases), continuously according to effect and need: 0.05 to 1.0 (up to a maximum of 5.0) µg/kg per min i. v.Bolus doses: 5–10 µg i. v.; with CPR: 1 mg i. v. every 3–5 min |
| Dobutamine*2 | Cardiogenic shock, all types of shock with insufficient ventricular pump function | Predominantly β1-receptor-mediated positive inotropic effect | Rise in heart rate ≥ 30/min, rise in BP ≥ 50 mmHg, headache, cardiac arrhythmias, possible drop in BP due to β2-receptor-mediated vasodilation | Continuously according to effect and need: 2.5 to 5 (up to a maximum of 10) µg/kg per min i. v. |
| Norepinephrine*2 | All types of shock with reduced peripheral resistance | Predominantly α1-receptor-mediated vasoconstriction, (low) positive inotropic effects | Peripheral ischemia, rise in BP, reflex bradycardia, cardiac arrhythmias | Continuously according to effect and need: 0.1–1.0 µg/kg per min i. v. Bolus administration: 5–10 µg i. v. |
| Milrinone*2 | Cardiogenic shock | PDE-3 inhibitor: positive inotropic and vasodilatory effect | Drop in BP due to vasodilation, ventricular ectopic beats and tachycardia, ventricular fibrillation, headache | Continuously according to effect and need: 0.375–0.75 µg/kg per min i. v. |
| Levosimendan*2 | Cardiogenic shock | Calcium sensitizer | Drop in BP due to vasodilation, ventricular tachycardia, headache, extrasystoles, atrial fibrillation, heart failure, myocardial ischemia, dizziness, gastrointestinal disorders | Single use only: 0.05–0.2 µg/kg per min/24 h i. v. |
| Vasopressin*3 | Shock states, especially septic shock, when norepinephrine alone does not achieve the required vasoconstriction and lost volume has been replaced | V1-mediated (catecholamine-independent) vasoconstriction | Ischemia, reduced cardiac output, bradycardia, tachyarrhythmia, hyponatremia, ischemia | Continuously according to effect and need: 0.01 up to max. 0.03 U/min i. v. |
| Cafedrine hydrochloride 200 mg Theodrenaline-hydrochloride 10 mg*4 | Neurogenic shock | β1-Receptor-mediated inotropy and α1-receptor-mediated vasoconstriction Rise in BP with peripheral resistance unchanged and moderately reduced heart rate | Palpitations, symptoms of angina pectoris, cardiac arrhythmias | ¼–1 ampoule (2 mL) usually diluted with NaCL 0.9% to a total of 10 mL i. v. Maximum: 3 ampoules/24 h |
| Glyceryl trinitrate*2 | Cardiogenic shock | Vasodilation to reduce preload in particular | Development of tolerance | Continuously according to effect and need: 0.3–4 µg/kg per min i. v. |
| Sodium nitroprusside*2 | Cardiogenic shock | Vasodilation to reduce afterload | Risk of cyanide toxicity | Initially: 0.1 µg/kg per min i. v., then: double the dose every 3–5 min up to 10 µg/kg per min i. v. |
| Anti-inflammatory and antiallergic drugs | ||||
| Dimetindene maleate*1 | Anaphylaxis/ anaphylactic shock | Blocks H1-receptor-mediated action of histamine | Drowsiness, fatigue, dizziness, nausea, dry mouth | 4–8 mg over 30 s/24 h i. v. |
| Methylprednisolone*1 | Anaphylaxis/ anaphylactic shock | Synthetic glucocorticoid, potent anti-inflammatory effect | Glucocorticoid-associated adverse effects only when given long-term | 0.5–1 g/24 h i. v. |
| Hydrocortisone*5, *6 | Septic shock with persistent instability after fluid and vasopressor therapy Adrenal insufficiency | Endogenous glucocorticoid, substituted in patients with reduced or no cortisol production | See Methylprednisolone | Initially: 100 mg over 10 min then: 200–500 mg/24 h i. v. |
| Fludrocortisone*7 | Neurogenic shockSeptic shock? | Mineralocorticoid | If given long-term: edema, hypertension, hypokalemia | 0.1–0.2 mg/24 h p. o. |
Sources of dosage recommendations:
*1 Guideline for acute therapy and management of anaphylaxis. S2 guideline (31), *2 German–Austrian S3 guideline “Infarction-related cardiogenic shock—diagnosis, monitoring, and therapy” (37), *3 drug information for Empressin® February 2015, *4 drug information for Akrinor® September 2016, *5 Angus and van der Poll 2013 (24), *6 drug information for Hydrocortison® March 2018, *7 drug information for Astonin-H® June 2014.
DIC, disseminated intravascular coagulation; RCC, red cell concentrates; FFP, fresh frozen plasma; HIT2, heparin-induced thrombocytopenia type 2; i. m., intramuscular;
i. v., intravenous; PC, platelet concentrates; TRALI, transfusion-related acute lung injury; PPSB, prothrombin, proconvertin, Stuart factor, and antihemophilic B factor; CPR, cardiopulmonary resuscitation; BP, blood pressure; PDE-3, phosphodiesterase 3
Surgical management should be undertaken as soon as possible using the damage control surgery (DCS) approach (12). Persisting hypotension, especially in patients with head trauma, should prompt administration of a vasconstrictor (e.g., norepinephrine) to achieve a systolic arterial pressure (SAP) = 90 mmHg (recommendation grade: B) (13).
Multidiscipinary treatment.
Multidisciplinary treatment includes early stabilization of coagulation by means of coagulation factors, either as individual factors or as fresh frozen plasma (FFP), together with surgical prevention of further blood loss.
In patients with controllable bleeding up to age-specific and comorbidity-specific hemoglobin threshold values, red cell concentrate (RCC) transfusions are given. Those with uncontrolled bleeding, irrespective of the current hemoglobin value, should receive transfusions of RCC, fresh frozen plasma (FFP), and platelet concentrates (PC). Patients with traumatic or peripartum bleeding should also be given 1 to 2 g tranexamic acid at an early stage (recommendation grade: A) (14– 16). Multidisciplinary treatment includes early stabilization of coagulation by means of coagulation factors, either as individual factors or as FFP, together with surgical prevention of further blood loss (17).
Distributive shock.
Distributive shock is a state of relative hypovolemia resulting from pathological redistribution of the absolute intravascular volume and is the most frequent form of shock.
In patients with gunshot or stab wounds to the body cavities or a ruptured aortic aneurysm, blood pressure shall be stabilized at a permissive hypotension (SAP = 70 to 80 mmHg) by norepinephrine infusion and moderate volume replacement until bleeding control is achieved (recommendation grade: B) (13).
For patients with large burns, the modified Brooke formula can give an indication of the volume replacement required in the first 24 h (18).
Distributive shock
Distributive shock is a state of relative hypovolemia resulting from pathological redistribution of the absolute intravascular volume and is the most frequent form of shock (table 1). The cause is either a loss of regulation of vascular tone, with volume being shifted within the vascular system, and/or disordered permeability of the vascular system with shifting of intravascular volume into the interstitium. The three subtypes are septic, anaphylactic/anaphylactoid, and neurogenic shock.
Septic shock
Sepsis is defined according to the current Sepsis-3 criteria as a dysregulated response by the body to an infection resulting in life-threatening organ dysfunctions. These are characterized and quantified by an increase in SOFA (Sequential Organ Failure Assessment) score by = 2 points (etable 2) (19). In the emergency care setting, the “Quick SOFA” (qSOFA) score can be used for screening, requiring only a preliminary examination of state of consciousness, respiration rate, and blood pressure. If there are pathological alterations of these parameters (obtunded consciousness, respiration rate = 22/min, systolic blood pressure = 90 mmHg), and if infection is suspected, the presence of sepsis may be assumed (20).
eTable 2. SOFA (Sequential Organ Failure Assessment) score as a basis for defining sepsis according to the ESCIM (European Society for Intensive Care Medicine) consensus.
| Points | ||||||
| Organ | Parameter | 1 | 2 | 3 | 4 | |
| Lung | PaO2/FiO2 | mmHg | <400 | <300 | <200 with respir. support |
<100 with respir. support |
| Kidney | Creatinine or urinary output |
mg/dL mL/day |
1.2–1.9 – |
2.0–3.4 – |
3.5–4.9 <500 |
≥ 5.0 <200 |
| Liver | Bilirubin | mg/dL | 1.2–1.9 | 2.0–5.9 | 6.0–11.9 | ≥ 12.0 |
| Cardiovascular system |
Blood pressure and catecholamines |
mmHg | Mean arterial pressure <70 |
Catechol. low* |
Catechol. moderate* |
Catechol. high* |
| Blood | Platelets | 1000/mm3 | <150 | <100 | <50 | <20 |
| CNS | Glasgow Coma Scale | 14–13 | 12–10 | 9–6 | <6 | |
*Catecholamine dose
low = dopamine = 5 or dobutamine (each dose) for at least 1 hour
moderate = dopamine >5 or epinephrine/norepinephrine = 0.1 µg/kg per min
high = dopamine >15 or epinephrine/norepinephrine >0.1 µg/kg per min
A lactate value above 2 mmol/L and persistent hypotension requiring the administration of vasopressors to keep mean arterial blood pressure (MAP) above 65 mmHg define septic shock (21). Hypovolemia as the sole cause of circulatory failure must be ruled out, for example by echocardiography (19, 21).
Pathogenesis and pathophysiology
Patients over the age of 65 years with immunosuppression or underlying malignant disease are disproportionately affected. In some patients the inflammatory response is small or nonexistent (19, 22, 23). In Germany about 280 000 patients annually are affected by sepsis; the incidence is rising every year by about 5.7%, and between 2007 and 2013 the mortality fell from 27.0% to 24.3% (20). About 35% of these patients suffer from septic shock, representing a total of about 100 000 patients per year (table 1).
Septic shock.
Sepsis is defined according to the current Sepsis-3 criteria as a dysregulated response by the body to an infection resulting in life-threatening organ dysfunctions.
The core of the pathophysiology is the endothelial dysfunction, which leads to dysregulation of vascular tone resulting in vasodilation, impaired distribution, and volume shifting in the macro- and microcirculation, and to a rise in vascular permeability (capillary leak syndrome) (22– 25). Frequently, biventricular impaired myocardial function is also present in the form of septic cardiomyopathy (26), which contributes to patient mortality (26, 27). Septic shock is a mixed form of a variety of pathologies (hypovolemia, vasodilation, impaired cardiac function, and mitochondrial dysfunction) and is usually associated with complex coagulopathies (22– 25).
Treatment
Prevalence.
In Germany about 280 000 patients are affected by sepsis every year; the incidence is rising every year by about 5.7%, and between 2007 and 2013 the mortality fell from 27.0% to 24.3%. About 35% of these patients suffer from septic shock.
Apart from an increased level of alertness and rapid diagnosis, septic shock requires treatment to support the circulation by the infusion of balanced crystalloid solutions (recommendation grade: A), administration of vasopressors (norepinephrine, vasopressin if needed), in some cases also inotropic drugs (e.g., dobutamine), and organ replacement therapy (recommendation grade: B) (table 2). Advanced invasive monitoring is indicated to allow tailored therapy for the impaired hemodynamics. Echocardiography has a central part to play here (22, 24, 28). In all sepsis patients, as soon as samples have been obtained for microbiological study, calculated broad-spectrum antibiotic therapy and (if possible) source control (causal treatment) should be started as soon as possible (recommendation grade: A) (29). Noninfectious disease involving extensive mediator activation (e.g., acute pancreatitis) may lead to a clinical presentation similar to that of septic shock. This is due to activation of the same mediator cascade by noninfectious molecular signals of soft tissue damage (22).
The pathophysiology and pathogenesis of toxic shock syndrome (TSS) are related to those of septic shock. TSS is characterized by fever, severe hypotension, and skin rash as the main symptoms. It is usually triggered by toxins from certain staphylococci. The incidence is 0.5 / 100 000, and mortality is between 2% and 11%. Treatment is the same as that recommended for septic shock.
Anaphylactic and anaphylactoid shock
Anaphylactic shock is characterized by massive histamine-mediated vasodilation and maldistribution with a shift of fluid from the intravascular to the extravascular space.
Anaphylactic and anaphylactoid shock.
Anaphylactic shock is characterized by massive histamine-mediated vasodilation and maldistribution with a shift of fluid from the intravascular to the extravascular space.
Pathogenesis and pathophysiology
Clinical presentation of anaphylactic shock.
The clinical presentation varies greatly from one individual to another according to the dose and site of entry of the antigen and the degree of sensitization. Initially, skin manifestations, abdominal symptoms, or respiratory symptoms may be prominent.
Anaphylaxis is an acute systemic reaction usually mediated by IgE-dependent hypersensitivity reactions. The central role is played by mast cells and the histamine they release. In Germany, the incidence of anaphylactic reactions is 50 per 100 000 / year; they are the reason for about 1% of emergency admissions. Lifetime prevalence is reported at 0.5% to 2% and mortality at 2% to 20%. On a conservative assumption that 10% of these patients suffer shock, this results in a total of 8000 shock patients a year. The most frequent trigger in children is food products (58%), whereas in adults it is insect venom (55%, of which 70% are wasp stings and 20% bee stings), followed by drugs (21%, two-thirds of these being diclofenac, acetylsalicylic acid, and antibiotics, and 1% being ACE inhibitors or beta-blockers). Intensifying factors include physical effort, stress, and acute infection.
Anaphylactoid shock is caused by physical, chemical, or osmotic hypersensitivity reactions that are IgE-independent. Mediators are released from mast cells and basophilic granulocytes independently of any antigen–antibody reaction or presensitization. Typical triggers are X-ray contrast media.
The clinical presentation varies greatly from one individual to another according to the dose and site of entry of the antigen and the degree of sensitization. Initially, skin manifestations, abdominal symptoms, or respiratory symptoms may be prominent. Anaphylactic reactions may resolve spontaneously or may progress despite appropriate therapy. In anaphylaxis with fatal outcome, thromboembolic events are seen as often as arrhythmias and ventricular dysfunction (30).
Treatment
Patients with severe anaphylactic reactions require constant monitoring, as late reactions including arrhythmias, myocardial ischemia, and respiratory failure may manifest as late as 12 hours after the initial event. In terms of drug treatment, for anaphylactic shock especially the administration of epinephrine (plus norepinephrine, if necessary) and forced fluid replacement are required (31). In patients with bronchospasm, ß-sympathomimetics and, as second-line treatment, glucocorticoids are indicated (as they are in patients with delayed progressive symptoms) (31). Histamine antagonists suppress the histaminergic effects (table 2). Treatment for anaphylactoid shock is the same as for anaphylactic shock.
Neurogenic shock
Neurogenic shock is a state of imbalance between sympathetic and parasympathetic regulation of cardiac action and vascular smooth muscle. The dominant signs are profound vasodilation with relative hypovolemia while blood volume remains unchanged, at least initially.
Pathogenesis and pathophysiology
Neurogenic shock.
Neurogenic shock is a state of imbalance between sympathetic and parasympathetic regulation of cardiac action and vascular smooth muscle. The dominant signs are profound vasodilation with relative hypovolemia while blood volume remains unchanged, at least initially.
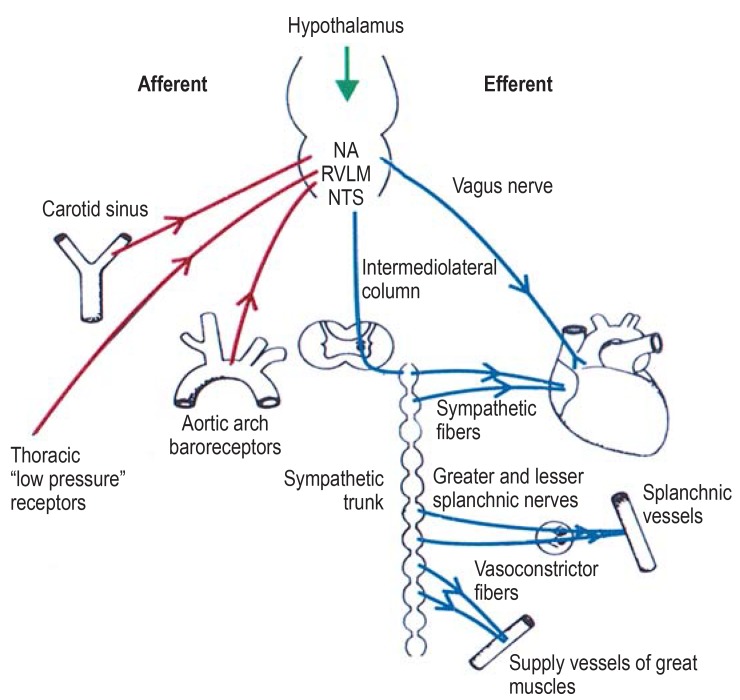
The pathomechanisms of neurogenic shock can be divided into three groups (efigure):
eFigure.
Pathomechanism of neurogenic shock: Connections in the autonomic system for heart rate and blood pressure regulation. NA, nucleus ambiguus; RVLM, rostral ventrolateral nucleus in the medulla; NTS, nucleus tractus solitarii
Direct injury to the centers for circulatory regulation due to compression (brainstem trauma), ischemia (e.g., basilar artery thrombosis), or the influence of drugs
Altered afferents to the circulatory center in the medulla oblongata due to fear, stress, or pain or dysregulated vagal reflexes
Interruption of the descending connection from the bulbar regulatory centers to the spinal cord, especially in patients who have sustained trauma above the middle of the thoracic spine (paraplegia).
At 15% to 20%, spinal cord injuries are the most common cause of neurogenic shock (32), followed by surgical intervention in the lumbar region (33). Neurogenic shock can occur due to cerebral ischemia, subarachnoid hemorrhage, meningitis, or, more rarely, during or after epileptic seizures, rapid onset of Guillain–Barré syndrome, pandysautonomia, or cerebral herniation. Occasionally, neurogenic shock can be triggered by stress or severe pain, or even after a karate kick.
Neurogenic shock is characterized by the sudden drop of SAP to <100 mmHg and heart rate to <60/min with obtunded consciousness (rapid onset in bulbar injury) and, in patients with high spinal cord injury, loss of spinal reflexes (34). The capacity of the splanchnic venous system and skeletal musculature rises while systemic venous pressure drops markedly. Mortality is around 20%.
Treatment
The critical element in treating neurogenic shock is the treatment of the cause. In addition to rapid fluid replacement, norepinephrine is given at increasing dosages until peripheral vascular resistance rises (table 1). To restore vascular tone, direct- or indirect-acting sympathomimetics can also be given (35). Mineralocorticoids to increase plasma volume are also a therapeutic option.
Cardiogenic shock
Cardiogenic shock.
Cardiogenic shock is primarily a disorder of cardiac function in the form of a critical reduction of the heart’s pumping capacity, caused by systolic or diastolic dysfunction leading to a reduced ejection fraction or impaired ventricular filling.
Cardiogenic shock is primarily a disorder of cardiac function in the form of a critical reduction of the heart’s pumping capacity, caused by systolic or diastolic dysfunction leading to a reduced ejection fraction or impaired ventricular filling. It is defined by SAP <90 mmHg or mean arterial blood pressure of 30 mmHg below the baseline value and cardiac index (CI) <1.8 L/min/m2 without pharmacologic or mechanical support or <2.0 L/min/m2 with support (36). According to the German–Austrian S3 guideline, cardiac index determination is not required for a clinical diagnosis of cardiogenic shock (37). In addition to these hemodynamic and clinical criteria, evidence of cardiac dysfunction is required, together with the exclusion of other types of shock (differential diagnosis).
Pathogenesis and pathophysiology
The cardiac dysfunction may be due to myocardial, rhythmologic, or mechanical causes (figure 1). With the myogenic form, reduction of pump function due to acute coronary syndrome (ACS) is the preeminent cause. Other causes include various cardiomyopathies, myocarditis, pharmacotoxicity, and blunt trauma to the heart. Mechanical causes include advanced acute and chronic valvular disease and mechanical complications after myocardial infarction or caused by intracavitary structures impeding flow (thrombi or tumors). Tachycardia and bradycardia may also result in the clinical picture of cardiogenic shock. Based on an average of 280 000 myocardial infarctions in Germany and an 8% incidence of cardiogenic shock among these cases, it can be estimated that 23 000 patients suffer cardiogenic shock every year (table 1). The main symptoms of cardiogenic shock are agitation, disturbed consciousness, cool extremities, and oliguria. Death in patients in cardiogenic shock is usually caused by hemodynamic instability, multiorgan failure, and systemic inflammation.
To maintain adequate cardiac output and hence sufficient organ perfusion, systemic counter-regulation mechanisms such as the sympathetic nervous system and neurohumoral, renal, and local vasoregulation are activated.
Treatment
Main symptoms of cardiogenic shock.
The main symptoms of cardiogenic shock are agitation, disturbed consciousness, cool extremities, and oliguria. Death in patients in cardiogenic shock is usually caused by hemodynamic instability, multiorgan failure, and systemic inflammation.
Echocardiography and invasive monitoring are the pillars of diagnosis. The primary goal of treatment is removing the cardiac causes of the shock. This includes the earliest possible coronary reperfusion in ACS by means of percutaneous coronary intervention (PCI) with the insertion of stents (bare metal stent, BMS; drug-eluting stent, DES) (recommendation grade: A), surgical or other interventional treatment of mechanical causes and structural heart disease, and surgical or interventional ablation, and pacemaker therapy (36, 38). In addition to this, symptomatic treatment is undertaken with the aim of improving end organ perfusion, microcirculation, and cellular oxygen utilization. This includes not just catecholamines such as dobutamine (recommendation grade: B), norepinephrine (recommendation grade: B), and epinephrine (recommendation grade: 0), vasodilators (recommendation grade: 0), calcium sensitizers (recommendation grade: 0), PDE3 inhibitors (recommendation grade: 0), antiarrhythmic drugs, and more (table 2), but also mechanical circulatory support such as intra-aortic balloon counterpulsation (recommendation grade: B), surgical and percutaneous interventional implantable ventricular support systems, and extracorporeal membrane oxygenation (ECMO) (37, 38).
Obstructive shock
Obstructive shock is a condition caused by the obstruction of the great vessels or the heart itself. Although the symptoms resemble those of cardiogenic shock, obstructive shock needs to be clearly distinguished from the latter because it is treated quite differently (39).
Pathogenesis and pathophysiology
Disorders involving impaired diastolic filling and reduced cardiac preload include vena cava compression syndrome, tension pneumothorax, pericardial tamponade, and high-PEEP ventilation. A pulmonary artery embolism or mediastinal space-occupying mass increases right-ventricular afterload, while at the same time left ventricular preload is reduced by obstructions in the pulmonary flow. The same mechanisms occur with an intracardial mass. Obstruction of the aortic flow can be distinguished from this, as it leads to a rise in left ventricular afterload (e.g., Leriche syndrome [aortoiliac occlusive disease], aortic dissection, and high-grade aortic valve stenosis). After trauma, especially, combined shock forms are seen, e.g., with tension pneumothorax and hemorrhage. No figures exist for the incidence of obstructive shock, but it is likely to be the rarest form of shock.
The pathophysiology of obstructive shock can be classified according to the location of the obstruction in the vascular system in relation to the heart (figure 1). Mechanical intra- or extravascular or luminal factors reduce blood flow in the great vessels or cardiac outflow with a critical drop in cardiac output and global oxygen supply. The result is a state of shock with tissue hypoxia in all organ systems. Common to all these obstructive states is the often rapid, massive drop in cardiac output and blood pressure.
Obstructive shock.
Obstructive shock is a condition caused by the obstruction of the great vessels or the heart itself. Although the symptoms resemble those of cardiogenic shock, obstructive shock needs to be clearly distinguished from the latter because it is treated quite differently.
The symptoms of obstructive shock are nonspecific and the condition is characterized by the compensatory autonomic response in the form of tachycardia, tachypnea, oliguria, and altered consciousness. Hypotension may be quite modest initially and this can lead to underestimation of the clinical situation (39). For the differential diagnosis, careful clinical examination is essential (auscultation, percussion, ultrasonography including echocardiography), but it must be accurate and prompt, because of the speed with which the state of shock progresses. Obstruction of intrathoracic blood flow can lead to cervical venous congestion or to atypical peripheral pulses. Tension pneumothorax may be associated with subcutaneous emphysema and deviation of the trachea visible in the neck, while aortic dissection or Leriche syndrome may cause pain in the chest or abdomen. The “4 H’s and 4 T’s” rule of reversible causes of cardiocirculatory arrest (40) involve three obstructive causes: pericardial tamponade, tension pneumothorax, and thromboembolism.
Treatment
Obstructive shock needs immediate causal treatment. Simple measures may suffice, such as changing the position of a patient with caval compression syndrome or adjusting the ventilation of the patient where the level of PEEP is too high. According to the underlying cause of the obstruction, a pulmonary embolism is treated with thrombolysis; tension pneumothorax or pericardial tamponade are relieved immediately by thoracic or pericardial drainage (recommendation grade: A); and Leriche syndrome is treated by surgical embolectomy.
Pathophysiology of obstructive shock.
The pathophysiology of obstructive shock can be classified according to the location of the obstruction in the vascular system in relation to the heart.
Treatment of obstructive shock.
Obstructive shock needs immediate causal treatment. Simple measures may suffice, such as changing the position of a patient with caval compression syndrome or adjusting the ventilation of the patient when the level of PEEP is too high.
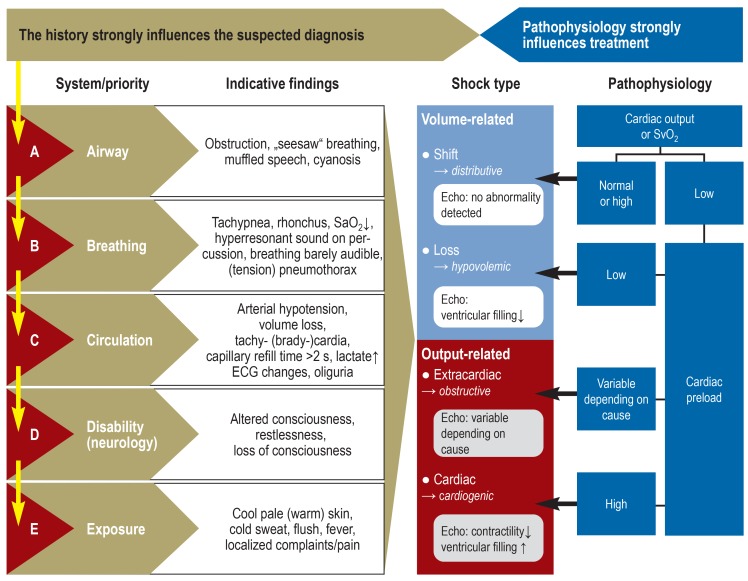
Figure 2.
Algorithm for differential diagnosis
as the basis for treatment of the different types of shock
SvO2, central venous oxygen blood saturation
Further information on CME.
-
Participation in the CME certification program is possible only over the Internet: cme.aerzteblatt.de. This unit can be accessed until 3 February 2019. Submissions by letter, e-mail or fax cannot be considered.
-
The following CME units can still be accessed for credit:
”The neurophysiology and treatment of motion sickness” (issue 41/2018) until 6 January 2019
”The diagnosis and treatment of anxiety disorders” (issue 37/2018) until 9 December 2018
”Arterial Hypertension” (issue 33–34) until 11 November 2018
-
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participants in the CME program can manage their CME points with their “uniform CME number” (einheitliche Fortbildungsnummer, EFN). The EFN must be stated during registration on www.aerzteblatt.de (“Mein DÄ”) or else entered in “Meine Daten,” and the participant must agree to communication of the results. The 15-digit EFN is found on the CME card (8027XXXXXXXXXXX).
CME credit for this unit can be obtained via cme.aerzteblatt.de until 3 February 2019.
Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What is the cause of hypovolemic shock?
Increased vasoregulation with volume shift
Inadequate organ perfusion caused by loss of intravascular volume, usually acute
Cardiac output and myocardial pump failure
Right heart–related circulatory failure due to obstruction
Decompensated valve stenosis
Question 2
What is a typical feature of hemorrhagic shock?
Acute hemorrhage
Pallor of the lower extremities
Raised body temperature
Microvascular dysfunction
Bradycardia
Question 3
Which of the following is often accompanied by traumatic hemorrhagic shock?
Persistent diarrhea
Acute cholera
Diabetic coma
Polytrauma sustained in a road traffic accident
Cirrhosis of the liver
Question 4
Which of the following is a typical cause of traumatic hypovolemic shock?
Gastrointestinal bleeding
Ruptured aneurysm
Hypothermia due to cold exposure
Myocardial infarction
Large surface burns
Question 5
Roughly how many people (including subgroups) develop hypovolemic shock every year in Germany?
5000
15 000
25 000
35 000
50 000
Question 6
In patients with large surface burns, which of the following can provide an indication of the fluid replacement needed in the first 24 hours?
Fick’s law of diffusion
Beer–Lambert law
Modified Brooke formula
HOMA Index
PROCAM Score
Question 7
What is the definition of sepsis according to the current Sepsis-3 criteria?
Dysregulated response by the body to an infection resulting in life-threatening organ dysfunctions
Inadequate organ perfusion caused by loss of intravascular volume
Primarily a disorder of cardiac function in the form of a critical reduction of the heart’s pumping capacity
Obstruction of the great vessels or the heart
State of imbalance between sympathetic and parasympathetic regulation
Question 8
Which of the following is a main symptom of toxic shock syndrome?
Hypertension
Tremor
Cardiac arrhythmias
Nonreactive pupils
Skin rash
Question 9
Which of the following patient groups has a disproportionately high incidence of septic shock?
Patients over the age of 65 who are immunosuppressed or have underlying malignant disease
Children up to the age of 10 with neuroblastoma
Adolescents up to the age of 20 who are dialysis-dependent
Pregnant women with HELPP syndrome
Men up to the age of 60 undergoing radiation therapy for prostate cancer
Question 10
What is the most common trigger of anaphylactic shock in adults?
Food products
Medical drugs
Insect venom
Physical effort
Acute infection
?Participation is possible only via the Internet: cme.aerzteblatt.de
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
Footnotes
Conflict of interest statement
Professor Annecke has received third-party funding or equipment for research projects or for carrying out clinical studies from CytoSorbents, Pulsion/Maquet, Corpuls, Köhler Chemie, Aerogen, and Medtronic.
Professor Standl has received lecture fees and reimbursement of conference fees and travel expenses from B. Braun, MSD, Pajunk, Grünenthal, and Fresenius.
The other authors declare that no conflict of interest exists.
References
- 1.Adams HA, Baumann G, Gänsslen A, et al. Die Definitionen der Schockformen. Intensivmed. 2001;38:541–553. doi: 10.1055/s-2001-18174. [DOI] [PubMed] [Google Scholar]
- 2.Adams HA, Baumann G, Cascorbi I, et al. Monographie Deutscher Ärzteverlag. Köln: 2010. Interdisziplinäre Behandlungspfade: Hypovolämischer Schock Eine Empfehlung der IAG Schock der DIVI. [Google Scholar]
- 3.Gänsslen A, Adams HA, Baumann G, et al. Hämostase im Schock Teil 4: Spezielle pathophysiologische Aspekte. Anästh Intensivmed. 2016;57:58–67. [Google Scholar]
- 4.Mutschler M, Nienaber U, Brockamp T, et al. Renaissance of base deficit for the initial assessment of trauma patients: a base deficit based classification for hypovolemic shock developed on data from 16,305 patients derived from the Trauma Register DGU. Crit Care. 2013;17 doi: 10.1186/cc12555. R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deitch E, Condon M, Feketeova E, et al. Trauma-hemorrhagic shock induces a CD36-dependent RBC endothelial-adhesive phenotype. Crit Care Med. 2014;42:e200–e210. doi: 10.1097/CCM.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 6.Slaughter A, Nunns G, Alessandro A, et al. The metabolopathy of tissue injury, hemorrhagic shock and resuscitation in a rat model. Shock. 2018;49:580–590. doi: 10.1097/SHK.0000000000000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karamercan M, Weiss S, Villarroel J, et al. Can peripheral blood mononuclear cells be used as a proxy for mitochondrial dysfunction in vital organs during hemorrhagic shock and resuscitation? Shock. 2013;40:476–484. doi: 10.1097/SHK.0000000000000026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song R, Bian H, Wang X, Huang X, Zhao K. Mitochondrial injury underlies hyporeactivity of arterial smooth muscle in severe shock. Am J Hypertension. 2011;24:45–51. doi: 10.1038/ajh.2010.184. [DOI] [PubMed] [Google Scholar]
- 9.Sherren PB, Hussey J, Martin R, Kundishora T, Parker M, Emerson B. Acute burn induced coagulopathy. Burns. 2013;39:1157–1161. doi: 10.1016/j.burns.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Mitra B, Wasiak J, Cameron PA, O’Reilly G, Dobson H, Cleland H. Early coagulopathy of major burns. Injury. 2013;44:40–43. doi: 10.1016/j.injury.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Lawton LD, Roncal S, Leonard E, et al. The utility of advanced trauma life support (ATLS) clinical shock grading in assessment of trauma. Emerg Med J. 2014;31:384–389. doi: 10.1136/emermed-2012-201813. [DOI] [PubMed] [Google Scholar]
- 12.Khan S, Davenport R, Raza I, et al. Damage control resuscitation using blood component therapy in standard doses has a limited effect on coagulopathy during trauma hemorrhage. Intensive Care Med. 2015;41:239–247. doi: 10.1007/s00134-014-3584-1. [DOI] [PubMed] [Google Scholar]
- 13.S3-Leitlinie Polytrauma/Schwerverletzten-Behandlung. AWMF Register-Nr. 012/019. Stand 7/2016 [Google Scholar]
- 14.Shakur H, Roberts I, Bautista R, Caballero J, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 15.Roberts I, Shakur H, Afolabi A, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377:1096–1101. doi: 10.1016/S0140-6736(11)60278-X. [DOI] [PubMed] [Google Scholar]
- 16.Shakur H, Roberts I, Fawole B, et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post- partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105–2116. doi: 10.1016/S0140-6736(17)30638-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossaint R, Bouillon B, Vladimir Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fourth edition. Critical Care. 2016;20 doi: 10.1186/s13054-016-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aoki K, Yoshino A, Yoh K, et al. A comparison of Ringer’s lactate and acetate solutions and resuscitative effects on splanchnic dysoxia in patients with extensive burns. Burns. 2010;36:1080–1085. doi: 10.1016/j.burns.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Singer M, Deutschmann CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleischmann C, Thomas-Rueddel DO, Hartmann M, et al. Hospital incidence and mortality rates of sepsis. Dtsch Arztebl Int. 2016;113:159–166. doi: 10.3238/arztebl.2016.0159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock. JAMA. 2016;315:775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gotts EJ, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353 doi: 10.1136/bmj.i1585. i1585. [DOI] [PubMed] [Google Scholar]
- 23.Delano MJ, Ward PA. The immune system`s role in sepsis progression, resolution, and long term outcome. Immunological Reviews. 2016;274:330–353. doi: 10.1111/imr.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 25.Vincent JL, De Backer D. Circulatory Shock. N Engl J Med. 2013;369:1726–1734. doi: 10.1056/NEJMra1208943. [DOI] [PubMed] [Google Scholar]
- 26.Antonucci A, Fiaccadori E, Donadello K, et al. Myocardial depression in sepsis: From pathogenesis to clinical manifestations and treatment. J Crit Care. 2014;29:500–511. doi: 10.1016/j.jcrc.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Werdan K, Oelke A, Hettwer S, et al. Septic cardiomyopathy: hemodynamic quantification, occurence, and prognostic implications. Clin Res Cardiol. 2013;100:661–668. doi: 10.1007/s00392-011-0292-5. [DOI] [PubMed] [Google Scholar]
- 28.Cecconi M, De Backer D, Antonelli M, et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795–1815. doi: 10.1007/s00134-014-3525-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campain: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43:302–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 30.Triggiani M, Montagni M, Parente R, Ridolo E. Anaphylaxis and cardiovascular diseases: a dangerous liaison. Curr Opin Allergy Clin Immunol. 20141;4:309–315. doi: 10.1097/ACI.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 31.Ring J, Beyer K, Biedermann T, et al. Guideline for acute therapy und management of anaphylaxis S2 guideline. Allergo J Int. 2014;23:96–112. doi: 10.1007/s40629-014-0009-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pastrana EA, Saavedra FM, Murray G, et al. Acute adrenal insufficiency in cervical spinal cord injury. World Neurosurg. 2012;77:561–563. doi: 10.1016/j.wneu.2011.06.041. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto T, Okuda S, Haku T, et al. Neurogenic shock immediately following posterior lumbar interbody fusion. Global Spine J. 2015;5:e13–e16. doi: 10.1055/s-0034-1395422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Summers RL, Baker SD, Sterling SA, et al. Characterization of the spectrum of hemodynamic profiles in trauma patients with neurogenic shock. J Critical Care. 2013;28:531.e1–531e5. doi: 10.1016/j.jcrc.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood GC, Boucher AB, Johnson JL, et al. Effectiveness of pseudoephedrine as adjunctive therapy for neurogenic shock after acute spinal cord injury: a case series. Pharmacotherapy. 2014;34:89–93. doi: 10.1002/phar.1335. [DOI] [PubMed] [Google Scholar]
- 36.Furer A, Wessler J, Burkhoff D. Hemodynamics of cardiogenic shock. Interv Cardiol Clin. 2017;6:359–371. doi: 10.1016/j.iccl.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Werdan K, Russ M, Buerke M, et al. Deutsch-österreichische S3-Leitlinie Infarktbedingter kardiogener Schock - Diagnose, Monitoring und Therapie. Kardiologe. 2011;5:166–224. [Google Scholar]
- 38.Nuding S, Werdan K, Prondzinsky R. Optimal course of treatment in acute cardiogenic shock complicating myocardial infarction. Expert Rev Cardiovasc Ther. 2018;16:99–112. doi: 10.1080/14779072.2018.1425141. [DOI] [PubMed] [Google Scholar]
- 39.Pich H, Heller AR. Obstruktiver Schock. Anaesthesist. 2015;64:403–419. doi: 10.1007/s00101-015-0031-9. [DOI] [PubMed] [Google Scholar]
- 40.Soar J, Nolan JP, Bottiger BW, et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 3 Adult advanced life support. Resuscitation. 2015;95:100–147. doi: 10.1016/j.resuscitation.2015.07.016. [DOI] [PubMed] [Google Scholar]