Abstract
A new selective and sensitive high-performance liquid chromatography (HPLC) method was developed for the quantification of diclofenac sodium (DS) in pharmaceutical dosage form using lidocaine as internal standard (IS). Chromatographic separation was achieved on a symmetry C18 column (4.6 mm × 150 mm, 3 μm spherical particles) using 0.05 M orthophosporic (pH 2.0) 35% and acetonitrile as 65%, as the mobile phase at a flow rate of 2.0 mL/min and monitored at 210 nm. The run time was 2 min.
The method was validated to fulfill International Conference on Harmonisation (ICH) requirements and this validation included specificity, linearity, limit of detection (LOD), limit of quantification (LOQ), accuracy, precision and robustness. The calibration curve was linear over the concentration range from 10 to 200 µg/ml, and lower limit of detection of 12.5 ng/ml. The accuracy and precision of the method were within the acceptable limit of ±20% at the lower limit of quantitation and ±15% at other concentrations. Diclofenac was unstable at room temperature it showed more than 25% loss after 24 h. While, DS is very stable at refrigerator 4 °C auto-sampler, freeze/thaw cycles and 30 days storage in a freezer at −35 ± 2 °C.
All results were acceptable and this confirmed that the method is suitable for its intended use in routine quality control and assay of drugs.
Keywords: Diclofenac, Reverse phase high performance liquid chromatography, HPLC
1. Introduction
Diclofenac sodium [N-piperidino-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-3-pyrazole-carboxamide] is one of the analgesic-antipyretic-nonsteroidal anti-inflammatory drug. Diclofenac is a widely used for the treatment of rheumatoid arthritis, osteoarthritis and ankylosing spondylitis osteoarthritis, musculoskeletal injuries, and post surgery analgesia in human and veterinary medicine (Bhattacharya et al., 2013). The molecule is practically water insoluble, but it is readily absorbed from the gastrointestinal tract as the salt form.
Various analytical techniques have been reported for the quantification of diclofenac sodium (DS) in different matrices. High-pressure liquid chromatography detection (HPLC) is the most common used method for the determination of DS in biological sample or dosage forms (Bhattacharya et al., 2013, Klimeš et al., 2001, Yan et al., 2014, Vieira et al., 2016, Mahdi et al., 2016, Cordery et al., 2017, Khan et al., 2016, Pireddu et al., 2015, Aldwaikat and Alarjah, 2015, Hegazy et al., 2015, Kasperek, 2008, Basusarkar, 2011, Nivsarkar et al., 2015, Korodi et al., 2012, Chaudhary et al., 2011, Kubala et al., 1993, Mulgund et al., 2009, Gaudiano et al., 2003).
Analytical methods keep on updating with time as per the requirements so as to develop a simple, reliable, cost effective, reproducible and above all a method bearing a high level of accuracy and precision.
Our study aimed to develop a rapid, robust, selective, sensitive, and precise HPLC method for the determination of DS. The assay method was validated using by USP 26 (United States Pharmacopeial Convention, 2003)or by the ICH guidelines (CPMP/ICH/381/95, 1994). The linearity, accuracy, precision, specificity, limit of detection (LOD), and limit of quantification (LOQ) and used for in determination of drug content of the DS in different pharmaceutical commercial products.
2. Materials and methods
2.1. Materials
Diclofenac was a gift from Spimaco (Riyadh, Saudi Arabia). All other reagents and chemicals were of HPLC analytical grade, and were used as received. Water was deionized and purified using a Milli-Q Reagent Grade water system (Millipore Cor), poration, Bedford, MX 01730, USA).
To determine the content of Diclofenac Sodium in conventional tablet available in Saudi market, randomly select the following products (Brand name: voltaren®, Clofen®, voltaic®, Rapidus® and Rofenac® label claim: 50 mg Diclofenac per tablet).
2.2. Liquid chromatography conditions
The HPLC system consisted of Waters 1525 binary pump Separation module (Waters, USA) fitted with C18 column (300 mm × 4.6 mm). The autosampler injection system (Waters 2707) used was a 10 µl sample loop. A Millipore Swinnex type filter (pore size = 0.45 µm) was obtained from Millipore (Bangalore, India). A Waters HPLC system equipped with a Waters 484 variable UV absorbance detector and a Waters 2707 plus autosampler was used. Waters 515 solvent delivery system was used to operate the gradient flow through a symmetry C 18 column (4.6 × 75 mm, 3.5 μm spherical particles). with 0.05 M orthophosporic pH 2.0 and acetonitrile as 35 and 65%, respectively as a mobile phase at a flow rate of 2.0 mL/min and the run time was 2 min. Degassing was achieved via filtration through a 0.45 μm Millipore membrane filter and sonication for 10 min. The injection volume was 20 µl and detection was at 210 nm. The HPLC system was operated at 25 °C. Data were collected with a Breeze Chromatography Manager Data Collection System. A daily standard calibration curve (6 standards ranging from 10 to 200 µg/ml was prepared to determine the unknown DS concentration.
2.3. Preparation of stock solutions
The stock standard solution of DS was prepared in methanol at a concentration of 0.5 mg/mL and stored in 4.0 mL glass vials in a refrigerator at 4 °C. Different working standard solutions of DS(10–200 µg/ml) were prepared by diluting of the above mentioned stock solution in pure methanol and were stored at 4 °C.
2.4. Validation of diclofenac HPLC assay
The RP-HPLC method for DS assay was validated in term of accuracy, reproducibility, linearity, specificity, LOD, LOQ, and robustness according to ICH Harmonized Tripartite Guidelines. Three standard calibration curves were prepared at different times (at least three months) to evaluate the linearity, precision, accuracy and stability.
2.4.1. Specificity
The specificity of the HPLC method was evaluated to ensure that there was no interference from the excipients present in the formulations. The specificity was studied by injecting the excipients.
2.4.2. System specificity
The system suitability was assessed by six replicate analyses of DS at a concentration of 20 μg/ml. The acceptance criterion was ±2% for the percent relative standard deviation (% RSD) for the peak area and retention times for DS.
2.4.3. Linearity and range
Linearity is the ability to obtain test results that are directly proportional to the concentration of the analyte. Linearity was determined by three injections of seven different DS concentrations (10, 20, 80, 120, 160 and 200 µg/ml). The average peak areas were plotted against concentrations. Then linearity was evaluated using the calibration curve to calculate coefficient of correlation, slope and intercept. In general, a value of correlation coefficient (r2) > 0.998 is considered as the evidence of an acceptable fit for the data to the regression line
2.4.4. Accuracy
The accuracy of an analytical method expresses the nearness between the expected value and the value found. It is obtained by calculating the percent recovery (R%) of the analyte recovered. In this case, to evaluate the accuracy of the developed method, successive analysis (n = 3) for three different concentrations (200 ng/ml, 120 ng/ml and 20 µg/ml) of standard DS solution were performed using the developed method. The data of the experiment were statistically analyzed using the formula [% Recovery = (Recovered conc. /Injected conc.) × 100] to study the recovery and validity of the developed method. The mean recovery should be within 90–110% to be accepted.
2.4.5. Precision
Precision of a measurable technique is the degree of agreement among individual tests, when the technique is applied repetitively to analyze multiple replicates in three different occasions. The intraday precision was assessed by analyzing the calibration curves of six replicates of different concentrations of DS within the same day. The inter-day precision was determined by analyzing of six replicates of different concentrations of DS on three different days. The total precision of the method was expressed as the relative standard deviation (%RSD). In the current method development and validation protocol, precision was determined by six replicate analyses at a concentration of 120 µg/mL of standard DS solution using the developed method and % RSD ≤ 2% was accepted.
2.4.6. Limit of detection and limit of quantification
LOD is the lowest concentration in a sample that can be detected, but not necessarily quantified under the stated experimental conditions. LOQ is the lowest concentration of analyte that can be determined with acceptable precision and accuracy. These two parameters were calculated using the formula LOD = 3.3 × SD/S and LOQ = 10 × SD/S, where SD = standard deviation of response (peak area) and S = slope of the calibration curve.
2.4.7. Robustness
The robustness of an analytical procedure is the measure of its capacity to remain unaffected by small, but deliberate variations in method parameters and provides an indication of its reliability during normal usage. The robustness was studied by evaluating the effect of small but deliberate variations in the chromatographic conditions.
2.4.8. Analysis of a marketed formulation
To determine the content of Diclofenac Sodium in conventional tablet (Brand name: voltaren®, Clofen®, voltaic®, Rapidus® and Rofenac® label claim: 50 mg Diclofenac per tablet), twenty tablets were weighed, their mean weight determined and finely powdered. The weight of the tablet triturate equivalent to 50 mg of Diclofenac Sodium was transferred into a 50 mL volumetric flask containing 30 mL methanol, sonicated for 30 min and diluted up to 50 mL with methanol. The resulting solution was centrifuged at 3000 rpm for 5 min and the drug content of the supernatant was determined (1000 μg/mL). Supernatant was taken and after suitable dilution the sample solution was then filtered using 0.45-µm filter (Millipore, Milford, MA). The above stock solution was further diluted to get sample solution of 50 μg/mL. A 20 μl volume of sample solution was injected into HPLC, six times, under the conditions described above. The peak areas were measured at 210 nm and concentrations in the samples were determined using multilevel calibration developed on the same HPLC system under the same conditions using linear regression equation.
2.5. Data and statistical analysis
In vitro results were expressed as mean ± SD of at least three replicates. The HPLC results of DS were calculated using linear regression without weighting, according to the equation: Y = 0.0225x + 0.8858, where Y is the area under the peak (AUP) ratio of the drug and X is the concentration of DICNA. The % RSD was calculated for all values. Student’s t-test was used to inspect the concentration difference at each day and one-way analysis of variance (ANOVA) was used to assess the reproducibility of the assay using IBMSPSS Statistics 21. The level of confidence was 95%.
3. Results
3.1. Development of HPLC method
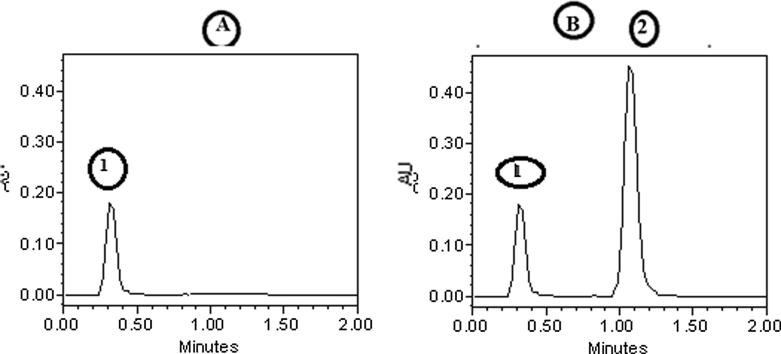
In Fig. 1, chromatogram A represents the blank mobile phase, and chromatogram B represents DS with an average retention time of 1.345 ± 0.127 min and with no interfering peaks. This is an indication of the specificity of the developed HPLC method. The retention time was comparable with the shorter published data for DS.
Fig. 1.
HPLC chromatograms of mobile phase spike with 50 µg/ml of lidocaine (1) (chromatogram A) and HPLC chromatograms of mobile phase containing 10 µg/ml DS(2) and 50 µg/ml of lidocaine (1) (chromatogram B).
3.1.1. System suitability
The system suitability was assessed by six replicate analyses of DS at a concentration of 20 µg/ml. The acceptance criterion was ±2% for the percent relative standard deviation (% RSD) for the peak area and retention times for DS (Table 1).
Table 1.
System suitability study.
| Concentration 20 μg/ml | Retention time (minutes) |
Peak area |
||
|---|---|---|---|---|
| Drug | IS | Drug | IS | |
| Mean (n = 6) | 1.345 | 0.32 | 1,772,863 | 1,212,927 |
| SD | 0.127 | 0.0023 | 49339.47 | 28878.47 |
| RSD‘ | 3.39 | 0.69 | 2.78 | 2.38 |
3.2. Specificity
The specificity of the method was monitored by analyzing the placebo and standard solution. No peak was detected close to the retention time of DS, which proved the high degree of specificity of the method (Fig. 1).
3.3. Linearity, limit of quantification, limit of detection
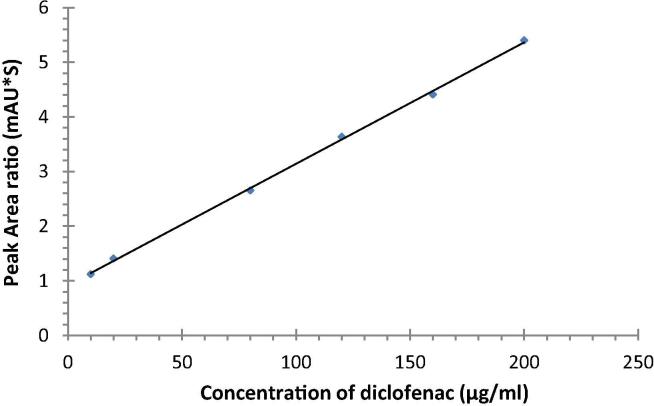
Linear relationship (r > 0.999) was observed between AUP of DS and the corresponding concentrations over 10–200 µg/mL (Fig. 2). The mean linear regression equation of the peak area ratios (Y) versus drug concentrations (X) of DS was typically of the form Y = (b ± S.D.) X ± (a ± S.D.) and it was Y = 0.0222 X − 0.9196 for DICNA. The LOQ of this assay was 3.9 ng/mL with a corresponding relative standard deviation of 4.8 and 4.0%. The LOD was 13.1 ng/mL at a signal-to-noise ratio of >3.
Fig. 2.
Standered calibration curve of Diclofenac ranging from 10 to 200 µg/ml in mobile phse. Each point represent the mean of 6 determination.
3.4. Precision and accuracy
The accuracy of the method was determined by recovery experiments. The recovery studies were carried out 6 times and the percentage recovery and % relative standard deviation was calculated. From the data obtained, recoveries of standard drugs were found to be accurate (Table 2). The %CV of interday and intraday precision obtained was less than 7% for the drugs as recommended by ICH guidelines
Table 2.
Inter- and Intra-day statistics.
| DICNA | Measured concentration (µg/mL) |
|||
|---|---|---|---|---|
| Nominal concentration (µg/mL) | ||||
| Day-1 | 20 | 120 | 200 | |
| 21 | 112 | 191 | ||
| 19 | 126 | 211 | ||
| 20 | 119 | 214 | ||
| 22 | 127 | 193 | ||
| 19 | 116 | 211 | ||
| Day-2 | 22 | 125 | 205 | |
| 23 | 125 | 210 | ||
| 21 | 128 | 191 | ||
| 20 | 118 | 204 | ||
| 20 | 114 | 194 | ||
| 18 | 126 | 194 | ||
| Day-3 | 22 | 117 | 207 | |
| 19 | 122 | 201 | ||
| 21 | 117 | 196 | ||
| 23 | 125 | 192 | ||
| 22 | 125 | 191 | ||
| 21 | 128 | 192 | ||
| n | 18 | 18 | 18 | |
| Inter-day statistics | Mean | 20.72222222 | 121.667 | 199.833 |
| SD | 1.447332457 | 5.09902 | 8.17636 | |
| Accuracy (%RSD) | 6.984446174 | 4.19097 | 4.09159 | |
| n | 6 | 6 | 6 | |
| Intra-day (on day 3), n = 6 | Mean | 20.16666667 | 120 | 203.333 |
| SD | 1.169045194 | 5.76194 | 10.0133 | |
| Accuracy | 5.79691832 | 4.80162 | 4.92459 | |
| (%RSD) | ||||
3.5. Recovery, accuracy, and precision
Within-day precision and accuracy of the method were determined from replicate analysis (n = 6) of DStest standards at concentrations within the linear range of the assay for each drug (Table 2). The reproducibility of the assay was evaluated by comparing the linear regressions of three standard plots prepared on three different days over a 3-week period. The mean correlation coefficient was >0.999 with % R.S.D. of the slopes of the three lines beign 4.3%. ANOVA of the data indicated no significant difference (p > 0.05) in the slopes, intra- and inter-day, of the calibration curves. The results confirmed the reproducibility of the assay method. The mean percentage recovery of 10–200 µg/mL DS was 95.2 ± 4.9%.
3.6. LOD and LOQ
Signal-to-noise ratios of 3:1 and 10:1 were obtained for the LOD and LOQ respectively. The LOD and LOQ were found to be 2 μg/mL and 4 μg/mL for Diclofenac Sodium.
3.7. Robustness
The robustness of the method was studied by deliberate changes in the method like alteration in pH of the mobile phase, percentage organic content, changes in the wavelength. It was observed that there was no marked changes in the chromatograms demonstrate that the HPLC methods have developed are robust.
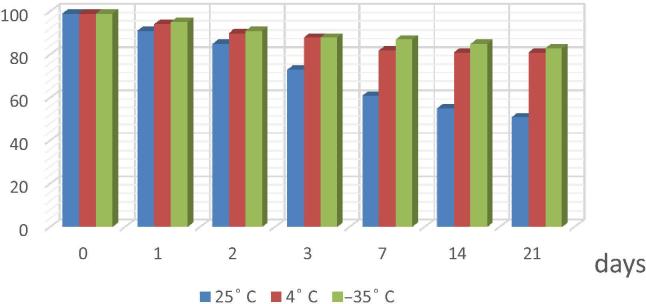
3.8. Stability studies
Fig. 3 shows that DS was stable in the processed samples held in the autosampler at 4° and −35 °C for three weeks. There were no evident changes observed in the elution profile and chromatographic reports. All the values of %CV were within the range provided in FDA guidelines (i.e., less than3%), indicating the fact that the developed method is stable (Fig. 1). However, the samples lost 26.3% (RSD of 6.7%) of its nominal concentration within 3 days if stored in the autosampler at room temperature. Therefore, it is not recommended to keep DS in the autosampler for longer than overnight to ensure reproducibility of the assay. The freeze–thaw temperature cycles did not significantly (p > 0.05) affect the stability of DS in first cycle, after 72 h, with the mean calculated values within 2.3% of the nominal concentration, while after the third cycle, 11.8% of DS was lost with an RSD of 5.6%. Unexpectedly, exposing DS to drastic conditions revealed that DS is stable in both 2 M HCl and 1 N NaOH solutions even after boiling, losing only 4.3% and 5.2% respectively (RSD of 5.3 and 6.6%) of its nominal value.
Fig. 3.
stability of diclofenac in different temperature for three weeks (n = 6)
3.9. Analysis of a diclofenac in the dosage form
Experimental results of the amount of Diclofenac Sodium in the selected commercial tablets, expressed as a percentage of label claim were in good agreement with the label claims thereby suggesting that there is no interference from any of the excipients which are normally present. The drug content was found to be 99.93% for Diclofenac Sodium five different lots of Diclofenac Sodium tablets were analyzed using the proposed procedures as shown in Table 3.
Table 3.
Analysis of commercial formulation Diclofenac Sodium (50 mg).
| Brand name (50 mg tablet | Mean ± SD (n = 6) | Recovery (%) |
|---|---|---|
| Voltaren® manufactured by Novartis kurtkoy in turkey for Novartis pharma AG, basle, Switzerland, lot number is K0392 | 49.92 ± 1.16 | 99.70 |
| Clofen® manufactured by Julphar Gulf pharmaceutical industries, Ras Al khaima U.A.E. Lot number :C402787A | 49.85 ± 1.06 | 98.5 |
| Voltaic® manufactured by Jamjoom Pharmaceuticals Co., Jeddah, Saudi Arabia. Lot number is B SF 0206 | 48.18 ± 2.13 | 98.3 |
| Rapidus® manufactured by Tabuk pharmaceutical manufacturing, Batch number (6 m × 391) | 48.75 ± 1.40 | 98.13 |
| Rofenac® Manufactured by Spimaco Al-Qassim Pharmaceutical Plant Saudi Arabia lot number 90217) | 49.82 ± 1.76 | 98.23 |
4. Discussion
Many studies reviewed the use of C18 for separation of the drug using acetonitrile as the main solvent. These HPLC methods reported in have several disadvantages, including unsatisfactory separation times, poor resolution, complicated solvent mixtures with gradient elution, and long analysis times. The aim of this study was to develop and validate a new simple and rapid analytical method for DS with short run time. The chromatographic runtime is also short. Therefore, the developed analytical method can be reliably employed as an assay method for pharmaceutical study of any dosage form containing DS.
5. Conclusions
A simple, rapid and sensitive analytical method was developed and validated for the analysis for DS. The chromatographic runtime was also short.
Statistical analysis proves that the method is suitable for the analysis of Diclofenac Sodium in pharmaceutical formulation without any interference from the excipients. It may be extended to study the degradation kinetics of Diclofenac Sodium and also for its estimation in plasma and other biological fluids.
Acknowledgments
Acknowledgement
The authors gratefully acknowledge Female Center for Scientific and Medical Colleges, Deanship of Scientific Research, King Saud University, Riyadh, Saudi Arabia for their support.
Conflict of interest
The authors have no conflict of interests to disclose other than what has been acknowledged above.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aldwaikat M., Alarjah M. Investigating the sonophoresis effect on the permeation of diclofenac sodium using 3D skin equivalent. Ultrason. Sonochem. 2015;22:580–587. doi: 10.1016/j.ultsonch.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Basusarkar A. Stability studies on diclofenac cream. Int. J. Adv. Pharm. Anal. 2011;1:1–3. [Google Scholar]
- Bhattacharya S.S., Banerjee S., Ghosh A.K., Chattopadhyay P., Verma A., Ghosh A. A RP-HPLC method for quantification of diclofenac sodium released from biological macromolecules. Int. J. Biol. Macromol. 2013;58:354–359. doi: 10.1016/j.ijbiomac.2013.03.065. [DOI] [PubMed] [Google Scholar]
- Chaudhary H., Kohli K., Amin S., Rathee P., Kumar V. Optimization and formulation design of gels of Diclofenac and Curcumin for transdermal drug delivery by Box-Behnken statistical design. J. Pharm. Sci. 2011;100:580–593. doi: 10.1002/jps.22292. [DOI] [PubMed] [Google Scholar]
- Cordery S., Pensado A., Chiu W., Shehab M., Bunge A., Delgado-Charro M., Guy R. Topical bioavailability of diclofenac from locally-acting, dermatological formulations. Int. J. Pharm. 2017;529:55–64. doi: 10.1016/j.ijpharm.2017.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CPMP/ICH/381/95 1994. Note for Guidance on Validation of Analytical Methods: Definitions and Terminology, Step 5.
- Gaudiano M., Valvo L., Bertocchi P., Manna L. RP-HPLC study of the degradation of diclofenac and piroxicam in the presence of hydroxyl radicals. J. Pharm. Biomed. Anal. 2003;32:151–158. doi: 10.1016/s0731-7085(03)00058-x. [DOI] [PubMed] [Google Scholar]
- Hegazy M.A., Abdelwahab N.S., Fayed A.S. A novel spectral resolution and simultaneous determination of multicomponent mixture of Vitamins B1, B6, B12, Benfotiamine and Diclofenac in tablets and capsules by derivative and MCR–ALS. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2015;140:524–533. doi: 10.1016/j.saa.2014.12.108. [DOI] [PubMed] [Google Scholar]
- Kasperek R. Determination of diclofenac sodium and papaverine hydrochloride in tablets by HPLC method. Acta Poloniae Pharm. 2008;65:403–408. [PubMed] [Google Scholar]
- Khan F., Ahmad I., Akhtar M., Rauf H.A., Altaf H., Hayat M.M. RP-HPLC method development and validation for simultaneous determination of esomeprazole and diclofenac sodium in pharmaceutical dosage forms. Pharm. Chem. J. 2016;49:788–794. [Google Scholar]
- Klimeš J., Sochor J., Doležal P., Körner J. HPLC evaluation of diclofenac in transdermal therapeutic preparations. Int. J. Pharm. 2001;217:153–160. doi: 10.1016/s0378-5173(01)00594-4. [DOI] [PubMed] [Google Scholar]
- Korodi T., Bukowski K., Lachmann B. Evaluation of a short stability-indicating HPLC method for diclofenac sodium gels. Die Pharmazie-Int. J. Pharm. Sci. 2012;67:980–983. [PubMed] [Google Scholar]
- Kubala T., Gambhir B., Borst S.I. A specific stability indicating HPLC method to determine diclofenac sodium in raw materials and pharmaceutical solid dosage forms. Drug Dev. Ind. Pharm. 1993;19:749–757. [Google Scholar]
- Mahdi M.H., Conway B.R., Mills T., Smith A.M. Gellan gum fluid gels for topical administration of diclofenac. Int. J. Pharm. 2016;515:535–542. doi: 10.1016/j.ijpharm.2016.10.048. [DOI] [PubMed] [Google Scholar]
- Mulgund S., Phoujdar M., Londhe S., Mallade P., Kulkarni T., Deshpande A., Jain K. Stability indicating HPLC method for simultaneous determination of mephenesin and diclofenac diethylamine. Ind. J. Pharm. Sci. 2009;71:35. doi: 10.4103/0250-474X.51950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nivsarkar M., Maroo S.H., Patel K.R., Patel D.D. Evaluation of skin penetration of diclofenac from a novel topical non aqueous solution: a comparative bioavailability study. J. Clin. Diagn. Res.: JCDR. 2015;9:FC11. doi: 10.7860/JCDR/2015/15690.6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pireddu R., Sinico C., Ennas G., Marongiu F., Muzzalupo R., Lai F., Fadda A.M. Novel nanosized formulations of two diclofenac acid polymorphs to improve topical bioavailability. Eur. J. Pharm. Sci. 2015;77:208–215. doi: 10.1016/j.ejps.2015.06.006. [DOI] [PubMed] [Google Scholar]
- United States Pharmacopeial Convention, i.e. (ed.) 2003. The United States Pharmacopeia, 26th Rev, and the National Formulary, Rockville.
- Vieira A.C., Serra A.C., Veiga F.J., Gonsalves A.M.D.A.R., Basit A.W., Murdan S. Diclofenac-β-cyclodextrin for colonic drug targeting: in vivo performance in rats. Int. J. Pharm. 2016;500:366–370. doi: 10.1016/j.ijpharm.2016.01.024. [DOI] [PubMed] [Google Scholar]
- Yan Y., Xing J., Xu W., Zhao G., Dong K., Zhang L., Wang K. Hydroxypropyl-β-cyclodextrin grafted polyethyleneimine used as transdermal penetration enhancer of diclofenac sodium. Int. J. Pharm. 2014;474:182–192. doi: 10.1016/j.ijpharm.2014.08.021. [DOI] [PubMed] [Google Scholar]