Abstract
Emerging antibiotic resistance necessitates the development of new therapeutic approaches. Many studies have reported the antimicrobial activity of diclofenac sodium (DIC) and chitosan nanoparticles (CNPs). Hence, this study aimed to prepare non-antibiotic DIC-loaded CNPs (DIC.CNPs) and characterize their in vitro antibacterial activity. DIC.CNPs were prepared from low and high molecular weight (LMW and HMW, respectively) chitosan using an ionic gelation method. Prepared NPs were characterized, and their antibacterial activity against gram-positive Staphylococcus aureus and Bacillus subtilis was evaluated using the agar diffusion and broth dilution methods. The particle size, polydispersity index (PDI), and encapsulation efficiency of the formulated DIC.CNPs increased with increasing MW of chitosan. The prepared NPs showed a narrow size distribution with low PDI values (0.18 and 0.24) and encapsulation efficiency (29.3% and 31.1%) for LMW.DIC.CNPs and HMW.DIC.CNPs, respectively. The in vitro release profile of DIC from the DIC.CNPs was biphasic with a burst release followed by slow release and was influenced by the MW of chitosan. DIC.CNPs exhibited significantly higher antibacterial activity against S. aureus (minimum inhibitory concentration [MIC90] LMW.DIC.CNPs = 35 µg/mL and MIC90 HMW.DIC.CNPs = 18 µg/mL) and B. subtilis (MIC90 LMW.DIC.CNPs = 17.5 µg/mL and MIC90 HMW.DIC.CNPs = 9 µg/mL) than DIC alone did (MIC90 DIC = 250 and 50 µg/mL against S. aureus and B. subtilis, respectively). The antibacterial activity was influenced by pH and the MW of chitosan. Collectively, these results may suggest the potential usefulness of DIC.CNPs as non-antibiotic antibacterial agent necessitating further future studies to asses the stability of DIC.CNPs prepared.
Abbreviations: DIC, diclofenac; DIC.CNPs, diclofenac-loaded chitosan nanoparticles; HMW, high-molecular weight; LMW, low-molecular weight
Keywords: Chitosan nanoparticles, Non-antibiotic, Antimicrobial, Low-molecular weight chitosan, High-molecular weight chitosan
1. Introduction
The emergence of antibiotic resistance is a major public health concern, which results from abuse and misuse of antibiotics, and limits their usefulness. This has prompted researchers to find alternative therapeutic approaches to overcome microbial antibiotic resistance. Among these approaches, drugs known as “non-antibiotics” have been used to manage microbial infections. These drugs are used for the management of non-infectious pathological conditions and have shown broad-spectrum antimicrobial activity both in vitro and in vivo against a variety of gram-positive and gram-negative bacteria (Kristiansen, 1992, Amaral et al., 2006). An example from this category is diclofenac sodium (DIC), which is a nonsteroidal anti-inflammatory drug that demonstrated antimicrobial activity in vitro and in vivo against both gram-positive and gram-negative bacteria (Annadurai et al., 1998, Salem-Milani et al., 2013, Padma and Yalavarthy, 2015).
Another approach to counteracting antimicrobial resistance is the use of nanotechnology for antimicrobial delivery. The development of nanoparticles (NPs) as liposomes, polymeric NPs, solid lipid NPs, and dendrimers loaded with antimicrobials improved the pharmacokinetics and therapeutic index of antimicrobial drugs (Zhang et al., 2010). Polymeric NPs are submicron-sized polymeric colloidal particles. Drugs can be encapsulated in the polymeric matrix or conjugated or adsorbed on the surface (Mahapatro and Singh, 2011). The formulation of antimicrobials in polymeric NPs provides several advantages such as prolongation of circulating half-life, as well as a modifiable particle size, zeta potential, and drug release behavior through the alteration of polymer length, type of organic solvents, and surfactants during preparation, and targetability to specific tissues (Zhang et al., 2010.). Polymers used in the preparation of NPs are either natural or synthetics. Chitosan is a natural, biodegradable, biocompatible polymer with a wide spectrum of antimicrobial activity against gram-positive and gram-negative bacteria (Rabea et al., 2003, Kong et al., 2010). Chitosan is a linear polysaccharide polymer composed of β-(1,4)-linked N-acetyl-d-glucosamine (Tikhonov et al., 2006). It is derived from the partial or total deacetylation of chitin (Tikhonov et al., 2006, Kong et al., 2010). According to its molecular weight (MW), chitosan is categorized into low-molecular weight (LMW, <50 kDa), medium MW (MMW, 50–150 kDa), and high MW (HMW, >150 kDa) (Rabea et al., 2003, Kong et al., 2010). Previous studies have demonstrated a correlation between the MW of chitosan and its antimicrobial activity (Rabea et al., 2003, Kong et al., 2010). The US Food and Drug Administration (FDA) has recognized chitosan derived from shrimp as a generally recognized as safe (GRAS) substance (Hjerde et al., 1997) for general use in foods (FDA, 2012). Chitosan also has been approved as a food additive in Japan and Korea since 1983 and 1995, respectively (Mahae et al., 2011).
The antimicrobial activity of chitosan has been explained by different theories; however, the exact mechanisms are still unknown (Rabea et al., 2003, Goy et al., 2009, Kong et al., 2010). Intracellular leakage is among the theories in which the positively charged chitosan binds to negatively charged bacterial surfaces such as lipopolysaccharides (LPS) (Rabea et al., 2003, Goy et al., 2009, Kong et al., 2010). This binding alters the bacterial membrane permeability causing leakage of the intracellular constituents and cell death (Rabea et al., 2003, Goy et al., 2009, Kong et al., 2010). Chitosan NPs (CNPs) showed high antibacterial activity, stability, and low toxicity to mammalian cells (de Campos et al., 2004, Qi et al., 2004).
Considering all the antimicrobial characteristics of DIC and CNPs, this study aimed to prepare DIC-loaded CNPs (DIC.CNPs) with LMW and HMW chitosan using ion gelation method and characterize their in vitro antibacterial properties against representative gram-positive bacteria, Staphylococcus aureus and Bacillus subtilis.
2. Materials and methods
2.1. Materials
LMW and HMW chitosan, tripolyphosphate (TPP), and acetic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). DIC was obtained from MP Biomedical (Solon, OH, USA). Mueller-Hinton Agar (MHB) and Mueller-Hinton Broth (MHB) were purchased from Merck (Armstadt, Germany). All the other solvents and chemicals were of analytical grade.
2.2. Preparation of NPs
CNPs were prepared using the ionic gelation method as described (Calvo et al., 1997) previously with minor modification. Solutions of LMW and HMW chitosan were prepared at a concentration of 0.5% (w/v) in 1% (v/v) acetic acid. Then, the pH was adjusted to 4.6 ± 0.2 using 10 N sodium hydroxide (NaOH). Then, 150 mg DIC was dissolved in distilled water containing 3% Tween 80 and added to the chitosan solution dropwise while the solution was mixed using a probe sonicator for 30 min. Then, 0.25% TPP was added dropwise to the chitosan solution at a ratio of 1:4 and rate of 1 mL/min under probe sonication for 15 min. After the addition of TPP, the NPs were formed spontaneously under probe sonication. The resultant NPs were collected by centrifugation at 9000 rpm for 30 min at 4 °C, the supernatant was discarded, and then the NPs were redispersed in distilled water.
2.3. Characterization of particle size and morphology of prepared NPs
The particle size and polydispersity index (PDI) were measured using dynamic light scattering with a Zeta sizer (Particle Sizing Systems, Port Richey, FL, USA). All measurements were performed in triplicate and reported as the means ± standard deviation (SD). The NP morphology was examined using the Tecnai transmission electron microscope (TEM) (OR, USA).
2.4. High-performance liquid chromatography (HPLC) assay of DIC
A high-performance liquid chromatography (HPLC) method similar to that reported by Alquadeib (2019) was used in this study for the detection of DIC. The concentration of DIC was measured using a Waters HPLC system, utilizing Waters 2707 autosampler delivery system and a Symmetry C18 column (10.3 × 1.0 cm) packed with 5-µm spherical particles. The mobile phase consisted of 0.05 M orthophosphoric acid (pH 2.0) and acetonitrile at 35 and 65%, respectively. The mobile phase was prepared daily, filtered through a 0.22-µm Millipore filter, and degassed under vacuum during the study. The flow rate of the mobile phase was 2.0 mL/min and the run time was 2.0 min. The injection volume was 20 µL, and detection was performed at 210 nm.
The data were analyzed using an Empower Pro chromatography manager data collection system. The HPLC system was operated at an ambient temperature. A stock solution containing 50 mg of drug in methanol was stored in 4.0 mL glass vials at −20 °C. A stock solution containing 5.0 mg/ml of lidocaine in methanol was used as the IS and stored at −20 °C. A daily standard calibration curve (n = 3) ranging from 10 to 200 µg/mL containing 25 µg/mL of IS was prepared to determine the unknown DS concentration for determining DEE and drug release. The standards were transferred to glass autosampler vials with pre-slit septum (Waters, USA), where 20 µL was injected into the HPLC system for analysis.
2.5. Entrapment efficiency (EE)
An indirect method was used to determine the percentage entrapment efficiency (EE%) of the DIC.CNPs. The prepared NPs were centrifuged at 9000 rpm for 30 min at 4 °C, and then the free drug present in the supernatant was analyzed using the HPLC method described in Section 2.4. By subtracting the free drug from the total amount added, the EE% was calculated according to the following equation:
2.6. In vitro drug release study
The in vitro release of DIC from the prepared NPs was analyzed using a previously described method (Agnihotri and Vavia, 2009) with minor adjustment. In brief, 2 mL of the DIC.CNP solution was placed into 15 mL tubes containing 8 mL phosphate buffer (pH 7.4), which were then placed on an electronic shaker set at 100 rpm. At predetermined time intervals, 2 mL of the release medium was withdrawn and replaced with the same volume of fresh medium. Isolated samples were centrifuged at 4400 rpm for 5 min and filtered through a 0.2-µm syringe filter. The amount of DIC in the withdrawn samples was determined using the HPLC method and the cumulative (%) drug amount released was calculated.
2.7. Drug release kinetics
Various dissolution models were used to determine the drug release kinetics of the DIC.CNP formulations, including the zero order, first order, Higuchi, and Korsmeyer-Peppas release models. The release profile data were processed and plotted according to the equations of different models, followed by regression analyses. The best goodness-of-fit (coefficient of determination [R2] values) were used to select the most appropriate model. The slope of each plot and release rate constant for each model were used to describe the release rate mechanism.
2.8. Microbiological assays
The antibacterial properties of the DIC.CNPs were investigated against S. aureus 25923 and B. subtilis 23857 using the agar diffusion and broth dilution methods. The tested bacterial strains were grown in Mueller-Hinton agar or broth (MHA or MHB, respectively) for 18 h at 37 °C.
2.8.1. Agar diffusion method
In the agar diffusion method, wells were created in the MHA plate inoculated with the test bacteria using a cork borer. Then, 50 μL of the different DIC.CNP formulations and DIC (pH = 7) were loaded into the wells with ampicillin as the positive control. All plates were incubated for 18 h at 37 °C, the diameter of the inhibition zones was measured in millimeters (mm), and all experiments were performed in triplicate.
2.8.2. Broth dilution method
The microdilution method was performed as described previously with some modification (Aleanizy et al., 2018). The test microorganisms were grown on MHB for 18 h at 37 °C and adjusted to 0.5 on the McFarland standard. Then, 100 µL of the bacterial broth suspension was inoculated into each well of a 100-well plate, containing 100 µL of serially diluted LMW.DIC.CNPs, HMW.DIC.CNPs, or DIC in MHB (pH = 5.5). The plate was then incubated for 18 h at 37 °C. The control wells contained bacterial suspension alone, and MHB supplemented with DIC.CNPs were left uninoculated. Bacterial growth was measured every 1 h using a microplate reader (Bioscreen C, Growth Curves USA, Piscataway, NJ, USA) set at an optical density (OD) of 600 nm for 18 h. Then, the minimum inhibitory concentration (MIC90) was determined.
2.9. Statistical analysis
The in vitro assay results were expressed as the means ± SD of at least three replicates. The HPLC results of DIC were calculated using linear regression without weighting according to the equation: Y = 0.0225X + 0.8858, where Y is the area under the peak (AUP) ratio of the drug to the internal standard (IS) and X is the concentration of DIC. The SD (%) was calculated for all values, the t-test was used to compare MIC90 values of different formulations, and a p < 0.05 was considered significant.
3. Results and discussion
3.1. Characterization of prepared nanoparticles
3.1.1. Mean particle size, PDI, and zeta potential
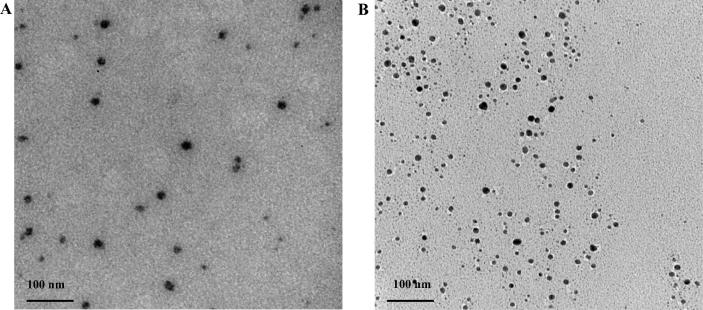
In the current study, the ionic gelation method was used to prepare various DIC.CNP formulations using different MW chitosan to investigate the effect of MW on the antimicrobial activity of the prepared NPs. As shown in Table 1, the mean particle size of the prepared NPs increased with increasing MW of the chitosan. Fig. 1 presents the TEM images of the DIC.CNPs revealing a spherical shape, smooth surface, and size range of approximately 150–336 nm.
Table 1.
Mean particle size, polydispersity index (PDI), and zeta potential of diclofenac chitosan loaded nanoparticles (DIC.CNPs).
| Formula | Chitosan concentration (mg/ml) | Particle size (nm, mean ± SD) | PDI (Mean ± SD) | Zeta potential (mV, mean ± SD) | EE% (Mean ± SD) |
|---|---|---|---|---|---|
| LMW.DIC.CNPs | 5 mg/ml | 295.33 ± 3.01 | 0.185 ± 0.016 | 29.3 ± 1.80 | 29.3 ± 4.3 |
| HMW.DIC.CNPs | 336 ± 22.1 | 0.246 ± 0.07 | 22.53 ± 1.76 | 31.1 ± 3.1 |
Values are means ± standard deviation (SD) of at least three experiments (n = 3). EE%, percentage entrapment efficiency; LMW.DIC.CNPs, diclofenac-loaded low-molecular weight chitosan nanoparticles; HMW.DIC.CNPs, diclofenac-loaded high-molecular weight chitosan nanoparticles.
Fig. 1.
Transmission electron microscopy (TEM) images of diclofenac-loaded chitosan nanoparticles (DIC.CNPs). Representative high molecular weight (MW) chitosan DIC-loaded NPs (HMW.DIC.CNPs) and (B) LMW.DIC.CNPs.
The prepared NPs showed a narrow size distribution with low PDI values (0.18 and 0.24) for the LMW.DIC.CNPs and HMW.DIC.CNPs, respectively, revealing the high homogeneity (Table 1). The mean zeta potential of the formulated NPs decreased with increasing MW chitosan (Table 1). This result was in agreement with that of other studies that reported an increase in the particle size, PDI, and zeta potential of CNPs with increasing chitosan MW (Gan et al., 2005, Ing et al., 2012).
3.1.2. Determination of EE%
As shown in Table 1, the EE% of the LMW.DIC.CNPs and HMW.DIC.CNPs was 29.3% and 31.1%, respectively. There was a slight increase in EE%, although the effect was not statistically significant when the MW of chitosan increased. These results corroborate the findings of previous studies (Xu and Du, 2003, Deng et al., 2008) where the EE of lysozymes increased with increasing MW of chitosan.
3.1.3. In vitro release studies
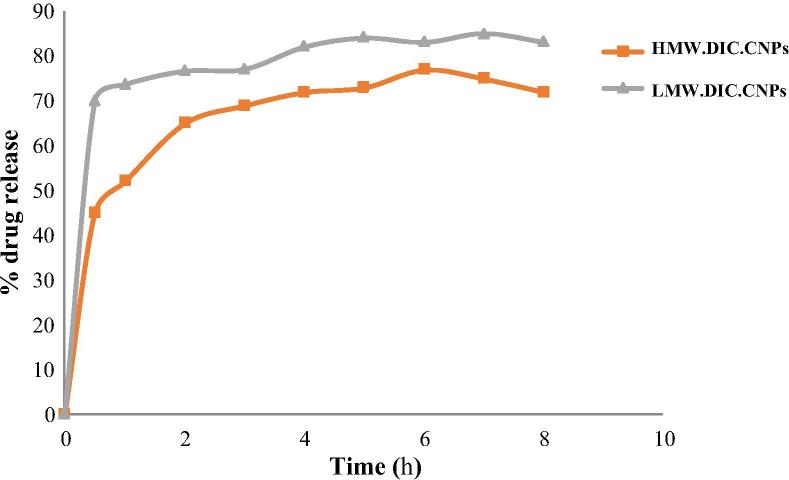
Fig. 2 shows the in vitro release of DIC from the CNPs, which was carried out using phosphate buffer at pH 7.4. Consistent with previous reports (Kouchak and Azarpanah, 2015), the release profile of DIC from the CNPs was biphasic (Fig. 2). In the first phase (0.5 h, r > 0.9) there was an initial rapid release of approximately 20–70% according to the formulation tested, followed by a slow release from 0.5 to 8 h when approximately 11–32% of the drug was released.
Fig. 2.
In vitro release behaviors of diclofenac (DIC) from chitosan nanoparticles (CNPs). In vitro release of DIC from low and high molecular weight chitosan NPs (LMW.DIC.CNPs and HMW.DIC.CNPs) in phosphate-buffered saline (PBS, pH 7.4). Value are means ± standard deviation (SD) of at least three experiments (n = 3).
The release rate of DIC decreased when the MW of chitosan increased as shown in Fig. 2, corroborating the findings of a previous study (Deng et al., 2008).
3.1.4. In vitro release kinetic study
The release data of DIC from the LMW.DIC.CNPs and HWW.DIC.CNPs were fitted to zero order, first order, Higuchi diffusion, and Korsmeyer-Peppas kinetic models (Table 2). The best fit with the highest R2 of both DIC formulations was achieved with the Korsmeyer-Peppas release model. This model described the drug release from the polymeric system through swelling and relaxation of the matrix. The release exponent (n) for the LMW.DIC.CNPs and HMW.DIC.CNPs was 0.07 and 0.174, respectively. The n value was <0.5, indicating a Fickian type of release (Korsmeyer and Peppas, 1984). Therefore, the release of DIC from the NPs was by Fickian diffusion.
Table 2.
Kinetic modeling of diclofenac (DIC) release from different chitosan nanoparticles (CNPs).
| Code | Zero-order |
First order |
Higuchi |
Korsmeyer Peppas |
|||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | K | R2 | K | R2 | K | R2 | K | n | |
| Low | −1.1903 | 14.677 | 0.5751 | 1.566 | 0.1928 | 37.797 | 0.9975 | 73.348 | 0.070 |
| High | −0.4429 | 12.864 | 0.4908 | 0.370 | 0.6051 | 32.690 | 0.9810 | 54.435 | 0.174 |
R2 is the coefficient of determination, K is the release rate constant for the respective model, and k is the release rate constants of respective equations.
3.2. Antibacterial activity of DIC.CNPs
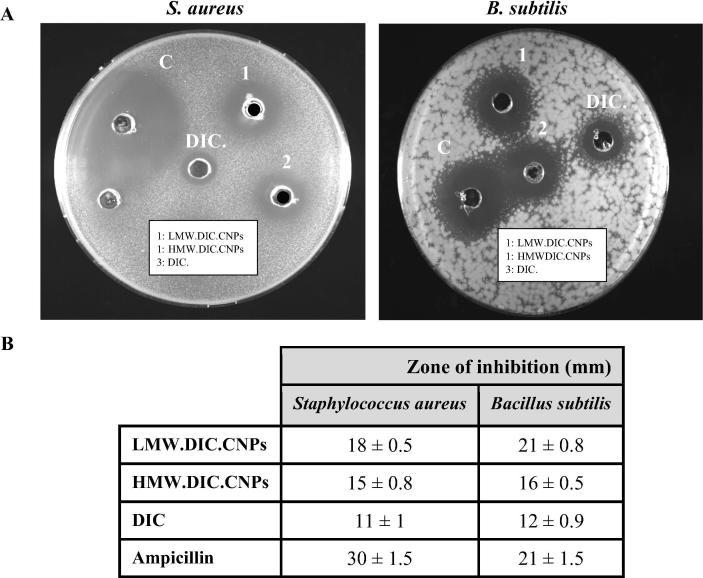
Several studies have reported the antimicrobial properties of DIC (Salem-Milani et al., 2013, Padma and Yalavarthy, 2015, Ahmed et al., 2017), and CNPs (Qi et al., 2004). Therefore, in this study, we investigated the antibacterial activities of DIC.CNPs using the agar diffusion method and the results are shown in Fig. 3. The LMW.DIC.CNPs and HMW.DIC.CNPs produced inhibition zones of 18 and 15 mm, respectively against S. aureus and 21 and 16 mm, respectively against B. subtilis. The zones of inhibition of DIC (100 µg) alone against S. aureus and B. subtilis were 11 and 12 mm, respectively. Both DIC.CNPs exhibited higher antibacterial activity than DIC alone did (Fig. 3). This revealed that the use of chitosan as polymer enhanced the antimicrobial activity of DIC. To the best of our knowledge, no previous study to date has investigated the antimicrobial activity of DIC.CNPs. The ampicillin produced inhibition zones of 30 and 21 against S. aureus and B. subtilis, respectively.
Fig. 3.
Inhibition zones of diclofenac-loaded chitosan nanoparticles (DIC.CNPs). (A and B) Antimicrobial activities of (1) LMW. DIC.CNPs (75 µg), (2) HMW. DIC.CNPs (75 µg), (3) DIC (100 µg), and (C) ampicillin (10 µg) as positive control were determined using agar diffusion method against Staphylococcus aureus and Bacillus subtilis after 24 h incubation. Value are mean inhibition zones ± standard deviation (SD) of at least three experiments (n = 3).
The microbroth dilution method was used to determine the MIC90 of DIC.CNPs against the test bacteria. The LMW.DIC.CNPs and HMW.DIC.CNPs showed significantly lower MIC90 values than that of free DIC against both S. aureus and B. subtilis (Table 3). These results revealed the synergistic antimicrobial activity of CNPs against S. aureus and B. subtilis following encapsulation with DIC. These results are in agreement with those of a previous study, which showed the increased antimicrobial activity of chitosan-capped Au NPs coupled with ampicillin against multidrug-resistant clinical isolates of Pseudomonas aeruginosa and Escherichia coli compared with free ampicillin (Chamundeeswari et al., 2010). Another study found that penicillin G-loaded CNPs exhibited greater antibacterial activity against Streptococcus pyogenes, B. subtilis, and S. aureus than penicillin did (Safhi et al., 2014). Moreover, a reduction in the MIC90 values of amoxicillin (Nguyen et al., 2017) and ciprofloxacin (Sobhani et al., 2017) loaded CNPs was reported compared with those of amoxicillin and ciprofloxacin alone.
Table 3.
Antibacterial activities of diclofenac-loaded chitosan nanoparticles (DIC.CNPs).
| Microorganism | MIC90 (µg/mL) |
||
|---|---|---|---|
| HMW.DIC.CNPs | LMW.DIC.CNPs | DIC | |
| Staphylococcus aureus | 18* ± 1.6 | 35* ± 2.2 | 250 ± 4 |
| Bacillus subtilis | 9* ± 1.2 | 17.5* ± 2.7 | 50 ± 3.1 |
P value <0.01, broth dilution method was used to determine the minimum inhibitory concentration (MIC90) of prepared NPs against S. aureus and B. subtilis after 18 h incubation at 37 °C. Values are means of MIC90 ± standard deviation (SD) of at least three experiments (n = 3).
It should be noted that the broth dilution experiment in our study was performed at pH = 5.5, because it has been reported that the antimicrobial activity of CNPs increased at lower pH (Qi et al., 2004). As shown in Table 3, the MIC90 value of HMW.DIC.CNPs was lower than that of LMW.DIC.CNPs (18 µg/mL versus 35 µg/mL for S. aureus and 9 µg/mL versus 17.5 µg/mL for B. subtilis). This revealed that the antibacterial activity of chitosan increased as the MW increased at pH = 5.5, which was reported previously (Qi et al., 2004). In contrast, at pH > 7 the chitosan activity increased when the MW decreased (Qi et al., 2004). This explains the higher antimicrobial activity of LMW.DIC.CNPs than HMW.DIC.CNPs observed in the agar diffusion method performed at pH 7.
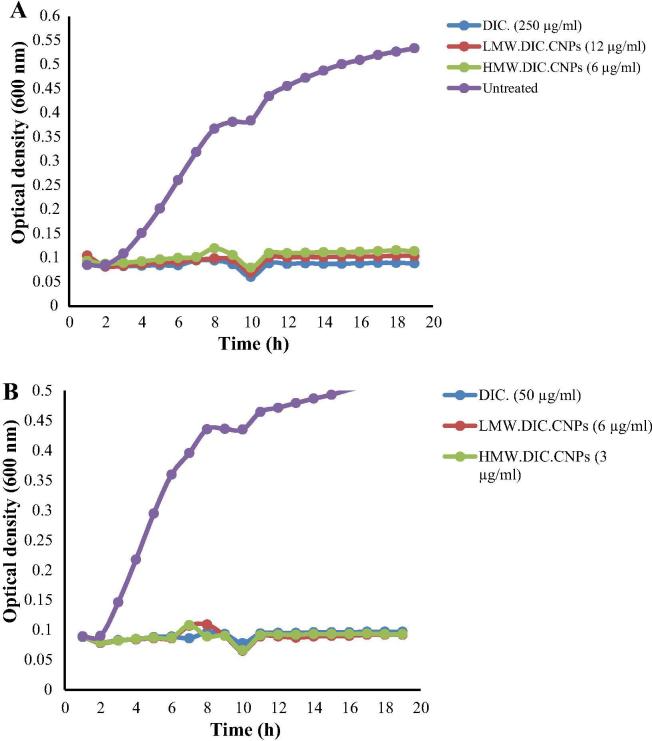
The growth of the tested bacteria in the presence and absence of the prepared NPs is presented in Fig. 4. The results clearly show that the pattern of bacterial inhibition mediated by HMW.DIC.CNPs and LMW.DIC.CNPs was similar to by DIC but with much lower MIC90 values. This indicates that the CNPs did not affect the antibacterial pattern of DIC but rather enhanced the efficacy.
Fig. 4.
Growth of bacteria in the presence and absence of diclofenac-loaded chitosan nanoparticles (DIC.CNPs). (A) Staphylococcus aureus and (B) Bacillus subtilis were grown in the absence (untreated) or presence of high and low molecular weight DIC-loaded CNPs (HMW.DIC.CNPs and LMW.DIC.CNPs, respectively) and DIC for 18 h at 37 °C. Points are means of three measurements.
4. Conclusion
The present study was designed to prepare DIC.CNPs and investigate their antibacterial activity against S. aureus and B. subtilis. The particle size, zeta potential, EE, and in vitro release behavior of the prepared NPs were characterized. The DIC.CNPs demonstrated higher antimicrobial activity, which depended on the MW of the chitosan and pH. Although, these results may suggest the potential usefulness of CNPs as a delivery carrier for DIC as an antimicrobial agent, however, further stability studies should be carried out in future to verify such implication.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No. RGP-1438-003.
Footnotes
Peer review under responsibility of King Saud University.
References
- Administration, F.D.A. 2012. Shrimp-derived Chitosan GRAS Notification. from Available: www.accessdata.fda.gov/scripts/fcn/gras_notices/GRN000443pdf.
- Agnihotri S.M., Vavia P.R. Diclofenac-loaded biopolymeric nanosuspensions for ophthalmic application. Nanomedicine. 2009;5:90–95. doi: 10.1016/j.nano.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Ahmed E.F., El-Baky R.M.A., Ahmed A.B.F., Waly N.G., Gad G.F.M. Antibacterial activity of some non-steroidal anti-inflammatory drugs against bacteria causing urinary tract infection. Am. J. Infect. Dis. Microbiol. 2017;5:66–73. [Google Scholar]
- Aleanizy F.S., Alqahtani F.Y., Shazly G., Alfaraj R., Alsarra I., Alshamsan A., Abdulhady H.G. Measurement and evaluation of the effects of pH gradients on the antimicrobial and antivirulence activities of chitosan nanoparticles in Pseudomonas aeruginosa. Saudi Pharm. J. 2018;26:79–83. doi: 10.1016/j.jsps.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral L., Viveiros M., Kristiansen J.E. Non-antibiotics: alternative therapy for the management of MDRTB and MRSA in economically disadvantaged countries. Curr. Drug Targets. 2006;7:887–891. doi: 10.2174/138945006777709539. [DOI] [PubMed] [Google Scholar]
- Annadurai S., Basu S., Ray S., Dastidar S.G., Chakrabarty A.N. Antibacterial activity of the antiinflammatory agent diclofenac sodium. Indian J. Exp. Biol. 1998;36:86–90. [PubMed] [Google Scholar]
- Alquadeib B.T. Development and validation of a new HPLC analytical method for the determination of diclofenac in tablets. Saudi Pharm. J. 2019;27(1):66–70. doi: 10.1016/j.jsps.2018.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo P., Remunan-Lopez C., Vila-Jato J.L., Alonso M.J. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm. Res. 1997;14:1431–1436. doi: 10.1023/a:1012128907225. [DOI] [PubMed] [Google Scholar]
- Chamundeeswari M., Sobhana S.S., Jacob J.P., Kumar M.G., Devi M.P., Sastry T.P., Mandal A.B. Preparation, characterization and evaluation of a biopolymeric gold nanocomposite with antimicrobial activity. Biotechnol. Appl. Biochem. 2010;55:29–35. doi: 10.1042/BA20090198. [DOI] [PubMed] [Google Scholar]
- de Campos A.M., Diebold Y., Carvalho E.L., Sanchez A., Alonso M.J. Chitosan nanoparticles as new ocular drug delivery systems: in vitro stability, in vivo fate, and cellular toxicity. Pharm. Res. 2004;21:803–810. doi: 10.1023/b:pham.0000026432.75781.cb. [DOI] [PubMed] [Google Scholar]
- Gan Q., Wang T., Cochrane C., McCarron P. Modulation of surface charge, particle size and morphological properties of chitosan-TPP nanoparticles intended for gene delivery. Colloids Surf. B. Biointerface. 2005;44:65–73. doi: 10.1016/j.colsurfb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Goy R.J., Douglas de Britto D., Assis A.B.G. A review of the antimicrobial activity of chitosan. Ciência e Tecnologia. 2009;19(3):241–247. [Google Scholar]
- Hjerde R.J.N., Varum K.M., Grasdalen H., Tokura S., Smidsrød O. Chemical composition of O-(carboxymethyl)-chitins in relation to lysozyme degradation rate. Carbohydr. Polym. 1997;34:131–139. [Google Scholar]
- Ing L.Y., Zin N.M., Sarwar A., Katas H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012;2012:632698. doi: 10.1155/2012/632698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int. J. Food Microbiol. 2010;144:51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Korsmeyer R.W., Peppas N.A. Solute and penetrant diffusion in swellable polymers. III. Drug release from glassy poly(HEMA-co-NVP) copolymers. J. Control. Release. 1984;1:89–98. [Google Scholar]
- Kouchak M., Azarpanah A. Preparation and in vitro evaluation of chitosan nanoparticles containing diclofenac using the ion-gelation method. Jundishapur J. Nat. Pharm. Prod. 2015;10:e23082. [Google Scholar]
- Kristiansen J.E. The antimicrobial activity of non-antibiotics. Report from a congress on the antimicrobial effect of drugs other than antibiotics on bacteria, viruses, protozoa, and other organisms. APMIS Suppl. 1992;30:7–14. [PubMed] [Google Scholar]
- Mahae N., Chalat C., Muhamud P. Antioxidant and antimicrobial properties of chitosan-sugar complex. Int. Food Res. J. 2011;18:1543–1551. [Google Scholar]
- Mahapatro A., Singh D.K. Biodegradable nanoparticles are excellent vehicle for site-directed in-vivo delivery of drugs and vaccines. J. Nanobiotechnol. 2011;9:55. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.V., Nguyen T.T.H., Wang S.-L., Vo T.P.K., Nguyen A.D. Preparation of chitosan nanoparticles by TPP ionic gelation combined with spray drying, and the antibacterial activity of chitosan nanoparticles and a chitosan nanoparticle–amoxicillin complex. Res. Chem. Intermed. 2017;43:3527–3537. [Google Scholar]
- Padma R., Yalavarthy P.D. Screening of diclofenac for antibacterial activity against pathogenic microorganisms. Int. J. Adv. Pharm. Biol. Chem. 2015;4:554–558. [Google Scholar]
- Qi L., Xu Z., Jiang X., Hu C., Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydr. Res. 2004;339:2693–2700. doi: 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Deng Q., Zhou C.-R., Luo B.-H. Preparation and characterization of chitosan nanoparticles containing lysozyme. Pharm. Biol. 2008;44:336–342. [Google Scholar]
- Rabea E.I., Badawy M.E., Stevens C.V., Smagghe G., Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4:1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- Safhi M.M., Sivakumar S.M., Jabeen A., Zakir F., Islam F., Barik B.B. Chitosan nanoparticles as a sustained delivery of penicillin G prepared by ionic gelation technique. J. Pharm. Res. 2014;88:1352–1354. [Google Scholar]
- Salem-Milani A., Balaei-Gajan E., Rahimi S., Moosavi Z., Abdollahi A., Zakeri-Milani P., Bolourian M. Antibacterial effect of diclofenac sodium on Enterococcus faecalis. J. Dent. (Tehran) 2013;10:16–22. [PMC free article] [PubMed] [Google Scholar]
- Sobhani Z., Samani S.M., Montaseri H., Khezri E. Nanoparticles of chitosan loaded ciprofloxacin: fabrication and antimicrobial activity. Adv. Pharm. Bull. 2017;7:427–432. doi: 10.15171/apb.2017.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikhonov V.E., Stepnova E.A., Babak V.G., Yamskov I.A., Palma-Guerrero J., Jansson H.-B., Lopez-Llorca L.V., Salinas J., Gerasimenko D.V., Avdienko I.D., Varlamov V.P. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydr. Polym. 2006;1:66–72. [Google Scholar]
- Xu Y., Du Y. Effect of molecular structure of chitosan on protein delivery properties of chitosan nanoparticles. Int. J. Pharm. 2003;250:215–226. doi: 10.1016/s0378-5173(02)00548-3. [DOI] [PubMed] [Google Scholar]
- Zhang L., Pornpattananangkul D., Hu C.M.J., Huang C.M. Development of nanoparticle for antimicrobial drug delivery. Curr. Med. Chem. 2010;17:585–594. doi: 10.2174/092986710790416290. [DOI] [PubMed] [Google Scholar]