Abstract
The actinobacterium strain ABH26 closely related to Saccharothrix xinjiangensis, isolated from an Algerian Saharan soil sample, exhibited highly antagonist activity against Gram-positive bacteria, yeasts and filamentous fungi. Its ability to produce antimicrobial compounds was investigated using several solid culture media. The highest antimicrobial activity was obtained on Bennett medium. The antibiotics secreted by strain ABH26 on Bennett medium were extracted by methanol and purified by reverse-phase HPLC using a C18 column. The chemical structures of the compounds were determined after spectroscopic (1H NMR, 13C NMR, 1H-1H COSY and 1H-13C HMBC spectra), and spectrometric (mass spectrum) analyses. Two new cyanogriside antibiotics named cyanogriside I (1) and cyanogriside J (2), were characterized along with three known caerulomycins, caerulomycin A (3), caerulomycin F (4) and caerulomycinonitrile (5). This is the first report of cyanogrisides and caerulomycins production by a member of the Saccharothrix genus. The minimum inhibitory concentrations (MIC) of these antibiotics were determined against pathogenic microorganisms.
Keywords: Antagonistic activity, Antimicrobial compounds, Caerulomycins, Cyanogrisides, Saccharothrix xinjiangensis
1. Introduction
Cyanogrisides and caerulomycins are bioactive secondary metabolites containing a 2,2′-bipyridine skeleton and produced by two mycelial actinobacteria species, Streptomyces caeruleus (Funk and Divekar, 1959) and Actinoalloteichus cyanogriseus (Fu et al., 2011). Cyanogrisides differ to caerulomycins by the presence of one or more sugars bonded to the bipyridine skeleton. At the time of writing, 9 cyanogrisides and 13 caerulomycins have been isolated and characterized. These compounds have been shown to have antibacterial, antifungal, antiparasitic and antitumor activities (Funk and Divekar, 1959, Chatterjee et al., 1984, Ambavane et al., 2014, Kujur et al., 2015).
Actinobacteria are Gram-positive bacteria with high (≥55%) guanine and cytosine content in their genomes. This group of microorganisms represents one of the most important sources of natural bioactive compounds. Berdy (2012) estimated that 62% of known bioactive microbial compounds are originally from actinobacteria. Saccharothrix spp. are relatively rare actinobacteria known to produce several antibiotics with different structures and biological activities, such as the antibacterial agent tianchimycins from Saccharothrix xinjiangensis NRRL B-24321 (Wang et al., 2013) and the antiviral agent fluvirucin from Saccharothrix mutabilis R516-16 (Naruse et al., 1991).
The arid soils of the Algerian Sahara have been shown to be rich in members of the Saccharothrix genus (Sabaou et al., 1998, Zitouni et al., 2005) including new species, such as Saccharothrix algeriensis NRRL B-24137T (Zitouni et al., 2004a), Saccharothrix hoggarensis DSM 45457T, Saccharothrix saharensis DSM 45456T, Saccharothrix tamanrassetensis DSM 45947T (Boubetra et al., 2013a, Boubetra et al., 2013b, Boubetra et al., 2015), Saccharothrix isguenensis DSM 46885T and Saccharotrix ghardaiensis DSM 46886T (Bouznada et al., 2016, Bouznada et al., 2017). In addition, many studies have shown the ability of Saccharothrix strains isolated from these soils to produce new or known antibiotics, such as dithiolopyrrolones (Lamari et al., 2002, Bouras et al., 2008, Merrouche et al., 2011), anthracyclines (Zitouni et al., 2004b) and chloramphenicol (Aouiche at al., 2012). These promising results emphasise the need to continue the research into Saccharothrix antimicrobial production.
In Algeria, we isolated a mycelial actinobacterium strain with strong antibacterial and antifungal activities designated as ABH26. Based on morphological, physiological and molecular characterization the strain was found to be related to Saccharothrix xinjiangensis (Lahoum et al., 2015). Initially, this strain had antimicrobial activity on solid and liquid ISP (International Streptomyces Project) 2 culture medium (Shirling and Gottlieb, 1966). However, the strain has subsequently lost its activity in liquid medium while this activity was conserved on solid medium (data not shown).
The aim of this study was to characterize the bioactive compounds secreted by strain ABH26 on various solid culture media, and to test the antimicrobial activity of these molecules against some representative pathogenic microorganisms.
2. Materials and methods
2.1. Microbial strains
The actinobacterial strain ABH26 was isolated from a Saharan soil sample collected in the Adrar region (Southern Algeria). It was found to be most closely related to Saccharothrix xinjiangensis (Lahoum et al., 2015). The target microorganisms used for antimicrobial testing are from our laboratory collection and are listed in Table 1. This list includes Gram-positive and Gram-negative bacteria, yeasts and filamentous fungi, most of which are pathogens for humans or plants.
Table 1.
Antimicrobial activity of the strain ABH26 on Bennett, ISP2, NA and ISP1 culture media.
| Target microorganism | Distance of inhibition (mm) |
|||
|---|---|---|---|---|
| Bennett | ISP2 | NA | ISP1 | |
| Aspergillus brasiliensis AB1 | 25 | 24 | 18 | 16 |
| A. carbonarius AC1 | 35 | 33 | 19 | 15 |
| A. carbonarius AC2 | 28 | 22 | 16 | 15 |
| A. flavus NRRL 3251 | 23 | 21 | 17 | 17 |
| A. parasiticus CBS 100,926 | 22 | 17 | 16 | 15 |
| A. westerdijkiae ATCC 3174 | 22 | 20 | 17 | 13 |
| Fusarium culmorum FC1 | 31 | 28 | 19 | 16 |
| F. equiseti FE1 | 25 | 24 | 16 | 14 |
| F. graminearum FG1 | 27 | 24 | 19 | 15 |
| F. oxysporum f. sp. albedinis Foa1 | 27 | 27 | 17 | 18 |
| F. o. f. sp. radicis-lycopersici Forl | 23 | 22 | 17 | 17 |
| F. solani Fsol | 28 | 27 | 18 | 17 |
| F. sporotrichioides FS1 | 27 | 26 | 18 | 15 |
| Rhizoctonia solani AG3 | 31 | 28 | 20 | 18 |
| Umbelopsis ramanniana NRRL 1829 | 38 | 32 | 21 | 19 |
| Candida albicans M2 | 25 | 22 | 12 | 4 |
| C. albicans M3 | 31 | 30 | 15 | 12 |
| C. albicans IPA200 | 24 | 23 | 13 | 5 |
| Saccharomyces cerevisiae ATCC 4226 | 34 | 32 | 16 | 14 |
| Bacillus subtilis ATCC 6633 | 22 | 20 | 19 | 19 |
| Corynebacterium diphtheriae CD1 | 28 | 31 | 22 | 20 |
| Enterococcus faecalis EF1 | 21 | 25 | 18 | 17 |
| Listeria monocytogenes ATCC 13,932 | 27 | 31 | 21 | 19 |
| Micrococcus luteus ATCC 9314 | 30 | 33 | 18 | 18 |
| Staphylococcus aureus ATCC 29,213 | 21 | 24 | 16 | 15 |
| S. aureus ATCC 43,300 | 19 | 20 | 14 | 11 |
| S. aureus MRSA 639c | 13 | 15 | 13 | 12 |
| Escherichia coli E52 | 0 | 0 | 0 | 0 |
| E. coli E195 | 0 | 0 | 0 | 0 |
| Klebsiella pneumoniae E40 | 0 | 0 | 0 | 0 |
| Pseudomonas aeruginosa IPA1 | 0 | 0 | 0 | 0 |
2.2. Antimicrobial activity of strain ABH26 on solid culture media
Antimicrobial activity was evaluated on ISP1 (tryptone-yeast extract agar), ISP2 (yeast extract-malt extract agar) (Shirling and Gottlieb, 1966), NA (nutrient agar) and Bennett (Waksman, 1961) media by the cross streak method. The actinobacterial strain was inoculated in a straight line on solid culture media and then incubated for 10 days at 30 °C. After the incubation period, target microorganisms were seeded in perpendicular streaks that cross the ABH26 streak. After further incubation at 30 °C for 24 h for bacteria and yeasts, and at 25 °C for 48 h for filamentous fungi, the antimicrobial activity was determined by measuring distance of inhibition between target microorganisms and the actinobacterial strain.
2.3. Kinetics of bioactive compound production on solid culture media
The spores of strain ABH26 were dislodged from the pre-culture media with a sterile loop and transferred to 10 ml of sterile distilled water. Spores were counted using a counting chamber and a 106 spores/ml solution was prepared. In Petri dishes (170 mm in diameter) filled with ISP1, ISP2, NA, or Bennett media, 1 ml of spore suspension was spread on the surface of each medium. The plates were incubated for 12 days at 30 °C. Agar plugs (10 mm in diameter) were regularly taken each day and used to determine antagonistic activity against Bacillus subtilis ATCC 6633, Candida albicans M3 and Umbelopsis ramanniana NRRL 1829. All the experiments were performed in duplicate.
2.4. Extraction and purification of antimicrobial products
Strain ABH26 was cultured on ISP1, ISP2, NA and Bennett solid media (150 ml of each medium in 170 mm diameter Petri dishes) as described above for 7 days at 30 °C. At the end of the incubation period, the medium was cut into small pieces and extracted with 100 ml of methanol on a magnetic stirrer set at 250 rpm for 2 h. The extracts were concentrated to dryness by a rotary evaporator under vacuum at 40 °C. The residues were dissolved in 1 ml of methanol. A 80 µl volume of each sample was injected into the HPLC system (Agilent 1260) using a reverse phase C18 column (200 × 10 mm; 5 µm) with a continuous linear gradient solvent system from 20 to 100% methanol in water. A total run time of 60 min was maintained at a flow rate of 1.2 ml/min under ambient temperature. The detection of products was carried out at 220 and 254 nm. The fractions corresponding to peaks were collected, concentrated then tested (6 mm paper disk diffusion method) against Bacillus subtilis ATCC 6633 and Umbelopsis ramanniana NRRL 1829 to detect the active fractions and distinguish them from the non-active fractions. Final purification of the antibiotics produced on Bennett medium was achieved after the second re-injection in the HPLC under the same conditions.
2.5. Spectroscopic and spectrometric analysis of antibiotics
These analyses were made with the pure bioactive compounds isolated from Bennett medium. The UV–visible spectra in methanol were determined with a Shimadzu UV 1605 spectrophotometer. The mass spectra were recorded on LCQ ion-trap mass spectrometer (Finnigan MAT, San Jose, CA) equipped with a nanospray ion electro-spray ionization source (positive and negative ion mode). 1H and 13C NMR spectroscopy were used for the final characterization of bioactive compounds. NMR samples were prepared by dissolving 3 mg of each compound in 600 µl of CD3OD. All spectra were recorded on a Bruker Avance 500 spectrometer equipped with a 5 mm triple resonance inverse Z-gradient probe (TBI 1H, 31P, BB). All chemical shifts for 1H and 13C are relative to TMS using 1H (residual) or 13C chemical shifts of the solvent as a secondary standard. The temperature was set at 298 K. All the 1H and 13C signals were assigned on the basis of chemical shifts, spin-spin coupling constants, splitting patterns and signal intensities, and by using 1H-1H COSY45, 1H-13C HSQC and 1H-13C HMBC experiments. Gradient-enhanced 1H COSY45 was performed and included 36 scans per increment. 1H-13C correlation spectra using a gradient-enhanced HSQC sequence (delay was optimised for 1JCH of 145 Hz) was obtained with 120 scans per increment. A gradient-enhanced HMBC experiment was performed allowing 62.5 ms for long-range coupling evolution (240 scans were accumulated). Typically, 2048 t2 data points were collected for 256 t1 increments.
2.6. Determination of minimum inhibitory concentrations
Minimum inhibitory concentrations (MIC) of pure antibiotics were determined using the conventional agar dilution method (Oki et al., 1990) on the target microorganisms listed in Table 1. The target strains were inoculated onto Mueller Hinton medium for bacteria and Sabouraud medium for fungi, containing different concentrations of each active compound (0.5, 1, 2, 3, 4, 5, 10, 15, 20, 25, 30, 40, 50, 60, 70, 80, 90 and 100 µg/ml). The same concentration of two standard antibiotics were used for comparison: nystatin (antifungal compound) and amoxicillin (antibacterial compound). After a growth period at 30 °C for 24 h for bacteria and yeasts and 48 h for filamentous fungi, the plates were examined for growth and the lowest antibiotic concentration that inhibited the growth of each organism was determined. Mueller Hinton and Sabouraud media inoculated with target organisms, but without active compounds were used as control treatments. All the experiments were performed in duplicate.
3. Results
3.1. Antimicrobial activity of strain ABH26 on solid media
The antimicrobial activity of strain ABH26 against various target microorganisms is shown in Table 1. The strain exhibited a strong ability to inhibit growth of Gram-positive bacteria, yeasts and filamentous fungi. However, this strain did not show any activity against Gram-negative bacteria. The highest antifungal activity was observed on Bennett medium, while the highest antibacterial activity was obtained on ISP2 medium. The strain showed less antimicrobial activity on ISP1 and NA media.
3.2. Kinetics of bioactive compound production on solid media
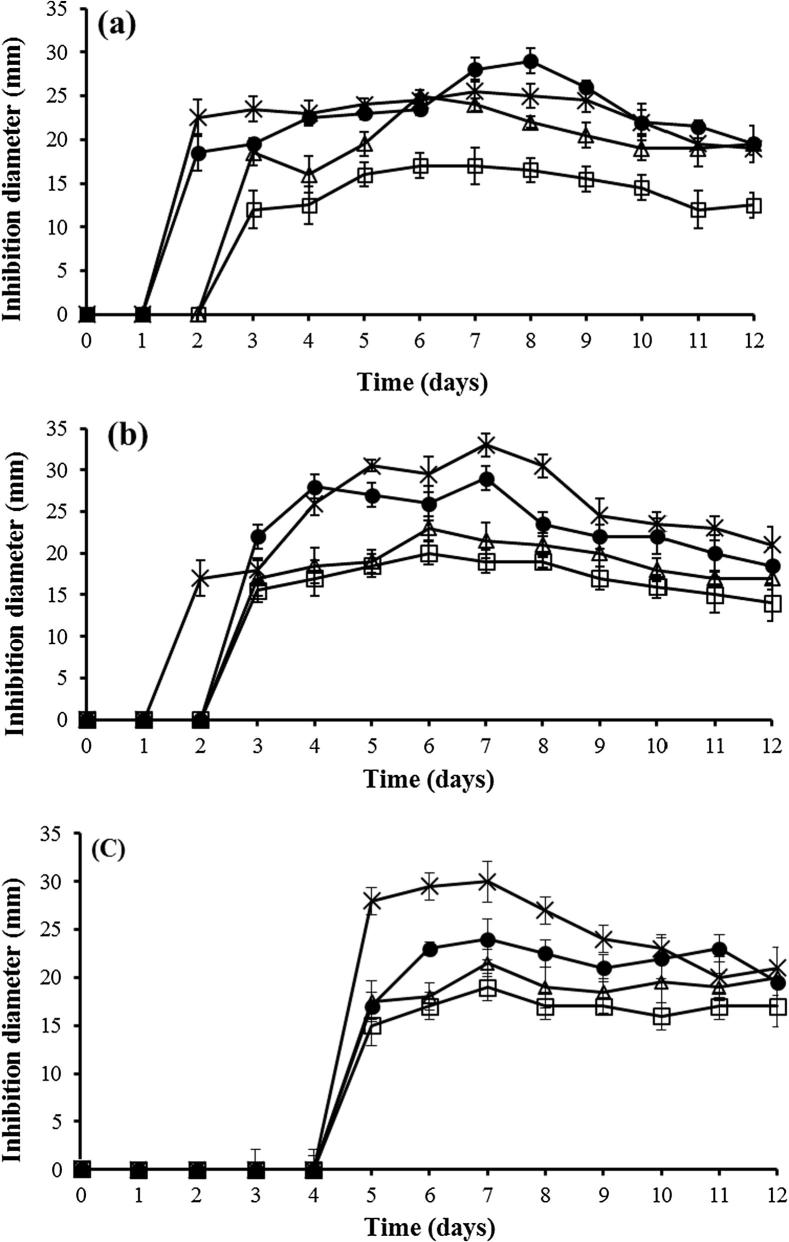
Antimicrobial activity was detected on all tested media, ISP1, ISP2, NA and Bennett (Fig. 1). The antibacterial activity started on the second or third day and reached a maximum after 7 or 8 days of incubation. The highest activity was observed in ISP2 medium then Bennett medium. The antifungal activity against Umbelopsis ramanniana started after 2 or 3 days of incubation and after 4 days for Candida albicans. Antifungal activity reached a maximum at 7 days on all tested media. The highest antifungal activity was observed in Bennett medium. Thus, Bennett medium was chosen as the production medium for purification of antimicrobial compounds.
Fig. 1.
Time course of antimicrobial activity of strain ABH26 on ISP1 (□), ISP2 (●), Bennett (×) and NA (△) solid culture media against Bacillus subtilis ATCC 6633 (a), Umbelopsis ramanniana NRRL 1829 (b) and Candida albicans M3 (c).
3.3. Detection of antimicrobial compounds by HPLC
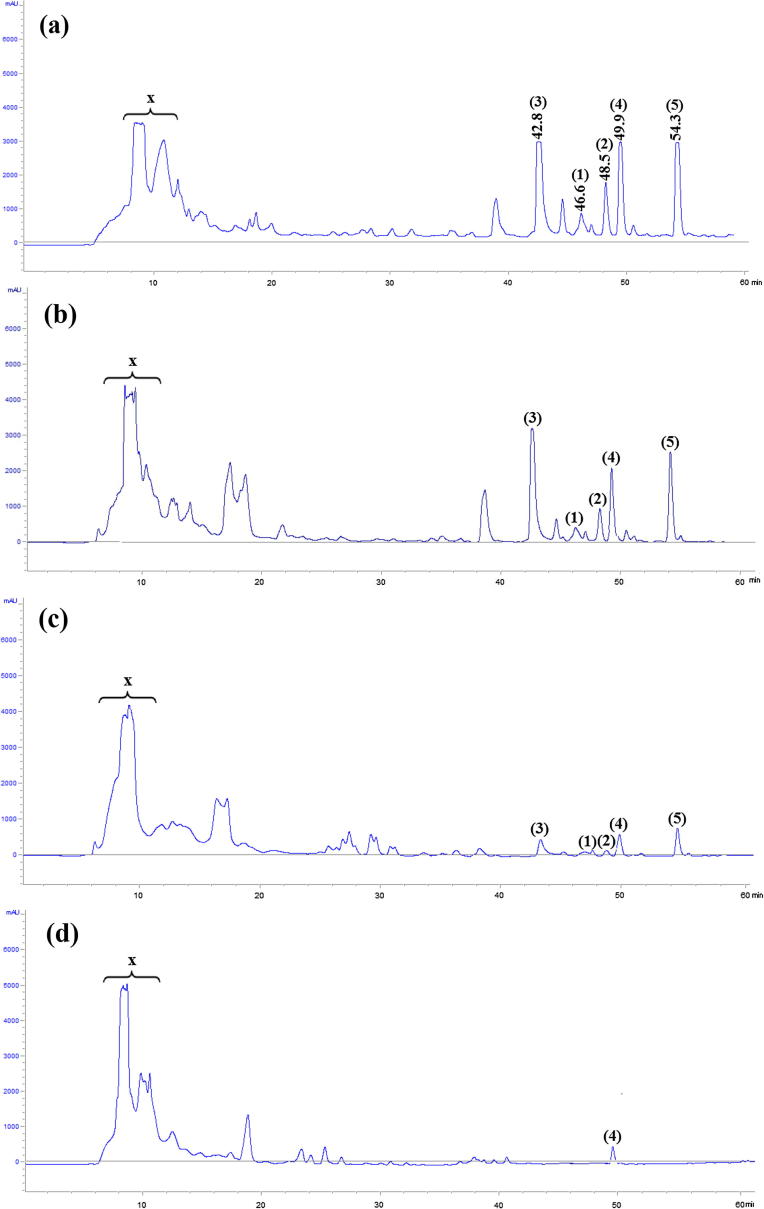
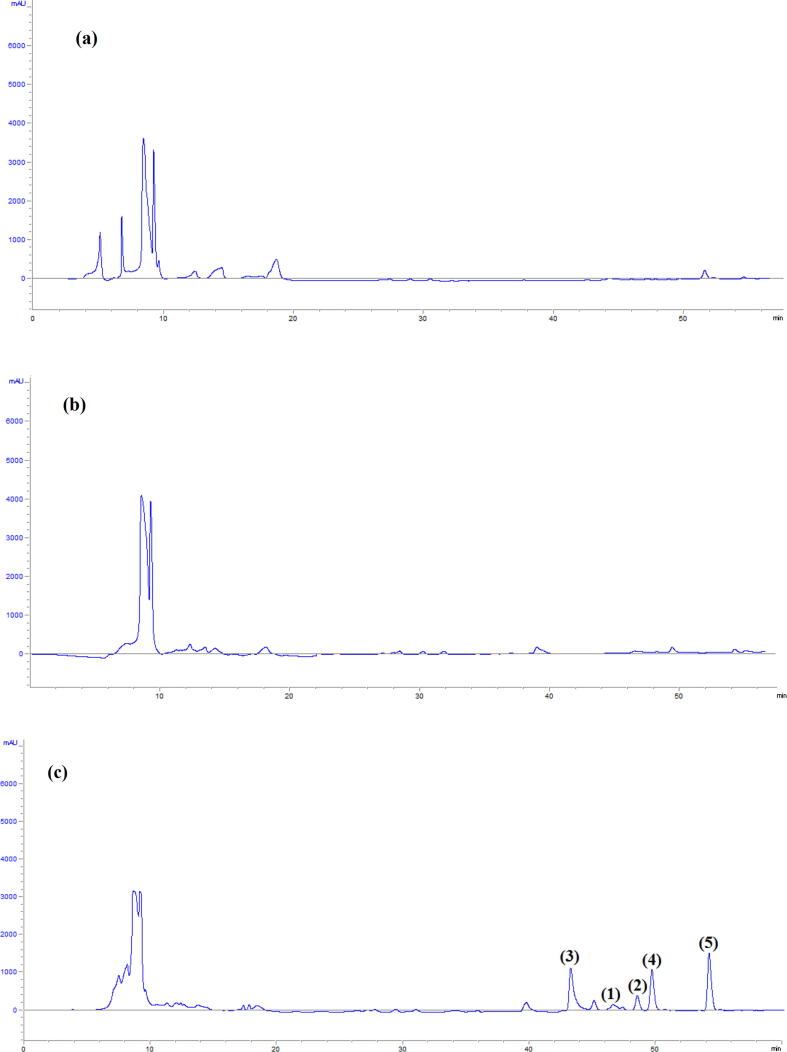
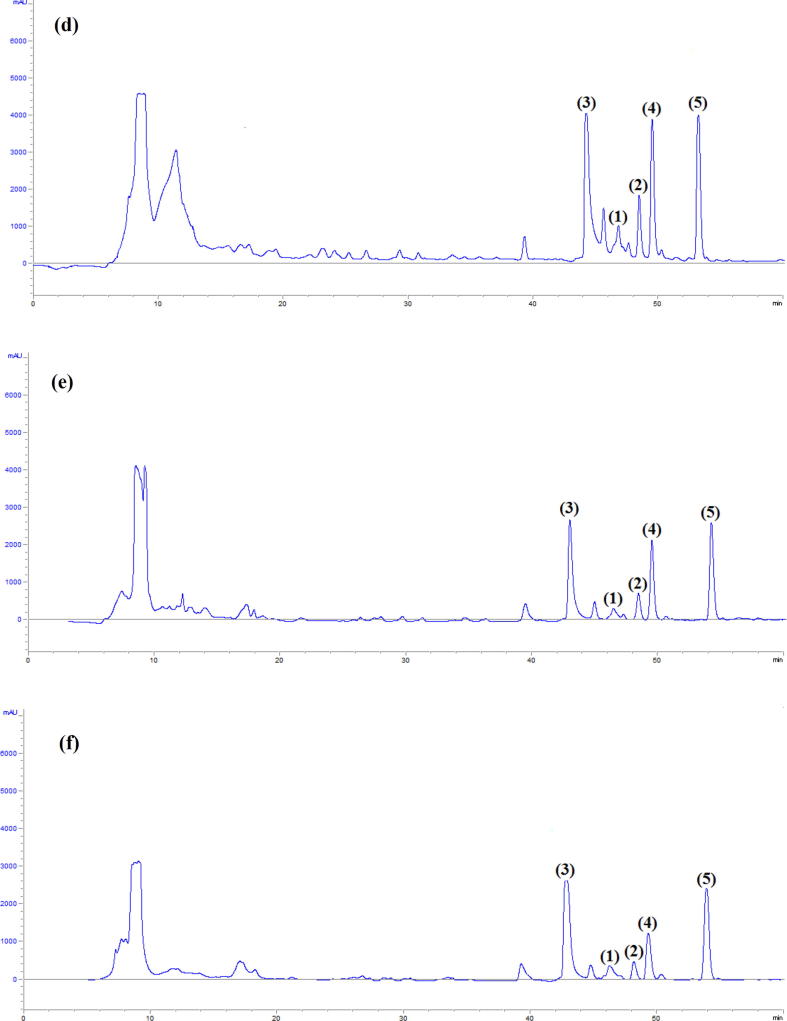
The HPLC profiles of methanolic extracts obtained after 7 days of incubation on ISP1, ISP2, NA and Bennett solid media are shown in Fig. 2. Five antimicrobial compounds (1–5) were collected from Bennett medium. The retention times of these compounds were 46.4 min (compound 1), 48.5 min (compound 2), 42.8 min (compound 3), 49.9 min (compound 4) and 54.3 min (compound 5). These compounds were also detected in the ISP2 methanolic extract. However, compounds 1–5 were not detected or produced in very small amounts in NA and ISP1 media. Compounds 1, 2, 3 and 5 were active only against Gram-positive bacteria. However, compound 4 showed antibacterial and antifungal activities. In addition of these five compounds, a hydrophilic unseparated complex (named X) was detected in all tested media. This complex has antibacterial and antifungal activity. The HPLC profiles from different days of incubation on Bennett medium (Fig. 3) showed that after 4 days the strain produced the five compounds. The major products were compounds 3, 4 and 5. After 7 days, the strain produced higher amounts of the five antimicrobial compounds. After 10 and 12 days, the amounts of the products were slightly reduced. We continued our study with products 1–5.
Fig. 2.
HPLC analysis of methanolic extracts of strain ABH26 cultured on Bennett (a), ISP2 (b), NA (c) and ISP1 (d) solid culture media. Numbers in brackets indicate the antimicrobial compounds secreted by the strain. Retention times were also indicated in Bennett medium.
Fig. 3.
HPLC analysis of methanolic extract of strain ABH26 cultured on Bennett medium for 0 day (a), 2 days (b), 4 days (c), 7 days (d), 10 days (e) and 12 days (f).
3.4. Spectroscopy and spectrometry analyses of antimicrobial products
Strain ABH26 was cultivated in 16 Petri dishes (170 mm in diameter), each containing 150 ml of Bennett solid medium at 30 °C for 7 days. The total volume of medium was 2.4 L.
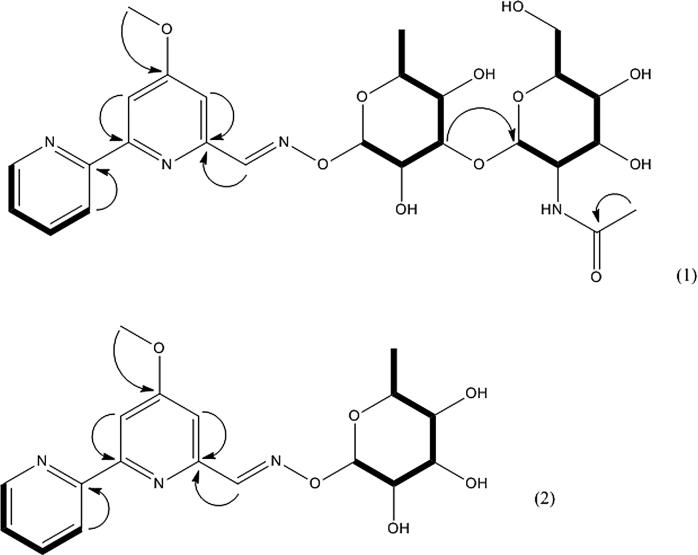
Compound 1 was obtained as a light purple oil. The UV spectrum showed the maximal absorbance at 234 and 266 nm. The ESIMS spectrum contained an ion peak at m/z 579.09 for [M-H]+. The 1H and 13C chemical shifts are given in Table 2. The 13C and HSQC spectra show 27 carbon signals. From the 13C data, it was possible to discern one amide group (δc 173.25), five hydroxyl groups (δc 61.0 to 74.56), one methoxy group (δc 54.90), 11 sp2–hybridized carbons (δc from 106.15 to 167.44) and 7 sp3-hybridized carbons (δc 16.63–102.74). The hydrogens of the hydroxyl and amide groups are not observed due to rapid exchange with CD3OD. The 2D 1H-1H and 1H-13C experiments and especially the long range 1H-13C couplings observed in the HMBC spectrum permitted to establish the connectivity between all the groups of the compound. The HMBC and COSY correlations of compound 1 are shown in Fig. 4, and the structure in Fig. 5. The NMR data suggested that compound 1 represents a new antibiotic belonging to the family of cyanogrisides, for which the name cyanogriside I was proposed. This compound is a methoxy-bipyridine linked (through a cyano radical C N) to two osidic derivatives, 2,4-dimethyl-α-L-mannose and N-acetylglucosamine. It differs from compound 4 by the presence of these sugars.
Table 2.
1H and 13C NMR data assignments of compounds 1 and 2 in CD3OD at 298 K. See Fig. 4, Fig. 5 for numbering of hydrogen and carbon atoms.
| 1H and 13C number |
1H chemical shift, ppm |
13C chemical shift, ppm |
||
|---|---|---|---|---|
| 1 | 2 | 1 | 2 | |
| 1 | 1.04 | 4.04 | 21.67 | 69.27 |
| 2 | – | 3.71 | 173.25 | 71.00 |
| 3 | – | 3.48 | – | 72.00 |
| 4 | 3.72 | 3.71 | 56.41 | 69.00 |
| 5 | 3.51 | 1.31 | 74.56 | 16.32 |
| 6 | 3.43 | 5.50 | 70.33 | 102.79 |
| 7 | 3.34 | 8.32 | 76.37 | 151.20 |
| 8 | 3.76–3.89 | – | 61.00 | 152.42 |
| 9 | 4.71 | 7.53 | 102.74 | 105.83 |
| 10 | 3.79 | – | 80.40 | 167.30 |
| 11 | 3.58 | 4.02 | 71.17 | 54.93 |
| 12 | 3.73 | 7.93 | 69.50 | 107.65 |
| 13 | 1.31 | – | 16.63 | 157.49 |
| 14 | 5.50 | – | 102.53 | 155.12 |
| 15 | 4.29 | 8.68 | 69.06 | 148.60 |
| 16 | 8.34 | 7.47 | 151.64 | 124.11 |
| 17 | – | 7.96 | 157.42 | 137.20 |
| 18 | 7.54 | 8.43 | 106.15 | 121.36 |
| 19 | – | – | 167.44 | – |
| 20 | 4.02 | – | 54.90 | – |
| 21 | 7.93 | – | 108.02 | – |
| 22 | – | – | 157.49 | – |
| 23 | – | – | 155.12 | – |
| 24 | 8.66 | – | 148.77 | – |
| 25 | 7.48 | – | 124.21 | – |
| 26 | 7.96 | – | 137.27 | – |
| 27 | 8.43 | – | 121.26 | – |
Fig. 4.
HMBC and COSY correlations of compounds 1 (1) and 2 (2).
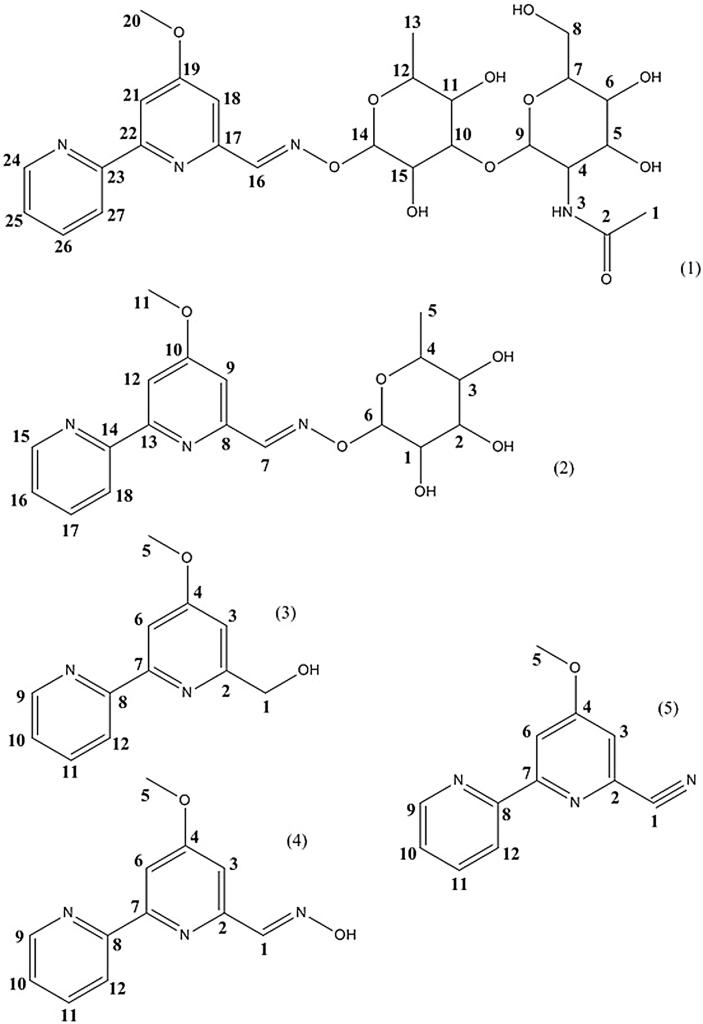
Fig. 5.
Structures of compounds 1 (1), 2 (2), 3 (3), 4 (4) and 5 (5).
Compound 2 was also obtained as a light purple oil. The UV spectrum showed the maximal absorbance at 238 and 272 nm. The ESIMS spectrum contained an ion peak at m/z 376.11 for [M-H]+. The 1H and 13C chemical shifts are given in Table 2. Eighteen carbon signals were present in the 13C and HSQC spectra. From the 13C data, it was possible to discern three hydroxyl groups (δc 69.27–72.00), one methoxy group (δc 54.93), 11 sp2–hybridized carbons (δc from 105.83 to 167.30) and 3 sp3-hybridized carbons (δc 16.32–102.79). Due to rapid exchange with CD3OD the hydrogens of the hydroxyl group are not observed. The 2D 1H-1H and 1H-13C experiments and especially the long range 1H-13C couplings observed in the HMBC spectrum permitted to establish the connectivity between all the groups of the compound. The HMBC and COSY correlations of compound 2 are shown in Fig. 4, and the structure in Fig. 5. The NMR data suggested that compound 2 represents a new antibiotic belonging to the family of cyanogrisides, for which the name cyanogriside J was proposed. This compound is a methoxy-bipyridine linked (through a cyano radical C N) to 2,4-dimethyl-α-L-mannose. It differs from the compound 4 by the presence of this sugar, and from the compound 1 by the absence of N-acetylglucosamine.
Compound 3 was obtained as white amorphous powder. The UV spectrum showed the maximal absorbance at 222 and 280 nm. The ESIMS spectrum contained an ion peak at m/z 217.16 for [M + H]+. The NMR data (Table 3) led to identify compound 3 as caerulomycin F (Fu et al., 2011). The structure of compound 3 is shown in Fig. 4. This compound is a methoxy-bipyridine containing a hydroxymethyl group CH2OH.
Table 3.
1H and 13C NMR data assignments of compounds 3, 4 and 5 in CD3OD at 298 K.
| 1H and 13C number |
1H chemical shift, ppm |
13C chemical shift, ppm |
||||
|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 3 | 4 | 5 | |
| 1 | 4.75 | 8.21 | – | 64.34 | 149.02 | 117.44 |
| 2 | – | – | – | 162.85 | 153.90 | |
| 3 | 7.14 | 7.45 | 7.53 | 106.28 | 105.35 | 115.81 |
| 4 | – | – | – | 167.86 | 167.31 | 167.50 |
| 5 | 4.00 | 4.00 | 4.05 | 54.62 | 54.58 | 55.80 |
| 6 | 7.75 | 7.87 | 8.21 | 105.33 | 107.15 | 109.47 |
| 7 | – | – | – | 156.84 | 156.82 | 156.80 |
| 8 | – | – | – | 155.82 | 155.38 | 155.65 |
| 9 | 8.65 | 8.66 | 8.73 | 148.65 | 148.54 | 149.22 |
| 10 | 7.46 | 7.47 | 7.53 | 123.94 | 123.96 | 125.00 |
| 11 | 7.95 | 7.96 | 8.00 | 137.27 | 137.20 | 137.63 |
| 12 | 8.35 | 8.41 | 8.43 | 121.63 | 121.60 | 121.49 |
Compound 4 was obtained as white powder. The UV spectrum showed the maximal absorbance at 225, 245 and 283 nm. The ESIMS spectrum contained an ion peak at m/z 230.11 for [M + H]+. The NMR data (Table 3) led to identify compound 4 as caerulomycin A (Funk and Divekar, 1959). The structure of compound 4 is shown in Fig. 4. This compound is a methoxy-bipyridine containing an oxime group CH N—OH.
Compound 5 was obtained as a light purple oil. The UV spectrum showed the maximal absorbance at 222, 246 and 280 nm. The ESIMS spectrum contained an ion peak at m/z 212.23 for [M+H]+. The NMR data (Table 3) led to identify compound 5 as caerulomycinonitrile (Fu et al., 2011). The structure of compound 5 is shown in Fig. 4. This compound is a methoxy-bipyridine linked to a nitrile group C N.
3.5. Minimum inhibitory concentrations of antimicrobial compounds
Minimum inhibitory concentrations of compounds 1–5 are summarized in Table 4. The most active product was compound 4. The MIC values of this compound were between 1 and 20 µg/ml for filamentous fungi, 2–10 µg/ml for yeasts and 3–50 µg/ml for Gram-positive bacteria. The strains of Gram-negative bacteria were resistant. The comparison of MIC values of compound 4 with those of the standard antibiotics used in this study indicated that the compound 4 showed a better activity than amoxicillin against the Gram positive bacteria Micrococcus luteus ATCC 9314, S. aureus ATCC 43,300 and S. aureus MRSA 639c, a same activity against four other Gram-positive bacteria, and a lower activity against all the Gram negative bacteria tested. For the antifungal activity, the compound 4 showed a better activity against nine filamentous fungi and yeasts by comparison with nystatin, a same activity against five fungi and a lower activity against five other fungi. Both the new compounds (1 and 2) and the known compounds (3 and 5) showed activity only against Gram-positive bacteria and this activity was less than that of compound 4. For these compounds, the most sensitive strains were Micrococcus luteus ATCC 9314, Corynebacterium diphtheriae CD1 and Listeria monocytogenes ATCC 13,932 (25 to 30 µg/ml), and the most resistant strain was Staphylococcus aureus MRSA 639c (100 µg/ml). The compounds 1, 2, 3 and 5 showed a better activity than amoxicillin against S. aureus ATCC 43,300 and S. aureus MRSA 639c, a same activity against Corynebacterium diphtheriae CD1, Enterococcus faecalis EF1 and Listeria monocytogenes ATCC 13,932 (except for the compound 5) and a lower activity against Micrococcus luteus ATCC 9314 and Staphylococcus aureus ATCC 29213.
Table 4.
Minimum inhibitory concentration (MIC) of compounds 1–5 produced by the strain ABH26.
| Target microorganism | Minimum inhibitory concentration (µg/ml) |
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Bacillus subtilis ATCC 6633 | 50 | 50 | 50 | 10 | 60 |
| Corynebacterium diphtheriae CD1 | 25 | 25 | 25 | 25 | 30 |
| Enterococcus faecalis EF1 | 50 | 50 | 50 | 50 | 60 |
| Listeria monocytogenes ATCC 13,932 | 25 | 25 | 25 | 25 | 30 |
| Micrococcus luteus ATCC 9314 | 25 | 25 | 25 | 3 | 30 |
| Staphylococcus aureus ATCC 29,213 | 50 | 50 | 50 | 50 | 60 |
| S. aureus ATCC 43,300 | 50 | 50 | 50 | 50 | 60 |
| S. aureus MRSA 639c | 100 | 100 | 100 | 20 | 100 |
| Escherichia coli E52 | >100 | >100 | >100 | >100 | >100 |
| E. coli E195 | >100 | >100 | >100 | >100 | >100 |
| Klebsiella pneumoniae E40 | >100 | >100 | >100 | >100 | >100 |
| Pseudomonas aeruginosa IPA1 | >100 | >100 | >100 | >100 | >100 |
| Aspergillus brasiliensis AB1 | >100 | >100 | >100 | 10 | >100 |
| A. carbonarius AC1 | >100 | >100 | >100 | 1 | >100 |
| A. carbonarius AC2 | >100 | >100 | >100 | 10 | >100 |
| A. flavus NRRL 3251 | >100 | >100 | >100 | 15 | >100 |
| A. parasiticus CBS 100,926 | >100 | >100 | >100 | 20 | >100 |
| A. westerdijkiae ATCC 3174 | >100 | >100 | >100 | 15 | >100 |
| Fusarium culmorum FC1 | >100 | >100 | >100 | 3 | >100 |
| F. equiseti FE1 | >100 | >100 | >100 | 10 | >100 |
| F. graminearum FG1 | >100 | >100 | >100 | 10 | >100 |
| F. oxysporum f. sp. albedinis Foa1 | >100 | >100 | >100 | 3 | >100 |
| F. o. f. sp. radicis-lycopersici Forl | >100 | >100 | >100 | 10 | >100 |
| F. solani Fsol | >100 | >100 | >100 | 3 | >100 |
| F. sporotrichioides FS1 | >100 | >100 | >100 | 5 | >100 |
| Rhizoctonia solani AG3 | >100 | >100 | >100 | 3 | >100 |
| Umbelopsis ramanniana NRRL 1829 | >100 | >100 | >100 | 2 | >100 |
| Candida albicans M2 | >100 | >100 | >100 | 10 | >100 |
| C. albicans M3 | >100 | >100 | >100 | 2 | >100 |
| C. albicans IPA200 | >100 | >100 | >100 | 10 | >100 |
| Saccharomyces cerevisiae ATCC 4226 | >100 | >100 | >100 | 2 | >100 |
4. Discussion
Strain ABH26 is related to Saccharothrix xinjiangensis (Lahoum et al., 2015). It has not been indicated in the literature that this species produces cyanogrisides and caerulomycins, but rather that it is a producer of tianchimycins, which are macrolides (Wang et al., 2013). This is the first report of cyanogrisides and caerulomycins production by a member of the Saccharothrix genus. The antibiotics belonging to these two families are known to be produced by Streptomyces caeruleus (Funk and Divekar, 1959) and Actinoalloteichus cyanogriseus (Fu et al., 2011), but are also manufactured synthetically because their important biological activity (Bobrov and Tyvorskii, 2010). The new cyanogrisides obtained in this study (products 1 and 2) only showed inhibitory activity against Gram-positive bacteria. In the literature, antimicrobial activity of cyanogrisides has never been reported. However, many works have described the antitumor activity of these products (Fu et al., 2011, Fu et al., 2014). In addition, Fu et al. (2014) reported that the cytotoxicity of cyanogrisides was lower than that of caerulomycin A. The previously identified caerulomycin A (product 4) exhibited an important activity. We noted that its antifungal activity was stronger than its antibacterial activity. The broad antifungal activity of caerulomycin A has been extensively reported (Chatterjee et al., 1984, Ambavane et al., 2014). Other studies have suggested the important role of caerulomycin A in the regulation of immune homeostasis (Gurram et al., 2014, Kujur et al., 2015). Product 3 (caerulomycin F) presented the same activity spectrum as the two new cyanogrisides, while product 5 (caerulomycinonitrile) has the lowest activity. In our study, we noted that the isolated products were not active against Gram-negative bacteria. The same observation was found by Boubetra et al. (2013c), who reported that the new antibiotics produced by Saccharothrix SA198 (isolated from Saharan soil) showed no activity against Gram-negative bacteria.
Compounds 1, 2, 3, 4 and 5 were secreted by strain ABH26 only on solid media, especially those containing glucose, such as Bennett medium (glucose, peptone, yeast extract and beef extract) and ISP2 medium (glucose, yeast extract and malt extract), while very small amounts were obtained on NA (peptone, yeast extract and beef extract) and ISP1 (tryptone and yeast extract) media. Thus, glucose seems to have an important role in the synthesis of these products. The production of compounds (1–5) started at 4 days in Bennett medium and reached a maximum after 7 days. Caerulomycins F (product 3) and A (product 4) and caerulomycinonitrile (product 5) are the major compounds. The new cyanogrisides (products 1 and 2) are produced in smaller quantities. The structure of the active product contained in the hydrophilic complex X could not be determined because of the difficulties encountered during its purification. However, this product appears to be the cause of activity observed in ISP1 and NA media (where the compounds 1–5 are not produced or produced in small amounts) and also the cause of activity observed during the second day of the kinetics study (compounds 1–5 being produced until the 4th day).
Moreover, we noted a correlation between the structure and antimicrobial activity of the compounds. Caerulomycin A (methoxy-bipyridine containing an oxime group CH N—OH) showed potent activity against Gram-positive bacteria, yeasts and filamentous fungi. The linkage of a sugar (2,4-dimethyl-α-L-mannose) or two sugars (2,4-dimethyl-α-L-mannose and N-acetylglucosamine) for formation of the new cyanogrisides (products 2 and 1 respectively), led to a considerable decrease in activity. This activity becomes weaker against Gram-positive bacteria and absent against filamentous fungi and yeasts. However, the presence of sugars may be also lead to decreased cytotoxicity to animal cells as reported by Fu et al. (2014). Similarly, we noted that the oxime group CH N—OH of product 4 had a very important role in the high activity of this product. Indeed, when this group is replaced by a hydroxymethyl group CH2OH (product 3) or by a nitrile group (product 5) the activity decreased considerably against Gram-positive bacteria and becomes absent against filamentous fungi and yeasts.
5. Conclusion
Strain ABH26, related to Saccharothrix xinjiangensis, produced five bipyridine antibiotics belonging to the caruleomycins and cyanogrisides families. Two new cyanogriside antibiotics, named cyanogriside I and cyanogriside J, were characterized along with three known caerulomycins: caerulomycin A, caerulomycin F and caerulomycinonitrile. The five compounds showed activity against several pathogenic Gram-positive bacteria and one of them also exhibited an interesting antifungal activity. The activity of the compounds was related to their chemical structure. The oxime group bonded to the bipyridine ring strongly increased activity while the presence of sugars decreased this activity. This is the first report of cyanogrisides and caerulomycins production by a member of the Saccharothrix genus.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgements
This work was gratefully supported by the « Comité mixte Franco-Algérien de l’Accord Programme TASSILI (PHC No. 15 MDU 932; Campus France: 33000WC) ».
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Nasserdine Sabaou, Email: sabaou@yahoo.fr.
Florence Mathieu, Email: Florence.Mathieu@ensat.fr.
References
- Ambavane A., Tokdar P., Parab R., Sreekumar E.S., Mahajan G., Mishra P.D., D’Souza L., Ranadive P. Caerulomycin A - an antifungal compound isolated from marine actinomycetes. Adv. Microbiol. 2014;4(9):567–578. [Google Scholar]
- Aouiche A., Sabaou N., Meklat A., Zitouni A., Bijani C., Mathieu F., Lebrihi A. Saccharothrix sp. PAL54, a new chloramphenicol-producing strain isolated from a Saharan soil. World J. Microbiol. Biotechnol. 2012;28(3):943–951. doi: 10.1007/s11274-011-0892-2. [DOI] [PubMed] [Google Scholar]
- Berdy J. Thoughts and facts about antibiotics: where we are now and where we are heading. J. Antibiot. 2012;65(8):385–395. doi: 10.1038/ja.2012.27. [DOI] [PubMed] [Google Scholar]
- Bobrov D.N., Tyvorskii V.I. Facile synthesis of caerulomycin E by the formation of 2,2′-bipyridine core via a 2-pyridyl substituted 4H-pyran-4-one. Formal synthesis of caerulomycin A. Tetrahedron. 2010;66(29):5432–5434. [Google Scholar]
- Boubetra D., Zitouni A., Bouras N., Mathieu F., Lebrihi A., Schumann P., Spröer C., Klenk H.P., Sabaou N. Saccharothrix hoggarensis sp. nov., an actinomycete isolated from Saharan soil. Int. J. Syst. Evol. Microbiol. 2013;63(2):549–553. doi: 10.1099/ijs.0.039099-0. [DOI] [PubMed] [Google Scholar]
- Boubetra D., Zitouni A., Bouras N., Mathieu F., Lebrihi A., Schumann P., Spröer C., Klenk H.P., Sabaou N. Saccharothrix saharensis sp. nov., an actinomycete isolated from Algerian Saharan soil. Int. J. Syst. Evol. Microbiol. 2013;63(10):3744–3749. doi: 10.1099/ijs.0.051839-0. [DOI] [PubMed] [Google Scholar]
- Boubetra D., Sabaou N., Zitouni A., Bijani C., Lebrihi A., Mathieu F. Taxonomy and chemical characterization of new antibiotics produced by Saccharothrix SA198 isolated from a Saharan soil. Microbiol. Res. 2013;168(4):223–230. doi: 10.1016/j.micres.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Boubetra D., Zitouni A., Bouras N., Schumann P., Spröer C., Klenk H.P., Sabaou N. Saccharothrix tamanrassetensis sp. nov., an actinomycete isolated from Saharan soil. Int. J. Syst. Evol. Microbiol. 2015;65(4):1316–1320. doi: 10.1099/ijs.0.000104. [DOI] [PubMed] [Google Scholar]
- Bouras N., Merrouche R., Lamari L., Mathieu F., Sabaou N., Lebrihi A. Precursor directed biosynthesis of new dithiolopyrrolone analogs by Saccharothrix algeriensis NRRL B-24137. Process Biochem. 2008;43(11):1244–1252. [Google Scholar]
- Bouznada K., Bouras N., Mokrane S., Chaabane Chaouch F., Zitouni A., Pötter G., Spröer C., Klenk H.P., Sabaou N. Saccharothrix isguenensis sp. nov., an actinobacterium isolated from desert soil. Int. J. Syst. Evol. Microbiol. 2016;66(11):4785–4790. doi: 10.1099/ijsem.0.001430. [DOI] [PubMed] [Google Scholar]
- Bouznada K., Bouras N., Mokrane S., Chaabane Chaouch F., Zitouni A., Pötter G., Spröer C., Klenk H.P., Sabaou N. Saccharothrix ghardaiensis sp. nov., an actinobacterium isolated from Saharan soil. Antonie van Leeuwenhoek. 2017;110(3):399–405. doi: 10.1007/s10482-016-0812-4. [DOI] [PubMed] [Google Scholar]
- Chatterjee D.K., Raether W., Iyer N., Ganguli B.N. Caerulomycin, an antifungal antibiotic with marked in vitro and in vivo activity against Entamoeba histolytica. Z. Parasitenkd. 1984;70(5):569–573. doi: 10.1007/BF00926587. [DOI] [PubMed] [Google Scholar]
- Fu P., Liu P., Li X., Wang Y., Wang S., Hong K., Zhu W. Cyclic bipyridine glycosides from the marine-derived actinomycete Actinoalloteichus cyanogriseus WH1-2216-6. Org. Lett. 2011;13(22):5948–5951. doi: 10.1021/ol202245s. [DOI] [PubMed] [Google Scholar]
- Fu P., Zhu Y., Mei X., Wang Y., Jia H., Zhang C., Zhu W. Acyclic congeners from Actinoalloteichus cyanogriseus provide insights into cyclic bipyridine glycoside formation. Org. Lett. 2014;16(16):4264–4267. doi: 10.1021/ol5019757. [DOI] [PubMed] [Google Scholar]
- Funk A., Divekar P.V. Caerulomycin, a new antibiotic from Streptomyces caeruleus Baldacci. I. Production, isolation, assay, and biological properties. Can. J. Microbiol. 1959;5(4):317–321. doi: 10.1139/m59-039. [DOI] [PubMed] [Google Scholar]
- Gurram R.K., Kujur W., Maurya S.K., Agrewala J.N. Caerulomycin A enhances the TGF-β-Smad3 signalling by suppressing IFN-γ-STAT1 signalling to expand Tregs. J. Biol. Chem. 2014;289(25):17515–17528. doi: 10.1074/jbc.M113.545871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujur W., Gurram R.K., Haleem N., Maurya S.K., Agrewala J.N. Caerulomycin A inhibits Th2 cell activity: a possible role in the management of asthma. Sci. Rep. 2015;5:15396. doi: 10.1038/srep15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahoum A., Bouras N., Bouznada K., Holtz M.D., Spröer C., Klenk H.P., Sabaou N. Saccharothrix sp. ABH26, a new actinobacterial strain from Algerian Saharan soil: isolation, identification and antimicrobial activity. J. Microbiol. Biotech. Food Sci. 2015;4(5):415–420. [Google Scholar]
- Lamari L., Zitouni A., Boudjella H., Badji B., Sabaou N., Lebrihi A. New dithiolopyrrolone antibiotics from Saccharothrix sp. SA 233 I. Taxonomy, production, isolation and biological properties. J. Antibiot. 2002;55(8):696–701. doi: 10.7164/antibiotics.55.696. [DOI] [PubMed] [Google Scholar]
- Merrouche R., Bouras N., Coppel Y., Mathieu F., Sabaou N., Lebrihi A. New dithiolopyrrolone antibiotics induced by adding sorbic acid to the culture medium of Saccharothrix algeriensis NRRL B-24137. FEMS Microbiol. Lett. 2011;318(1):41–46. doi: 10.1111/j.1574-6968.2011.02246.x. [DOI] [PubMed] [Google Scholar]
- Naruse N., Tenmyo O., Kawano K., Tomita K., Ohgusa N., Miyaki T., Konishi M., Oki T. Fluvirucins A1, A2, B1, B2, B3, B4 and B5, new antibiotics active against influenza A virus. I. Production, isolation, chemical properties and biological activities. J. Antibiot. 1991;44(9):733–740. doi: 10.7164/antibiotics.44.733. [DOI] [PubMed] [Google Scholar]
- Oki T., Tenmyo O., Tomatsu K., Kamei H. Pradimicins A, B and C: new antifungal antibiotics. II. In vitro and in vivo biological activities. J. Antibiot. 1990;43(7):763–770. doi: 10.7164/antibiotics.43.763. [DOI] [PubMed] [Google Scholar]
- Sabaou N., Boudjella H., Bennadji A., Mostefaoui A., Zitouni A., Lamari L., Bennadji H., Lefèbvre G., Germain P. Les sols des oasis du Sahara algérien, source d’actinomycètes rares producteurs d’antibiotiques. Sécheresse. 1998;9(2):147–153. [Google Scholar]
- Shirling B., Gottlieb D. Methods for characterization of Streptomyces species. Int. J. Syst. Bacteriol. 1966;16(3):3313–3340. [Google Scholar]
- Waksman S.A. Williams & Wilkins; Baltimore: 1961. Classification, identification, and descriptions of genera and species. The actinomycetes. [Google Scholar]
- Wang X., Tabudravu J., Jaspars M., Deng H. Tianchimycins A-B, 16-membered macrolides from the rare actinomycete Saccharothrix xinjiangensis. Tetrahedron. 2013;69(30):6060–6064. [Google Scholar]
- Zitouni A., Lamari L., Boudjella H., Badji B., Sabaou N., Gaouar A., Mathieu F., Lebrihi A., Labeda D.P. Saccharothrix algeriensis sp. nov., isolated from Saharan soil. Int. J. Syst. Evol. Microbiol. 2004;54(4):1377–1381. doi: 10.1099/ijs.0.02679-0. [DOI] [PubMed] [Google Scholar]
- Zitouni A., Boudjella H., Mathieu F., Sabaou N., Lebrihi A. Mutactimycin PR, a new anthracycline antibiotic from Saccharothrix sp. SA 103. I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 2004;57(6):367–372. doi: 10.7164/antibiotics.57.367. [DOI] [PubMed] [Google Scholar]
- Zitouni A., Boudjella H., Lamari L., Badji B., Mathieu F., Lebrihi A., Sabaou N. Nocardiopsis and Saccharothrix genera in Saharan soils in Algeria: isolation, biological activities and partial characterization of antibiotics. Res. Microbiol. 2005;156(10):984–993. doi: 10.1016/j.resmic.2005.05.006. [DOI] [PubMed] [Google Scholar]