Abstract
The 8-hydroxyquinoline core is a privileged scaffold for drug design explored to afford novel derivatives endowed with biological activity. Our research aimed at clarifying the antifungal mechanism of action of clioquinol, 8-hydroxy-5-quinolinesulfonic acid, and 8-hydroxy-7-iodo-5-quinolinesulfonic acid (three 8-hydroxyquinoline derivatives). The antifungal mode of action of these derivatives on Candida spp. and dermatophytes was investigated using sorbitol protection assay, cellular leakage effect, ergosterol binding assay, and scanning electron microscopy. Clioquinol damaged the cell wall and inhibited the formation of pseudohyphae by C. albicans. The 8-hydroxy-5-quinolinesulfonic acid derivatives compromised the functional integrity of cytoplasmic membranes. To date no similar report was found about the antifungal mechanism of 8-hydroxyquinolines. These results, combined with the broad antifungal spectrum already demonstrated previously, reinforce the potential of 8-hydroxyquinolines for the development of new drugs.
Keywords: Clioquinol, 8-Hydroxyquinoline derivatives, Mechanism of action, Candida spp., Dermatophytes
1. Introduction
The discovery of new antifungal agents is modest when compared to antibacterial drugs. Similarities between fungi and mammals eukaryotic cells limit their development. Besides, the traditionally smaller number of fungal compared to bacterial infections also slowed the discovery of antifungals (Vandeputte et al., 2012, Scorzoni et al., 2017). However, in recent decades, a dramatic increase of fungal infections has been noticed and diseases related to them are now considered a global public health problem (Vandeputte et al., 2012).
Fungi are significantly affecting the growing population of patients with weakened immune system and the systemic fungal infections are increasingly important causes of high morbidity and mortality. Additionally to life-threatening systemic infection, such as those caused by Candida spp., superficial and cutaneous mycoses can persist requiring continuous treatment (Scorzoni et al., 2017). In addition, resistance to antifungal drugs has become an important problem and further intensifies the need for new treatment strategies (Lukaszuk et al., 2017).
It is clear that the discovery of new antimicrobials is essential nevertheless more detailed research of molecules with known antifungal activity and structural modifications to improve the action seem to be faster alternatives to this end (Ngo et al., 2016). 8-Hydroxyquinoline derivatives are a subclass of quinolines with a wide variety of biological effects. The 8-hydroxyquinoline derivatives emerged as a privileged scaffold for drug design being widely explored (Prachayasittikul et al., 2013) for several biological functions such as: neuroprotection (Suwanjang et al., 2016), anticancer (Chan-On et al., 2015), anti-HIV, antimicrobial (Abouelhassan et al., 2017, Cherdtrakulkiat et al., 2016) and antifungal effects (Oliveri and Vecchio, 2016).
The most known member of this family is clioquinol (5-chloro-7-iodo-8-hydroxyquinoline, 1) (Oliveri and Vecchio, 2016). Clioquinol was marketed in 1934 by Ciba-Geigy (now Novartis) as an antimicrobial agent for the treatment of a wide range of intestinal diseases. In the 1970s, it was withdrawn from the market as an oral agent due to association with a neurodegenerative syndrome known as subacute myelooptic neuropathy (SMON) (Mao and Schimmer, 2008, Nakae et al., 1973, Oliveri and Vecchio, 2016). Clioquinol is currently available as a topical formulation for the treatment of skin infections (Mao and Schimmer, 2008), and in the 2000s the interest in this drug reappeared as a potential treatment of non-infectious pathologies such as: Alzheimer’s, Parkinson’s and Huntington’s disease (Finkelstein et al., 2016, Franklin et al., 2016, Huntington Study Group Reach2HD Investigators, 2015, Zhang et al., 2013).
Although the antifungal activity of clioquinol is well known and presents rare reports of resistance (Alsterholm et al., 2010), its mechanism of action into fungal cells is poorly understood. Some groups have proposed 8-hydroxyquinoline derivatives as new antifungals but without mentioning how these compounds act on fungal cells (Gershon et al., 2001, Pippi et al., 2017).
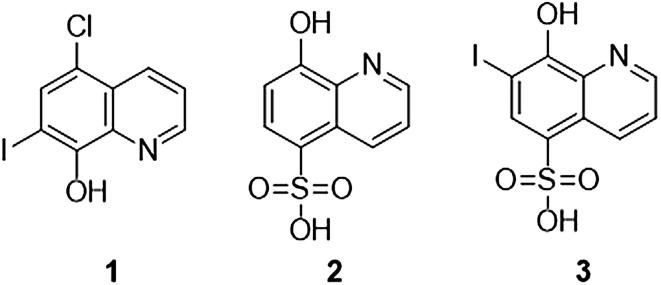
Thus, herein it is presented some insights into the mechanism of action of clioquinol (derivative 1) and two other 8-hydroxyquinoline derivatives: 8-hydroxy-5-quinolinesulfonic acid (derivative 2) and 8-hydroxy-7-iodo-5-quinolinesulfonic acid (derivative 3) (Fig. 1). In vitro methods were employed to analyze alterations of fungal morphology, cell wall damage, cellular leakage, and ergosterol complexation. Since modifying and/or repurposing current treatments represent valid ways of seeking new antifungal agents, our research aimed to gain information about the mode of action in order to rationalize the design of new antifungal derivatives.
Fig. 1.
Chemical structures of 8-hidroxyquinoline derivatives 1–3.
2. Material and methods
2.1. Fungal strains
A total of 27 isolates were included in this study: three strains of Candida albicans (ATCC 18804, CA 01, CA DEB 05), C. glabrata (CG RL12m, CG RL24, CG RL49), C. krusei (CK 03, CK CVB 42, CK Den 43), C. parapsilosis (CP RL11m, CP RL27m, CP RL 38), C. tropicalis (ATCC 750, CT 57A, CT 72A), Microsporum canis (MCA 01, MCA 29, MCA 40), M. gypseum (MGY 42, MGY 50, MGY 58), Trichophytum mentagrophytes (TME 16, TME 32, TME 40) and T. rubrum (TRU 43, TRU 50, TRU 51). All isolates are deposited into the Mycology Collection of Universidade Federal do Rio Grande do Sul (Porto Alegre, Brazil) and were obtained from the National Program of Quality Control of Brazil and clinical samples. Standard strains of C. albicans (ATCC 18804) and C. tropicalis (ATCC 750) were obtained from ATCC (American Type Culture Collection, Manassas, VA, USA) and were included as controls.
2.2. Antifungal compounds
Clioquinol (1), 8-hydroxy-5-quinolinesulfonic acid (2), and 8-hydroxy-7-iodo-5-quinolinesulfonic acid (3) were purchased from Sigma-Aldrich (St. Louis, MO, USA). These compounds were dissolved in DMSO (Sigma-Aldrich) and diluted in a medium assay to obtain a maximum concentration of 2% DMSO in the experiments.
2.3. Sorbitol protection assay
The effect of the 8-hydroxyquinoline derivatives on the integrity of the fungal cell wall was evaluated by sorbitol protection assay. Minimum inhibitory concentrations (MICs) of 8-hydroxyquinoline derivatives 1–3 were determined by the standard broth microdilution (CLSI M27-A3 for Candida spp.; CLSI M38-A2 for dermatophytes) (Clinical and Laboratory Standards Institute, 2008a, Clinical and Laboratory Standards Institute, 2008b) in the absence and presence of 0.8 M sorbitol (Sigma-Aldrich) added to the RPMI 1640 growth medium (Gibco) as an osmoprotectant. Microplates were incubated at 35 °C and 30 °C for Candida spp. and dermatophytes, respectively. Experiments were carried out in duplicate for all three strains of each species. Anidulafungin (Pfizer, New York, USA) was used as positive control. MICs were measured after 2 and 7 days for Candida spp.; and after 4 and 7 days for dermatophytes (Escalante et al., 2008).
2.4. Cellular leakage effect
The cell leakage was assessed by measuring 260-nm-absorbing materials released to the medium, primarily representing nucleotides of which uracil-containing compounds exhibited the strongest absorbance (Lunde and Kubo, 2000). The measurement of cellular leakage was carried out with one representative strain of each species studied (C. albicans ATCC 18804, C. glabrata CG RL24, C. krusei CK Den 43, C. parapsilosis CP RL38, C. tropicalis ATCC 750, M. canis MCA 01, M. gypseum MGY 50, T. mentagrophytes TME 40 and T. rubrum TRU 43) and the procedures were conducted as previously described with some modifications (Escalante et al., 2008, Lunde and Kubo, 2000). Candida spp. were cultured on SDA (Sabouraud Dextrose Agar; HiMedia; India) at 35 °C for 48 h. After incubation, the cells were washed three times (3000 rpm for 10 min, Spinlab SL-5M, Ribeirão Preto, Brazil), suspended and adjusted to approximately 1–5 × 106 cells/mL with cold 0.16 M MOPS buffered at pH 7. A 1:10 dilution of fungal suspension was made in cold MOPS buffer with the desired amount of 8-hydroxyquinoline derivatives 1–3 in order to obtain the final concentration equal to MIC. This dilution resulted in a suspension with fungal inoculum of 0.1–0.5 × 106 cells/mL. For dermatophytes, a suspension of each fungus was prepared after 10 days at 30 °C on PDA (potato dextrose agar; HiMedia; India) and adjusted to 1–3 × 103 cells/mL with cold MOPS buffer and derivatives 1–3. Untreated cells incubated with MOPS buffer were prepared as negative control and cells treated with amphotericin B (6.25 µg/mL) and SDS (2%) (Sodium dodecyl sulfate; Neon, São Paulo, Brazil) were used as positive control. Solutions and controls were tested in triplicate. Incubation was performed at 35 °C (Candida spp.) or 30 °C (dermatophytes). Aliquots were taken at time intervals (6, 24 and 48 h for Candida spp.; 6, 24, 48 and 96 h for dermatophytes) and centrifuged at 10,000 rpm for 10 min (Biosystems MCD2000, Curitiba, Brazil). The absorbance of supernatants was analyzed at 260 nm in a spectrophotometer (Agilent Technologies 8453, Santa Clara, EUA). Solutions of SDS (2%), amphotericin B (6.25 µg/mL), and 8-hydroxyquinoline derivatives 1–3 (MIC) without fungal inoculum were used as blank for absorbance readings. The results were expressed as means ± standard deviation. Absorption obtained for derivatives 1–3, SDS and amphotericin B were compared to the absorption of the untreated control and analyzed by one-Way ANOVA as well as Dunnett’s multiple comparisons test. P < 0.05 was considered statistically significant.
2.5. Ergosterol binding assay
The ability of the 8-hydroxyquinoline derivatives to complex with ergosterol in the fungal membrane was evaluated by ergosterol binding assay. The MICs of 8-hydroxyquinoline derivatives 1–3 were determined by the standard broth microdilution (Clinical and Laboratory Standards Institute, 2008a, Clinical and Laboratory Standards Institute, 2008b) in the absence and presence of different concentrations (50–250 µg/ml) of external ergosterol (Sigma-Aldrich, St. Louis, MO, USA) added to the RPMI 1640 growth medium. The plates were incubated at 35 °C and 30 °C for Candida spp. and dermatophytes, respectively. Experiments were carried out in triplicate and tested for all three strains of each species. Amphotericin B (União Química, São Paulo, Brazil) was used as positive control. MICs were measured after 48 h of incubation for Candida spp. and 96 h for dermatophytes (Escalante et al., 2008).
2.6. Scanning electron microscopy (SEM)
Morphological changes in C. albicans ATCC 18804, T. mentagrophytes TME 40 and M. canis MCA 01 grown in the presence of 8-hydroxyquinoline derivatives 1–3 were determined using the macro broth dilution method (CLSI M27-A3; CLSI M38-A2). Treated and untreated (control) cells were analyzed by SEM. After the incubation period (C. albicans: 48 h at 35 °C; M. canis and T. mentagrophytes: 96 h at 30 °C), fungal cells treated with the derivatives 1–3 (MIC/2) and untreated cells were washed three times with PBS (3000 rpm for 5 min. Biosystems MCD2000, Curitiba, Brazil). After washing, cells were fixed in 1 ml of modified Karnovsky's fixative adapted from Joubert et al. (2015). Then, the wells were washed three times (3000 rpm for 5 min) in 0.1 M sodium cacodylate buffered at pH 7.2 containing 0.2 M sucrose, and 2 mM MgCl2 with the aid of two pipettes, which were used for addition and concurrent removal to avoid air exposure. Cells were adhered in coverslips previously functionalized with poly-L-lysine for 1 h. Adhered cells were dehydrated in a series of freshly made solutions of graded acetone 30, 50, 70, 95 (5 min) and 100% (10 min). Samples were then subjected to critical point drying (EM CPD 300, Leica), mounted on metallic stubs, sputter-coated with a 15–20 nm gold-palladium layer and visualized in a scanning electron microscope (Carl Zeiss EVO® MA10 Carl, Oberkochen, Germany) operating at 10 kV.
3. Results
3.1. Sorbitol protection assay
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jsps.2018.07.017.
When Candida spp. were treated with 8-hydroxyquinoline derivatives 1–3 in a medium supplemented with sorbitol, MICs increased after seven days of incubation compared to MIC in medium without sorbitol. MICs of clioquinol 1 increased for all dermatophytes tested. MICs to the derivative 2 also increased for dermatophytes, except for T. rubrum. Derivative 3 had the MIC increased for M. canis and M. gypseum isolates. However, the MICs for T. rubrum and T. mentagrophytes remained constant. After addition of sorbitol into the medium, the MIC values of anidulafungin (positive control) increased 2–128-fold, 2–16-fold for clioquinol 1, and until 8 and 4-fold for the derivative 2 and 3, respectively (Tables S1 and S2).
Supplementary data 1.
3.2. Cellular leakage effect
It was noted that clioquinol 1 did not cause nucleic acid leakage from fungal cells, while the derivatives 2 and 3 occasioned it to all Candida spp. (C. albicans, C. glabrata, C. krusei, C. parapsilosis and C. tropicalis) and dermatophytes (M. canis, M. gypseum, T. mentagrophytes and T. rubrum).
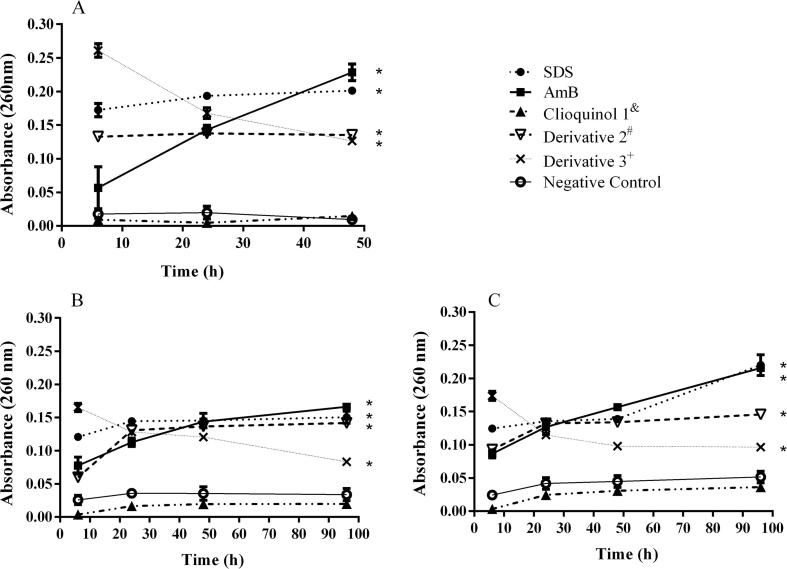
Significant (P < 0.05) release of cellular material was observed for derivatives 2 and 3, as well as positive controls (SDS and amphotericin B). All results were compared to untreated control. On the other hand, clioquinol 1 was not statistically different from the untreated control. Fig. 2 shows leakage from cells over 48 h (Candida albicans; Fig. 2A) and 96 h (M. canis, Fig. 2B; T. mentagrophytes; Fig. 2C) period due to 8-hydroxyquinoline derivatives 1–3, SDS, amphotericin B, and untreated control. A representative isolate of each genus studied was selected to compose Fig. 2, but all other isolates had the same effect (data not shown).
Fig. 2.
Cellular leakage of 260-nm-absorbing materials of Candida albicans ATCC 18804 (A), Microsporum canis MCA 01 (B) and Trichophyton mentagrophytes TME 40 (C) treated with MIC of 8-hydroxyquinoline derivatives 1–3 by time intervals (6, 24 and 48 h for C. albicans; 6, 24, 48 and 96 h for dermatophytes). Amphotericin B (6.25 µg/ml) and SDS (2%) are the positive controls. Untreated cells are negative control. The asterisks indicate statistical difference compared to the negative control (* p < 0.05). & (1) clioquinol MIC: 0.250 µg/ml (ATCC 18804), 0.250 µg/ml (MCA 01) and 0.500 µg/ml (TME 40); # (2) 8-hydroxy-5-quinolinesulfonic MIC: 64 µg/ml (ATCC 18804), 128 µg/ml (MCA 01) and 64 µg/ml (TME 40); + (3) 8-hydroxy-7-iodo-5-quinolinesulfonic acid MIC: 32 µg/ml (ATCC 18804), 256 µg/ml (MCA 01) and 256 µg/ml (TME 40).
3.3. Ergosterol binding assay
MICs of 8-hydroxyquinoline derivatives 1–3 against Candida spp. (Table S3) and dermatophytes (Table S4) did not increase after adding different concentrations of ergosterol. As expected, a 16–128-fold increase of MIC values was observed for amphotericin B (positive control). All isolates tested behaved similarly.
3.4. Scanning electron microscopy (SEM)
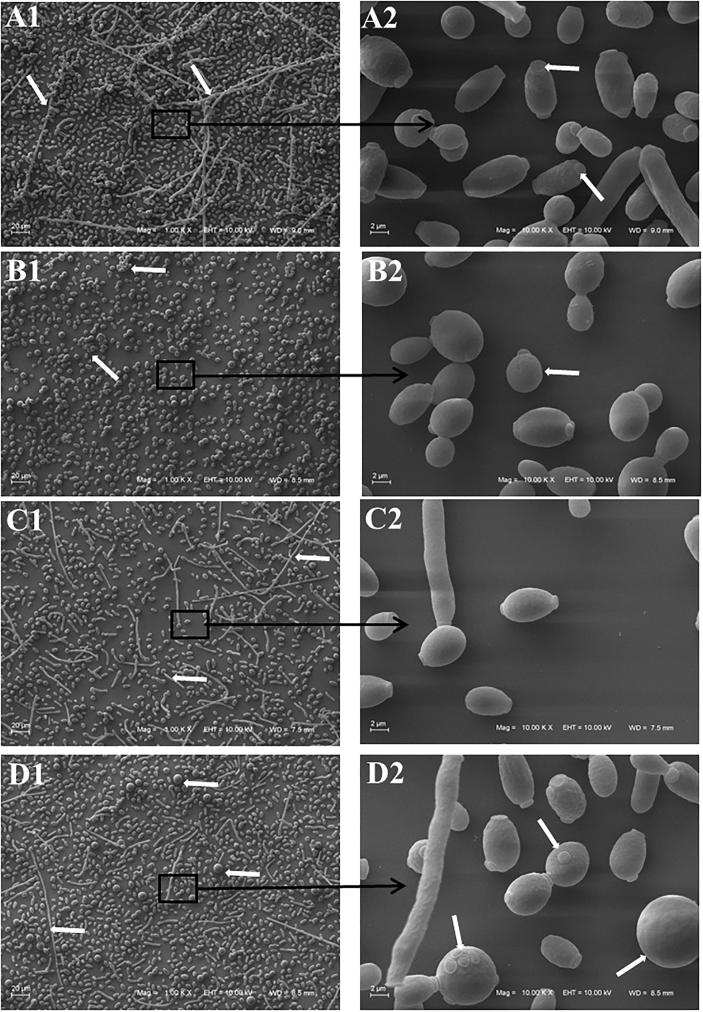
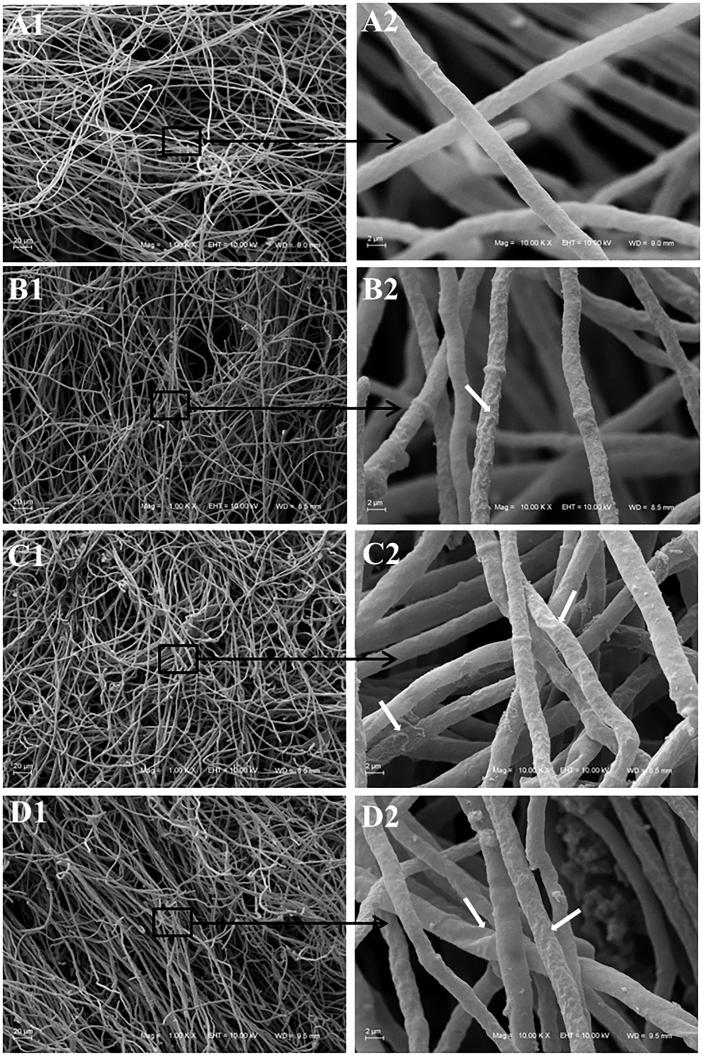
SEM images of C. albicans (ATCC 18804) depict important morphological changes (Fig. 3) in the presence of the 8-hydroxyquinoline derivatives 1–3. Untreated cells present its natural smooth oval-shape and polar bud scars bearing blastoconidia as well as pseudohyphae. Addition of clioquinol 1 shrinks the cell surface modifying its format to circular. Cell clusters and blastoconidia are still observed, although without pseudohyphae. C. albicans grown in the presence of derivative 2 revealed the shrinkage of cell and the presence of pseudohyphae but with less blastoconidia and a few chlamydospores. Similarly, cells treated with derivative 3 demonstrate pseudohyphae with less blastoconidia. Larger round-cells and non-polar bud scars are widely noted.
Fig. 3.
Scanning electron microscopy of Candida albicans ATCC 18804 treated with sub-inhibitory concentration of 8-hydroxyquinoline derivatives 1–3 (1: clioquinol; 2: 8-hydroxy-5-quinolinesulfonic acid; 3: 8-hydroxy-7-iodo-5-quinolinesulfonic acid) and untreated cells. A. Untreated cells; A1 (Bar = 20 µm): white arrows indicate pseudohyphae; A2 (Bar = 2 µm): white arrows indicate polar bud scars. B. Hyphal cells treated with clioquinol 1; B1 (Bar = 20 µm): white arrows indicate cell clusters; B2 (Bar = 2 µm): white arrow indicates rounded cells. C. Hyphal cells treated with derivative 2; C1 (Bar = 20 µm): white arrows indicate pseudohyphae with few blastoconidia and chlamydospore; C2 (Bar = 2 µm). D. Hyphal cells treated with derivative 3; D1 (Bar = 20 µm): white arrows indicate pseudohyphae with few blastoconidia and larger round cells; D2 (Bar = 2 µm): white arrows indicate pseudohyphae with few blastoconidia and larger round cells.
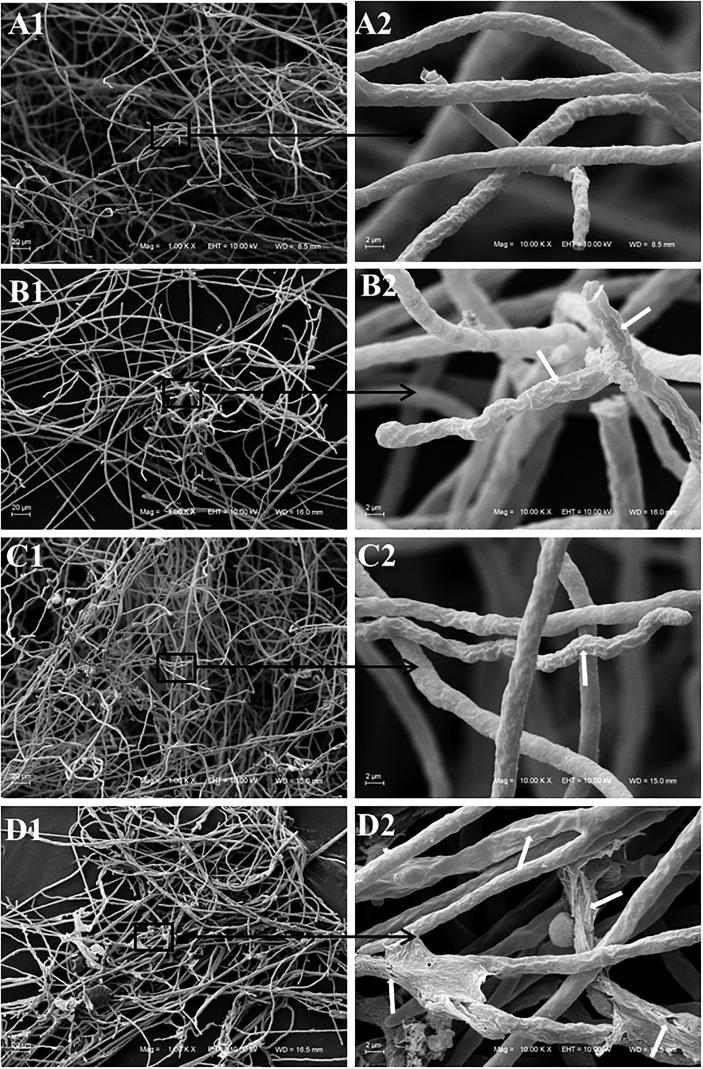
Both M. canis MCA 01 (Fig. 4) and T. mentagrophytes TME 40 (Fig. 5) incubated with 8-hydroxyquinoline derivatives 1–3 show irregular, rough and groove cell wall. Some hyphal cells of M. canis treated with derivative 3 exhibited extensive degeneration. The fungal wall displays notable pitting and tears with apparent extravasation of the intracellular material.
Fig. 4.
Scanning electron microscopy of Microsporum canis MCA 01 treated with sub-inhibitory concentration of 8-hydroxyquinoline derivatives 1–3 (1: clioquinol; 2: 8-hydroxy-5-quinolinesulfonic acid; 3: 8-hydroxy-7-iodo-5-quinolinesulfonic acid) and untreated cells. A. Untreated cells; A1 (Bar = 20 µm); A2 (Bar = 2 µm). B. Hyphal cells treated with clioquinol 1; B1 (Bar = 20 µm); B2 (Bar = 2 µm): white arrow indicates irregular and rough cell walls with grooves. C. Hyphal cells treated with derivative 2; C1 (Bar = 20 µm); C2 (Bar = 2 µm): white arrow indicates irregular and rough cell walls with grooves. D. Hyphal cells treated with derivative 3; D1 (Bar = 20 µm); D2 (Bar = 2 µm): white arrows indicate irregular and rough cell walls with grooves, pitting and tears.
Fig. 5.
Scanning electron microscopy of Trichophyton mentagrophytes TME 40 treated with sub-inhibitory concentration of 8-hydroxyquinoline derivatives 1–3 (1: clioquinol; 2: 8-hydroxy-5-quinolinesulfonic acid; 3: 8-hydroxy-7-iodo-5-quinolinesulfonic acid) and untreated cells. A. Untreated cells; A1 (Bar = 20 µm); A2 (Bar = 2 µm). B. Hyphal cells treated with clioquinol 1; B1 (Bar = 20 µm); B2 (Bar = 2 µm): white arrow indicates irregular and rough cell walls with grooves. C. Hyphal cells treated with derivative 2; C1 (Bar = 20 µm); C2 (Bar = 2 µm): white arrows indicate irregular and rough cell walls with grooves. D. Hyphal cells treated with derivative 3; D1 (Bar = 20 µm); D2 (Bar = 2 µm): white arrows indicate irregular and rough cell walls with grooves, pitting and tears.
4. Discussion
Based on the results of the sorbitol protection assay, we observed that the 8-hydroxyquinoline derivatives 1–3 could target the fungal cell wall. After adding sorbitol, increased MICs were observed, indicating some effect of the derivatives 1–3 on the fungal cell wall. This effect was species dependent. Candida spp. and dermatophytes exhibit damage to the wall when treated with clioquinol 1. In the same way, derivative 2 showed action on the wall, except for T. rubrum cells. Derivative 3 also damages the cell wall, although T. rubrum and T. mentagrophytes appear not to suffer this action.
Further additional level of evidence on the mode of action was assessed by the measurement of cellular leakage for detection of cell membrane as a possible target site. Data obtained by measuring these UV-absorbing materials revealed that the sulfonic quinolines (derivatives 2 and 3) enhanced the cell permeability of Candida spp. and dermatophytes (Fig. 2). The high absorbance observed suggests that nucleic acids were lost through cytoplasmic membrane irreversible damage (Khan et al., 2013). This effect was also observed for amphotericin B and SDS (positive controls) which are membrane disruptive-agents that affect cell permeability and show lethal action against fungal cells. These observations indicate that the fungal membrane is a possible site of action for 8-hydroxyquinoline-sulfonic acids. In contrast, small values of absorbance were read when fungi were treated with clioquinol 1 indicating that cells were not lysed. Therefore, clioquinol 1 does not appear to target the fungal-cell membrane.
Due to the acidity of sulfonic acids, 8-quinolinol sulfonic acids (derivatives 2 and 3) are deprotonated at the culture medium (pH = 7). Thus, active species of these derivatives present ionic characters which penetrate the fungal cells in the same manner (Gershon et al., 2001). Clioquinol 1 does not bear a sulfonic acid group, still it presents a phenolic proton with pKa around 7.4 which can be removed at the pH of the culture medium. However, less than half of the clioquinol molecules also will be at the ionic form (Völgyi et al., 2007).
Antifungal agents exhibiting membrane-damaging effects can target ergosterol which is a structural component of the membrane, forming transmembrane pores that lead to alterations on the membrane permeability with loss of intracellular content and consequent fungal membrane disruption. If the activity of the antifungal agent is a consequence of binding to ergosterol, external ergosterol would prevent the binding, l. consequently, the MIC of the antifungal agent would increase (Escalante et al., 2008). Amphotericin B is a well-known membrane-disruptive agent lethal to fungi. This drug complex with membrane-ergosterol forming stable pores into the membrane, allowing permeability of ions such as potassium and proton (Flevari et al., 2013, Lunde and Kubo, 2000, Scorzoni et al., 2017). Thus, the possible binding of 8-hydroxyquinoline derivatives 1–3 to ergosterol was verified. However, unchanged MICs for derivatives 1–3 in the presence of exogenous ergosterol suggested that they do not act by binding to the membrane ergosterol. Therefore, the destabilization of the membrane that occurs by the action of derivatives 2 and 3 is not by direct binding to the plasma membrane. Thus, other targets as enzyme of the ergosterol biosynthesis pathway may be involved.
Next, morphological changes in fungal cells after treatment with 8-hydroxyquinoline derivatives 1–3 were evaluated by SEM. Fungal cells were subjected to sub-minimal inhibitory concentration (sub-MIC) of 1–3 since malformations were previously detected at this dilution (Escalante et al., 2008).
The demonstration that clioquinol 1 inhibits pseudohyphae formation of C. albicans (Fig. 3, Fig. 4, Fig. 5) is an important finding. Studies suggest that pseudohyphae are an intermediate state between yeast cells and hyphae (Lu et al., 2014). The ability to switch between yeast, pseudohyphal, and hyphal growth forms is one of the most investigated virulence factors of C. albicans (Albertin and Marullo, 2012, Whiteway and Bachewich, 2007). The morphological transition from yeast to filamentous form represents an essential aspect of the pathogen’s biology and is linked to important properties for interaction with the host, such as: adhesion to epithelial and endothelial cells, escape from phagocytes and immune evasion. The capacity of hyphal growth is believed to be essential for pathogenicity at both superficial and systemic levels (Lu et al., 2014). Although the commercially available antifungals have the plasma membrane and the cell wall as main targets (Ngo et al., 2016), recent studies have focused on the inhibition of virulence factors to develop new therapies (Lu et al., 2014, Scorzoni et al., 2017).
The cell envelope of Candida sp. appeared to be damaged by the 8-hydroxyquinoline derivatives 1–3 as evidenced by the shrinkage of the cell surfaces. Chlamydospores that were present when Candida cells were treated with derivative 2 are often formed when the fungus is under extreme growth conditions (Fig. 3, Fig. 4, Fig. 5). Indeed, the formation of chlamydospore is associated with the microorganism mechanism of defense under adverse conditions (Whiteway and Bachewich, 2007). Numerous non-polar bud scars noted on cells treated with derivative 3 (Fig. 3, Fig. 4, Fig. 5) suggest that the normal yeast division process may have been affected resulting in single cells with multiple attempts to divide but not increasing the viable number of cells (Khan et al., 2013). The presence of the large round cells (Fig. 3, Fig. 4, Fig. 5) reinforce this hypothesis and can indicate variation in their genome size. The endopolyploidy phenomenon (somatic polyploidy) can occur in C. albicans, through the fusion of their diploid cells resulting in tetraploids cells. Endopolyploidy arises by recurrent cycles of DNA replication without cellular division via either endoreduplication or endomitosis processes. Environmental factors, such as antifungal treatments, have been shown to induce variation of genome content and chromosomal complement in various fungal species (Albertin and Marullo, 2012). In addition, the presence of the large round cells could also indicate change in cell permeability.
Similarly, irregular and rough dermatophytes cell walls are seen after treatment with 8-hydroxyquinoline derivatives 1–3 (Figs. 4 and 5). These modifications may also be a consequence of membrane permeability. The damage caused by derivative 3 on the M. canis wall was more clearly identified, being pitting and tears easily visualized (Fig. 4, Fig. 5).
5. Conclusion
Clioquinol 1 inhibits the formation of pseudohyphae in C. albicans cells and appears to act more effectively on the cell wall than 8-hydroxyquinoline derivatives containing sulfonic acid. On the other hand, 8-hydroxyquinoline-sulfonic acids 2 and 3 compromised the functional integrity of cytoplasmic membranes suggesting that their antifungal mechanism occur mainly due to membrane damage. This demonstrates that changing the substitution pattern of the nucleus of 8-hydroxyquinoline is possible to obtain different mechanisms for antifungal activity. Rational drug design can accelerate the development of new antifungal agents by optimizing activity through appropriate structural modifications. 8-Hydroxyquinolines are strong candidates for this purpose. This study is very important because without adequate identification of the mechanism of action we cannot design new drugs effectively. In addition, we emphasize that to date no similar report was found about the antifungal mechanism of 8-hydroxyquinolines. However, additional studies including a larger number of isolates should be performed in order to confirm the data found here. These results, combined with the broad antifungal spectrum already demonstrated previously by us, reinforce the potential of this class of substances to develop antimicrobial drugs.
Acknowledgments
Acknowledgements
The authors thank the Brazilian agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul ((FAPERGS – EDITAL 04/2016 – PRONUPEQ 2016) for financial support and research fellowships.
Conflict of interest
No conflict of interest declared.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Saulo Fernandes Andrade, Email: saulo.fernandes@ufrgs.br.
Alexandre Meneghello Fuentefria, Email: alexandre.fuentefria@ufrgs.br.
References
- Abouelhassan Y., Yang Q., Yousaf H., Nguyen M.T., Rolfe M., Schultz G.S. Nitroxoline: a broad-spectrum biofilm-eradicating agent against pathogenic bacteria. Int. J. Antimicrob. Agents. 2017;49:247–251. doi: 10.1016/j.ijantimicag.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Albertin W., Marullo P. Polyploidy in fungi: evolution after whole-genome duplication. Proc. Biol. Sci. 2012;279:2497–2509. doi: 10.1098/rspb.2012.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsterholm M., Karami N., Faergemann J. Antimicrobial activity of topical skin pharmaceuticals – an in vitro study. Acta. Derm. Venereol. 2010;90:239–245. doi: 10.2340/00015555-0840. [DOI] [PubMed] [Google Scholar]
- Chan-On W., Huyen N.T., Songtawee N., Suwanjang W., Prachayasittikul S., Prachayasittikul V. Quinoline-based clioquinol and nitroxoline exhibit anticancer activity inducing FoxM1 inhibition in cholangiocarcinoma cells. Drug Des. Devel. Ther. 2015;9:2033–2047. doi: 10.2147/DDDT.S79313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherdtrakulkiat R., Boonpangrak S., Sinthupoom N., Prachayasittikul S., Ruchirawat S., Prachayasittikul V. Derivatives (halogen, nitro and amino) of 8-hydroxyquinoline with highly potent antimicrobial and antioxidant activities. Biochem. Biophys. Rep. 2016;6:135–141. doi: 10.1016/j.bbrep.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI), 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard – Third Edition. CLSI Document M27-A3. Clinical Laboratory Standards Institute, Wayne, PA, USA.
- Clinical and Laboratory Standards Institute (CLSI), 2008. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous fungi; Approved Standard – Second Edition. CLSI Document M38-A2. Clinical Laboratory Standards Institute, Wayne, PA, USA.
- Escalante A., Gattuso M., Perez P., Zacchino S. Evidence for the mechanism of action of the antifungal Phytolaccoside B Isolated from Phytolacca tetramera Hauman. J. Nat. Prod. 2008;71:1720–1725. doi: 10.1021/np070660i. [DOI] [PubMed] [Google Scholar]
- Finkelstein D.I., Hare D.J., Billings J.L., Sedjahtera A., Nurjono M., Arthofer E. Clioquinol improves cognitive, motor function, and microanatomy of the alpha-Synuclein hA53T transgenic mice. ACS Chem. Neurosci. 2016;7:119–129. doi: 10.1021/acschemneuro.5b00253. [DOI] [PubMed] [Google Scholar]
- Flevari A., Theodorakopoulou M., Velegraki A., Armaganidis A., Dimopoulos G. Treatment of invasive candidiasis in the elderly: a review. Clin. Interv. Aging. 2013;8:1199–1208. doi: 10.2147/CIA.S39120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R.B., Zou J., Zheng Y., Naslund M.J., Costello L.C. Zinc ionophore (clioquinol) inhibition of human ZIP1-deficient prostate tumor growth in the mouse ectopic xenograft model: a zinc approach for the efficacious treatment of prostate cancer. Int. J. Cancer Clin. Res. 2016;3:1–11. doi: 10.23937/2378-3419/3/1/1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon H., Gershon M., Clarke D.D. Antifungal activity of substituted 8-quinolinol-5- and 7-sulfonic acids: a mechanism of action is suggested based on intramolecular synergism. Mycopathologia. 2001;155:213–217. doi: 10.1023/a:1021166500169. [DOI] [PubMed] [Google Scholar]
- Huntington Study Group Reach2HD Investigators Safety, tolerability, and efficacy of PBT2 in Huntington’s disease: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015;14:39–47. doi: 10.1016/S1474-4422(14)70262-5. [DOI] [PubMed] [Google Scholar]
- Joubert L.M., Ferreira J.A.G., Stevens D.A., Cegelsk L. Aspergillus fumigatus biofilms: a comparison of processing techniques for scanning electron microscopy of fungal mycelium and extracellular matrix. Microsc. Microanal. 2015;21:934–936. [Google Scholar]
- Khan M.S., Ahmad I., Cameotra S.S. Phenyl aldehyde and propanoids exert multiple sites of action towards cell membrane and cell wall targeting ergosterol in Candida albicans. AMB Exp. 2013;3:54. doi: 10.1186/2191-0855-3-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Su C., Liu H. Candida albicans hyphal initiation and elongation. Trends Microbiol. 2014;22:707–714. doi: 10.1016/j.tim.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszuk C., Krajewska-Kułak E., Kułak W. Retrospective observation of drug susceptibility of Candida strains in the years 1999, 2004, and 2015. Peer J. 2017;5:1–12. doi: 10.7717/peerj.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunde C.S., Kubo I. Effect of polygodial on the mitochondrial ATPase of Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 2000;44:1943–1953. doi: 10.1128/aac.44.7.1943-1953.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X., Schimmer A.D. The toxicology of clioquinol. Toxicol. Lett. 2008;182:1–6. doi: 10.1016/j.toxlet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Nakae K., Yamamoto S., Shigematsu I., Kono R. Relation between subacute myelo-optic neuropathy (S.M.O.N.) and clioquinol: nationwide survey. Lancet. 1973;1:171–173. doi: 10.1016/s0140-6736(73)90004-4. [DOI] [PubMed] [Google Scholar]
- Ngo H.X., Garneau-Tsodikova S., Green K.D. A complex game of hide and seek: the search for new antifungals. Medchemcomm. 2016;7:1285–1306. doi: 10.1039/C6MD00222F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveri V., Vecchio G. 8-Hydroxyquinolines in medicinal chemistry: a structural perspective. Eur. J. Med. Chem. 2016;120:252–274. doi: 10.1016/j.ejmech.2016.05.007. [DOI] [PubMed] [Google Scholar]
- Pippi B., Reginatto P., Machado G.D., Bergamo V.Z., Lana D.F., Teixeira M.L. Evaluation of 8-hydroxyquinoline derivatives as hits for antifungal drug design. Med. Mycol. 2017;55:763–773. doi: 10.1093/mmy/myx003. [DOI] [PubMed] [Google Scholar]
- Prachayasittikul V., Prachayasittikul S., Ruchirawat S., Prachayasittikul V. 8-Hydroxyquinolines: a review of their metal chelating properties and medicinal applications. Drug Des. Devel. Ther. 2013;7:1157–1178. doi: 10.2147/DDDT.S49763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorzoni L., de Paula E., Silva A.C., Marcos C.M., Assato P.A., de Melo W.C., de Oliveira H.C. Antifungal therapy: new advances in the understanding and treatment of mycosis. Front. Microbiol. 2017;8:1–23. doi: 10.3389/fmicb.2017.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwanjang W., Prachayasittikul S., Prachayasittikul V. Effect of 8-hydroxyquinoline and derivatives on human neuroblastoma SH-SY5Y cells under high glucose. Peer J. 2016;4:e2389. doi: 10.7717/peerj.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte P., Ferrari S., Coste A.T. Antifungal resistance and new strategies to control fungal infections. Int. J. Microbiol. 2012;2012:1–26. doi: 10.1155/2012/713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völgyi G., Ruiz R., Box K., Comer J., Bosch E., Takács-Novák K. Potentiometric and spectrophotometric pKa determination of water-insoluble compounds: validation study in a new cosolvent system. Anal. Chim. Acta. 2007;583:418–428. doi: 10.1016/j.aca.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Whiteway M., Bachewich C. Morphogenesis in Candida albicans. Annu. Rev. Microbiol. 2007;61:529–553. doi: 10.1146/annurev.micro.61.080706.093341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.H., Raymick J., Sarkar S., Lahiri D.K., Ray B., Holtzman D. Efficacy and toxicity of clioquinol treatment and A-beta42 inoculation in the APP/PSI mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2013;10:494–506. doi: 10.2174/1567205011310050005. [DOI] [PubMed] [Google Scholar]