Abstract
Background/Objective
Adventure racing is an ultra-endurance activity that imposes a unique multifaceted stress on the human body. The purpose of this field study was to examine the physiological responses to a 5-day adventure race.
Methods
Eight competitors, two teams (1 female each) in the 2012 GODZone adventure race volunteered. Competitors trekked, cycled and paddled ∼326 km in ∼116 hours. Continuous glucose was measured the day before and throughout. Body mass, urinary solutes, and blood pressure and heart rate during resting, standing, and repeated squat-stand conditions, were assessed pre and post.
Results
Despite no changes in mean blood glucose levels, there was increased glycemic variability (Standard deviation glucose; Pre: 0.5 ± 0.1 vs Race: 1.0 ± 0.2 mmol/L, p = 0.02) and periods of hypoglycemia (i.e., Min glucose Pre: 4.1 ± 0.3 vs Race: 3.6 ± 0.5 mmol/L, p = 0.05) during the race. After the race, the blood pressure during resting, standing and squat-stand conditions was significantly lower, by 14 ± 14 mmHg, 16 ± 15 mmHg and 18 ± 15 mmHg (all p < 0.05), respectively, with no change in heart rate. During five-days of adventure racing there is increased glycemic variability and more frequent periods of low blood glucose levels. Additionally, following the race pronounced hypotension is observed in competitors.
Conclusion
We observed more frequent glucose fluctuations, lower glucose levels and significant perturbations in blood pressure control. Further research is warranted to examine the long-term impact of adventure racing on metabolic and cardiovascular function.
Keywords: Ultraendurance, Glucose, Exercise, Adventure racing, Orthostatic
Highlights
-
•
Five-days of adventure racing can cause blood pressure pertubations.
-
•
Adventure racing results in fluctuations of blood glucose.
-
•
There are periods of hypoglycemia during an adventure race which maynot be captured by pre-post- measures.
Introduction
Adventure racing imposes significant physiological demands.1 Competitors typically endure 3–5 days of near continuous exercise of various modalities (paddling, trekking and cycling). Unpredictable weather (with over 12 km of ascent) meaning temperatures can fluctuate from sunny and hot to below zero degrees, rough terrain, and sleep and nutrient deprivation add to the physiological strain. Consequently, tightly regulated variables, including blood glucose and blood pressure (BP) are expected to be challenged by the constant loading.1 For example, energy-expenditures of 30–45 MJ per day (resulting in energy deficits of over 4 MJ) and less than 2 h sleep a night, were reported in two previous field-based studies.2,3 In this regard, competitors often report periods of hypoglycemia and hallucinations during prolonged ultraendurance events. Despite this, studies to date report no significant changes in blood glucose levels when measured at the end of an adventure race. However, using continuous glucose monitoring (CGM) Helge et al.4 revealed significant hypoglycemic excursions in two adventure racing athletes during the Southern Traverse (a multiday supported adventure race); interestingly these corresponded with the kayak and paddle portions of the race.
There is a growing interest and participant in adventure racing. For example, in 2017 the number of teams in the GODZone adventure race more than doubled; from 33 teams in 2012 to 71 teams in 2017. Of interest, more than 60% of the competitors in 2017 had never done an expedition length adventure race before. However, despite growing interest and participation in adventure racing there is a paucity of field-based studies investigating the physiological responses to adventure races. Simulated adventure races lack external validity and field studies are often limited to pre- and post-race measurements.2,3,5 Accordingly, the aim of this field study was to examine the blood glucose responses during, and the hemodynamic and autonomic changes after, a multiday expedition-style adventure race. Using continuous glucose monitoring, we explored the impact of adventure racing on the daily mean and variability of blood glucose. This is the first non-simulated field study to examine continuous blood glucose across the entire race, and to study the effects of a multiday adventure race on orthostatic tolerance.
Methods
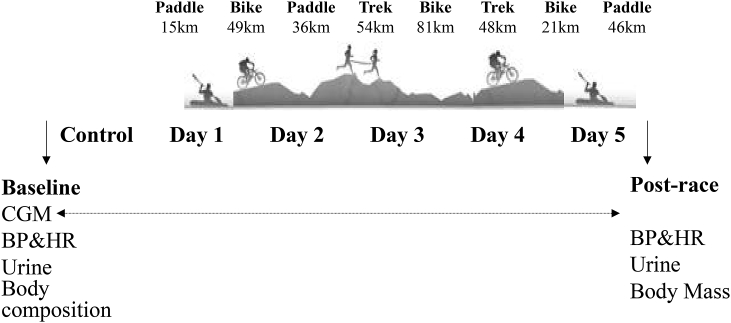
The race. The 2012 GODZone adventure race was a ∼500 km unsupported expedition-style adventure race with eight stages and four disciplines (kayak, canoe, mountain bike and trekking) lasting 4–7 days. In brief, Day 1 involved kayaking, cycling and canoeing, Day 2 trekking, Day 3 cycling, Day 4 trekking and Day 5 a long kayak leg. Teams (four competitors) were provided with the course map just hours before the start of the race.
Participants. Ethical approval was obtained from the University of Otago Ethics Committee. Two teams (eight participants; one female per team) provided informed written consent. One competitor withdrew from the race due to injury, thus not included in analyses. Baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics and race data collected from the two participating teams that completed the GODZone adventure race.
| Age (y) | Body fat (%) | Distance covered (km) | Time (h) | Time spent in each discipline (h) | |
|---|---|---|---|---|---|
| Team 1 (3 M:1F) | 55 ± 3 | 9 ± 2 | ∼350 | 128 (5.3 days) | Kayak/Canoe: 14 h 41 min |
| Road/mountain Bike: 40 h 57 min | |||||
| Trekking: 34 h 38 min | |||||
| Team 2 (3 M:1F) | 35 ± 7 | 11 ± 6 | ∼302 | 115 (4.8 days) | Kayak/Canoe: 17 h 50 min |
| Road/mountain Bike: 34 h 10 min | |||||
| Trekking: 53 h 00 min |
Data Collection. Before the race, body composition was assessed via the sum of eight skinfolds.6 Body mass was measured before and after the race using portable electronic scales wearing light, dry clothing only. During the race, competitors were monitored and recorded via a GPS satellite-tracking device. The morning after the race (within 24-h), all baseline measures (except skinfolds) were repeated.
Metabolic Measures. A continuous glucose monitor (CGM) was positioned in the subcutaneous layer of the abdomen to measure glucose the day before, and throughout the race (Fig. 1). Data are validated against capillary samples obtained during the race (Carelink, iPro Medtronic). Glycemic variability variables were analysed via spreadsheet (EasyGV, Oxford, UK). Urine samples were collected and measured for hydration (USG), electrolytes (Na+, K+) using the Ion Selective Electrode technique, and urine glucose, protein, leukocytes and ketones (Combur10 Test®, Roche Diagnostics).
Fig. 1.
Schematic of the different stages in the 2012 GODZone adventure race, and the timing of physiological outcomes before, during and after the race.
Cardiovascular Measures. Beat-to-beat heart rate (HR: 3-lead ECG) and BP (Finapres Medical Systems, Netherlands) were assessed for five min in a supine position following 20 min of rest. Following this, orthostatic tolerance and baroreflex sensitivity were assessed via: 1) a single supine-to-stand maneuver (stood rapidly and remained standing still for 1 min), followed by 2) repeated squat-standing for 2 min (5-s cycles at each posture; i.e., 5-s in a half squat position followed by 5-s of upright standing), synchronized to a metronome.7 Mean HR and BP, power spectrum analysis of heart rate variability (HRV)8 and cardiac baroreflex sensitivity (BRS)9 were calculated for each block using LabChart 7.1 and the HRV2.0 and BRS macros (v7.1, ADInstruments, NZ). Beat-to-beat BP was calibrated by a manual sphygmomanometer.
Statistical Analyses. Statistical analyses and normality were assessed using SPSS (IBM, IL, USA), with results reported as means ± standard deviations and/or 95% confidence intervals. Glucose data (Table 2) were analysed using one-way repeated measures ANOVA, whereas paired t-tests compared the glucose data during the race (average of 5 race days) compared to pre-race, and pre-post changes in body mass, BP, HR, HRV and BRS. Effect sizes and magnitude-based inferences (MBI) for the true effect are reported based on the methods by Hopkins and Batterham.10 MBI give the probability that an outcome effect is positive, trivial or negative based on whether the change is clinically, practically or mechanistically meaningful and are reported as a % beneficial/trivial/adverse.10 For blood glucose, −1.1 mmol/L (based <3.9 mmol/L ADA criteria11) was deemed a meaningful change. An increase in the Low Blood Glucose Index (LBGI), of 2.5 increments was used based on hypoglycemia and hospitalization.12 For MAP, a change >13 mmHg upon standing based on the criteria for orthostatic hypotension.13 For other variables, the default value of 0.20 Cohen units was used.
Table 2.
Continuous glucose data (n = 5) before (Pre) and for each day during the adventure race.
| Pre-race | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
|---|---|---|---|---|---|---|
| Glucose mean (mmol/L) | 4.9 ± 0.3 | 5.0 ± 0.5 | 4.8 ± 0.5 | 5.1 ± 0.7 | 4.7 ± 0.3 | 4.8 ± 0.6 |
| Glucose Min (mmol/L) | 4.1 ± 0.3 | 3.6 ± 0.6 | 3.6 ± 0.5 | 3.6 ± 0.7 | 3.5 ± 0.4 | 3.7 ± 0.2* |
| SD Glucose (mmol/L) | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1* | 1.2 ± 0.5* | 0.6 ± 0.0 | 0.7 ± 0.3* |
| Glucose Max (mmol/L) | 6.3 ± 0.1 | 6.7 ± 0.7 | 6.7 ± 0.5 | 8.8 ± 1.4 | 6.4 ± 0.6 | 6.4 ± 1.1 |
| LBGI | 2.3 ± 0.3 | 2.9 ± 1.2 | 3.8 ± 2.4 | 4.4 ± 3.6 | 3.5 ± 1.0 | 4.4 ± 1.4* |
| MAG | 0.7 ± 0.2 | 0.8 ± 0.1 | 1.1 ± 0.1* | 1.1 ± 0.3* | 0.9 ± 0.1 | 0.9 ± 0.4 |
LBGI = low blood glucose index, MAG = mean absolute glucose change per hour.
*p < 0.05 vs Pre-race.
Results
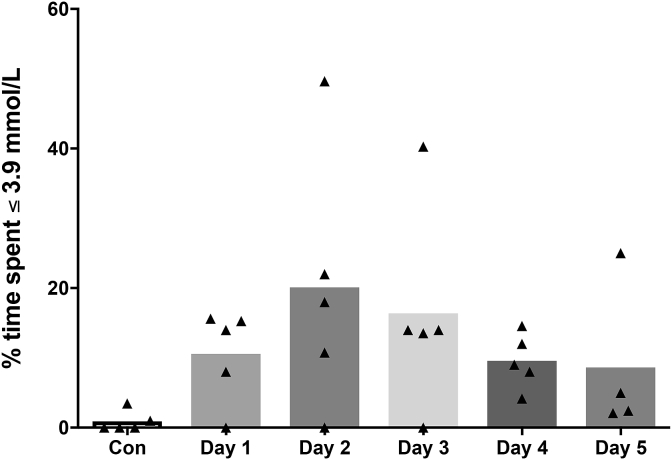
Continuous glucose data. CGM data was analysed from 5/8 participants (3 dislodged during the race), with time spent below 3.9 mmol/L is shown in Fig. 2. Mean glucose during the race was not significantly different compared to pre-race (p = 0.56, Cohens d 0.4, Table 2). The variability in 24-h glucose, measured using standard deviation (SD) was significantly higher during the race compared to pre-race (p = 0.02, Table 2). The chance of the true effect being beneficial/trivial/adverse was 1/1/98%, (Cohens d 1.7). Minimum glucose during the race was significantly lower than pre-race (p = 0.05, Table 2). The true effect for the change in minimum glucose was 0/98/2% (Cohens d 1.3). The LBGI was not statistically different, however the true effect was 0/88/12%, (Cohens d 1.2, Table 2).
Fig. 2.
Percent time spent below 3.9 mmol/L for group mean (bars) and individual (symbols) continuous blood glucose data across the control (pre-race) day and each day throughout the race.
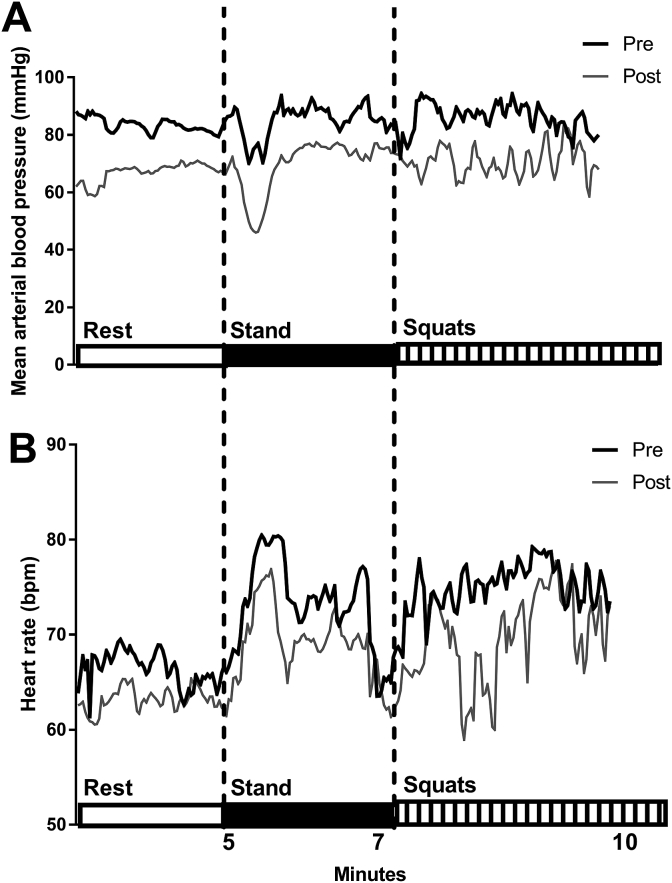
Autonomic function. The average MAP during resting, standing and repeated squat-stand manoeuvres was significantly lower after the race, −14 mmHg (95% CI: −25 to −3 mmHg, p = 0.02, Cohens d 1.1, Fig. 3), 16 mmHg (95% CI: −27 to −3 mmHg, p = 0.02, Cohens d 1.0) and 18 mm Hg (95% CI: −31 to −4 mmHg, p = 0.02, Cohens d 0.9), respectively. The true effect for the change in MAP on standing was 1/21/79% (Cohens d 1.0). HR, HRV and baroreflex sensitivity were not significantly different after the race (all p > 0.25, Table 3).
Fig. 3.
Mean (of n = 7) beat-to-beat heart rate and blood pressure responses during rest, standing and repeated squat-stand manoeuvres pre and post-race.
Table 3.
Pre- and post-race measures (n = 7) of heart rate, blood pressure, heart rate variability and baroreceptor sensitivity during rest, stand, and repeated squat-standing maneuvers.
| Rest |
Stand |
Squat-stand |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| HR (bpm) | 65 ± 12 | 63 ± 12 | 75 ± 10 | 70 ± 13 | 76 ± 12 | 70 ± 8 |
| MAP (mm Hg) | 82 ± 12 | 68 ± 20* | 85 ± 11 | 69 ± 16* | 88 ± 14 | 70 ± 18* |
| LF (ms2) | 705 ± 448 | 744 ± 516 | 1220 ± 489 | 902 ± 573 | 563 ± 392 | 863 ± 1211 |
| LF (n.u) | 66 ± 9 | 67 ± 15 | 86 ± 9 | 85 ± 14 | 70 ± 15 | 72 ± 16 |
| HF (ms2) | 419 ± 561 | 427 ± 460 | 157 ± 90 | 262 ± 372 | 124 ± 73 | 193 ± 224 |
| HF (n.u) | 30 ± 10 | 29 ± 16 | 11 ± 7 | 14 ± 13 | 22 ± 11 | 23 ± 14 |
| LF/HF | 2.5 ± 1.1 | 3.2 ± 2.4 | 11.5 ± 8.8 | 14.5 ± 15.8 | 4.1 ± 2.3 | 4.9 ± 4.3 |
| BRS gain (ms/mmHg) | 3.8 ± 3.2 | 2.9 ± 3.1 | 2.7 ± 1.2 | 2.3 ± 1.6 | 3.7 ± 2.8 | 2.1 ± 1.7 |
HR = Heart Rate, MAP = Mean Arterial Blood Pressure, LF = Low Frequency, HF = High Frequency, BRS = Baroreflex Sensitivity.
*p < 0.05 vs pre-race.
Body weight, hydration and urine analysis. Body mass and hydration status (USG) were not significantly different from baseline (Table 4). Dipstick urine analysis showed traces of proteins (7/8 competitors), elevated leukocytes (4/8) and ketones (3/8). Urine glucose remained in the normal range.
Table 4.
Body mass, hydration and electrolytes for competitors (n = 7) before and after the GODZone adventure race.
| Pre | Post | |
|---|---|---|
| Body mass (Kg) | 74 ± 11 | 76 ± 11 |
| USG | 1.018 ± 0.001 | 1.020 ± 0.001 |
| [Na+], (mmol·L−1) | 105 ± 59 | 120 ± 70 |
| [K+], (mmol·L−1) | 49 ± 29 | 38 ± 19 |
| PH | 5.6 ± 0.9 | 6.1 ± 1.0 |
USG = Urine Specific Gravity, Na+ = Sodium, K+ = Potassium.
Discussion
The major findings of this field study were: a) Competitors experienced higher variability in blood glucose and lower glucose concentrations; and b) Blood pressure was significantly lower after the race, 16–18 ± 15 mmHg during resting, standing and squatting. Although, future research with a larger sample size is required it is possible that the increase in glycemic variability and hypotention observed in the present study may, in part, explain reports of low energy, fatigue and orthostatic intolerance/syncope after prolonged expedition-style adventure races.
Adventure racing poses unique, multifaceted stress on the body. The maintenance of blood glucose levels within the normal range is critical for optimal physiological functioning and exercise performance.14 Although glucose is not the predominant fuel oxidized during prolonged low-intensity exercise (typical of adventure racing4) glucose is necessary for fatty acid metabolism, high intensity bursts of effort and for arm exercise.15, 16, 17 We observed a two-fold increase in glycemic variability and periods of hypoglycemia during the race, however the mean glucose levels did not significantly deviate from pre-race levels, highlighting the importance of during race measures. The increase in glycemic variability observed is likely to due to the increased energy requirements and glucose uptake that accompany prolonged ultraendurance exercise.2,4 In addition, poor nutrition patterns may in part explain the increased glycemic variability. For example, anecdotally after the race the athletes mentioned gorging on sweets only after feeling ‘low on energy’ as opposed to having a constant energy intake (particularly during cycle and paddle stages). Increased glycemic variability and periods of hypoglycemia is attributed to negative mood swings, impaired cognition and low energy.18,19 This is in agreement with previous reports after prolonged exercise.17 “Unfortunately, because of the remote unsupported nature of this event and poor reporting/recall post-race we were unable to gain any valuable insight into the competitors' nutrition habits during the race. Furthermore, our sample size was reduced to five after three monitors dislodged during the race. Future research is required to investigate the effects of dietary intakes on blood glucose fluctuations during such a race”.
Additionally, a marked ∼17% decrease in BP was observed, this is much greater than seen following an ironman,20 but similar to a simulated 24-h adventure race.21 We also show that the BP response to orthostatic challenges (postural perturbations) was significantly lower after the race, and lower HR responses were seen in 6/7 competitors. This is believed to be due to reduced vagal activation and compromised control of vagal tone, however the mechanisms following prolonged ultraendurance exercise remain to be fully elucidated.20,21 This is the first study to report the changes in orthostatic tolerance and BP after an expedition style adventure race. Although, the timing of measurements post-race were not able to be standardized. Both teams finished at various times throughout the night, and measures were obtained the following morning at their first convenience. Thus, our cardiovascular measures may be confounded by the circadian influence of blood pressure22 Regardless, five days of almost continuous exercise with variable and limited sleep, is likely to disrupt the circadian rhythm.
Conclusions. This field study provided a unique opportunity to study physiological responses to one of the most popular, gruelling, and unpredictable ultra-endurance endeavours; the “GODZone” adventure race. We observed more frequent glucose fluctuations, lower glucose levels and significant perturbations in BP control.
Author contributions
All authors declare no conflict of interest.
M.E.F., S.D.C., N.M.W., S.J.E.L. and K.E.B. were responsible for conception and design of the research. M.E.F., S.D.C., N.M.W., and K.E.B performed the experiments. M.E.F., S.D.C., N.M.W., S.J.E.L. and K.E.B. collected, assembled and analysed data. M.E.F., S.D.C., S.J.E.L. and K.E.B. interpreted the results of the experiments. M.E.F. drafted the paper. M.E.F., S.D.C., N.M.W., S.J.E.L. and K.E.B. edited and critically revised the paper.
All authors have approved the final version of the paper and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jesf.2018.07.002.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Lucas S.J., Helge J.W., Schütz U.H., Goldman R.F., Cotter J.D. Moving in extreme environments: extreme loading; carriage versus distance. Extreme Physiol Med. 2016;5(1):1. doi: 10.1186/s13728-016-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enqvist J.K., Mattsson C.M., Johansson P.H., Brink-Elfegoun T., Bakkman L., Ekblom B.T. Energy turnover during 24 hours and 6 days of adventure racing. J Sports Sci. 2010;28(9):947–955. doi: 10.1080/02640411003734069. [DOI] [PubMed] [Google Scholar]
- 3.Lucas S.J., Anglem N., Roberts W.S. Intensity and physiological strain of competitive ultra-endurance exercise in humans. J Sports Sci. 2008;26(5):477–489. doi: 10.1080/02640410701552872. [DOI] [PubMed] [Google Scholar]
- 4.Helge J.W., Rehrer N., Pilegaard H. Increased fat oxidation and regulation of metabolic genes with ultraendurance exercise. Acta Physiol. 2007;191(1):77–86. doi: 10.1111/j.1748-1716.2007.01709.x. [DOI] [PubMed] [Google Scholar]
- 5.Zimberg I.Z., Crispim C.A., Juzwiak C.R. Nutritional intake during a simulated adventure race. Int J Sport Nutr Exerc Metabol. 2008;18(2):152. doi: 10.1123/ijsnem.18.2.152. [DOI] [PubMed] [Google Scholar]
- 6.Marfell-Jones M.J., Stewart A., de Ridder J. 2012. International Standards for Anthropometric Assessment. [Google Scholar]
- 7.Zhang R., Claassen J.A., Shibata S. Arterial-cardiac baroreflex function: insights from repeated squat-stand maneuvers. Am J Physiol Regul Integr Comp Physiol. 2009;297(1):R116–R123. doi: 10.1152/ajpregu.90977.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardiology TFotESo Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- 9.Bertinieri G., Di Rienzo M., Cavallazzi A., Ferrari A., Pedotti A., Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl. 1985;3(3):S79–S81. [PubMed] [Google Scholar]
- 10.Batterham A.M., Hopkins W.G. Making meaningful inferences about magnitudes. Int J Sports Physiol Perform. 2006;1(1):50–57. [PubMed] [Google Scholar]
- 11.Seaquist E.R., Anderson J., Childs B. Hypoglycemia and diabetes: a report of a workgroup of the american diabetes association and the endocrine society. J Clin Endocrinol Metab. 2013;98(5):1845–1859. doi: 10.1210/jc.2012-4127. [DOI] [PubMed] [Google Scholar]
- 12.Kovatchev B.P., Cox D.J., Gonder-Frederick L.A., Young-Hyman D., Schlundt D., Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care. 1998;21(11):1870–1875. doi: 10.2337/diacare.21.11.1870. [DOI] [PubMed] [Google Scholar]
- 13.Freeman R., Wieling W., Axelrod F.B. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 14.Suh S.-H., Paik I.-Y., Jacobs K. Regulation of blood glucose homeostasis during prolonged. Mol cells. 2007;23:272–279. [PubMed] [Google Scholar]
- 15.Rauch H., Gibson A.S.C., Lambert E., Noakes T. A signalling role for muscle glycogen in the regulation of pace during prolonged exercise. Br J Sports Med. 2005;39(1):34–38. doi: 10.1136/bjsm.2003.010645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahlborg G., Jensen-Urstad M. Metabolism in exercising arm vs. leg muscle. Clin Physiol. 1991;11(5):459–468. doi: 10.1111/j.1475-097x.1991.tb00818.x. [DOI] [PubMed] [Google Scholar]
- 17.Noakes T., Nathan M., Irving R. Physiological and biochemical measurements during a 4-day surf-ski marathon. S Afr Med J. 1985;67:212–216. [PubMed] [Google Scholar]
- 18.Owens D.S., Parker P.Y., Benton D. Blood glucose and subjective energy following cognitive demand. Physiol Behav. 1997;62(3):471–478. doi: 10.1016/s0031-9384(97)00156-x. [DOI] [PubMed] [Google Scholar]
- 19.Penckofer S., Quinn L., Byrn M., Ferrans C., Miller M., Strange P. Does glycemic variability impact mood and quality of life? Diabetes Technol Therapeut. 2012;14(4):303–310. doi: 10.1089/dia.2011.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gratze G., Rudnicki R., Urban W., Mayer H., Schlögl A., Skrabal F. Hemodynamic and autonomic changes induced by Ironman: prediction of competition time by blood pressure variability. J Appl Physiol. 2005;99(5):1728–1735. doi: 10.1152/japplphysiol.00487.2005. [DOI] [PubMed] [Google Scholar]
- 21.Lucas S.J., Cotter J.D., Murrell C. Mechanisms of orthostatic intolerance following very prolonged exercise. J Appl Physiol. 2008;105(1):213–225. doi: 10.1152/japplphysiol.00175.2008. [DOI] [PubMed] [Google Scholar]
- 22.Millar-Craig M., Bishop C., Raftery E. Circadian variation of blood-pressure. Lancet. 1978;311(8068):795–797. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.