Abstract
Background
Ginseng is believed to have antitumor activity. Autophagy is largely a prosurvival cellular process that is activated in response to cellular stressors, including cytotoxic chemotherapy; therefore, agents that inhibit autophagy can be used as chemosensitizers in cancer treatment. We examined the ability of Korean Red Ginseng extract (RGE) to prevent autophagic flux and to make hepatocellular carcinoma (HCC) cells become more sensitive to doxorubicin.
Methods
The cytotoxic effects of total RGE or its saponin fraction (RGS) on HCC cells were examined by the lactate dehydrogenase assay in a dose- or time-dependent manner. The effect of RGE or RGS on autophagy was measured by analyzing microtubule-associated protein 1A/1B-light chain (LC)3-II expression and LC3 puncta formation in HCC cells. Late-stage autophagy suppression was tested using tandem-labeled green fluorescent protein (GFP)–monomeric red fluorescent protein (mRFP)–LC3.
Results
RGE markedly increased the amount of LC3-II, but green and red puncta in tandem-labeled GFP–mRFP–LC3 remained colocalized over time, indicating that RGE inhibited autophagy at a late stage. Suppression of autophagy through knockdown of key ATG genes increased doxorubicin-induced cell death, suggesting that autophagy induced by doxorubicin has a protective function in HCC. Finally, RGE and RGS markedly sensitized HCC cells, (but not normal liver cells), to doxorubicin-induced cell death.
Conclusion
Our data suggest that inhibition of late-stage autophagic flux by RGE is important for its potentiation of doxorubicin-induced cancer cell death. Therapy combining RGE with doxorubicin could serve as an effective strategy in the treatment of HCC.
Keywords: autophagic flux, cell death, ginseng extract
1. Introduction
Korean Red Ginseng extract (RGE) derived from heat-processed Panax ginseng Meyer is characterized by numerous steroidal saponins with considerable inhibitory activity against important signaling enzymes; this extract is used in traditional oriental medicine to increase energy [1]. Reports indicate that RGE may make chemotherapy more potent by inhibiting both cancer cell propagation and metastasis [1], [2], [3]. Mechanisms have been suggested for the anticancer functions of RGE; RGE reportedly leads to decreased vascular endothelial growth factor expression and inhibitory effects on nuclear factor-κB activity [4], [5], [6], [7], [8], [9]. Our previous study suggested that RGE promotes tumor-necrosis-factor-related apoptosis-inducing ligand (TRAIL)-induced cell death in hepatocellular carcinoma (HCC) cells by inducing upregulation of death receptor 5 expression downstream of increased expression of CCAAT-enhancer-binding protein homologous protein (CHOP) [10], [11], indicating that RGE could potentially be further utilized as a chemosensitizer for anticancer drugs.
Autophagy is important in many physiological and pathological processes, and has dual roles in cancer: it is thought to inhibit cancer development at early stages, while having a procancer role in tumor progression at later stages [12], [13]. At present, autophagy is typically thought to be a prosurvival process that is activated by cancer chemotherapeutics; therefore, inhibitors of autophagy often sensitize to cancer cell death under various stresses. A variety of autophagy inhibitors are currently under development as novel cancer therapeutic agents, either alone or in combination with other therapies [14], [15].
HCC is a prevalent solid tumor type; the high death rate from HCC is largely due to the lack of efficacious therapies [16]. Currently, the multikinase inhibitor sorafenib is one of few effective therapies among targeted agents [17]. However, resistance to this drug often occurs, hence, new therapies for HCC are needed. Recently, combination treatments are being utilized more often as a strategy in treating HCC [17], [18]. According to recent reports, a blockade of autophagic signaling may particularly be beneficial in making HCC cells sensitive to classical cytotoxic chemotherapies [19], [20]. Previously, we also suggested that the ginseng compound 20(S)-ginsenoside Rg3 inhibits late stage autophagy [21]. Therefore, combined chemotherapy with autophagy inhibiting agents can be one of the effective alternative treatments for HCC therapy.
In the present study, we investigated the effect of RGE and red ginseng sapoinin (RGS) on modulation of autophagy in HCC cell lines to evaluate whether effects on autophagy are relevant to RGE- and RGS-potentiated doxorubicin-induced cytotoxicity. We show RGE inhibits late-stage autophagic flux, thus sensitizing HCC cells to doxorubicin cytotoxicity. The combination of RGE or RGS and doxorubicin synergized to kill HCC cell lines, suggesting that RGE and RGS may possibly be utilized as a potent inhibitor of autophagy to chemosensitize cancer cells to cytotoxic chemotherapy: such a combination may work as an effective approach in the treatment of HCC.
2. Materials and methods
2.1. Reagents
Anti-Beclin-1, anti-p62, anti-Atg5, and anti-Vps34 antibodies were obtained from Cell Signaling (Danvers, MA, USA). The anti-LC3 antibody was from Sigma (St. Louis, MO, USA). Chloroquine and doxorubicin were from Calbiochem (San Diego, CA, USA). RGE and RGS were provided as a powder by the Korea Ginseng Corporation in (Gangnam-Gu, Seoul, Korea).
2.2. Chemical profiling
The ginsenoside components in RGE and the RGS fraction were determined by an Agilent 1260 Infinity HPLC system equipped with an evaporative light scattering detector (Sedex 80; Sedere, Alfortville, France). An Zorbax Eclipse Plus C18 column (4.6 mm I.D. × 150 mm L, 3.5 μm particle size) (Agilent, Santa Clara, CA, USA) was used for separation, and the mobile phase consisted of water (Phase A) and acetonitrile (Phase B). The flow rate was 1 mL/min, and the temperature of the stationary phase was kept at 30°C. The following gradient condition was used: 0–11 min (21% B), 11–16 min (21-29% B), 16–21 min (29% B), 21–37 min (29–32% B), 37–60 min (32–50% B), 60–67 min (50–60% B), 67–72 min (60–100% B). The evaporative light scattering detector settings optimized were as follows: nebulizer gas pressure 3.0 bar, drift tube temperature 40°C, and detector gain 8. Ginsenosides in RGE and RGS were extracted with 80% methanol three times, and the total solution was adjusted to an appropriate concentration. The solution was filtered through a 0.45-μm membrane filter prior to HPLC analysis.
2.3. Cell culture
HepG2, Hep3B, Hur-7, and SK-Hep1 cells were grown in RPMI 1640 (#31800-022, Invitrogen) supplemented with 10% fetal bovine serum (#1600044, Invitrogen), 2mM glutamine (# 25030081, Invitrogen), and penicillin/streptomycin (#10378016, Invitrogen).
2.4. Western blotting
M2 buffer [20mM Tris (pH 7.0), 250mM NaCl, 0.5% NP-40, 3mM egtazic acid, 3mM EDTA, 2mM dithiothreitol, 1 μg/mL leupeptin, 0.5mM phenylmethane sulfonyl fluoride, 1mM sodium vanadate, and 20mM β-glycerol phosphate] was used to lyse cells. Cell extract proteins were resolved by SDS-PAGE (12% or 15%) and detected by enhanced chemiluminescence (Amersham, Little Chalfont, Bucks, UK) after western blotting with the appropriate antibodies.
2.5. Transfection
Transfection was performed using Lipofectamine PLUS (#11514, Invitrogen, Waltham, MA, USA). The green fluorescent protein (GFP)–microtubule-associated protein 1A/1B-light chain (LC)3 construct was transfected by this method. The monomeric red fluorescent protein (mRFP)–GFP tandem LC3 (tfLC3) plasmid was previously described [28] and was obtained from Dr T. Yoshimori (Osaka University).
2.6. Confocal microscopy
Cells were added in a chamber slide. After the described treatments, GFP–LC3 puncta and tfLC3 were observed under a confocal microscope (LSM710; Carl Zeiss, Jena, Germany). Data shown are representative of three independent experiments at minimum.
2.7. Cytotoxicity assay
Lactate dehydrogenase (LDH) activity released by dying cells was measured at 490 nm using a Promega LDH kit (Madison, WI, USA). A tetrazolium colorimetric test (MTT test) was also used to measure cell death with absorbance read at 570 nm. Pictures of cell morphology in dying cells were produced by phase-contrast microscopy. All data were from at least three separate experiments.
2.8. Short hairpin RNA lentivirus
Short hairpin RNAs for Beclin-1 (NM-_003766), ATG5 (NM-_004849), Vps34 (NM-_002647), and non-targeting control (SHC002) were obtained from Sigma–Aldrich (St. Louis, MO, USA). Lentivirus was produced in 293TN (System Biosciences, Palo Alto, CA, USA) after transfection using Lipofectamine 2000 (#11668019, Invitrogen, Carlsbad, CA, USA). Knock-down was confirmed by immunoblotting.
2.9. Statistical analysis
Results are expressed as the mean ± standard deviation. Statistical analyses were conducted using analysis of variance and unpaired Student's t test. A p < 0.01 was considered statistically significant.
3. Results
3.1. RGE and RGS have no cytotoxic effect on HCC cells
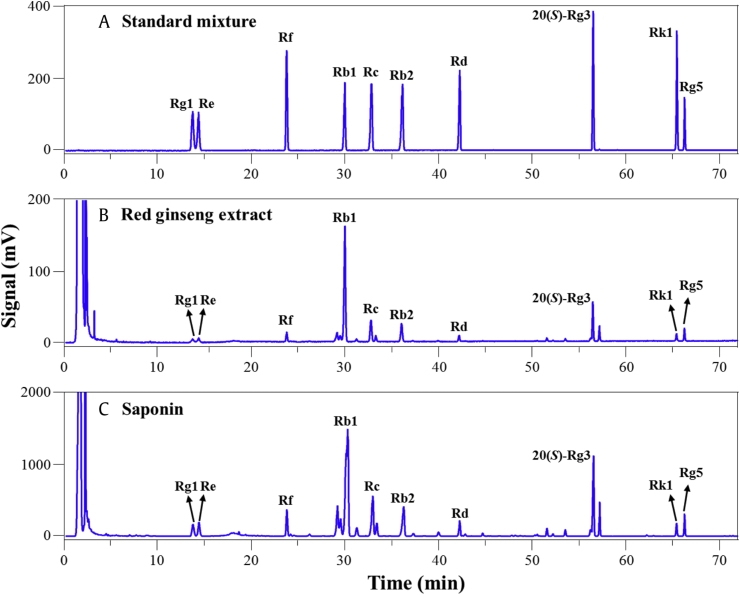
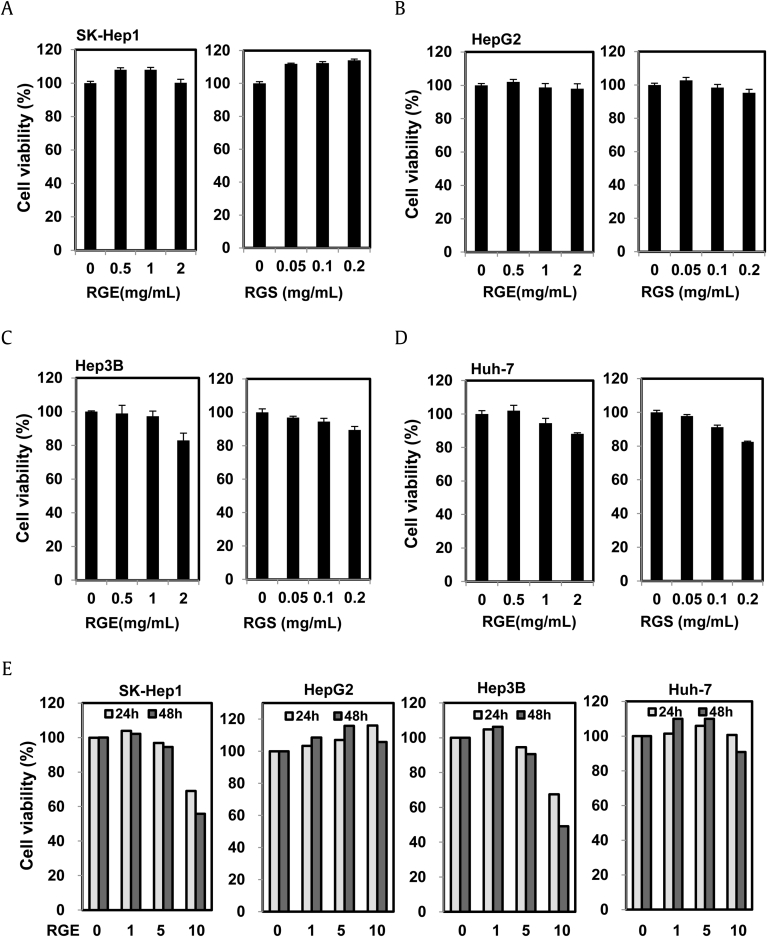
Previous reports have proposed that ginsenoside Rg3, from the root of Panax ginseng, can increase the efficacy of cancer chemotherapy; related ginsenosides Rb1 and Rk from ginseng also have antitumor properties [21], [22], [23]. However, the ways in which these compounds have these effects are unknown. To understand the antitumor activities of ginseng, we examined the effect of RGE and the RGS fraction on cancer cell death. We investigated the concentrations of the 10 major ginsenosides, Rg1, Re, Rf, Rb1, Rc, Rb2, Rd, 20(S)-Rg3, Rk1, and Rg5, in RGE and in the RGS fraction. The composition of the ginsenosides and the patterns of the chromatograms (Fig. 1 and Table 1) confirmed that both RGE and RGS were from red ginseng. The contents of the 10 ginsenosides in RGE accounted for about 2.4% of the total weight, whereas those in RGS accounted for about 18.7% of the total weight, indicating that the ginsenosides were concentrated in RGS during the purification process. As shown in Fig. 2A–D, RGE or RGS alone had no obvious effects on cancer cell death at the indicated concentrations for 24 h in four different HCC cell lines. A small amount of cytotoxicity was found in SK-Hep1 and Hep3B cells treated with a high concentration of RGE for 48 h, but this minimal amount toxicity did not occur in HepG2 and Huh-7 cells (Fig. 2E). Taken together, RGE or RGS alone had limited cytotoxic effects on HCC cell lines.
Fig. 1.
Analysis of the ginsenosides in RGE and RGS by HPLC–ELSD. The chromatogram of (A) the ten ginsenoside standards, (B) RGE, and (C) RGS. ELSD, evaporative light scattering detector; RGE, red ginseng extract; RGS, red ginseng saponin fraction.
Table 1.
Contents of 10 ginsenosides in RGE and RGS
| Contents (mg/g) |
Rg1 | Re | Rf | Rb1 | Rc | Rb2 | Rd | 20(S)-Rg3 | Rk1 | Rg5 |
|---|---|---|---|---|---|---|---|---|---|---|
| RGE | 1.12 | 1.21 | 1.35 | 7.76 | 2.98 | 2.73 | 1.09 | 3.79 | 1.20 | 1.25 |
| RGS | 13.32 | 13.73 | 12.91 | 49.44 | 22.78 | 20.75 | 10.35 | 25.89 | 8.61 | 8.97 |
RGE, red ginseng extract; RGS, red ginseng saponin fraction
Fig. 2.
Effects of RGE and RGS on viability of hepatocellular carcinoma cells. (A–E) The indicated concentration of RGE or RGS was used to treat (A) SK-Hep1, (B) HepG2 , (C) Hep3B, and (D) Huh-7, for 24 h. (E) The indicated concentration of RGE was also used to treat SK-Hep1, HepG2, Hep3B, and Huh-7 cells for 24 h and 48 h. Cell viability was quantitated by tetrazolium colorimetric test. Data represent the mean ± standard error of the mean. RGE, red ginseng extract; RGS, red ginseng saponin fraction.
3.2. Accumulation of LC3-II conversion in response to RGE or RGS
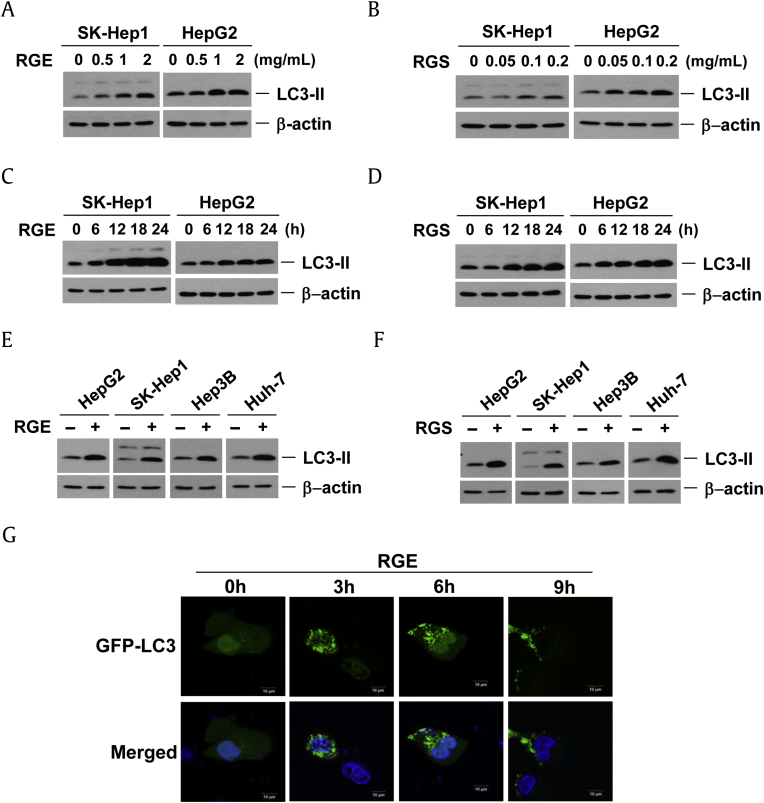
We measured the effects of RGE on autophagy by analyzing the production of LC3-II in HCC cells. Similar to 20(S)-ginsenoside Rg3 [21], RGE or RGS treatment in SK-Hep1 and HepG2 cells led to an increase in the amount of LC3-II in a time- and dose-dependent fashion (Fig. 3A–D). We tested four different HCC cell lines and all behaved similarly, suggesting that RGE and RGS could initiate autophagy in HCC cells (Fig. 3E, 3F). We investigated whether RGE treatment also increased the formation of GFP-LC3 puncta in HepG2 cells (Fig. 3G). RGE treatment markedly increased GFP-LC3 puncta formation, indicating that RGE treatment affects the autophagic process of HCC cells (Fig. 3F).
Fig. 3.
RGE and RGS induce the LC3-II production in a time- and dose-dependent fashion. (A and B) SK-Hep1 and HepG2 cells were treated with different amounts of (A) RGE, or (B) RGS, for 24 h. The protein samples were resolved in 15% SDS-PAGE and then probed with anti-LC3 antibody. SK-Hep1 and HepG2 cells were treated with (C) 2 mg/mL RGE or (D) 0.2 mg/mL RGS for the indicated times. Four hepatocellular carcinoma cells were treated with € 2 mg/mL RGE, or (F) 0.2 mg/mL RGS, for 24 h and the protein samples were analyzed with anti-LC3 antibody. (G) RGE treatment increases GFP–LC3 punctuation. Confocal images display GFP–LC3-expressing (4′,6-diamidino-2-phenylindole costained) HepG2 cells treated with RGE as indicated. GFP, green fluorescent protein; LC3, microtubule-associated protein 1A/1B-light chain; RGE, red ginseng extract; RGS, red ginseng saponin fraction.
3.3. RGE inhibits late-stage autophagy
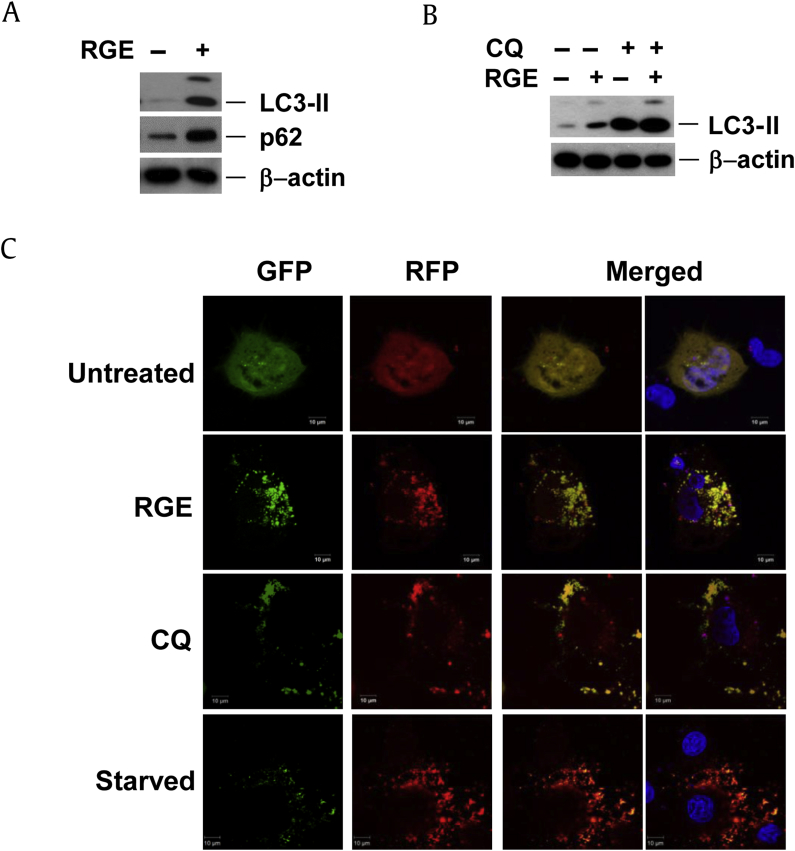
Increases in LC3-II may signify either an increased production of autophagosomes or the inhibition of autophagic flux at a late stage, which prevents LC3II degradation [24]. The autophagy adapter cargo protein, p62/SQSTM1, is recruited to the autophagosomal membrane and then degraded within the lysosomes along with LC3II [25]. Thus, downregulation of p62 protein may signify increased autophagy [26], [27]. To determine if the increased amount of LC3-II is due to increased early-stage autophagy or reduced late-stage autophagy, cells were treated with RGE for 24 h. RGE treatment led to an increase in p62, rather than a decrease (Fig. 4A). Upon chloroquine (CQ) treatment, which prevents autophagy by inhibiting the lysosome function required for autophagic degradation, RGE did not further augment LC3-II or p62 accumulation (Fig. 4B), suggesting that the increased LC3-II conversion in RGE-treated cells is not from increasing early-stage autophagy, but is consistent with late-stage obstruction of autophagic flux.
Fig. 4.
RGE prevents autophagic flux. (A) HepG2 cells were treated with 2 mg/mL RGE for 24 h and western blotting was performed on cell lysates. (B) HepG2 cells were pretreated with CQ (10μM) for 30 min and then with 2 mg/mL RGE for 24 h; cell lysates were subjected to western blotting. (C) GFP–mRFP–LC3 expressing HepG2 cells were treated for 7 h with 2 mg/mL RGE or CQ (10μM) or were treated with Hank's Balanced Salt Solution for 8 h. Green and red fluorescence was then observed using a confocal microscope. CQ, chloroquine; GFP, green fluorescent protein; LC3, microtubule-associated protein 1A/1B-light chain; mRFP, monomeric red fluorescent protein; RFP, red fluorescent protein; RGE, red ginseng extract; RGS, red ginseng saponin fraction.
To confirm that RGE suppresses late stage autophagy, we used tfLC3, a tandem labeled GFP–mRFP–LC3 that is useful in differentiating actual increases in autophagy from late-stage inhibition [28], [29], since increased flux leads to the preferential quenching of the GFP signal in the lysosome, resulting in red-only puncta. RGE treatment of HepG2 cells transfected with tfLC3 led to puncta that were both GFP and mRFP positive, and thus appear yellow, rather than red, indicating that RGE treatment leads to a reduction in autophagosomal–lysosomal fusion or function (Fig. 4C). As expected, CQ treatment (which blocks autophagic flux at a late stage) led to similar yellow puncta, while starvation-induced autophagy led to red-only puncta. These data are consistent with the conclusion that RGE actually prevents autophagic flux at a late stage, rather than increasing flux.
3.4. Autophagy induced by doxorubicin protects against cell death in HCC cells
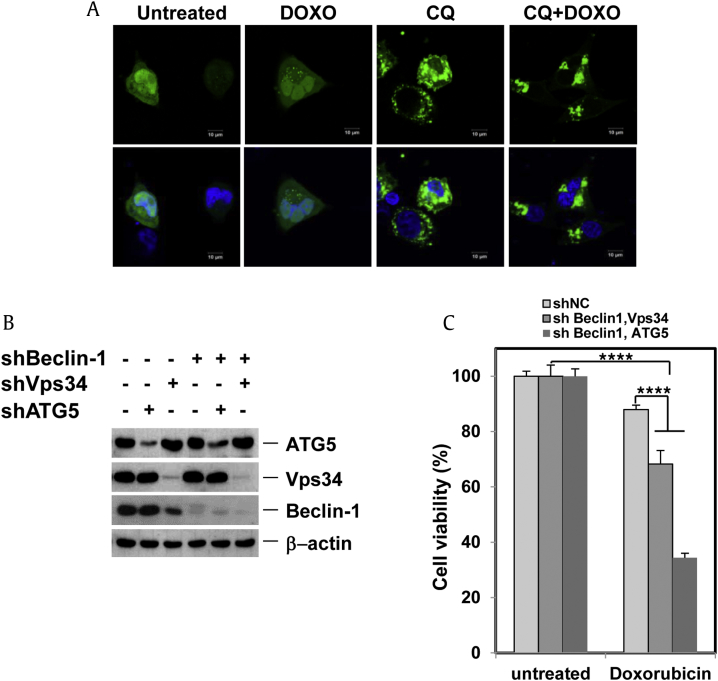
Based on the common perception that autophagy serves as an important prosurvival mechanism in cancer cells when under stress [13], [30], we explored whether RGE would promote doxorubicin-induced cell death in HCC cells since RGE suppresses autophagic flux. Doxorubicin induced GFP-LC3 puncta formation and LC3-II production; CQ further enhanced the amount of GFP-LC3 puncta, indicating that doxorubicin caused increased autophagic flux and is capable of inducing autophagy in HCC cells (Fig. 5A). Double knock-down of Beclin-1 and ATG5 or Beclin-1 and Vps34 (Fig. 5B) in HepG2 cells noticeably enhanced doxorubicin-induced toxicity, indicating that autophagy protects against doxorubicin-stimulated cytotoxicity (Fig. 5C).
Fig. 5.
Doxorubicin induced GFP–LC3 puncta formation. (A) HepG2 cells were treated for 7 h with 2.5μM doxorubicin or 10μM CQ in addition to doxorubicin; cells were examined for GFP–LC3 punctuation/aggregation by confocal microscopy. (B) HepG2 cells were infected with combinations of short hairpin RNA lentiviruses. After puromycin selection, knockdown was examined by western blotting. (C) Cells from (B) were treated with 2.5μM doxorubicin for 18 h; cell quantity was then analyzed by tetrazolium colorimetric test. Error bars are mean ± standard error of the mean. ****p < 0.001. CQ, chloroquine; DOXO, doxorubicin; GFP, green fluorescent protein; LC3, microtubule-associated protein 1A/1B-light chain; RGE, red ginseng extract; RGS, red ginseng saponin fraction.
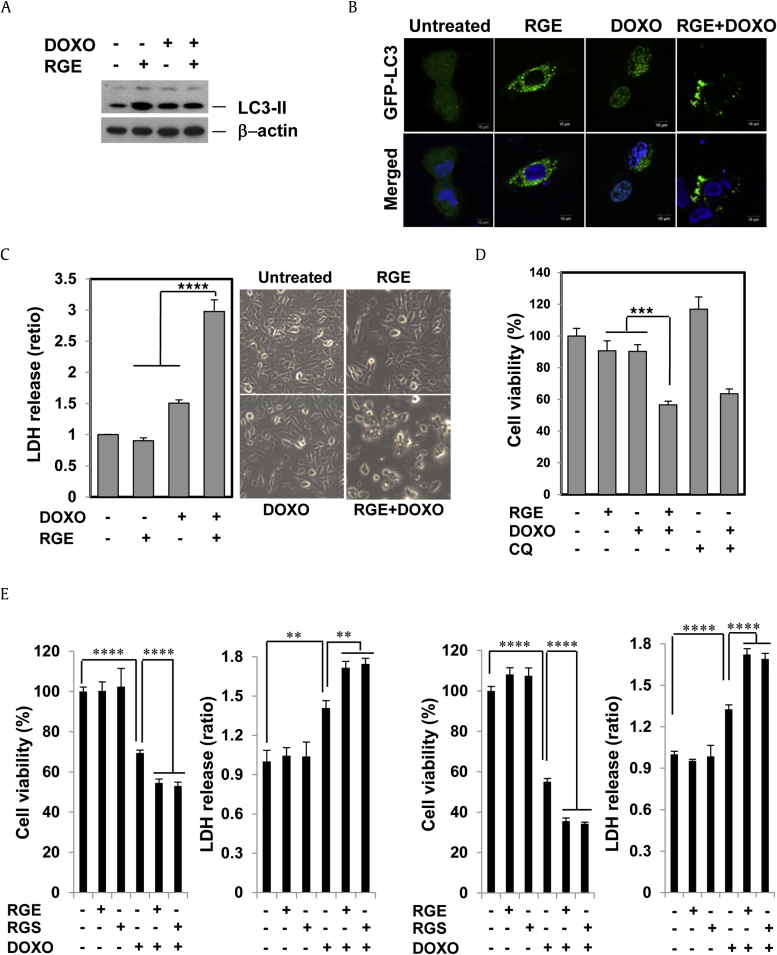
3.5. RGE enhances the cytotoxic effect of doxorubicin in HCC cells
Based on the data obtained above, autophagy is a prosurvival process that prevents doxorubicin cytotoxicity, whereas RGE inhibits autophagy. We consequently investigated whether RGE suppression of autophagy would make cells sensitive to doxorubicin. As expected, RGE augmented doxorubicin-stimulated LC3-II production (Fig. 6A) as well as the formation of LC3 puncta (Fig. 6B), suggesting that RGE blocks doxorubicin-induced autophagic flux. When cells were treated with RGE for 1 h before doxorubicin treatment for 24 h, doxorubicin-induced cell death was dramatically enhanced (Fig. 6C). Notably, both CQ and RGE chemosensitized to doxorubicin individually (Fig. 6D). This is consistent with the idea that RGE and CQ chemosensitize by similar means. RGS showed an effect similar to RGE with regard to sensitization of doxorubicin-induced cell death (Fig. 6E). Taken together, our data suggest that RGE potentiates doxorubicin-stimulated cell death via suppressing autophagy.
Fig. 6.
RGE and RGS enhance the cytotoxic effect of doxorubicin in hepatocellular carcinoma cells. (A) Western blots of cell lysates from HepG2 cells pretreated with RGE for 30 min, then treated with doxorubicin (2.5μM) for an additional 12 h. (B) HepG2 cells were treated with 2.5μM doxorubicin, RGE, or RGE in combination with doxorubicin, for 7 h. Fluorescence was then observed with a confocal microscope. SK-Hep1 (C) and HepG2 (D) cells were pretreated with RGE for 30 min before addition of doxorubicin for another 24 h and cell number was examined by MTT. Error bars are mean ± SEM. ***p < 0.005, ****p < 0.001. (E) SK-Hep1 and HepG2 cells were pretreated with 2 mg/ml RGE or 0.2 mg/mL RGS for 1 h and then 2.5μM doxorubicin was treated for an additional 24 h. The cell viability was analyzed by MTT assay and lactate dehydrogenase release assay. Data represent the mean ± SEM. **p < 0.01 and ****p < 0.001. CQ, chloroquine; DOXO, doxorubicin; LDH, lactate dehydrogenase; MTT, tetrazolium colorimetric test; RGE, red ginseng extract; RGS, red ginseng saponin fraction; SEM = standard error of the mean.
4. Discussion
A formulated RGE is a food and a dietary supplement that is consumed worldwide and has been reported to possess antioxidant and antitumor properties. The RGS fraction isolated from ginseng is known to induce Cu/Zn-superoxide dismutase mRNA transcription and to protect low-density lipoproteins from oxidation [31]. Ginsenosides have effects on numerous signaling pathways but effects on autophagy are still largely uncharacterized. Here, we showed that RGE or RGS inhibits autophagy, which leads to its increases doxorubicin-induced cytotoxicity in HCC cells. Although the means by which RGE affects autophagy needs further investigation, we have presented evidence suggesting that RGE affects autophagy comparably to CQ. CQ is currently being investigated in > 40 cancer clinical trials [30], [32], [33]. RGE may also have promise as a novel autophagy inhibitor for cancer therapy, especially useful in the treatment of HCC.
In the present study, we found that doxorubicin induces autophagy, and that autophagy induction performs a prosurvival role. This is consistent with previous observations regarding induction of autophagy by doxorubicin in cancer cells. For instance, Xu et al [34] suggested that inhibition of autophagy by deguelin, which is a retinoid extracted from Mundulea sericea (Wild), sensitizes pancreatic cancer cells to doxorubicin. They demonstrated that autophagy suppression by deguelin strikingly enhances doxorubicin-driven cell death in pancreatic carcinoma cells. Inhibition of autophagic flux by RGE markedly augments doxorubicin-induced cell death, therefore, autophagy inhibition by RGE could be an effectual approach in combination therapy to augment the chemotherapeutic effectiveness of doxorubicin and overcome chemoresistance in HCC. At present, since HCC has a high mortality rate due to the lack of effective treatment and sorafenib is the only effective targeted agent, autophagy inhibitors may be particularly valuable in sensitizing HCC cells to classical chemotherapy.
Acknowledgments
We are grateful to Dr Yoshimori for providing us with the mRFP–GFP tandem LC3 construct (tfLC3). This work was supported by the 2014 grant from the Korean Society of Ginseng. and this work was also supported by the National Research Foundation of Korea grant funded by the Korea government (No. 2011-0030043); funding was also provided from a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health & Welfare, Republic of Korea (No. HI15C0554).
Conflicts of interest
All contributing authors declare no conflicts of interest.
References
- 1.Jia L., Zhao Y., Liang X.J. Current evaluation of the millennium phytomedicine- ginseng (II): collected chemical entities, modern pharmacology, and clinical applications emanated from traditional Chinese medicine. Curr Med Chem. 2009;16:2924–2942. doi: 10.2174/092986709788803204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seo E.Y., Kim W.K. Red ginseng extract reduced metastasis of colon cancer cells in vitro and in vivo. J Ginseng Res. 2011;35:315–324. doi: 10.5142/jgr.2011.35.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S., Wang Z., Huang Y., O'Barr S.A., Wong R.A., Yeung S., Chow M.S. Ginseng and anticancer drug combination to improve cancer chemotherapy: a critical review. Evid Based Complement Alternat Med. 2014;2014:1–13. doi: 10.1155/2014/168940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu T.G., Huang Y., Cui D.D., Huang X.B., Mao S.H., Ji L.L., Song H.B., Yi C. Inhibitory effect of ginsenoside Rg3 combined with gemcitabine on angiogenesis and growth of lung cancer in mice. BMC Cancer. 2009;9:250. doi: 10.1186/1471-2407-9-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q.Y., Kang X.M., Zhao W.H. Antiangiogenic effect of low-dose cyclophosphamide combined with ginsenoside Rg3 on Lewis lung carcinoma. Biochem Biophys Res Comm. 2006;342:824–828. doi: 10.1016/j.bbrc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 6.Keum Y.S., Han S.S., Chun K.S., Park K.K., Park J.H., Lee S.K., Surh Y.J. Inhibitory effects of the ginsenoside Rg3 on phorbol ester-induced cyclooxygenase-2 expression, NF-kappaB activation and tumor promotion. Mutat Res. 2003;523–524:75–85. doi: 10.1016/s0027-5107(02)00323-8. [DOI] [PubMed] [Google Scholar]
- 7.Chen Q.J., Zhang M.Z., Wang L.X. Gensenoside Rg3 inhibits hypoxia-induced VEGF expression in human cancer cells. Cell Physiol Biochem. 2010;26:849–858. doi: 10.1159/000323994. [DOI] [PubMed] [Google Scholar]
- 8.Kim J.W., Jung S.Y., Kwon Y.H., Lee J.H., Lee Y.M., Lee B.Y., Kwon S.M. Ginsenoside Rg3 attenuates tumor angiogenesis via inhibiting bioactivities of endothelial progenitor cells. Cancer Biol Ther. 2012;13:504–515. doi: 10.4161/cbt.19599. [DOI] [PubMed] [Google Scholar]
- 9.Xu T.M., Xin Y., Cui M.H., Jiang X., Gu L.P. Inhibitory effect of ginsenoside Rg3 combined with cyclophosphamide on growth and angiogenesis of ovarian cancer. Chinese Med J-Peking. 2007;120:584–588. [PubMed] [Google Scholar]
- 10.Lee Y.S., Lee D.G., Lee J.Y., Kim T.R., Hong S.S., Kwon Kim YS. A formulated red ginseng extract upregulates CHOP and increases TRAIL-mediated cytotoxicity in human hepatocellular carcinoma cells. Int J Oncol. 2013;43:591–599. doi: 10.3892/ijo.2013.1964. [DOI] [PubMed] [Google Scholar]
- 11.Lee J.Y., Jung K.H., Morgan M.J., Kang Y.R., Lee H.S., Koo G.B., Hong S.S., Kwon S.W., Kim Y.S. Sensitization of TRAIL-induced cell death by 20(S)-ginsenoside Rg3 via CHOP-mediated DR5 upregulation in human hepatocellular carcinoma cells. Mol Cancer Ther. 2013;12:274–285. doi: 10.1158/1535-7163.MCT-12-0054. [DOI] [PubMed] [Google Scholar]
- 12.White E. The role for autophagy in cancer. J Clin Invest. 2015;125:42–46. doi: 10.1172/JCI73941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiuri M.C., Zalckvar E., Kimchi A., Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 14.Shen H.M., Codogno P. Autophagic cell death: Loch Ness monster or endangered species? Autophagy. 2011;7:457–465. doi: 10.4161/auto.7.5.14226. [DOI] [PubMed] [Google Scholar]
- 15.Rosenfeldt Mathias T., Ryan Kevin M. The multiple roles of autophagy in cancer. Carcinogenesis. 2011;32:955. doi: 10.1093/carcin/bgr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzanti R., Arena U., Tassi R. Hepatocellualr carcinoma: where are we? World J Exp Med. 2016;20:21–36. doi: 10.5493/wjem.v6.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takimoto C.H., Awada A. Safety and anti-tumor activity of sorafenib (Nexavar®) in combination with other anti-cancer agents: a review of clinical trials. Cancer Chemother Pharmacol. 2008;61:535–548. doi: 10.1007/s00280-007-0639-9. [DOI] [PubMed] [Google Scholar]
- 18.Cabibbo G., Latteri F., Antonucci M., Craxì A. Multimodal approaches to the treatment of hepatocellular carcinoma. Nat Clin Pract Gastroenterol Hepatol. 2009;6:159–169. doi: 10.1038/ncpgasthep1357. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y.H., Ding Z.B., Zhou J., Hui B., Shi G.M., Ke A.W., Wang X.Y., Dai Z., Peng Y.F., Gu C.Y. Targeting autophagy enhances sorafenib lethality for hepatocellular carcinoma via ER stress-related apoptosis. Autophagy. 2011;7:1159–1172. doi: 10.4161/auto.7.10.16818. [DOI] [PubMed] [Google Scholar]
- 20.Ding Z.B., Hui B., Shi Y.H., Zhou J., Peng Y.F., Gu C.Y., Yang H., Shi G.M., Ke A.W., Wang X.Y. Autophagy activation in hepatocellular carcinoma contributes to the tolerance of oxaliplatin via reactive oxygen species modulation. Clin Cancer Res. 2011;17:6229–6238. doi: 10.1158/1078-0432.CCR-11-0816. [DOI] [PubMed] [Google Scholar]
- 21.Kim D.G., Jung K.H., Lee D.G., Yoon J.H., Choi K.S., Kwon S.W., Shen H.M., Morgan M.J., Hong S.S., Kim Y.S. 20(S)-Ginsenoside Rg3 is a novel inhibitor of autophagy and sensitizes hepatocellular carcinoma to doxorubicin. Oncotarget. 2014;5:4438–4451. doi: 10.18632/oncotarget.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Semenov D., Lushnikova E., Nepomnyashchikh L. Anthracycline-induced cardiomyopathy is manifested in decreased protein synthesis, impaired intracellular regeneration, and non-necrotic death of cardiomyocytes. Bull Exp Biol Med. 2001;131:505–510. doi: 10.1023/a:1017956922385. [DOI] [PubMed] [Google Scholar]
- 23.Park J.Y., Choi P., Lee D., Kim T., Jung E.B., Hwang B.S., Kang K.S., Ham J. Effect of amino acids on the generation of ginsenoside Rg3 epimers by heat processing and the anticancer activities of epimers in A2780 human ovarian cancer cells. Evid Based Complement Alternat Med. 2016;20:1–6. doi: 10.1155/2016/3146402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur J., Debnath J. Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol. 2015;16:461–472. doi: 10.1038/nrm4024. [DOI] [PubMed] [Google Scholar]
- 25.Pankiv S., Clausen T., Lamark T., Brech A., Bruun J., Outzen H., Overvatn A., Bjorkoy G., Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 26.Mizushima N., Yoshimori T. How to interpret LC3 immunoblotting. Autophagy. 2007;3:542–545. doi: 10.4161/auto.4600. [DOI] [PubMed] [Google Scholar]
- 27.Bjørkøy G., Lamark T., Brech A., Outzen H., Perander M., Øvervatn A., Stenmark H., Johansen T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura S., Noda T., Yoshimori T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy. 2007;3:452–460. doi: 10.4161/auto.4451. [DOI] [PubMed] [Google Scholar]
- 29.Katayama H., Yamamoto A., Mizushima N., Yoshimori T., Miyawaki A. GFP-like proteins stably accumulate in lysosomes. Cell Struct Funct. 2007;33:1–12. doi: 10.1247/csf.07011. [DOI] [PubMed] [Google Scholar]
- 30.Yang Z.J., Chee C.E., Huang S., Sinicrope F.A. The role of autophagy in cancer: therapeutic implications. Mol Cancer Ther. 2011;10:1533–1541. doi: 10.1158/1535-7163.MCT-11-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang M.S., Lee S.G., Rho H.M. Transcriptional activation of Cu/Zn superoxide dismutase and catalase genes by panaxadiol ginsenosides extracted from Panax ginseng. Phytother Res. 1999;13:641–644. doi: 10.1002/(sici)1099-1573(199912)13:8<641::aid-ptr527>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 32.Sui X., Chen R., Wang Z., Huang Z., Kong N., Zhang M., Han W., Lou F., Yang J., Zhang Q. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death Dis. 2013;10:e838. doi: 10.1038/cddis.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morgan M.J., Gamez G., Menke C., Hernandez A., Thorburn J., Gidan F., Staskiewicz L., Morgan S., Cummings C., Maycotte P. Regulation of autophagy and chloroquine sensitivity by oncogenic RAS in vitro is context-dependent. Autophagy. 2014;10:1814–1826. doi: 10.4161/auto.32135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X.D., Zhao Y., Zhang M., He R.Z., Shi X.H., Guo X.J., Shi C.J., Peng F., Wang M., Shen M. Inhibition of autophagy by deguelin sensitizes pancreatic cancer cells to doxorubicin. Int J Mol Sci. 2017;18:1–13. doi: 10.3390/ijms18020370. [DOI] [PMC free article] [PubMed] [Google Scholar]