Abstract
Background/objective
Significant biomechanical differences were found among deadlift variations. However, little is known about the differences between the conventional and the Romanian deadlifts. Therefore, the purpose of this study was to determine which deadlift technique is a better training protocol between the conventional and the Romanian deadlifts as indicated by the greater demand in muscle activities and joint kinetics.
Methods
21 males performed each deadlift with 70% of the Romanian deadlift one repetition maximum (1RM) determined using a 1RM testing. Myoelectric activities of the rectus femoris, biceps femoris, and gluteus maximus and lower extremity net joint torque (NJT) were compared. The variables were extracted through an electromyography system (EMG) and a three-dimensional motion analysis. The EMG values were normalized to the peak EMG activation from a submaximal non-isometric voluntary contraction. A two-way repeated measures analysis of variance was conducted for statistical analysis. The level of significance was set at 0.05.
Results
Significantly greater normalized EMG values were found from the rectus femoris and gluteus maximus (58.57 ± 13.73 and 51.52 ± 6.08 %peak) of the conventional deadlift than those of the Romanian deadlift (25.26 ± 14.21 and 46.88 ± 7.39 %peak). The conventional deadlift indicated significantly greater knee and ankle NJTs (0.21 ± 0.13 and −0.33 ± 0.08 Nm/kg cm) than those of the Romanian deadlift (−0.28 ± 0.1 and −0.29 ± 0.06 Nm/kg cm).
Conclusion
The conventional deadlift would be a better technique for training the rectus femoris and gluteus maximus than the Romanian deadlift as indicated by the greater EMG and NJT values.
Keywords: Biomechanics, Electromyography, Joint kinetics, Resistance training
Introduction
The deadlift is a free weight exercise in which the barbell is lifted from the floor by extending the knees, hips, and ankles.1 Typically, the term deadlift is associated with the conventional deadlift (CD) characterized by a shoulder width stance of the feet and the arms outside of the thighs.2 Due to its ability to develop full body strength, the deadlift has been called one of the “big three” (squat, bench press, and deadlift) in gaining total body strength.2 When performing deadlifts, multiple agonists are activated at the same time, specifically at the knee and hip, where knee flexors and extensors and hip extensors are activated throughout the lift.3 For example, the hamstrings serve as knee stabilizers and hip extensors while the quadriceps work as knee extensors in the ascending phase. The CD attempts to activate a multitude of lower body musculature in developing strength. However, several variations of the deadlift exist in order to target specific muscle groups.2
The variations of the deadlift are the sumo deadlift (SD), the stiff-legged deadlift (SLD), and the Romanian deadlift (RD).4 The SD is a technique often performed in the powerlifting competition of the deadlift using a wider stance than the CD. The SLD involves keeping the legs almost straight as the bar is brought straight down to the floor. The RD is performed with approximately 15° of knee flexion employed4; however, unlike the SLD, as the barbell descends towards the ground, it remains in close contact with the legs during the RD. It was reported that the RD and the SLD are useful techniques to train gluteals and hamstrings5, 6, 7, 8 while the SD and the CD are performed to train the entire lower extremity muscles.9,10
Several studies compared the above-mentioned variations to identify the biomechanical differences, and significant biomechanical differences were found among the deadlift variations.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 A previous study compared the myoelectric activities of the lower extremity muscles between the CD and the SLD and reported that the CD showed a significantly higher vastus lateralis activity while the SLD exhibited a significantly greater medial gastrocnemius activity.5 Another study compared lower extremity joint kinetics between the CD and the SD and indicated that the CD had significantly greater plantarflexor and knee flexor torques while the SD had greater dorsiflexor and knee extensor torques.13 For achieving desired training goals, understanding biomechanical differences among the deadlift exercises is important for coaches and athletes to select the most appropriate deadlift technique.
Potential biomechanical differences also exist between the CD and the RD because of the technical differences between the lifts. However, little is known about the potential differences between the CD and the RD. The RD was compared only with exercises performed to train hamstrings and gluteals.6 It may be because the RD is considered one of the deadlift variations performed for training the posterior part of the leg. However, the hamstrings and gluteals are also agonists during the CD since those muscles serve as knee stabilizers and hip extensors.3,13 Furthermore, in order to identify in-depth biomechanical differences between the variations of the deadlift, joint kinetics, such as net joint torque (NJT), need to be concurrently analyzed with the myoelectric activities of the lower extremity muscles. This is because joint kinetics enables us to identify muscle dominance throughout the deadlift.13 It is expected that the CD will show different muscle dominance as well as myoelectric activities of the lower extremity muscles from those of the RD. Thus, in-depth electromyographic and kinetic comparisons between the CD and the RD need to be performed to provide coaches and athletes with a better insight into the deadlift variation. There was also one issue on the use of terminology. The RD was used but the SLD was actually performed in the literature.6 Piper and Waller4 also indicated that the RD is similar to the SLD with the exception of 15° of knee flexion employed. But, the 15° of knee flexion is a slight knee bend position that was used as the SLD in some studies.5,7,8
Therefore, the purpose of the present study was to determine which deadlift technique is a better training protocol between the CD and the RD as indicated by the greater demand in the muscle activities and joint kinetics. To achieve the purpose, electromyographic and kinetic variables between the CD and the RD were compared. It was hypothesized that (1) the CD would show significantly greater overall myoelectric activities of the lower extremity muscles including the rectus femoris (RF), biceps femoris (BF), and gluteus maximus (GM) than the RD, (2) the CD would show significantly greater knee and ankle NJTs than the RD, and (3) the CD would show different muscle dominance at the knee from the RD.
Methods
Participants
Twenty-one males (age: 22.4 ± 2.2 years; body mass: 82.5 ± 13.0 kg; height: 176.0 ± 7.1 cm) were recruited for the present study based on a priori G-power analysis with 6 repetitions, a power of 0.80, an alpha level of 0.05, and a medium effect size of 0.25.15 Participants volunteered to participate in the present study as this study was not supported by any grant. Participants had at least three years of the CD and the RD experience. They performed resistance training at least twice a week, and no one had powerlifting experience. All the participants reported no musculoskeletal injuries within the last 12 months that would prevent them from performing either deadlift technique. Before participating in the study, the participants were informed of the benefits and risks of the investigation prior to signing an institutionally approved informed consent document to participate.
Trial conditions
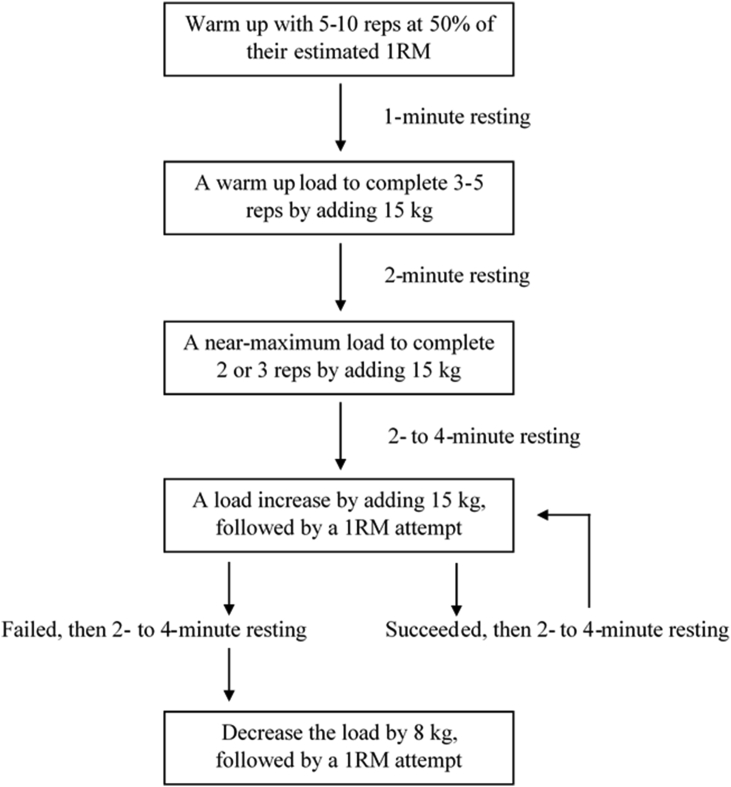
On day one, each participant's one repetition maximum (1RM) of the RD defined as the maximum weight one can lift once for an exercise was determined using a 1RM testing protocol (Fig. 1).16 The same absolute 1RD measured was used for both the CD and the RD because the CD and the RD were assumed to the same exercise with technique variations in this study.14 Participants were asked to perform the RD with about 15° of knee flexion.4 The 15° of knee flexion was measured using a goniometer with fulcrum, stabilization arm, and movement arm being the lateral epicondyle, the lateral midline of the lateral thigh, and the lateral middle line of the lateral shank, respectively. One week later, participants returned for actual deadlift testing. Participants performed an active warm up on a stationary bike for 5 min, followed by multiple practice deadlift trials. The practice deadlift trials consisted of 12 repetitions of each deadlift with a weighted barbell (20.4 kg) only and then 8 repetitions of each deadlift with the barbell plus additional weight plates (61.2 kg). For actual deadlift testing, participants were randomly assigned to either the CD or the RD, then performed a total of 10 deadlift trials (two techniques × five trials per technique) at 70% of RD 1RM in alternating order because it is often used when performing resistance training.5 A medium stance (100% of pelvic width) and a double overhand grip were used for both deadlift trials. A self-selected speed was used because participants reported the unnaturalness of the movement under a time-controlled condition. Participants were then asked to perform the deadlift as consistently as possible in terms of speed and grip width across all trials. A minimum rest period of 2 min between trials was allowed to minimize fatigue.
Fig. 1.
A one repetition maximum protocol.16.
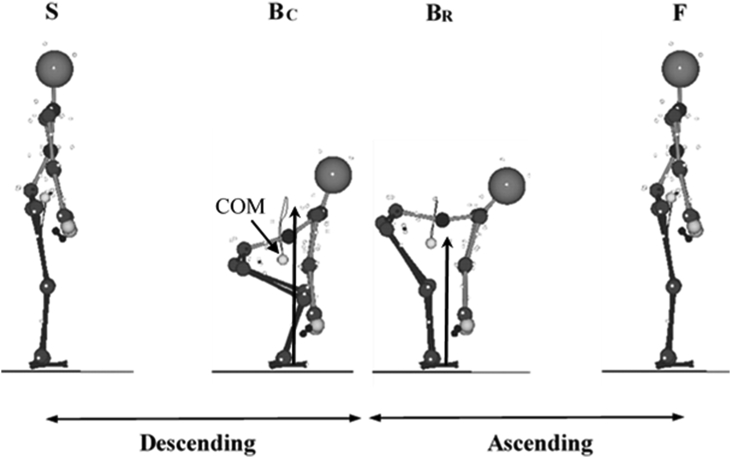
To facilitate data analysis, the deadlift movement was broken down into descending and ascending phases using the whole body center-of-mass movement (Fig. 2). The participants were asked to touch the floor with the bar while descending during both the CD and the RD to minimize biomechanical discrepancies attributed to the different vertical displacements of the bar (Fig. 2). To assess the reliability of the speed between the CD and the RD,12 the intraclass correlation coefficients (ICCs) for the durations of descending, ascending, and total movement were analyzed (ICCdescending: 0.84; ICCascending: 0.71; ICCtotal: 0.82). The durations of descending and ascending were measured using time elapsed while descending and ascending, respectively (Fig. 2).
Fig. 2.
Sagittal view of deadlift showing events and phases defined: S (Start of Deadlift), BC (Bottom of CD), BR (Bottom of RD), and F (Finish of Deadlift). The GRF vectors at the BC and BR and whole body center-of-mass (COM) were also presented in the figure.
Experimental setup
Fifty-nine markers were placed on participants' anatomical landmarks (Table 1). A 200-Hz six-camera VICON motion capture system (Model Bonita 3 and 10; VICON, Centennial, CO, USA) was used to capture the markers placed. A static T-pose trial was initially captured to define the markers placed, and a group of additional secondary points (13 joint centers) were also defined using the captured markers to facilitate analysis (Table 1). A group of markers (ASISs, medial knee epicondyles, medial malleolis, xiphoid, medial elbow epicondyles, and radial styloid processes) expected to interfere with the deadlift performance were removed in the deadlift trials. The static T-pose trial was imported into the deadlift trials during data processing to locate the markers removed. Ground reaction force (GRF) data was also collected using two AMTI force plates (Model Optima; Advanced Mechanical Technology, Inc., Watertown, MA, USA) at a sampling frequency of 1000-Hz. Participants oriented in the direction of the positive X-axis of the laboratory reference frame (global reference frame). The positive Y-axis pointed leftward perpendicular to the X-axis. The positive Z-axis was vertical.
Table 1.
Markers and defined secondary points.
| Segment | Markers/defined secondary points | Description |
|---|---|---|
| Pelvis | Markers (7) | Right and left ASISs, PSISs, iliac crests, and sacrum. |
| Defined (5) | Hip joints, lumbar 4/5, mid-ASIS, and mid-hip. The hip joints were calculated using the ‘Tylkowski-Andriacchi Method’.17,18 The lumbar 4/5 was computed using the ‘MacKinnon Method’.19 The mid-ASIS and mid-hip were the mid-points of right and left ASISs and the hip joints, respectively. | |
| Trunk | Markers (6) | Right and left acromions, sternum, C7, T10, and xiphoid. |
| Defined (2) | Mid-thorax and mid-abdomen. The mid-thorax and mid-abdomen were the mid-points of C7 and sternum and T10 and xiphoid, respectively. | |
| Legs | Markers (9 × 2) | Right and left greater trochanters, lateral thighs, lateral and medial epicondyles, lateral shanks, lateral and medial malleoli, and heels and toes. |
| Defined (2 × 2) | Knee and ankle joints. Knee and ankle joint centers were the mid-points of lateral and medial femoral epicondyles and lateral and medial malleoli, respectively. | |
| Arms | Markers (12 × 2) | Right and left anterior and posterior shoulders, three upper arm markers, lateral and medial epicondyles, two forearm markers, radial and ulnar styloid processes, and hand markers. |
| Defined (3 × 2) | Shoulder, elbow, and wrist joints. The shoulder, elbow, and wrist joint centers were computed using the ‘Rigid body Method’.20 | |
| Head | Markers (4) | Four head markers (right, left, forehead, and tophead). |
| Defined (1) | Head center. Head center is mid-point of the right and left head markers |
Note. ASIS: anterior superior iliac spines; PSIS: posterior superior iliac spines; C7: 7th cervical vertebra; T10: 10th thoracic vertebra.
For electromyography (EMG) analysis, a three-channel wireless EMG system (Desktop DTS; Noraxon, AZ, USA) was used to collect the myoelectric activities of the predetermined muscles at a sampling frequency of 1500 Hz. Three muscle groups (RF, BF, and GM) were selected in the present study21 and Ag/AgCL surface electrodes (2 cm × 2 cm) were unilaterally attached to those muscles on each participant's right side. Prior to electrode placement, the skin was cleaned with an alcohol swab and gently shaved using a razor to ensure that the resistance between the skin and the electrode is low which reduces interference in the recorded EMG signal. The surface electrodes were placed on the muscles by an experienced athletic trainer who was proficient in musculoskeletal structures for all the participants. For improving reliability and validity, the electrodes were placed on the skin over the muscles by a certified athletic trainer (fourth author) who had expertise in musculoskeletal structures for all the participants. The locations of the electrodes were determined based on a three-dimensional (3D) muscle map provided by the EMG analysis software (MyoMuscle MR3 3.8.6; Noraxon, AZ, USA).
Trial processing
Initial data processing was conducted through VICON Nexus program to acquire data files in the form of C3D files. The C3D files were then imported into Kwon3D Motion Analysis Suite (Version XP; Visol Inc., Seoul, Korea) for subsequent data processing and analysis. The raw 3D coordinates of the markers were filtered using a Butterworth zero phase-lag fourth-order low-pass filter with a cut-off frequency of 6-Hz determined by using the residual method22 to eliminate the noise generated due to experimental errors.
Sixteen body segments (pelvis, abdomen, chest, thighs, shanks, feet, upper arms, forearms, hands, and head) were defined based on the captured makers for computing subsequent data. Segmental reference frames were defined for all the lower extremity segments. The X-, Y-, and Z-axis of the segments were aligned with the mediolateral, anteroposterior, and longitudinal axes of the segments, respectively. For the computation of lower extremity NJT, the body segment parameters measured by de Leva23 were employed in an inverse dynamics procedure using the joint coordinate system.24 The computed NJT were normalized to total mass (body mass + barbell mass) × height. The positive and negative signs of the NJT mean the direction of the NJT acting on the segment at the joint is counterclockwise and clockwise, respectively.22 Flexion angles and angular accelerations of the lower extremity joints were additionally extracted to explain the results of electromyographic and kinetic variables. The flexion angles were calculated as the relative orientation angle of a distal segment to its proximal segment using a Cardan sequence of XYZ (mediolateral-anteroposterior-longitudinal). The first orientation angle about the mediolateral axis was used as the flexion angle. The relative orientation angles computed were then used to calculate the relative angular accelerations of a distal segment to its proximal segment by taking the second time-derivative of the orientation angles.
Raw EMG data of the first CD trial was initially rectified and smoothed using the full-wave rectification and the root mean square method with a 20-millisecond moving window. The peak EMG activation obtained during the task (i.e. the first CD trial) performed at a submaximal non-isometric voluntary contraction (i.e. 70% of 1RM) was used as the normalization value.25 Although the EMG normalization method used in this study is known to be reliable, muscle activation between muscles was not compared because this method is not valid to compare muscle activation between muscles within the task.25 Therefore, the main effect of the muscles was not included in data analysis. The mean amplitude of the normalized data during the CD and the RD was extracted for statistical analysis.
Statistical analysis
A two-way repeated measures analysis of variance (ANOVA) was conducted to compare electromyographic variables between the CD and the RD with the type of the deadlift (within-subject: CD and RD) and the myoelectric activities of the lower extremity muscles (within-subject: RF, BF, and GM) being factors. A two-way repeated measures ANOVA was also performed to compare kinetic variables with the type of the deadlift (within-subject: CD and RD) and the lower extremity NJT (within-subject: hip NJT, knee NJT, and ankle NJT) being factors. To explain the results of the electromyographic and kinetic variables, the flexion angles and angular accelerations of the lower extremity joints were compared between the CD and the RD using a two-way repeated measures ANOVA. For significant factor effect or interaction, post-hoc tests were performed with Bonferroni adjustment. The IBM SPSS Statistics version 24 (IBM, New York) was used. The level of significance was set at 0.05 for all statistical tests.
Prior to running a two-way repeated measures ANOVA, several statistical assumptions required for a two-way repeated measures ANOVA including significant outliers, normality, and Sphericity were checked using box plot, Shapiro-Wilk test and normal Q-Q plot, and Mauchly's test of Sphericity, respectively.
Results
The CD revealed significantly greater overall EMG activities of the lower extremity muscles than the RD (p < 0.001) (Table 2). Specifically, the RF and GM activities of the CD were significantly greater than those of the RD (RF: p < 0.001, GM: p = 0.021) (Table 2).
Table 2.
Summary of the lower extremity NJT and EMG activity results.
| Variables | CD∗ | RD | ||||
|---|---|---|---|---|---|---|
| EMG (%Peak) |
RF† | BF | GM† | RF | BF | GM |
| 58.57 ± 13.73 |
57.45 ± 6.34 |
51.52 ± 6.08 |
25.26 ± 14.21 |
56.66 ± 18.56 |
46.88 ± 7.39 |
|
| NJT (Nm/kg cm) | Hip | Knee† | Ankle† | Hip | Knee | Ankle |
| −0.85 ± 0.08 | 0.21 ± 0.13 | −0.33 ± 0.08 | −0.86 ± 0.07 | −0.28 ± 0.1 | −0.29 ± 0.06 | |
Note. ∗ significantly (p < 0.05) different overall EMG activity from the RD; † significantly different EMG activity and NJT from the matching variable of the RD; CD: conventional deadlift; RD: Romanian deadlift; NJT: net joint torque; EMG: electromyography: RF: rectus femoris; BF: biceps femoris; GM: gluteus maximus.
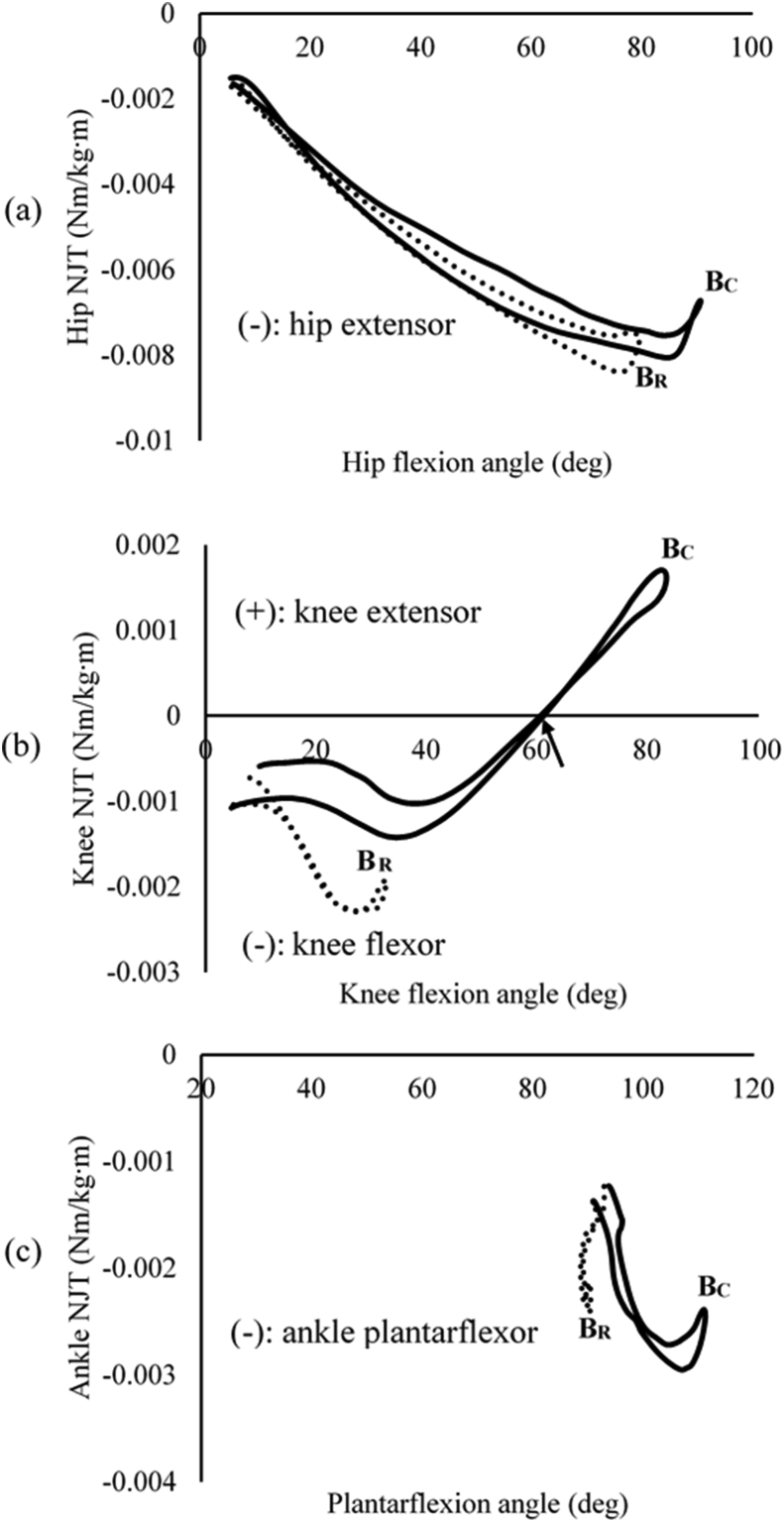
The CD indicated significantly greater knee and ankle NJTs than the RD (knee: p < 0.001, ankle: p = 0.022) (Table 2). While hip and ankle NJTs showed single muscle dominance (i.e. extensor for the hip and plantarflexor for the ankle) throughout the CD and the RD, knee NJT showed both knee flexor and extensor during the CD (Fig. 3).
Fig. 3.
(a) hip net joint torque (NJT) as a function of the hip flexion angle, (b) knee NJT as a function of the knee flexion angle, and (c) ankle NJT as a function of plantarflexion angle. The solid and dotted lines represent the conventional deadlift (CD) and the Romanian deadlift (RD), respectively. BC and BR represents the bottoms of the CD and the RD, respectively. The arrow in the knee NJT indicated the event when the GRF vector was posteriorly located to the knee joint.
Discussion
One of the key findings of this study is that the RF and GM activities of the CD were significantly greater than those of the RD (Table 2). These results can be explained by significantly increased knee and hip flexions observed during the CD (Table 3). The increased knee and hip flexions were likely to cause the extensors, including the RF and GM, to work harder during the CD. In addition, it is anecdotally believed that the RD is performed primarily for training the hamstrings. But, our study showed no significant difference in the BF between the CD and the RD. Although no significant difference in the BF was observed, the role of the BF for the knee might be different between the CD and the RD based on muscle dominance (Fig. 3). The BF worked primarily as the knee flexors during the RD since only knee flexor NJT occurred during the RD; however, the BF served as both the knee flexors and stabilizers during the CD because both knee flexor and extensor NJTs were observed during the CD. Therefore, it can be determined that the CD would be a better technique for training the RF and GM than the RD, and the RD works differently between the CD and the RD. An in-depth biomechanical analysis of the CD and the RD performed in this study is expected to help coaches and athletes to select a better deadlift exercise for achieving their desired goals.
Table 3.
Summary of the kinematics of the lower extremity joints.
| Variables | CD | RD | ||||
|---|---|---|---|---|---|---|
| Flexion (º) |
Hip∗ | Knee∗ | Ankle∗ | Hip | Knee | Ankle |
| 90.63 ± 8.39 |
85.17 ± 11.71 |
111.89 ± 6.68 |
79.97 ± 15.85 |
33.86 ± 12.59 |
90.47 ± 4.82 |
|
| Angular acc. (deg/s2) | Hip∗ | Knee∗ | Ankle∗ | Hip | Knee | Ankle |
| 631.14 ± 240.49 | 795.29 ± 223.29 | 312.51 ± 80.99 | 464.99 ± 136.82 | 362.77 ± 124.95 | 163.76 ± 67.68 | |
Note. ∗ significantly (p < 0.05) different from the matching variable of the RD; CD: conventional deadlift; RD: Romanian deadlift; acc.: acceleration.
No comparable EMG data exists; however, a previous study that compared the myoelectric activities of the lower extremity muscles between the CD and the SLD reported similar results to those of the current study.5 The study found that the CD showed a greater EMG activity of the vastus lateralis than the SLD with no significant difference in the BF. The previous study also showed a significant difference in EMG activity of the gastrocnemius that might also be in line with the finding of the current study. The current study did not collect EMG activity of the gastrocnemius but examined the ankle plantarflexor torque to identify muscle dominance, showing that the CD showed a greater ankle plantarflexor torque than the RD that is likely to increase the myoelectric activity of the gastrocnemius (Table 2).
Another key finding of this study is that the CD revealed significantly greater knee and ankle NJTs than the RD. It was most likely due to significantly greater flexion angles of the lower extremity joints observed during the CD than during the RD (Table 3). The greater flexion angles of the lower extremity joints, especially the knee and ankle, are likely to increase angular accelerations of the knee and ankle (Table 3). Consequently, the increased angular accelerations of the knee and ankle would result in the greater knee and ankle NJTs during the CD. In addition, although all of the participants were asked to touch the floor with the bar while descending, some participants were unable to perform it during the RD. It may be due to the tightness of the hamstrings that are two-joint muscles with a limited length. The failure of touching the floor might lead to the reduced vertical displacement of whole body center-of-mass that caused the knee and ankle joints to generate decreased NJTs during the RD.
In interpreting muscle dominance, the CD showed both negative and positive knee NJTs (Fig. 3). The negative knee NJT (knee flexor torque) occurred in the beginning of descending and the end of ascending during the CD while the positive one (knee extensor torque) occurred after which the knee was flexed about 60°. The RD, however, exhibited negative knee NJT only throughout the lift (Fig. 3). These results were consistent with those reported by the previous studies analyzing NJT during the CD.3,13 The studies reported that both knee extensor and flexor torques occurred during the CD. The point of the GRF vector can explain these results (Fig. 1). The GRF vector was located anterior to the knee that enforced the knee to flex in the beginning of descending and the end of ascending during the CD and posteriorly located to the knee joint after which the knee was flexed around 60°. The GRF vector, however, was located anterior to the knee throughout the RD that enforced the knee to flex.
This study also compared the knee flexions between the RD and the SLD because the terminology was not clearly distinguished between the RD and the SLD in some studies. McAllister et al.6 referred to the fact that the RD is similar to the SLD in their studies. They used the RD but the SLD was actually performed with a near full extension of the knee. Pipe and Waller4 also reported that the RD is similar to the SLD with the exception of the 15° of knee flexion employed during the RD. The 15° of knee flexion means the knees are in a slightly flexed position that was defined as the SLD in some studies.5,7,8 The 15° of knee flexion reported was inconsistent with the average knee flexion angle (33°) observed during the RD in the current study (Table 3). This discrepancy may be due to different vertical barbell displacements. In the previous study, participants descended until the barbell was located right below the knee joint. In the current study, however, participants were asked to descend until touching the floor. The increased knee flexion observed in the current study may be attributed to an adaptive strategy to release hamstrings tightness. Based on the results of the current study and previous literature, therefore, the RD can be performed with the knee flexion of 15–33° while the SLD is performed with a near full extension of the knee.
This study was the first study that compared electromyographic and kinetic variables between the CD and the RD. In spite of the novelty, several limitations were observed in this study. First, using a submaximal load (70% of RD 1RM) during data collection may produce different results when compared to maximal loads that are often used during competitive lifting. Further investigations are warranted on the effects of different loads on biomechanical differences between the CD and the RD. Second, 70% of the RD 1 RM was used for both deadlifts despite the relative intensities being not the same for each deadlift. Third, despite the potential differences in EMG activity among the hamstrings including the BF, the semitendinosis, and the semimembranosis, the RF was only examined in this study.
Conclusion
The purpose of this study was to determine which deadlift technique is a better training protocol between the CD and the RD as indicated by the greater demand in the muscle activities and joint kinetics. To achieve the purpose, electromyographic and kinetic variables between the CD and the RD were compared. Based on the findings of this study, the following conclusions were derived:
-
•
The RF and GM activities of the CD were significantly greater than those of the RD.
-
•
The CD indicated significantly greater knee and ankle NJTs than the RD.
Practically, the CD would be a better technique for training lower extremity muscles, including the RF and GD, than the RD as indicated by the greater EMG and NJT values observed during the CD.
Acknowledgements
Authors would like to thank participants for volunteering this study. The authors have no conflicts of interest relevant to this article. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Berglund L., Aasa B., Hellqvist J. Which patients with low back pain benefit from deadlift training? J Strength Condit Res. 2015;29:1803–1811. doi: 10.1519/JSC.0000000000000837. [DOI] [PubMed] [Google Scholar]
- 2.Bird S., Barrington-Higgs B. Exploring the deadlift. Strength Condit J. 2010;32:46–51. [Google Scholar]
- 3.Thompson B.J., Stock M.S., Shields J.E. Barbell deadlift training increases the rate of torque development and vertical jump performance in novices. J Strength Condit Res. 2015;29:1–10. doi: 10.1519/JSC.0000000000000691. [DOI] [PubMed] [Google Scholar]
- 4.Piper T.J., Waller M.A. Variations of the deadlift. Strength Condit J. 2001;23:66–73. [Google Scholar]
- 5.Bezerra E.S., Simao R., Fleck S.J. Electromyographic activity of lower body muscles during the deadlift and still-legged deadlift. J Exerc Physiol. 2013;16:30–39. [Google Scholar]
- 6.McAllister M.J., Hammond K.G., Schilling B.K. Muscle activation during various hamstring exercises. J Strength Condit Res. 2014;28:1573–1580. doi: 10.1519/JSC.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 7.Schoenfeld B.J., Contreras B., Tiryaki-Sonmez G. Regional differences in muscle activation during hamstrings exercise. J Strength Condit Res. 2015;29:159–164. doi: 10.1519/JSC.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 8.Wright G.A., Delong T.H., Gehlsen G. Electromyographic activity of the hamstrings during performance of the leg curl, stiff-leg deadlift, and back squat movements. J Strength Condit Res. 1999;13:168–174. [Google Scholar]
- 9.Escamilla R.F., Lowry T.M., Osbahr D.C. Biomechanical analysis of the deadlift during the 1999 special olympics world games. Med Sci Sports Exerc. 2001;33:1345–1353. doi: 10.1097/00005768-200108000-00016. [DOI] [PubMed] [Google Scholar]
- 10.McGuigan M.R., Wilson B.D. Biomechanical analysis of the deadlift. J Strength Condit Res. 1996;10:250–255. [Google Scholar]
- 11.Camara K.D., Coburn J.W., Dunnick D.D. An examination of muscle activation and power characteristics while performing the deadlift exercise with straight and hexagonal barbells. J Strength Condit Res. 2016;30:1183–1188. doi: 10.1519/JSC.0000000000001352. [DOI] [PubMed] [Google Scholar]
- 12.Chulvi-Medrano I., García-Massó X., Colado J.C. Deadlift muscle force and activation under stable and unstable conditions. J Strength Condit Res. 2010;24:2723–2730. doi: 10.1519/JSC.0b013e3181f0a8b9. [DOI] [PubMed] [Google Scholar]
- 13.Escamilla R.F., Francisco A.C., Fleisig G.S. A three-dimensional biomechanical analysis of sumo and conventional style deadlifts. Med Sci Sports Exerc. 2000;32:1265–1275. doi: 10.1097/00005768-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Nijem R.M., Coburn J.W., Brown L.E. Electromyographic and force plate analysis of the deadlift performed with and without chains. J Strength Condit Res. 2016;30:1177–1182. doi: 10.1519/JSC.0000000000001351. [DOI] [PubMed] [Google Scholar]
- 15.Faul F., Erdfelder E., Lang A.G. *Power 3: a flexible statistical power analysis program for the social, behavioural, and biomedical sciences. Behav Res Meth. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 16.Coburn J.W., Malek M.H. second ed. Human Kinetics; Champaign, IL: 2012. NSCA's Essentials of Personal Training. [Google Scholar]
- 17.Andriacchi T.P., Andersson G.B., Fermier R.W. A study of lower-limb mechanics during stair climbing. J Bone Joint Surg Am. 1980;62:749–757. [PubMed] [Google Scholar]
- 18.Tylkowski C.M., Simon S.R., Mansour J.M. Internal rotation gait in spastic cerebral palsy. Hip. 1982:89–125. [PubMed] [Google Scholar]
- 19.MacKinnon C.D., Winter D.A. Control of whole body balance in the frontal plane during human walking. J Biomech. 1983;26:633–644. doi: 10.1016/0021-9290(93)90027-c. [DOI] [PubMed] [Google Scholar]
- 20.Kwon Y.H., Como C., Singhal K. Assessment of planarity of the golf swing based on the functional swing plane of the clubhead and motion planes of the body points in golf. Sports BioMech. 2012;11:127–148. doi: 10.1080/14763141.2012.660799. [DOI] [PubMed] [Google Scholar]
- 21.Snyder B.J., Cauthen C.P., Senger S.R. Comparison of muscle involvement and posture between the conventional deadlift and a "Walk-In" style deadlift machine. J Strength Condit Res. 2017;31:2859–2865. doi: 10.1519/JSC.0000000000001723. [DOI] [PubMed] [Google Scholar]
- 22.Winter D.A. fourth ed. John Wiley & Sons; New York, NY: 2009. Biomechanics and Motor Control of Human Movement. [Google Scholar]
- 23.de Leva P. Adjustments to Zatsiorsky-Seluyanov’s segment inertia parameters. J Biomech. 1996;29:1223–1230. doi: 10.1016/0021-9290(95)00178-6. [DOI] [PubMed] [Google Scholar]
- 24.Grood E.S., Suntay W.J. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 25.Burden A. How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25 years of research. J Electromyogr Kinesiol. 2010;20:1023–1035. doi: 10.1016/j.jelekin.2010.07.004. [DOI] [PubMed] [Google Scholar]