Abstract
Objective
Carnitine palmitoyltransferase 1C (CPT1C) is implicated in central regulation of energy homeostasis. Our aim was to investigate whether CPT1C in the ventromedial nucleus of the hypothalamus (VMH) is involved in the activation of brown adipose tissue (BAT) thermogenesis in the early stages of diet-induced obesity.
Methods
CPT1C KO and wild type (WT) mice were exposed to short-term high-fat (HF) diet feeding or to intracerebroventricular leptin administration and BAT thermogenesis activation was evaluated. Body weight, adiposity, food intake, and leptinemia were also assayed.
Results
Under 7 days of HF diet, WT mice showed a maximum activation peak of BAT thermogenesis that counteracted obesity development, whereas this activation was impaired in CPT1C KO mice. KO animals evidenced higher body weight, adiposity, hyperleptinemia, ER stress, and disrupted hypothalamic leptin signaling. Leptin-induced BAT thermogenesis was abolished in KO mice. These results indicate an earlier onset leptin resistance in CPT1C KO mice. Since AMPK in the VMH is crucial in the regulation of BAT thermogenesis, we analyzed if CPT1C was a downstream factor of this pathway. Genetic inactivation of AMPK within the VMH was unable to induce BAT thermogenesis and body weight loss in KO mice, indicating that CPT1C is likely downstream AMPK in the central mechanism modulating thermogenesis within the VMH. Quite opposite, the expression of CPT1C in the VMH restored the phenotype.
Conclusion
CPT1C is necessary for the activation of BAT thermogenesis driven by leptin, HF diet exposure, and AMPK inhibition within the VMH. This study underscores the importance of CPT1C in the activation of BAT thermogenesis to counteract diet-induced obesity.
Keywords: CPT1C, Hypothalamus, Thermogenesis, Brown adipose tissue, Diet-induced obesity
Highlights
-
•
Diet- and leptin-induced thermogenesis are impaired in CPT1C KO mice.
-
•
Expression of CPT1C in the VMH restores short-term diet-induced thermogenesis in CPT1C KO mice.
-
•
AMPK inhibition in the VMH does not restore the activation of BAT in CPT1C KO mice.
-
•
CPT1C is essential in the activation of BAT thermogenesis to counteract diet-induced obesity.
1. Introduction
Obesity is ultimately the result of a sustained imbalance between energy intake and energy expenditure. A key mechanism to maintain body weight homeostasis against an overload of energy is diet-induced thermogenesis [1], [2]. Brown adipose tissue (BAT) is considered a major site for the regulation of diet-induced thermogenesis through the sympathetic nervous system (SNS), and it is precisely orchestrated by the hypothalamus [3], [4]. In fact, an intact hypothalamic function will ensure a fine-tune activation of BAT thermogenesis in response to short-term high fat (HF) diet or leptin to counteract excessive body weight gain [2], [5], [6]. Despite this evidence, to date, little is known about the exact molecular hypothalamic pathways regulating thermogenesis under conditions of nutrient surplus [7]. In light of the current obesity epidemic, identification of the hypothalamic pathways and potential targets mediating short-term activation of thermogenesis in response to nutritional status would provide valuable information about obesity development and progression [2], [7], [8], [9].
Recent findings have demonstrated that hypothalamic AMPK is a major regulator of BAT thermogenesis through its modulation of the SNS [3], [10]. Particularly, it has revealed AMPK activity in the ventromedial nucleus of the hypothalamus (VMH) on thermogenic response. Remarkably, selective inactivation of AMPK within the VMH increased ventral hypothalamic malonyl-CoA levels and BAT activity and promoted weight loss, in a feeding-independent manner [10], [11]. Although this pathway constitutes a canonical circuit that mediates the effect of several thermogenic molecules (e.g. T3 or leptin) [3], [10], [12], further studies are necessary to explore the sub-cellular mechanisms and neuronal networks involved in the AMPK(VMH)-SNS-BAT axis. In this regard, recent data have demonstrated that selective ablation of the isoform AMPKα1 in steroidogenic factor 1 (SF1) neurons of the VMH promotes BAT activation and subsequently a leaner, feeding-independent and obese-resistant phenotype [12], [13].
The acetyl-CoA (ACC)/malonyl-CoA pathway is one of the most important signaling pathways downstream AMPK [14]. Within the hypothalamus, malonyl-CoA levels fluctuate in response to the nutritional status, acting as a canonical signal of energy surplus [15], [16]. Malonyl-CoA is the physiological inhibitor of carnitine palmitoyltransferase 1 (CPT1) enzymes, which catalyze the transport of long chain fatty acids into the mitochondria [16]. Among CPT1s, the neuron-specific CPT1C isoform is the most puzzling carnitine acyltransferase [17], [18]. In contrast to the canonical isoforms (CPT1A and CPT1B), CPT1C is located in the endoplasmic reticulum (ER) of neurons, instead of the mitochondrial membrane, and has insignificant CPT1 activity [19]. Nevertheless, it is still able to bind malonyl-CoA with similar affinity than CPT1A [20], suggesting that CPT1C could act as a sensor of this lipid intermediary in the hypothalamus [16].
The expression of CPT1C in the brain has been found particularly high in neurons of hypothalamic areas involved in the regulation of feeding and energy expenditure including arcuate nucleus (ARC), paraventricular hypothalamus (PVH) and VMH [17], [21]. Studies from our group and others have demonstrated that CPT1C within these areas plays a major role in the modulation of energy balance. For example, hypothalamic CPT1C mediates that central effects of leptin and ghrelin on feeding behavior [22], [23]. Hypothalamic CPT1C also determines fuel selection and food preference during fasting [24], [25]. Moreover, CPT1C KO mice are more prone to become obese when chronically fed a HF diet with a reduced peripheral fatty acid oxidation [20], [21], [26], [27]. In these studies, the expression of CPT1C, especially in the mediobasal hypothalamus (MBH) was found to be crucial in mediating the effects in energy homeostasis [21], [24]. However, the possible role of CPT1C in the hypothalamic regulation of BAT thermogenesis is totally unknown.
Here, we show that the obese phenotype and metabolic inflexibility that characterizes to CPT1C KO mice is related to an impaired BAT thermogenesis following a short-term HF diet exposure and central leptin injection. We also demonstrate that the lack of CPT1C disrupts the canonical pathway of AMPK(VMH)-SNS-BAT-mediated thermogenesis. Our data thus uncover CPT1C as a key downstream factor of the hypothalamic AMPK/ACC pathway in the control of brown fat thermogenesis.
2. Materials and methods
2.1. Animals
Male (8–10 week old) CPT1C KO mice and their wild-type (WT) littermates with the same genetic background (C57BL/6J) were used for the experiments [24]. All animals were housed on a 12 h/12 h light/dark cycle (light on at 8 am, light off at 8 pm) in a temperature- and humidity-controlled room. The animals were allowed free access to water and standard laboratory chow, unless otherwise specified. For HF diet studies, animals were placed on an HF diet (60% kcal from fat, D12492) or standard diet (SD) (10% kcal from fat, D12450B, Research Diets, New Brunswick, USA) for 3, 7, or 14 days. At the end of the studies, animals were sacrificed and tissues collected for further molecular and biochemical analysis as further detailed. All animal procedures were performed in agreement with European guidelines (2010/63/EU) and approved by the University of Barcelona Local Ethical Committee (Procedure ref. 9606 from the Generalitat de Catalunya).
2.2. Intracerebroventricular administration of leptin
Chronic cannulae were stereotaxically implanted into the lateral cerebral ventricle under ketamine/xylazine intraperitoneal anesthesia (ketamine 75 mg/kg body weight plus xylazine 10 mg/kg body weight). The coordinates were 0.58 mm posterior to Bregma, 1 mm lateral to the midsagittal suture, and 2.2 mm deep. Mice were individually caged and allowed to recover for 5 days before the experiment. Prior to the experiment, cannula placement was verified by a positive dipsogenic response to angiotensin II (1 nmol in 1 ml; Sigma–Aldrich). On experimental day, WT and CPT1C KO mice received an intracerebroventricular (ICV) administration of 2 μl of either leptin (0.1 μg/μl) (PeproTech, London, UK) or vehicle (aqueous buffer containing 0.1% BSA), 3 h after lights-on. 200 min after the injection, mice were sacrificed by cervical dislocation and MBH and BAT were collected for further analysis.
2.3. Stereotaxic microinjection and viral vectors
The lentiviral vectors pWPI-IRES-GFP, and pWPI-CPT1C-IRES-GFP were produced and titrated as previously described [24]. In addition, a lentiviral vector with a mutated isoform of CPT1C insensitive to malonyl-CoA, pWPI-CPT1CM589S-IRES-GFP, was produced. Mouse malonyl-CoA CPT1C sensitive site was identified by sequence homology with CPT1A. The homologous mutation in CPT1A (M593S) abolishes malonyl-CoA sensitivity while maintaining CPT1 activity [28]. CPT1C mutant M589S was constructed using the Q5 Site-Directed mutagenesis procedure (New England BioLabs) with the pWPI-IRES-CPT1C plasmid as template. The primers were obtained from the online design software NEBaseChanger and designed with 5′ ends phosphorylated and annealing back-to-back: forward 5′-GAGTCAGCCAGTACCCGACTGTTC-3′ and reverse 5′-ATAAGTCAGGCAGAATTGAC-3’ (the mutated nucleotide were underlined). The appropriate substitutions as well as the absence of unwanted mutations were confirmed by sequencing the inserts in both directions.
Adenoviral vectors (GFP and AMPKα1-dominant negative + AMPKα2-dominant negative, AMPK-DN; Viraquest; North Liberty, IA, USA) were kindly provided by Dr. Miguel Lopez [12]. Stereotaxic surgery to target the VMH was performed in mice under ketamine/xylazine anesthesia. Purified lentivirus (1 × 109 pfu ml−1) or adenovirus (1 × 1012 pfu ml−1) in artificial cerebrospinal fluid were injected bilaterally in the VMH over 10 min through a 33-gauge injector connected to a Hamilton Syringe and an infusion pump (0.5 μl per injection site) [24]. The injections were directed to the following stereotaxic coordinates: 1.6 mm posterior from Bregma, ±0.4 mm lateral to midline, and 5.6 mm deep. Mice underwent 6 days (adenovirus) or 7 days (lentivirus) of recovery before other experiments were performed. Correct bilateral infection was confirmed by western blot and histologically by GFP fluorescence in brain slices.
2.4. BAT temperature measurements
Skin temperature surrounding BAT was visualized using a high-resolution infrared camera (FLIR Systems) and analyzed with a specific software package (FLIR-Tools-Software, FLIR; Kent, UK), as previously described [12]. For ICV administration of leptin, images were recorded and analyzed every 10 min during 220 min. For the rest of experiments, thermal images were acquired the day of sacrifice.
2.5. Sample collection and processing
Mice were killed by cervical dislocation. For each animal, either the whole brain (for histology) or the MBH, as well as blood (for plasmatic determinations), liver, interscapular BAT, visceral and subcutaneous WAT were collected, weighed, and stored at −80 °C until further processing. To dissect the MBH, brains were placed in a coronal brain matrix and sectioned from Bregma (−1 to −2.5 mm) and the MBH was obtained using a tissue collector measuring 1 mm in diameter.
2.6. Plasma analysis
Plasma was obtained after blood centrifugation (2000 g, 15 min). Plasma levels of leptin were determined by mouse ELISA kit (Crystal Chem, Zaandam, Netherlands), following the manufacturer's instructions.
2.7. Tissue morphology
Interscapular BAT and visceral and subcutaneous WAT were fixed overnight in 10% PBS-buffered formalin. Histological samples were paraffin-embedded and stained with hematoxylin and eosin (H&E), as previously described [29]. Tissue sections were captured by light microscopy (Olympus, Hamburg, Germany) at 20X magnification and using NIS-Elements software (Nikon, Japan).
2.8. Liver triglycerides (TG) quantification
Liver samples were homogenized, and lipids were extracted as previously described [30]. TG were measured in the lipid extract using a commercial kit (Sigma, Madrid, Spain), following the manufacturer's instructions.
2.9. RNA preparation and quantitative RT-PCR
Total RNA was extracted from tissues using Trizol Reagent (Fisher Scientific, Madrid, Spain). Retrotranscription and quantitative RT-PCR (qPCR) was performed as previously described [24]. Proprietary SYBR Green or Taqman Gene Expression assay primers used (IDT DNA Technologies, Leuven, Belgium) are detailed in the supplementary material (Table S.I). Relative mRNA levels were measured using the CFX96 Real-time System, C1000 Thermal Cycler (BioRad).
2.10. Western blotting
Western blot was performed as previously described [24]. Briefly, tissue was homogenized in RIPA buffer (Sigma–Aldrich, Madrid, Spain) containing protease and phosphatase inhibitor cocktails. Protein extracts were separated on SDS-PAGE, transferred into Immobilion-PVDF membranes (Merck Millipore, Madrid, Spain) and probed with antibodies against: ACC, AMPKα, pACC (Ser79), pAMPKα (Thr172), pSTAT3 (Tyr705) (Cell Signaling; Danvers, MA, USA); GAPDH, UCP1 (Abcam, Cambridge, UK) β-actin (Fisher Scientific, Madrid, Spain) and α-tubulin (Sigma, Madrid, Spain). Each membrane was then incubated with the corresponding horseradish peroxidase-conjugated secondary antibody, anti-mouse or anti-rabbit (DAKO, Glostrup, Denmark), and developed using LuminataForte Western HRP substrate (Merck Millipore). Images were collected by GeneTools software (Syngene, Cambridge, UK) and quantified by densitometry using ImageJ-1.33 software (NIH, Bethesda, MD, USA). GAPDH or β-actin was used as an endogenous control to normalize protein expression levels. In all the figures showing images of gels, all the bands for each picture come from the same gel, although they may be spliced for clarification.
2.11. Statistical analysis
All results are expressed as mean ± SEM. Statistical analysis was conducted using GraphPad Prism 5 Software (GraphPad Software, La Jolla, CA, USA). Statistical analysis was determined by ANOVA (more than 2 groups were compared) followed of post hoc two-tailed Bonferroni test. P < 0.05 was considered significant. The number of animals used in each experiment is specified in each figure legend.
3. Results
3.1. CPT1C KO mice show impaired diet-induced thermogenesis
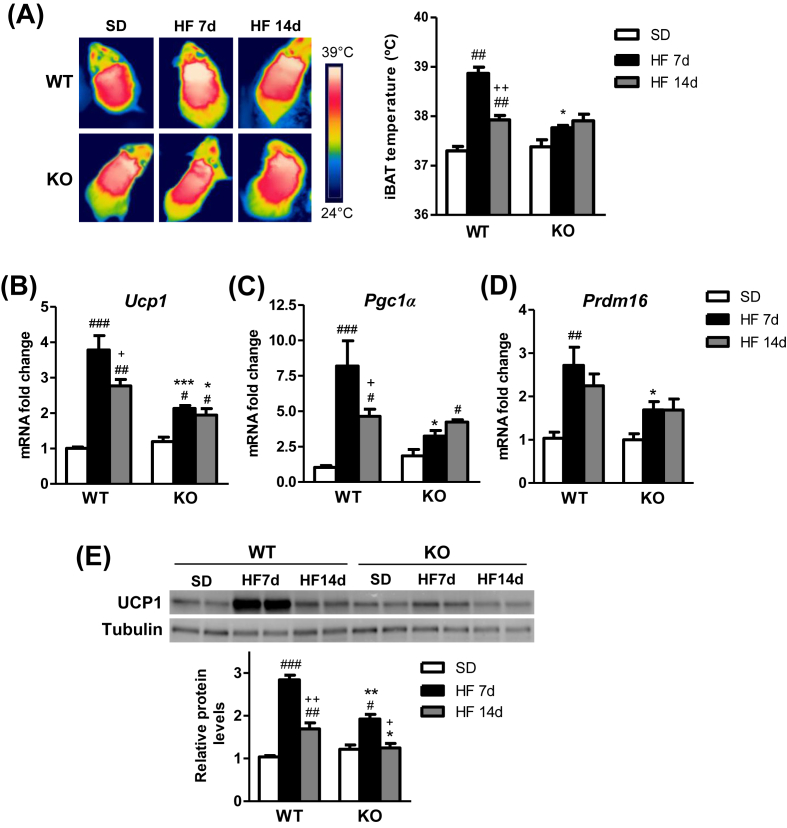
The induction of thermogenesis in the interscapular BAT of mice was first analyzed after short-term exposure to HF diet, compared to SD. To identify the maximum activation peak of diet-induced thermogenesis over time, experiments were performed in response to 3, 7, and 14 days HF diet feeding (Figure 1 and Fig. S.1). The peak in the thermogenesis was reached in interscapular temperature (Figure 1A), gene expression of thermogenic markers (Figure 1B–D, Fig. S.1A) and UCP1 protein expression (Figure 1E) in the BAT of WT mice after 7 days of HF diet when compared to other timings and SD. Of note, HF feeding over 7 days resulted in significantly ameliorated responses in CPT1C KO mice, when compared to WT mice (Figure 1A–E).
Figure 1.
Impaired diet-induced thermogenesis in CPT1C KO mice. (A) Representative infrared thermal images and quantification of interscapular temperature adjacent to the BAT depot of WT and CPT1C KO mice fed a standard diet (SD) or a high fat (HF) diet for 7 and 14 days. (B–D) Relative mRNA expression of the thermogenic markers UCP1 (B), PGC1α (C) and PRDM16 (D) in BAT of WT and KO mice fed SD or HF diet. (E) Protein levels of UCP1 in BAT of WT and KO fed SD or HF diet for 7 and 14 days. Data are expressed as mean ± SEM (n = 5–9). *P < 0.05, **P < 0.01, ***P < 0.001 versus WT with the same diet; #P < 0.05, ##P < 0.01, ###P < 0.001 versus SD within the same genotype; +P < 0.05, ++P < 0.01 versus HF 7d within the same genotype.
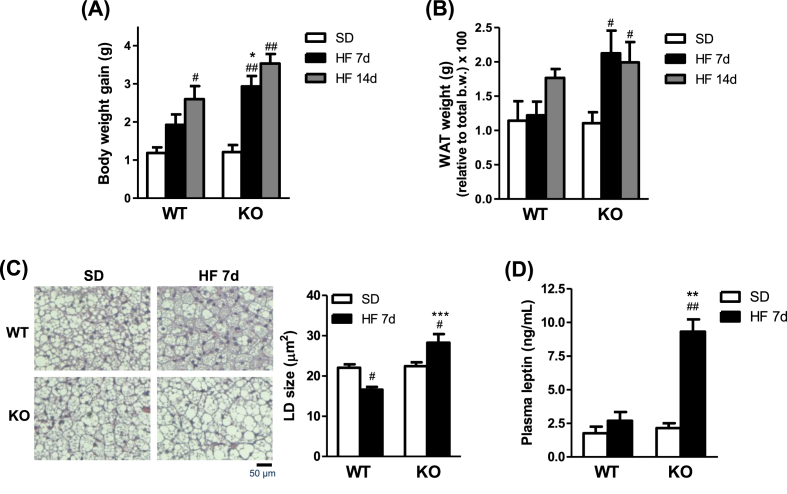
In WT mice, a significant increase in body weight gain was not appreciated until 14 days of administration of a HF diet (Figure 2A). In contrast, CPT1C KO mice already revealed higher body and visceral WAT weight over 7 days of HF diet compared to SD (Figure 2A,B; Fig. S.2A). The induction of thermogenesis and body weight gain of WT and CPT1 KO mice were associated with a reduction in the size of unilocular lipid droplets observed after 7 days of HF diet feeding in histological sections of BAT (Figure 2C). As illustrated in Figure 2D, HF feeding over 7 days resulted in a substantial increase of leptin levels in plasma of CPT1C KO mice, whereas these levels remained unaltered in WT mice. Altogether, these data indicate that CPT1C KO mice show an earlier obesogenic phenotype in response to acute HF diet administration, likely due to an impaired activation of BAT thermogenesis, compared to WT. This is also supported by the fact that food intake, measured during 7 and 14 days of HF feeding, was comparable in the WT and KO mice (Fig. S.2B), indicating that the obesogenic phenotype observed in CPT1C KO mice is not due to alterations in food intake.
Figure 2.
CPT1C KO mice show an earlier obesogenic phenotype compared to WT. (A and B) Body weight gain (A) and visceral WAT weight (B) of WT and KO mice fed a standard diet (SD) or a high fat (HF) diet for 7 and 14 days. (C) Representative histological H&E staining and quantification of the unilocular lipid droplets (LD) size of interscapular BAT of WT and KO mice fed a SD or a HF diet for 7 days. (D) Plasma leptin levels of WT and KO mice fat a SD or HF diet for 7 days. Data are expressed as mean ± SEM (n = 5–7). *P < 0.05, **P < 0.01, ***P < 0.001 versus WT with the same diet; #P < 0.05, ##P < 0.01 versus SD within the same genotype.
3.2. The thermogenic response to central leptin is impaired in CPT1C KO mice
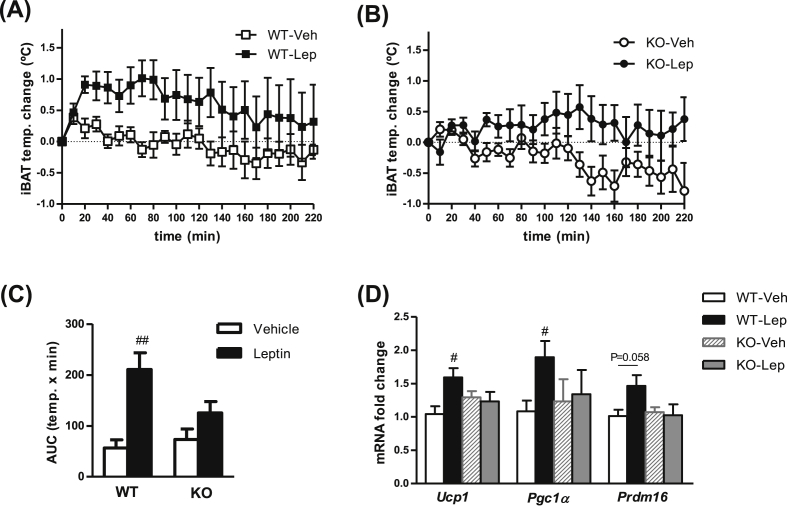
In another set of experiments, activation of BAT thermogenesis was also evaluated after central administration of leptin (Figure 3). We found an increase of BAT interscapular temperature by central leptin that was maintained for at least 3 h in WT mice (Figure 3A,C). This effect was confirmed by an increase in gene expression of thermogenic markers in BAT of WT (Figure 3D). However, these acute leptin-induced responses were significantly attenuated in CPT1C KO mice (Figure 3B–D).
Figure 3.
Impaired leptin-induced thermogenesis in CPT1C KO mice. (A–C) Quantification of interscapular temperature changes adjacent to the BAT depot (iBAT) after ICV leptin treatment in WT (A) and CPT1C KO mice (B) compared with ICV vehicle. (C) Area under the curve (AUC) of iBAT temperature during 220 min. (D) Gene expression analysis of thermogenic markers in BAT of WT and KO mice after ICV leptin. Data are expressed as mean ± SEM (n = 5–8). #P < 0.05, ##P < 0.01 versus Vehicle within the same genotype.
3.3. CPT1C KO mice display an altered expression of hypothalamic leptin signaling markers and ER stress after short-term HF diet feeding
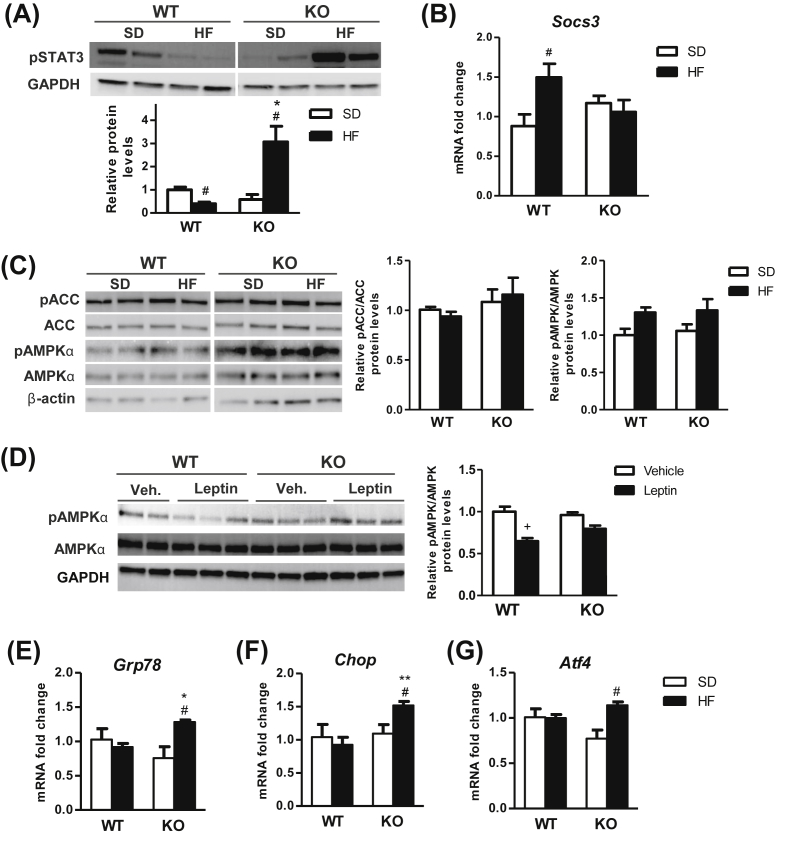
The hyperleptinemia after 7 days of HF diet administration and the impaired central leptin-induced thermogenesis observed in CPT1C KO mice suggest an earlier onset in the disruption of leptin signaling in these mice compared to WT. Therefore, the expression of proteins involved in leptin signaling in the MBH was evaluated. First, expression levels of pSTAT3 and SOCS3, important transcription factors in leptin signaling, were analyzed in MBH of WT and KO mice fed a HF diet for 7 days. MBH of WT mice showed a reduced phosphorylation of STAT3 (Figure 4A) and increased mRNA levels of SOCS3 (Figure 4B) with HF diet compared to SD. Conversely, CPT1C KO mice fed a HF diet exhibited a substantial increase in pSTAT3 without changes in SOCS3 (Figure 4A,B).
Figure 4.
CPT1C KO mice show an altered expression of markers of leptin signaling and ER stress in the mediobasal hypothalamus after short-term administration of a HF diet. (A–C) Protein levels of pSTAT3 (A), mRNA levels of SOCS3 (B) and protein expression of pAMPKα, pACC, AMPK, and ACC (C) in the mediobasal hypothalamus of WT and CPT1C KO mice fed a standard diet (SD) or a high fat (HF) diet for 7 days. (D) Protein levels of pAMPKα and AMPK in the mediobasal hypothalamus of WT and CPT1C KO mice after ICV administration of leptin or vehicle. (E) mRNA levels of ER stress markers in the mediobasal hypothalamus of WT and CPT1C KO mice fed a SD or a HF diet for 7 days. Data are expressed as mean ± SEM (n = 5–7). *P < 0.05, **P < 0.01 versus WT with the same diet; #P < 0.05 versus SD within the same genotype; +P < 0.01 versus vehicle within the same genotype.
Next, we evaluated the impact of HF and/or CPT1C ablation on the AMPK signaling in the MBH. No significant alterations in the expression of pAMPK and pACC were detected in either genotype after 7 days of HF diet exposure (Figure 4C). Remarkably, the leptin-induced inhibition of pAMPK in the MBH was suppressed in CPT1C KO mice, indicating that a normal CPT1C function is required for a normal leptin hypothalamic signaling (Figure 4D).
Finally, since CPT1C is located in the ER, and hypothalamic ER stress has been strongly related to leptin signaling disruption and obesity, as well as on the central control of thermogenesis [31], [32], [33], ER stress markers were analyzed in MBH. Whereas no changes in mRNA expression levels for ER stress markers were appreciated in WT mice-fed HF diet for 7 days, significant increases were shown in MBH of CPT1C KO mice (Figure 4E–G), in keeping with their impaired thermogenic responses, leptin signaling, and more prone obese phenotype.
3.4. Expression of CPT1C in the VMH is enough to restore short-term diet-induced response in CPT1C KO mice
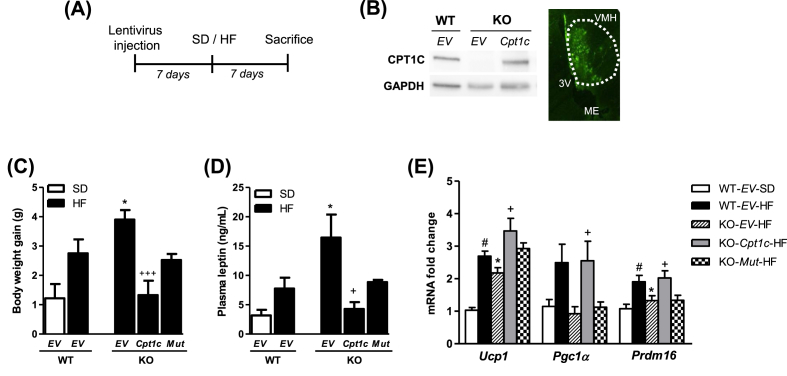
Due to the importance of CPT1C in the MBH region (which includes the VMH) in the regulation of energy homeostasis [21], [24], and considering the crucial role of the VMH in the control of BAT thermogenesis [10], we evaluated if the expression of CPT1C in this hypothalamic area was able to restore the phenotype observed after short-term HF feeding in KO mice. Lentiviral vectors expressing CPT1C-GFP or empty vector (EV)-GFP were microinjected in the VMH of WT and CPT1C KO mice and, after 7 days, mice were fed SD or HF diet for 7 days (see experimental protocol illustrated in Figure 5A). Injection site was confirmed by direct fluorescence of GFP in brain sections or by CPT1C expression analysis by western blot in the MBH (Figure 5B). The expression of CPT1C in the VMH was enough to reverse the body weight gain (Figure 5C), the hyperleptinemia (Figure 5D) and the expression of gene thermogenic markers in BAT (Figure 5E) of KO mice fed a HF diet for 7 days. Stereotaxic injection of adeno-associated viruses expressing the EV-GFP or CPT1C in the VMH of CPT1C KO mice (see Supplementary Methods) also revealed a significant restoration of iBAT temperature and body weight gain in response to 7 days HF diet feeding (Fig. S.3).
Figure 5.
Expression of CPT1C in the VMH restores short-term diet-induced response in CPT1C KO mice. (A) GFP (empty vector, EV) or CPT1C-GFP (Cpt1c)-expressing lentiviruses were microinjected in the VMH of WT and CPT1C KO mice and after 1 week, mice were fed a standard diet (SD) or a high fat (HF) diet for 7 days. (B) Injection site was confirmed by direct fluorescence of GFP in brain sections or by CPT1C expression analysis by western blot in the ventral hypothalamus. (C–E) Body weight gain (C), plasma leptin (D) and gene expression analysis of thermogenic markers in BAT of WT-EV, KO-EV, and KO expressing CPT1C (KO-Cpt1c) or CPT1CM589S (KO-Mut) fed SD or HF diet for 7 days. Data are expressed as mean ± SEM (n = 6–8). *P < 0.05 versus WT-EV-HF; #P < 0.05 versus WT-EV-SD; +P < 0.05, +++P < 0.001 versus KO-EV-HF.
These data confirm a key role of CPT1C in this hypothalamic area during diet-induced thermogenesis.
We also investigated the role of malonyl-CoA in the impaired hypothalamic function of CPT1C null mice. For this purpose, lentiviral vectors expressing a mutant CPT1C insensitive to malonyl-CoA (CPT1CM589S, see Methods), were used and compared to vectors expressing EV and CPT1C. Expression of the mutated isoform of CPT1CM589S in VMH of KO mice was not able to fully restore body weight gain (Figure 5C), leptinemia (Figure 5D), and expression of gene thermogenic markers in BAT (Figure 5E) in response to HF diet. These data indicate that malonyl-CoA sensing by CPT1C is relevant to regulate short-term diet-induced responses.
3.5. Selective inactivation of AMPKα in the VMH was not able to induce BAT thermogenesis and body weight loss in CPT1C KO mice
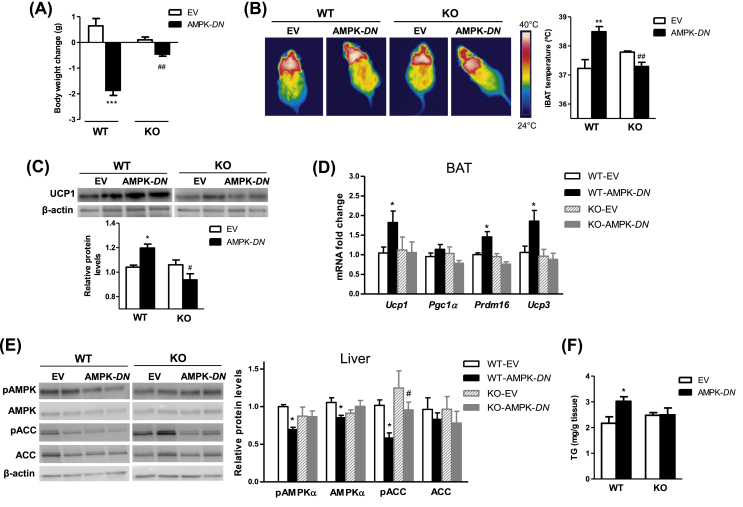
To assess whether CPT1C is a downstream factor in the AMPKα-mediated regulation of energy balance, we selectively inactivated AMPK in VMH of WT and KO mice by stereotaxic delivery of a dominant-negative AMPKα1+α2 isoforms (AMPK-DN) [11], [12], [13], [34]. This inactivation was confirmed by reduced hypothalamic protein levels of pACC (Fig. S.4) [34]. Previous data demonstrated that AMPK-DN delivery into the VMH increased malonyl-CoA concentrations in the ventral hypothalamus, inducing weight loss and increased expression of BAT thermogenic markers, without altering food intake [11], [12]. As illustrated in Figure 6, selective inactivation of AMPK in the VMH of WT mice involved a substantial reduction of body weight gain (Figure 6A) with a significant increase in interscapular temperature adjacent to the BAT depot (Figure 6B), elevated UCP1 protein expression levels (Figure 6C) and increased gene expression of thermogenic markers in BAT (Figure 6D). Notably, CPT1C KO mice showed a significant attenuation in all these parameters compared to WT mice (Figure 6A–D).
Figure 6.
CPT1C KO mice show impaired AMPK-mediated effects within the VMH on body weight change, BAT thermogenesis and liver. (A) Body weight change of WT and CPT1C KO mice treated with adenoviruses encoding GFP (Empty vector, EV) or AMPK-DN in the VMH. (B–D) Representative infrared thermal images and quantification of interscapular temperature adjacent to the BAT depot (B), protein levels of UCP1 in BAT (C) and gene expression analysis of thermogenic markers in BAT of WT and KO mice treated with EV or AMPK-DN in the VMH (D). (E and F) Protein levels of the AMPK pathway (E) and TG levels in the liver of mice treated with EV or AMPK-DN in the VMH (G). Data are expressed as mean ± SEM (n = 6–7). *P < 0.05, **P < 0.01, ***P < 0.001 versus WT-EV; #P < 0.05, ##P < 0.01 versus WT-AMPK-DN.
Recent data from our group have shown that the inhibition of AMPK in the VMH promotes decreased hepatic AMPK signaling through the vagus nerve and subsequently increased lipogenesis [12]. Our data showed that while in WT recapitulated that response, it was totally blunted in CPT1C KO mice (Figure 6E–F). Altogether, AMPK-DN-mediated effects within the VMH in body weight change, BAT and liver were impaired in mice lacking CPT1C. This is of importance because, it has been recently demonstrated that increased lipogenesis after VMH inhibition of AMPK is demanding for BAT thermogenesis. Therefore, CPT1C KO mice, which show impaired BAT function, also display altered associated liver responses.
4. Discussion
Development of and progression to obesity are mediated by short-term neurological changes in response to nutritional status that progressively impair hypothalamic neuronal functions and therefore body weight regulation. In the last few years, several investigations have been directed towards the identification of proteins involved in the temporal dysregulation of neuronal functions to control aspects of energy balance beyond food intake, during the development of diet-induced obesity [2], [7], [8], [9].
The present research demonstrates that the neuron-specific CPT1 isoform, CPT1C, plays a critical role in hypothalamic regulation of BAT thermogenesis, particularly in response to metabolic challenges activating BAT, such as short-term diet and central leptin. Considering the importance of the canonical pathway dependent on AMPK in the VMH to regulate BAT thermogenesis during the development of diet-induced obesity [10], [12], [13], our study also reveals that CPT1C might be a crucial factor in this canonical pathway.
Although CPT1C is still the most unknown CPT1, and its neuronal function is uncertain, our group and others have demonstrated its critical role in energy homeostasis [18]; also, it has been suggested to be a key indicator of the energetic status of neurons by sensing malonyl-CoA, a canonical signal of energy surplus [16], [35]. The present study reveals that the obesogenic phenotype and acute alterations in metabolic flexibility already described in CPT1C KO mice [20], [22], [23], [24] are related to impaired hypothalamic regulation of BAT thermogenesis, as exposed in response to short-term diet or central leptin administration. These metabolic challenges imply an increase in hypothalamic levels of malonyl-CoA [16], [35], [36] that need to be sensed by CPT1C. We show that, under short-term HF diet feeding (7 days), a robust activation peak of BAT thermogenesis was appreciated in WT mice, which helps mice to maintain normal body weight, adiposity and leptinemia, thus counteracting obesity development. Previous studies analyzing initial hypothalamic events during development of diet-induced obesity in mice [7], [8], [9], [37] have also demonstrated that C57BL/6J mice show hypothalamic compensatory changes at early time points in response to HF diet (from 2 days to 7 days), but they may not be able to maintain them (from 14 days onwards). This could contribute to their obese phenotype after a prolonged period of HF diet. Our study describes a pronounced activation of BAT thermogenesis after 7 days of feeding a HF diet, which could be directly related to the short-term hypothalamic compensatory changes that have been previously described to counteract obesity. In contrast, HF feeding over 7 days resulted in a diminished activation of BAT thermogenesis, higher body weight gain, hyperleptinemia, and adiposity in CPT1C KO mice. This indicates that the lack of neuronal CPT1C determines an early obesogenic phenotype in response to fat-rich diets. In relation to this result, acute activation of BAT thermogenesis in response to central leptin administration was also attenuated in mice lacking CPT1C. These data could correlate with the fact that CPT1C KO mice are resistant to the satiety effect of central leptin (Fig. S.5 and [23]).
Development of obesity has been linked to increased plasma levels of leptin that positively correlate to high adiposity and body weight gain and an altered hypothalamic leptin signaling [38]. Evaluation of molecular mediators of leptin signaling during initial exposure to HF diets in MBH revealed that WT mice showed transient reduced levels of pSTAT3 with increased levels of SOCS3 levels after 7 days of HF diet. These results are in line with previous findings analyzing hypothalamic responses after short-term administration of fat-rich diets [8], [9]. Transitory hypothalamic changes observed in WT animals could be more related to a compensatory response to the positive energy surplus that contributes to maintaining stable body weight during initial stages of fat-rich diets administration, as suggested by others [7], [8], [9]. In contrast to WT mice, CPT1C KO mice fed a HF diet for 7 days had increased hypothalamic pSTAT3 levels and unchanged expression of SOCS3. The opposite response in the hypothalamus of KO mice during initial stages of diet-induced obesity could indicate a lack of compensatory changes at early time points of HF diet feeding and therefore an earlier obesogenic phenotype.
In addition to these findings, MBH of mice deficient in CPT1C fed a HF diet showed significant increases in ER stress markers. The hypothesis that hypothalamic ER stress is causally linked with leptin resistance and obesity has gained substantial support in the recent years [39]. Although the exact mechanisms by which HF diet feeding can directly perturb hypothalamic neuronal function remain unclear, a number of investigations associate hypothalamic lipotoxicity derived from exposure to HF diet with ER stress as a possible explanation for the onset of obesity [31], [32], [33], [40], [41]. CPT1C is suggested to act as a sensor of hypothalamic malonyl-CoA levels fluctuations and also as a main regulator of the metabolism of complex lipids such as ceramides in neurons [18], [20], [42]. In addition, hypothalamic ER stress induced by lipotoxicity has been shown to impair the BAT thermogenic process [31], [32], [33]. A plausible hypothesis would be that the lack of CPT1C is determining an inaccurate lipid sensing, leading to hypothalamic lipotoxicity and subsequently ER stress, an idea that will require data to be confirmed.
It is known that the AMPK pathway is dysregulated in hypothalamus in obese states resulting from chronic HF feeding and that lack of dynamic responsiveness of this pathway is crucial in the pathophysiology of leptin resistance during diet-induced obesity [37]. In our study, administration of a HF diet during 7 days did not induce significant changes in pAMPK and pACC in MBH of WT or KO mice. This result agrees with previously reported data, showing that short-term administration of a HF diet (1–3 weeks) to rats did not modify hypothalamic AMPK phosphorylation [43], [44]. Longer periods of HF feeding (from 3 weeks onwards) induced increased levels of the active phosphorylated form of AMPK in the hypothalamus of rats [43] and mice [37], mediating the interplay between hypothalamic and peripheral response to diet. When analyzing central administration of leptin, we found a significant attenuation of pAMPK expression levels in MBH of WT mice after leptin injection, whereas this attenuation was not evidenced in CPT1C KO mice. Considering the findings that leptin has a role in SNS-mediated activation of BAT thermogenesis [45], and that inhibition of hypothalamic AMPK activity by leptin implies sympathetic activation to BAT and WAT [6], we suggest that the lack of changes in pAMPK in the MBH of CPT1C KO mice could be related to the impaired leptin-induced thermogenesis in these animals.
To further demonstrate if CPT1C, particularly in the VMH, is a factor involved in the AMPK-SNS-BAT axis, specific strategies were achieved in this study. Firstly, considering the importance of the VMH in the control of BAT thermogenesis [6], [10], we showed that the lentiviral expression of CPT1C in the VMH was enough to restore the phenotype observed after short-term HF feeding in KO mice. In addition, the phenotype was not fully restored when expressing the mutated isoform of CPT1C insensitive to malonyl-CoA in the VMH. This result suggests that sensing malonyl-CoA by CPT1C is relevant to regulate short-term diet-induced responses in this hypothalamic area. Secondly, our virogenetic approaches showed that BAT thermogenesis and body weight of CPT1C KO mice did not respond to selective inactivation of AMPK in the VMH, indicating that CPT1C is a crucial factor in the AMPKα(VMH)-mediated regulation of BAT thermogenesis. Our data are in line with recent investigations proposing CPT1C as a downstream factor of AMPK in different hypothalamic nuclei to regulate feeding. A study from our group demonstrated the existence of a downstream pathway to SIRT1/p53/pAMPK axis in response to ghrelin, involving CPT1C, triggering acute changes in ceramide levels to regulate food intake by the modulation of NPY/AgRP expression in the ARC [22]. Interestingly, a recent investigation from Minokoshi's group [25] found that activation of an AMPK-CPT1C pathway in a subset of CRH-positive neurons in the PVH mediates the fasting-induced increase in high-carbohydrate diet selection. Our current investigation shows for the first time a role of CPT1C in the AMPK-brown fat axis to regulate thermogenic program in the VMH. These data suggest CPT1C as a downstream factor of hypothalamic AMPK to maintain energy homeostasis. Despite these results, using a whole-body CPT1C KO mouse is a limitation in our study, and therefore the importance of other hypothalamic nuclei (e.g. PVH or ARC) in these thermogenic responses cannot be excluded. Further work will be necessary to determine the specific neuronal VMH population mediating these effects. An interesting candidate could be SF1 neurons, as we have recently demonstrated that the specific ablation of AMPKα1 at these levels promotes a lean feeding-independent, but thermogenic-dependent phenotype that protects against HF-induced obesity [12], [13].
Overall, the present investigation demonstrates that CPT1C in the VMH is necessary for the activation of BAT thermogenesis in response to central leptin and short-term HF diet administration. Also, we demonstrate that the role of CPT1C in adaptive thermogenesis is throughout the canonical pathway dependent on AMPK in the VMH. This study underscores the importance of CPT1C to provide metabolic adaptation during short-term consumption of fat-rich diets and during obesity development.
5. Conclusions
A better understanding of the neuronal pathways mediating short-term hypothalamic changes in response to nutritional status would provide valuable information about obesity development and progression. Therefore, identification of potential targets involved in these hypothalamic pathways to control aspects of energy balance beyond food intake, such as the BAT thermogenic activity, has gained relevance in the last few years.
The neuron-specific CPT1C, the most enigmatic CPT1 isoform, seems to play a key role in central regulation of energy homeostasis, mostly in terms of fuel selection and food preference during fasting or in response to ghrelin by AMPK-dependent mechanisms. The present investigation reveals that mice lacking CPT1C show an impaired activation of BAT thermogenesis in response to short-term HF feeding and central leptin administration. In this phenotype, expression of CPT1C, by sensing malonyl-CoA, in the VMH is enough to restore diet-induced thermogenesis and counteract body weight gain. Considering the importance of the canonical pathway dependent on AMPK in the VMH to regulate BAT thermogenesis during the development of diet-induced obesity, our study also demonstrates for the first time that CPT1C is a crucial factor in this canonical pathway. The link between hypothalamic CPT1C and adaptive thermogenesis by the AMPK-brown fat axis could explain the obesogenic phenotype characteristic of CPT1C KO mice and also emphasize the role of CPT1C in the VMH to provide metabolic adaptation during short-term consumption of fat-rich diets. Altogether, this study underscores the importance of CPT1C in the development and progression of obesity and could add insight into the understanding of the mechanisms underlying diet-induced obesity.
Financial support
This work was supported by the Ministerio de Economía, Industria y Competitividad (MINECO), Agencia Estatal de Investigación (AEI) and Fondo Europeo de Desarrollo Regional (FEDER) (Grants SAF2014-52223-C2-2-R to NC, SAF2017-83813-C3-3-R to NC and RR-R, SAF2014-52223-C2-1-R to DS, SAF2017-83813-C3-1-R to DS and LH, SAF2015-71026-R to ML, TEC2014-51903-R to MV and XP, and Ramón y Cajal RYC-2010-07434 to XP), the Centro de Investigación Biomédica en Red Fisiopatología de la Obesidad y la Nutrición (CIBEROBN) (Grant CB06/03/0001 to DS), the Generalitat de Catalunya (2014SGR465 to DS and NC, 2017SGR1384 to MV and XP), Fundació La Marató de TV3 (Grant 87/C/2016 to DS and NC), and by Projectes de Recerca per a investigadors novells (2015) to RR-R. AF is the recipient of a fellowship from the Agència de Gestió d’Ajuts Universitaris i de la Recerca (AGAUR) in Catalonia.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2018.10.010.
Contributor Information
Rosalía Rodríguez-Rodríguez, Email: rrodriguez@uic.es.
Núria Casals, Email: ncasals@uic.es.
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lowell B.B., Spiegelman B.M. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404(6778):652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 2.Kim K.W., Zhao L., Donato J., Kohno D., Xu Y., Elias C.F. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proceedings of the National Academy of Sciences. 2011;108(26):10673–10678. doi: 10.1073/pnas.1102364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lage R., Ferno J., Nogueiras R., Dieguez C., Lopez M. Contribution of adaptive thermogenesis to the hypothalamic regulation of energy balance. Biochemical Journal. 2016;473(22):4063–4082. doi: 10.1042/BCJ20160012. [DOI] [PubMed] [Google Scholar]
- 4.Bachman E.S., Dhillon H., Zhang C.-Y., Cinti S., Bianco A.C., Kobilka B.K. Beta AR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297(5582):843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- 5.Whittle A.J., López M., Vidal-Puig A. Using brown adipose tissue to treat obesity - the central issue. Trends in Molecular Medicine. 2011;17(8):405–411. doi: 10.1016/j.molmed.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Contreras C., Nogueiras R., Diéguez C., Rahmouni K., López M. Traveling from the hypothalamus to the adipose tissue: the thermogenic pathway. Redox Biology. 2017;12:854–863. doi: 10.1016/j.redox.2017.04.019. (April) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olofsson L.E., Unger E.K., Cheung C.C., Xu A.W. Modulation of AgRP-neuronal function by SOCS3 as an initiating event in diet-induced hypothalamic leptin resistance. Proceedings of the National Academy of Sciences. 2013;110(8):E697–E706. doi: 10.1073/pnas.1218284110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziotopoulou M., Mantzoros C.S., Hileman S.M., Flier J.S. Differential expression of hypothalamic neuropeptides in the early phase of diet-induced obesity in mice. American Journal of Physiology Endocrinology and Metabolism. 2000;279(4):E838–E845. doi: 10.1152/ajpendo.2000.279.4.E838. [DOI] [PubMed] [Google Scholar]
- 9.Thaler J.P., Yi C.-X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O. Obesity is associated with hypothalamic injury in rodents and humans. Journal of Clinical Investigation. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.López M., Nogueiras R., Tena-Sempere M., Diéguez C. Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nature Reviews Endocrinology. 2016;12(7):421–432. doi: 10.1038/nrendo.2016.67. [DOI] [PubMed] [Google Scholar]
- 11.Lopez M., Varela L., Vazquez M.J., Rodriguez-Cuenca S., Gonzalez C.R., Velagapudi V.R. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nature Medicine. 2010;16(9):1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martínez-Sánchez N., Seoane-Collazo P., Contreras C., Varela L., Villarroya J., Rial-Pensado E. Hypothalamic AMPK-ER stress-JNK1 Axis mediates the central actions of thyroid hormones on energy balance. Cell Metabolism. 2017;26(1):212–229. doi: 10.1016/j.cmet.2017.06.014. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seoane-Collazo P., Roa J., Rial-Pensado E., Liñares-Pose L., Beiroa D., Ruíz-Pino F. SF1-Specific AMPKα1 deletion protects against diet-induced obesity. Diabetes. 2018:db171538. doi: 10.2337/db17-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardie D.G., Ross F.A., Hawley S.A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature Reviews Molecular Cell Biology. 2012;13(4):251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lane M.D., Wolfgang M., Cha S.-H., Dai Y. Regulation of food intake and energy expenditure by hypothalamic malonyl-CoA. International Journal of Obesity (2005) 2008;32(Suppl. 4):S49–S54. doi: 10.1038/ijo.2008.123. [DOI] [PubMed] [Google Scholar]
- 16.Wolfgang M.J., Lane M.D. Hypothalamic malonyl-CoA and CPT1c in the treatment of obesity. FEBS Journal. 2011:552–558. doi: 10.1111/j.1742-4658.2010.07978.x. [DOI] [PubMed] [Google Scholar]
- 17.Price N., van der Leij F., Jackson V., Corstorphine C., Thomson R., Sorensen A. A novel brain-expressed protein related to carnitine palmitoyltransferase I. Genomics. 2002;80(4):433–442. doi: 10.1006/geno.2002.6845. [DOI] [PubMed] [Google Scholar]
- 18.Casals N., Zammit V., Herrero L., Fado R., Rodriguez-Rodriguez R., Serra D. Carnitine palmitoyltransferase 1C: from cognition to cancer. Progress in Lipid Research. 2016;61:134–148. doi: 10.1016/j.plipres.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Sierra A.Y., Gratacós E., Carrasco P., Clotet J., Ureña J., Serra D. CPT1c is localized in endoplasmic reticulum of neurons and has carnitine palmitoyltransferase activity. Journal of Biological Chemistry. 2008;283(11):6878–6885. doi: 10.1074/jbc.M707965200. [DOI] [PubMed] [Google Scholar]
- 20.Wolfgang M.J., Kurama T., Dai Y., Suwa A., Asaumi M., Matsumoto S. The brain-specific carnitine palmitoyltransferase-1c regulates energy homeostasis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(19):7282–7287. doi: 10.1073/pnas.0602205103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai Y., Wolfgang M.J., Cha S.H., Lane M.D. Localization and effect of ectopic expression of CPT1c in CNS feeding centers. Biochemical and Biophysical Research Communications. 2007;359(3):469–474. doi: 10.1016/j.bbrc.2007.05.161. [DOI] [PubMed] [Google Scholar]
- 22.Ramirez S., Martins L., Jacas J., Carrasco P., Pozo M., Clotet J. Hypothalamic ceramide levels regulated by CPT1C mediate the orexigenic effect of ghrelin. Diabetes. 2013;62(7):2329–2337. doi: 10.2337/db12-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao S., Zhu G., Gao X., Wu D., Carrasco P., Casals N. Important roles of brain-specific carnitine palmitoyltransferase and ceramide metabolism in leptin hypothalamic control of feeding. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(23):9691–9696. doi: 10.1073/pnas.1103267108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pozo M., Rodríguez-Rodríguez R., Ramírez S., Seoane-Collazo P., López M., Serra D. Hypothalamic regulation of liver and muscle nutrient partitioning by brain-specific carnitine palmitoyltransferase 1C in male mice. Endocrinology. 2017;158(7):2226–2238. doi: 10.1210/en.2017-00151. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto S., Sato T., Tateyama M., Kageyama H., Maejima Y., Nakata M. Activation of AMPK-regulated CRH neurons in the PVH is sufficient and necessary to induce dietary preference for carbohydrate over fat. Cell Reports. 2018;22(3):706–721. doi: 10.1016/j.celrep.2017.11.102. [DOI] [PubMed] [Google Scholar]
- 26.Wolfgang M.J., Cha S.H., Millington D.S., Cline G., Shulman G.I., Suwa A. Brain-specific carnitine palmitoyl-transferase-1c: role in CNS fatty acid metabolism, food intake, and body weight. Journal of Neurochemistry. 2008;105(4):1550–1559. doi: 10.1111/j.1471-4159.2008.05255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao X.F., Chen W., Kong X.P., Xu a. M., Wang Z.G., Sweeney G. Enhanced susceptibility of Cpt1c knockout mice to glucose intolerance induced by a high-fat diet involves elevated hepatic gluconeogenesis and decreased skeletal muscle glucose uptake. Diabetologia. 2009;52(5):912–920. doi: 10.1007/s00125-009-1284-0. [DOI] [PubMed] [Google Scholar]
- 28.Morillas M., Gómez-Puertas P., Bentebibel A., Sellés E., Casals N., Valencia A. Identification of conserved amino acid residues in rat liver carnitine palmitoyltransferase I critical for malonyl-CoA inhibition: mutation of methionine 593 abolishes malonyl-CoA inhibition. Journal of Biological Chemistry. 2003;278(11):9058–9063. doi: 10.1074/jbc.M209999200. [DOI] [PubMed] [Google Scholar]
- 29.Justo M.L., Claro C., Zeyda M., Stulnig T.M., Herrera M.D., Rodríguez-Rodríguez R. Rice bran prevents high-fat diet-induced inflammation and macrophage content in adipose tissue. European Journal of Nutrition. 2016;55(6) doi: 10.1007/s00394-015-1015-x. [DOI] [PubMed] [Google Scholar]
- 30.Justo M.L., Rodriguez-Rodriguez R., Claro C.M., Alvarez De Sotomayor M., Parrado J., Herrera M.D. Water-soluble rice bran enzymatic extract attenuates dyslipidemia, hypertension and insulin resistance in obese Zucker rats. European Journal of Nutrition. 2013;52(2):789–797. doi: 10.1007/s00394-012-0385-6. [DOI] [PubMed] [Google Scholar]
- 31.Contreras C., González-García I., Martínez-Sánchez N., Seoane-Collazo P., Jacas J., Morgan D.A. Central ceramide-induced hypothalamic lipotoxicity and ER stress regulate energy balance. Cell Reports. 2014;9(1):366–377. doi: 10.1016/j.celrep.2014.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Contreras C., González-García I., Seoane-Collazo P., Martínez-Sánchez N., Liñares-Pose L., Rial-Pensado E. Reduction of hypothalamic endoplasmic reticulum stress activates browning of white fat and ameliorates obesity. Diabetes. 2017;66(1):87–99. doi: 10.2337/db15-1547. [DOI] [PubMed] [Google Scholar]
- 33.Liñares-Pose L., Rial-Pensado E., Estévez-Salguero Á., Milbank E., González-García I., Rodríguez C. Genetic targeting of GRP78 in the VMH improves obesity independently of food intake. Genes. 2018;9(7):357. doi: 10.3390/genes9070357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez de Morentin P.B., González-García I., Martins L., Lage R., Fernández-Mallo D., Martínez-Sánchez N. Estradiol regulates Brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metabolism. 2014;20(1):41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfgang M.J., Lane M.D. The role of hypothalamic malonyl-CoA in energy homeostasis. Journal of Biological Chemistry. 2006;281(49):37265–37269. doi: 10.1074/jbc.R600016200. [DOI] [PubMed] [Google Scholar]
- 36.Wolfgang M.J., Cha S.H., Sidhaye A., Chohnan S., Cline G., Shulman G.I. Regulation of hypothalamic malonyl-CoA by central glucose and leptin. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19285–19290. doi: 10.1073/pnas.0709778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin T.L., Alquier T., Asakura K., Furukawa N., Preitner F., Kahn B.B. Diet-induced obesity alters AMP kinase activity in hypothalamus and skeletal muscle. Journal of Biological Chemistry. 2006;281(28):18933–18941. doi: 10.1074/jbc.M512831200. [DOI] [PubMed] [Google Scholar]
- 38.Münzberg H., Morrison C.D. Structure, production and signaling of leptin. Metabolism. 2015;64(1):13–23. doi: 10.1016/j.metabol.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramírez S., Claret M. Hypothalamic ER stress: a bridge between leptin resistance and obesity. FEBS Letters. 2015;589(14):1678–1687. doi: 10.1016/j.febslet.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 40.Posey K.A., Clegg D.J., Printz R.L., Byun J., Morton G.J., Vivekanandan-Giri A. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. American Journal of Physiology Endocrinology and Metabolism. 2009;296(5):E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mayer C.M., Belsham D.D. Palmitate attenuates insulin signaling and induces endoplasmic reticulum stress and apoptosis in hypothalamic neurons: rescue of resistance and apoptosis through adenosine 5′ monophosphate-activated protein kinase activation. Endocrinology. 2010;151(2):576–585. doi: 10.1210/en.2009-1122. [DOI] [PubMed] [Google Scholar]
- 42.Gao S., Moran T.H., Lopaschuk G.D., Butler A.A. Hypothalamic malonyl-CoA and the control of food intake. Physiology and Behavior. 2013;122:17–24. doi: 10.1016/j.physbeh.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cavaliere G., Viggiano E., Trinchese G., De Filippo C., Messina A., Monda V. Long feeding high-fat diet induces hypothalamic oxidative stress and inflammation, and prolonged hypothalamic AMPK activation in rat animal model. Frontiers in Physiology. 2018;9:818. doi: 10.3389/fphys.2018.00818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viggiano E., Mollica M.P., Lionetti L., Cavaliere G., Trinchese G., De Filippo C. Effects of an high-fat diet enriched in lard or in fish oil on the hypothalamic amp-activated protein kinase and inflammatory mediators. Frontiers in Cellular Neuroscience. 2016;10:150. doi: 10.3389/fncel.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanida M., Yamamoto N., Shibamoto T., Rahmouni K. Involvement of hypothalamic AMP-activated protein kinase in leptin-induced sympathetic nerve activation. PLoS One. 2013;8(2):e56660. doi: 10.1371/journal.pone.0056660. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.