Abstract
Background
Herbal medicine has been widely utilized by pregnant women despite the limited available evidence regarding the safety and efficacy of that practice. The current available studies, from different countries, estimated that the use of herbal medicine during pregnancy range from 7% up to 96%. The aim of this study is to determine the prevalence, attitude, source of information, and reasoning behind the use of herbal medicine among pregnant women in Saudia Arabia.
Methods
A cross-sectional study conducted using a convenience sample including pregnant women who visited the obstetric clinics at King Abdulaziz Medical City in Riyadh, Saudi Arabia. A survey was administered in order to evaluate the prevalence and perception toward herbal medicine use among pregnant women in Saudi Arabia.
Results
A total of 297 pregnant women completed the survey. The results showed that 56% of the respondents have used some type of herbal medicine during their pregnancy. Olive oil was utilized in 26% of the respondents followed by cumin 20% and garlic 15%. In addition, 37% of the respondents used herbal medicine by their own initiative, while 33% and 12% used herbal medicine based on recommendations from their families and friends, respectively. Furthermore, 19% of the respondents reported a positive attitude toward herbal medicine use during pregnancy. In addition, the percentage of women with positive attitude was marginally higher among respondents with lower educational level.
Conclusion
The prevalence of using herbal medicine is considerably high among pregnant women in Saudi Arabia. Unfortunately, the majority of the users relied on informal sources to use herbal medicine during pregnancy.
Keywords: Herbal medicine, Supplements, Pregnancy, Prevalence, Attitudes, Saudi Arabia
1. Introduction
The use of complementary and alternative medicine (CAM) has been widely utilized all over the world (Eisenberg et al., 1998, MacLennan et al., 1996, Fisher and Ward, 1994, Posadzki et al., 2013, Elolemy and Albedah, 2012). This practice includes but is not limited to massage therapy, acupuncture, and herbal medicine. Herbal medicine has been used by different populations for various diseases and conditions (Alaaeddine et al., 2012, Klafke et al., 2012, Angsten, 2000, Pallivalappila et al., 2013, Yang et al., 2015). One of these conditions is pregnancy. In many published studies, the use of herbal medicine was observed among pregnant women from different backgrounds and countries (Furlow et al., 2008, Gaffney and Smith, 2004, Holst et al., 2009a). The usage of CAM during pregnancy varies among these studies and is estimated as low as 1% and as high as 87% (Hall et al., 2011).
A multinational study measured the prevalence of herbal medicine used during pregnancy on four different continents (North America, South America, Europe, and Australia) and showed that about half of the population used herbs (Kennedy et al., 2013). The previous study found that ginger, cranberry, valerian, raspberry and chamomile were the most commonly used herbs among pregnant women in these countries. Other studies have found that the estimated usage of herbal medicine during pregnancy also varied from 7% to 96% (Gibson et al., 2001, Tsui et al., 2001, Forster et al., 2006). This variation has been noticed in the Middle East as well, where it ranged from 22.3% to 82.3% with the highest consumption of herbs occurred during the first trimester of pregnancy (John and Shantakumari, 2015). In addition, a recent study conducted at King Khalid University Hospital, in Riyadh, showed that 30.7% of pregnant women participating in the study used herbal medicine during their pregnancy, but the study did not thoroughly investigate the use of herbal supplements during pregnancy (Alsubaie et al., 2017).
Women who have used herbal medicine during their pregnancy have demonstrated positive attitudes toward these products (Nordeng and Havnen, 2004, Lapi et al., 2010, Pallivalappila, 2014, Nordeng and Havnen, 2005, Kim Sooi and Lean, 2013). They believe that herbal medicinal products are safer and more effective as compared to conventional medicine (Nordeng and Havnen, 2004, Lapi et al., 2010, Pallivalappila, 2014, Nordeng and Havnen, 2005, Kim Sooi and Lean, 2013). These attitudes and beliefs are not limited to western countries. In Saudi Arabia, there is also a positive attitude towards the complementary and alternative medicine practices, specifically the herbal medicine (Elolemy and Albedah, 2012, Abdullah Al-Rowais et al., 2012, AlBedah et al., 2012, Al-Eidi et al., 2016, Jazieh et al., 2012). However, none of these studies included pregnant women.
Unfortunately, many studies have found that a significant number of women do not inform their healthcare providers about their use of herbal medicine during pregnancy (Furlow et al., 2008, Holst et al., 2009a, Holst et al., 2009b). Moreover, a considerable number of pregnant women depend mainly on family and friends to obtain information regarding the use of herbs during pregnancy (Forster et al., 2006, John and Shantakumari, 2015, Strouss et al., 2014, Mothupi, 2014). Despite their lack of knowledge, a modest number of pregnant women are using these herbs (Nordeng and Havnen, 2004). The increased number of pregnant women seeking medical attention, medications, and even herbal medicine is well-documented, due to some pregnancy-related symptoms such as nausea, vomiting, urinary tract infections, fatigue, and backache (Hollyer et al., 2002, Close et al., 2014, Lacasse et al., 2009, Jepson et al., 2012).
Unlike conventional medications, there is a lack of solid evidence regarding the safety and efficacy of herbal medicine during pregnancy (Ernst, 2002). Some herbs have shown to be useful during pregnancy. For example, Willetts et al. (2003) found a significant reduction in nausea among pregnant women who took ginger. In addition, the follow-up period of the study showed normal ranges of birth weight, gestational age and Apgar score. The findings of this study confirmed results from previous reports which found that ginger was effective in reducing the nausea and vomiting episodes during pregnancy (Vutyavanich et al., 2001). Furthermore, a systematic review study showed that ginger can be considered as one of the beneficial intervention to reduce nausea and vomiting in early pregnancy although this conclusion is not based on a good quality evidence (Matthews et al., 2014).
On the other hand, some herbs have negative effects on the fetus. A study found that almond oil consumption during pregnancy increased the risk of pre-term delivery significantly (Facchinetti, 2012). However, chamomile consumption during pregnancy was associated with an increased risk of low birth weight infants but was not statistically significant (Facchinetti, 2012).
In addition, there are many herbs that are contraindicated during pregnancy. Papaya and motherwort are examples of such herbs, since they are associated with fetal malformations (Kennedy et al., 2016). Although there is no evidence of harmful effects, other herbs such as raspberry and valerian should be used with caution during pregnancy because of limited available data (Kennedy et al., 2016). Indeed, a multinational study showed that healthcare providers recommended contraindicated herbs more often than other sources of recommendations (Kennedy et al., 2016).
Vitamin and mineral supplements have some established evidence for use in pregnant women, as compared to herbal medicine (Organization, 2012, Organization, 2013). The World Health Organization (WHO) recommends taking folic acid and iron supplements prior to and during pregnancy, to reduce the risk of birth defects, low birth weight and iron deficiency (Organization, 2012). Moreover, supplementation of calcium during pregnancy reduces the risk of pre-eclampsia (Organization, 2013).
To the best of our knowledge, very limited data is available regarding the use of herbal, vitamin, and mineral supplements among pregnant women in Saudi Arabia. Thus, the primary aim of this study is to determine the prevalence, attitudes, sources of information, and the reasoning behind the use of herbs among pregnant women in Saudi Arabia. The secondary aim of this study is to assess the adherence of pregnant women to recommended supplements.
2. Material and Methods
A cross-sectional survey study was conducted, between October 2016 - January 2017, using two structured and self-administered questionnaires that explored the prevalence and attitudes of pregnant women’s use of herbal, vitamin, and mineral supplements. The questionnaires were administered to a convenience sample of pregnant women who visited the Obstetric/Gynecological (Ob/Gyn) clinics at King Abdulaziz Medical City (KAMC) in Riyadh, Saudi Arabia. The first questionnaire, which was adapted from an Australian study, consists of 49 items and is divided into two sections: (Section 1) demographic and pregnancy related information (19 items) including, but not limited to, age, education, household income, city of residence, cigarette use, pregnancy week, number of children and previous miscarriages (Section 2) herbal, vitamin, and mineral supplements information (30 items) including type, dose or amount, duration, the source of recommendation, indications, and efficacy (Forster et al., 2006). The second questionnaire was adapted from a Malaysian study to gather information on pregnant women’s perception toward usage of herbal supplements (7 items) (Ab Rahman et al., 2009). Responses to items assessing attitudes were measured on a 5-point Likert scale (‘Strongly Agree,’ ‘Agree,’ ‘Not Sure,’ ‘Disagree,’ and ‘Strongly Disagree’); and were scored as ‘4,’ ‘3,’ ‘2,’ ‘1,’ and ‘0,’ respectively. “Attitude” was categorized according to mean scores. A score greater than or equal to 19 was considered as “negative attitude;” whereas a score less than 19 was considered as “positive attitude” based on the original questionnaire. The validity and reliability of the modified questionnaires were established.
Subjects were approached by the study administrators, provided with a description of the study and its objectives. Then an informed consent was distributed to all participants, and those who signed the informed consent were provided with a hard copy of the questionnaires and were given time to complete. The completed questionnaires were collected and safely stored. Data was uploaded and saved into an appropriately designed Excel spread sheet. In addition, data was processed in accordance with the best practices for raw data management, to identify any inaccuracies or incompleteness prior to statistical analyses. Responses to all items in the questionnaires were checked and compared against the possible minimum and maximum values of each variable and items with implausible values were flagged. A similar process was applied to demographic variables to identify any potential anomalies by running general frequency analyses.
Statistical Analysis: Descriptive statistical analyses were performed for the study participants. Continuous variables were summarized using mean and standard deviation (SD), and proportions were used for categorical variables. Herbal, vitamin, and mineral supplements usage, as well as scores measuring attitudes and perceptions were analyzed and compared by demographic and pregnancy related variables. Comparisons were made using the chi-square test. Statistical significance was considered at P < 0.05. All statistical analyses were performed using SPSS 21.0 (Release 21.0.0.0, IBM, USA).
Ethics: Ethical approval was obtained from the Ethical Review Board at King Abdullah International Medical Research Centre (KAIMRC), Riyadh, Saudi Arabia. Informed consent was obtained from all participants.
3. Results
A total of 297 women completed the survey. Mean age ± SD was 31.5 ± 6.0 years. Forty four percent of respondents were from Riyadh and 69% had an education level of high school or above. About 54% of respondents were in their third trimester and the majority of them (76%) were expecting their second or third child. Moreover, approximately 33% of the respondents had unplanned pregnancy.
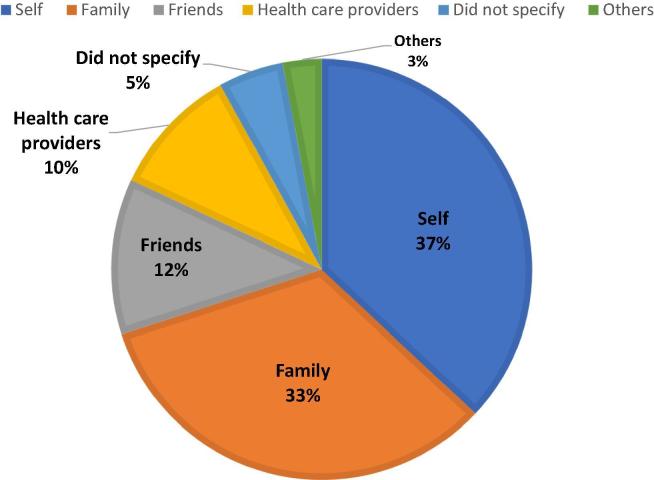
In addition, 56% of respondents used some type of herbs. Fig. 1 shows that the most common herbs used during pregnancy was highest for topical or oral olive oil (25.5%) followed by cumin (19.5%), garlic (15.4%), ginger (13.4%), cranberry juice (13.4%), black cumin (11.6%) umeboshi plums (10.4%), cinnamon (10.4%), fenugreek (7.7%), and chamomile (4.3%). Furthermore, 98% of respondents used some type of vitamin and mineral supplements; prior to their pregnancy, some (38%) used folic acid and others (18%) used multivitamins. However, during pregnancy, the most common vitamin and mineral supplements used were highest for folic acid (97%) followed by iron (85%), calcium (74%), multivitamins (65%) and vitamin B6 (16%). In addition, 37% of the respondents used herbs by their own initiative, while 33% and 12% used herbs based on recommendations from their families and friends, respectively (Fig. 2).
Fig. 1.
Distribution of herbs usage among pregnant women. N = 297.
Fig. 2.
. Sources of recommendation of herbs.
Results for overall herbal supplements usage, or usage by demographic and pregnancy-related factors are shown in Table 1. Herbal supplements use was marginally higher among women in their third trimester [60% vs. 41% and 50% for those in the second and first trimester, respectively; p = 0.051]. No significant association was found between other respondents’ demographic factors and usage of herbal supplements.
Table 1.
Descriptive statistics for herbal supplements usage, overall and by demographic and pregnancy-related factors. N = 297.
| All respondents n | Users n (%)* | p-value† | |
|---|---|---|---|
| 297 | 165 (55.6%) | ||
| Factor | |||
| Age (years) mean ± SD | 31.5 ± 6.0 | ||
| median (IQR) | 31 (27–36) | ||
| Level of Education | 0.69 | ||
| High school | 206 | 113 (54.9%) | |
| Lower education level | 50 | 29 (58.0%) | |
| Missing | 41 | ||
| Region | 0.14 | ||
| Riyadh | 131 | 67 (51.1%) | |
| Other | 138 | 83 (60.1%) | |
| Missing | 28 | ||
| Trimester | 0.051 | ||
| First | 42 | 21 (50.0%) | |
| Second | 51 | 21 (41.2%) | |
| Third | 160 | 96 (60.0%) | |
| Missing | 44 | ||
| First child | 0.92 | ||
| Yes | 61 | 34 (55.7%) | |
| No | 225 | 127 (56.4%) | |
| Missing | 11 | ||
| Household monthly income (SAR) | 0.075 | ||
| Less than 20,000 | 113 | 66 (58.4%) | |
| 20,000–30,000 | 48 | 19 (39.6%) | |
| More than 30,000 | 106 | 60 (56.6%) | |
| Missing | 30 | ||
Based on the chi-square test.
Users: women who used at least one type of herbal supplements during pregnancy.
The results in Table 2 show the percentage of respondents with positive attitudes toward herbal supplements, overall and by respondents’ demographic and practice-related characteristics. Positive attitudes are indicated by a score of less than 19. Significantly or marginally different rates of positive attitudes toward herbal supplements use were observed by trimester and level of education. That is, first trimester respondents had significantly higher rates of positive attitudes toward herbal supplements use, than those in the second or third trimester (36% vs. 15–16%, p = 0.028). In addition, marginally higher rates of positive attitudes toward herbal supplements were observed among respondents with lower education levels (30% vs. 17% among those with high school education; p = 0.084). No significant differences in attitudes were observed by region, first child or household monthly income (p > 0.05).
Table 2.
Pregnant women with positive attitudes toward herbal supplements by respondents’ characteristics.
| All respondents* n | Women with positive attitudes** n | % | p-value† | |
|---|---|---|---|---|
| 243 | 46 | 18.9% | ||
| Level of education | 0.084 | |||
| High school | 173 | 30 | 17.3% | |
| Lower education level | 37 | 11 | 29.7% | |
| Region | 0.81 | |||
| Riyadh | 113 | 21 | 18.6% | |
| Other | 111 | 22 | 19.8% | |
| Trimester | 0.028 | |||
| First | 33 | 12 | 36.4% | |
| Second | 46 | 7 | 15.2% | |
| Third | 131 | 22 | 16.8% | |
| First child n (%) | 0.75 | |||
| Yes | 53 | 11 | 20.8% | |
| No | 181 | 34 | 18.8% | |
| Household monthly income (SAR) n (%) | 0.85 | |||
| Less than 20,000 | 94 | 19 | 20.2% | |
| 20,000–30,000 | 43 | 7 | 16.3% | |
| More than 30,000 | 85 | 17 | 20.0% | |
All respondents: participants who filled the attitude questionnaire completely.
Indicated by a score of less than 19 (based on the original questionnaire).
Based on the chi-square test.
4. Discussion
Among the different modalities of complementary and alternative medicine, herbal medicine has been commonly used by pregnant women all over the word (Hall et al., 2011, Kennedy et al., 2013, Gibson et al., 2001, Tsui et al., 2001, Forster et al., 2006, John and Shantakumari, 2015). Based on western published studies, some herbs have been found to be effective to manage a variety of pregnancy-related symptoms (Hollyer et al., 2002, Close et al., 2014, Lacasse et al., 2009, Jepson et al., 2012). Others were found to be associated with an increased risk of pre-term delivery and low birth weight. (Facchinetti et al., 2012, Kennedy et al., 2016). Since there is a lack of data in Saudi Arabia regarding the use of herbal, vitamin, and mineral supplements during pregnancy, the present study intends to provide answers for the questions related to the use of herbal, vitamin, and mineral supplements in pregnant women in Saudi Arabia.
The first question in this study sought to determine the prevalence of using herbal supplements among pregnant Saudi women. The estimated prevalence of using herbal supplements during pregnancy varies greatly in the studies and among countries (Hall et al., 2011, Kennedy et al., 2013, Gibson et al., 2001, Tsui et al., 2001, Forster et al., 2006, John and Shantakumari, 2015). However, according to a recent multinational study which involved 4 different continents, the overall reported prevalence was 28.9%, and it ranged from 4.3% to 69% (Kennedy et al., 2013). Furthermore, the reported prevalence in Middle East countries ranged from 22.3% to 82.3% (John and Shantakumari, 2015). Our findings are consistent with the global and Middle East ranges.
To our knowledge, our findings are the first that specifically investigated the use of herbal, vitamin, and mineral supplements during pregnancy in Saudi Arabia. Compared with the limited local data, our findings are contrary to that of Zaki and Albarraq (2014) who reported a low percentage of herbal supplements usage during pregnancy, which was only 4.6%. This discrepancy could be attributed to the major differences in the objectives and methodology between this study and ours. The present study focused on the attitudes and the prevalence of using herbal supplements during pregnancy, while Zaki et al. focused on the attitudes towards medications use among pregnant women. Additionally, this study used questionnaires specifically pertaining to the use of herbal supplements in pregnant women which may account for this variation between the percentages.
In addition, a recent study conducted by Alsubaie et al. (2017) showed that (30%) of women used herbal supplements during their pregnancy although the previous study included a small number of pregnant women.
The high prevalence of herbal supplements use can be further explained by the fact that there is a positive attitude towards the complementary and alternative medicine practices, specifically the use of herbal supplements in Saudi Arabia (Elolemy and Albedah, 2012, Abdullah Al-Rowais et al., 2012, AlBedah et al., 2012, Al-Eidi et al., 2016). Those positive attitudes were reported in the general population, diabetic patients, as well as, healthcare providers in the Riyadh region. Besides that, Suleiman et al revealed that roughly 80% of participants, from the Riyadh region, believed that herbal supplements did not pose any harm (Suleiman et al., 2014). In the present study, only 19% of women had a positive attitude towards using herbal supplements during pregnancy. This result was unexpected since more than half of the participants consumed herbal supplements. This unexpected result may be explained by the presence of totally two different questionnaires. As a matter of fact, the questionnaire we used to assess the attitude includes seven different items, and only one of them is about willingness of using herbs during pregnancy. Furthermore, the positive attitude was significantly observed among pregnant women in the first trimester and women with lower educational level. This might indicate that the pregnant Saudi women with positive attitude are willing to use herbal supplements and they will encourage other women to use these products during pregnancy. These results are in agreement with those obtained from another Middle East study, in which the highest prevalence of herbal supplements use was in the first trimester of pregnancy (John and Shantakumari, 2015).
Pregnant women suffer from different symptoms that accompany pregnancy and they tend to seek for treatment for these symptoms (Hollyer et al., 2002, Close et al., 2014, Lacasse et al., 2009, Jepson et al., 2012). Among the different types of herbal products, olive oil was found to be the most commonly used among pregnant women in this study population. Cumin comes in second with an approximate percentage of 20%, followed by garlic, ginger, and cranberry juice with approximate percentages of 15%, 13%, and 13%, respectively. Compared with another Middle East study (John and Shantakumari, 2015), garlic and ginger were also reported among the most consumed herbs during pregnancy, whereas in international studies, ginger and cranberry were reported among the top five consumed herbs (Kennedy et al., 2013).
Our study is the first one that revealed the high consumption of olive oil among pregnant women. It was reported previously in an Iraqi study with a low percentage. (Hwang et al., 2016) A possible explanation for this might be that olive oil is part of the traditional healing remedies in the Islamic culture (Rassool et al., 2014). It was among the top dietary and herbal products in a previous Saudi study that investigated the use and prevalence of complementary and alternative medicine among cancer patients. (Jazieh et al., 2012) Unfortunately, this study was not able to identify how olive oil was used by the pregnant women. Interestingly, there is inconsistent evidence regarding the dietary consumption of olive oil during pregnancy. One study found olive oil to be beneficial in reducing the wheezing in the first year of the new born child’s life (Castro-Rodriguez et al., 2010). Another study, found it less effective compared with fish oil in reducing persistent wheeze (Bisgaard et al., 2016). However, the inconsistencies of the findings require further studies to confirm the potential role of either dietary or therapeutic use of olive oil during pregnancy.
Ginger was the only common herb that is reported in both International and Middle Eastern, studies as one of the most used herbs during pregnancy (Kennedy et al., 2013, John and Shantakumari, 2015). This may be explained by the fact that ginger was found to be safe and beneficial in the prevention of nausea and vomiting during pregnancy according to several studies (Willetts et al., 2003, Vutyavanich et al., 2001). Cumin, fenugreek, chamomile and cinnamon were used in the present study with different percentages. Despite the lack of solid evidence, these herbs were used by pregnant women for several reasons in previous studies, including but is not limited to pain, constipation, flatulence, insomnia and facilitation of the delivery (Adawi, 2012, Orief et al., 2014, Amasha and Jarrah, 2012, Bishop et al., 2011). For cranberry, it is classified as “safe for pregnancy” and it is used mainly for urinary tract infection prophylaxis and treatment (Kennedy et al., 2016).
Although herbal supplements come with benefits, its risks must be evaluated for any harm to the mother and her child. With this being said, the available evidence is not conclusive to rule out risk. Thus, it is crucial to keep in mind that the adverse effects of herbal supplements are affected by different factors including the amount of herb consumed and other factors like age, concomitant medications use, and gestational age. Garlic is one of the herbs that is associated with risk of bleeding in general population, in addition to some herb-drug interactions (Izzo and Ernst, 2009). Likewise, chamomile was associated with an increased risk of miscarriage (Cuzzolin et al., 2010) and was considered unsafe in 6% of the studies included in a literature survey by Wilkinson et al. (2000) Cinnamon is another example of a herb that poses some risk if taken during the first months of pregnancy since it is a uterine stimulant (Ernst, 2002).
It is important to carefully choose the source of recommendation for treatment. One of the main aspects of this study was to collect data about the source of recommendation for using herbal and complementary medicines. Most Saudi women depend on non-professional sources such as self-initiated, family, friends, and internet (Alsubaie et al., 2017). In the present study, only 10% of herbal supplements users received their recommendations from healthcare providers. These findings are consistent with the global and Middle Eastern studies (Kennedy et al., 2013, John and Shantakumari, 2015). Furthermore, our findings are consistent with another Saudi study, in the Riyadh region, which showed that most of the general population received their recommendations for using herbal and complementary medicines from non-professional sources (Suleiman, 2014). A Saudi study, conducted in Riyadh and included 1113 physicians from 412 primary care hospitals, reported a lack of knowledge regarding the use of herbal and complementary medicines (Abdullah Al-Rowais et al., 2012). Only 8.2% of those physicians had attended any educational activity regarding CAM. This necessitates the need to encourage the healthcare providers to improve their knowledge about using CAM modalities in order to educate the general population, including pregnant women, about the benefits and risk of using such herbs.
The present study extended the scope of previous studies by measuring the prevalence of using vitamin and mineral supplements among pregnant women. The WHO and National Institute for Health and Care Excellence (NICE) recommend 400 mcg of daily folic acid from the planning of pregnancy until the end of the first trimester, to reduce the incidence of neural tube defects (NTD) (Organization, 2012, Health and Excellence, 2008). Our findings showed that 97% of the responders had been taking folic acid during pregnancy and only 38% of women took it before becoming pregnant. Folic acid was taken by nearly all the pregnant women based on healthcare providers’ recommendations. These findings are expected since folic acid has strong evidence in pregnant women to reduce neural tube defects (Berry et al., 1999). However, nearly 33% of the pregnancies were unplanned and this may explain why the percentage of using folic acid before pregnancy is reduced in the present study.
Approximately 85% of participants used iron during pregnancy which makes it the second most consumed supplement after folic acid. According to the WHO, daily oral iron is recommended for pregnant women in order to decrease the risk of low birth weight, and iron deficiency (Organization, 2012). On the other hand, NICE indicated that there is no need for iron as routine supplementation for all pregnant women, since it associated with bothersome gastrointestinal symptoms (Health and Excellence, 2008). It worth to mentation that a Cochrane review found that the response to iron supplementation may not lead to the same result, since it affected by several factors during pregnancy (Peña-Rosas et al., 2015). Calcium was the third most used supplement in the present study, with 74%. 1 – 2 g of elemental Calcium is recommended by the WHO from week 20 until the end of pregnancy in women who are at high risk of hypertension in order to prevent preeclampsia (Organization, 2013).
Lastly, multivitamins were consumed by more than half of participants in this study. The latest evidence in the literature showed modest benefit from Vitamin B6 in the management of nausea and vomiting (Matthews, 2014). In addition, Vitamin B12 during pregnancy and early lactation was found to improve the B12 status in breast milk, mothers and infants (Duggan et al., 2014, Siddiqua et al., 2016). On the other hand, Vitamin C and E are not recommended during pregnancy based on systematic reviews (Rumbold et al., 2015, Rumbold et al., 2015). Also, Vitamin A, in general, has no clear benefits in pregnancy outcomes and it might be teratogenic with high doses (Health and Excellence, 2008, McCauley et al., 2015).
This study has some limitations. First, it was conducted in a single center which may limit the generalizability of the findings. However, only 44% of the participants were from Riyadh and the rest were from other regions in Saudi Arabia. Second, most of participants did not specify the exact amount, duration, reasons and efficacy of the consumed herbs. Lastly, the participants may did not differentiate well between regular food and herbal supplements although the description and objectives of this study were defined to all participants before filling the questionnaires.
5. Conclusion
This study has shown that the prevalence of herbal supplements is considerably high among pregnant Saudi women. Furthermore, the percentage of women with positive attitude was significantly higher among respondents in the first trimester and marginally higher among respondents with lower educational level. The majority of the women relied on informal sources to use herbal supplements during pregnancy. These findings should capture the healthcare providers’ attention to strive to increase the awareness of pregnant women regarding the safety and efficacy of using herbal vitamin, and mineral supplements during their pregnancy. In addition, clinicians should assess the need of using these supplements during pregnancy and follow the most recent evidence.
6. Declarations of interest
None.
Acknowledgement
The authors would like to thank King Abdullah International Medical Research Center (KAIMRC) for their support and assistance with this project. In addition, the authors would like to thank Dr. Gregory Poff and Mr. Billy McGowan for their valuable help in reviewing this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ab Rahman A. Women's attitude and sociodemographic characteristics influencing usage of herbal medicines during pregnancy in Tumpat District, Kelantan. Southeast Asian J. Trop. Med. Publ. Health. 2009;40(2):330. [PubMed] [Google Scholar]
- Abdullah Al-Rowais N. Knowledge and attitudes of primary health care physicians towards complementary and alternative medicine in the Riyadh region, Saudi Arabia. Forschende Komplementärmedizin/Research in Complementary. Medicine. 2012;19(1):7–12. doi: 10.1159/000335814. [DOI] [PubMed] [Google Scholar]
- Adawi, D.H., Prevalence and Predictors of Herb Use during Pregnancy (A study at Rafidia Governmental Hospital/Palestine). 2012, Faculty of Graduate Studies Prevalence and Predictors of Herb Use during Pregnancy (A study at Rafidia Governmental Hospital/Palestine) By Deema Hilmi Adawi Supervisor Dr. Rowa’Al-Ramahi Co-supervisor Dr. Nidal Jarradat This thesis is submitted in Partial Fulfillment of the Requirements for the Degree of Master of Clinical Pharmacy, Faculty of Graduate Studies, An-Najah National University.
- Alaaeddine N. Use of complementary and alternative therapy among patients with rheumatoid arthritis and osteoarthritis. J. Clin. Nurs. 2012;21(21–22):3198–3204. doi: 10.1111/j.1365-2702.2012.04169.x. [DOI] [PubMed] [Google Scholar]
- AlBedah A.M., El-Olemy A.T., Khalil M.K. Knowledge and attitude of health professionals in the Riyadh region, Saudi Arabia, toward complementary and alternative medicine. J. Family Commun. Med. 2012;19(2):93. doi: 10.4103/2230-8229.98290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Eidi S. Knowledge, attitude and practice of patients with type 2 diabetes mellitus towards complementary and alternative medicine. J. Integr. Med. 2016;14(3):187–196. doi: 10.1016/S2095-4964(16)60244-3. [DOI] [PubMed] [Google Scholar]
- Alsubaie S.F., Alshehri M.G., Ghalib R.H. Use awareness and attitude towards herbal medicines among saudi women-cross sectional study. Imperial J. Interdiscipl. Res. 2017;3(2) [Google Scholar]
- Amasha H., Jarrah S. The use of home remedies by pregnant mothers as a treatment of pregnancy related complaints: An exploratory study. Med. J. Cairo Univ. 2012;80(2) [Google Scholar]
- Angsten J. Use of complementary and alternative medicine in the treatment of asthma. Adolescent medicine. (Philadelphia, Pa.) 2000;11(3):535–546. [PubMed] [Google Scholar]
- Berry R., Li Z., Erickson J., Li S., Moore C., Wang H., Mulinare J., Zhao P., Wong L., Gindler J., Hong S., Hao L., Gunter E., Correa A. Prevention of Neural-Tube Defects with Folic Acid in China. N. Engl. J. Med. 1999;341(20):1485–1490. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- Bisgaard H. Fish oil-derived fatty acids in pregnancy and wheeze and asthma in offspring. N. Engl. J. Med. 2016;375(26):2530–2539. doi: 10.1056/NEJMoa1503734. [DOI] [PubMed] [Google Scholar]
- Bishop Jackie L. The use of complementary and alternative medicine in pregnancy: data from the Avon Longitudinal Study of Parents and Children (ALSPAC) Complement. Therap. Med. 2011;19(6):303–310. doi: 10.1016/j.ctim.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Castro-Rodriguez J.A. Olive oil during pregnancy is associated with reduced wheezing during the first year of life of the offspring. Pediat. Pulmonol. 2010;45(4):395–402. doi: 10.1002/ppul.21205. [DOI] [PubMed] [Google Scholar]
- Close C. A systematic review investigating the effectiveness of Complementary and Alternative Medicine (CAM) for the management of low back and/or pelvic pain (LBPP) in pregnancy. J. Adv. Nurs. 2014;70(8):1702–1716. doi: 10.1111/jan.12360. [DOI] [PubMed] [Google Scholar]
- Cuzzolin L. Use of herbal products among 392 Italian pregnant women: focus on pregnancy outcome. Pharmacoepidemiol. Drug Saf. 2010;19(11):1151–1158. doi: 10.1002/pds.2040. [DOI] [PubMed] [Google Scholar]
- Duggan C. Vitamin B-12 supplementation during pregnancy and early lactation increases maternal, breast milk, and infant measures of vitamin B-12 status. J. Nutr. 2014;144(5):758–764. doi: 10.3945/jn.113.187278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D.M. Trends in alternative medicine use in the United States, 1990–1997: results of a follow-up national survey. JAMA. 1998;280(18):1569–1575. doi: 10.1001/jama.280.18.1569. [DOI] [PubMed] [Google Scholar]
- Elolemy A.T., Albedah A. Public knowledge, attitude and practice of complementary and alternative medicine in Riyadh region Saudi Arabia. Oman. Med. J. 2012;27(1):20–26. doi: 10.5001/omj.2012.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst E. Herbal medicinal products during pregnancy: are they safe? BJOG: Int. J. Obstet. Gynaecol. 2002;109(3):227–235. doi: 10.1111/j.1471-0528.2002.t01-1-01009.x. [DOI] [PubMed] [Google Scholar]
- Facchinetti F. Herbal supplements in pregnancy: unexpected results from a multicentre study. Hum Reprod. 2012;27(11):3161–3167. doi: 10.1093/humrep/des303. [DOI] [PubMed] [Google Scholar]
- Fisher P., Ward A. Complementary medicine in Europe. BMJ. Br. Med. J. 1994;309(6947):107. doi: 10.1136/bmj.309.6947.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster D.A. Herbal medicine use during pregnancy in a group of Australian women. BMC Pregnancy Childbirth. 2006;6:21. doi: 10.1186/1471-2393-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlow M.L. Physician and patient attitudes towards complementary and alternative medicine in obstetrics and gynecology. BMC Complement. Altern. Med. 2008;8(1):1. doi: 10.1186/1472-6882-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffney L., Smith C. The views of pregnant women towards the use of complementary therapies and medicines. Birth Issues. 2004;13:43–50. [Google Scholar]
- Gibson P.S., Powrie R., Star J. Herbal and alternative medicine use during pregnancy: a cross-sectional survey. Obstet. Gynecol. 2001;97(4):S44–S45. [Google Scholar]
- Hall H.G., Griffiths D.L., McKenna L.G. The use of complementary and alternative medicine by pregnant women: a literature review. Midwifery. 2011;27(6):817–824. doi: 10.1016/j.midw.2010.08.007. [DOI] [PubMed] [Google Scholar]
- Health, N.I.F., Excellence C. Antenatal care for uncomplicated pregnancies. NICE clinical guidelines. Updated edition. London, 2008.
- Hollyer T. The use of CAM by women suffering from nausea and vomiting during pregnancy. BMC Complement. Altern. Med. 2002;2(1):5. doi: 10.1186/1472-6882-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst L. Use of herbal preparations during pregnancy: focus group discussion among expectant mothers attending a hospital antenatal clinic in Norwich UK. Complement. Therap. Clin. Pract. 2009;15(4):225–229. doi: 10.1016/j.ctcp.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Holst L. The use and the user of herbal remedies during pregnancy. J. Altern. Complement. Med. 2009;15(7):787–792. doi: 10.1089/acm.2008.0467. [DOI] [PubMed] [Google Scholar]
- Hwang J.H. Use of complementary and alternative medicine in pregnancy: a cross-sectional survey on Iraqi women. BMC Complement. Altern. Med. 2016;16(1):191. doi: 10.1186/s12906-016-1167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo A.A., Ernst E. Interactions between herbal medicines and prescribed drugs. Drugs. 2009;69(13):1777–1798. doi: 10.2165/11317010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Jazieh A.R. Use of complementary and alternative medicine by patients with cancer in Saudi Arabia. J. Altern. Complement. Med. 2012;18(11):1045–1049. doi: 10.1089/acm.2011.0266. [DOI] [PubMed] [Google Scholar]
- Jepson R.G., Williams G., Craig J.C. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2012:10(10). doi: 10.1002/14651858.CD001321.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John L.J., Shantakumari N. Herbal medicines use during pregnancy: a review from the Middle East. Oman Med. J. 2015;30(4):229. doi: 10.5001/omj.2015.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.A. Herbal medicine use in pregnancy: results of a multinational study. BMC Complement. Altern. Med. 2013;13(1):355. doi: 10.1186/1472-6882-13-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy D.A. Safety classification of herbal medicines used in pregnancy in a multinational study. BMC Complement. Altern. Med. 2016;16(1):102. doi: 10.1186/s12906-016-1079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Sooi L., Lean Keng S. Herbal medicines: Malaysian women’s knowledge and practice. Evidence-Based Complement. Altern. Med. 2013;2013 doi: 10.1155/2013/438139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klafke N. Prevalence and predictors of complementary and alternative medicine (CAM) use by men in Australian cancer outpatient services. Ann. Oncol. 2012;23(6):1571–1578. doi: 10.1093/annonc/mdr521. [DOI] [PubMed] [Google Scholar]
- Lacasse A. Epidemiology of nausea and vomiting of pregnancy: prevalence, severity, determinants, and the importance of race/ethnicity. BMC Pregnancy Childbirth. 2009;9(1):26. doi: 10.1186/1471-2393-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapi F. Use, attitudes and knowledge of complementary and alternative drugs (CADs) among pregnant women: a preliminary survey in Tuscany. Evidence-Based Complement. Altern. Med. 2010;7(4):477–486. doi: 10.1093/ecam/nen031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan A.H., Wilson D.H., Taylor A.W. Prevalence and cost of alternative medicine in Australia. The Lancet. 1996;347(9001):569–573. doi: 10.1016/s0140-6736(96)91271-4. [DOI] [PubMed] [Google Scholar]
- Matthews Anne. Interventions for nausea and vomiting in early pregnancy. Cochrane Database Syst Rev. 2014;3 doi: 10.1002/14651858.CD007575.pub3. [DOI] [PubMed] [Google Scholar]
- McCauley M., van den Broek N., Dou L., Othman M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database System. Rev. 2015 doi: 10.1002/14651858.CD008666.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothupi M.C. Use of herbal medicine during pregnancy among women with access to public healthcare in Nairobi, Kenya: a cross-sectional survey. BMC Complement. Altern. Med. 2014;14(1):432. doi: 10.1186/1472-6882-14-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeng H., Havnen G.C. Use of herbal drugs in pregnancy: a survey among 400 Norwegian women. Pharmacoepidemiol. Drug Saf. 2004;13(6):371–380. doi: 10.1002/pds.945. [DOI] [PubMed] [Google Scholar]
- Nordeng H., Havnen G.C. Impact of socio-demographic factors, knowledge and attitude on the use of herbal drugs in pregnancy. Acta Obstet. et Gynecol. Scandinavica. 2005;84(1):26–33. doi: 10.1111/j.0001-6349.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Organization W.H. World Health Organization; 2012. Guideline daily iron and folic acid supplementation in pregnant women. [PubMed] [Google Scholar]
- Organization, W.H., Guideline: calcium supplementation in pregnant women, in Guideline: Calcium supplementation in pregnant women. 2013. p. 30–30.
- Orief Y.I., Farghaly N.F., Ibrahim M.I.A. Use of herbal medicines among pregnant women attending family health centers in Alexandria. Middle East Fertil. Soc. J. 2014;19(1):42–50. [Google Scholar]
- Pallivalappila A.R. Complementary and alternative medicines use during pregnancy: a systematic review of pregnant women and healthcare professional views and experiences. Evid. Complement. Alternat. Med. 2013;2013 doi: 10.1155/2013/205639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallivalappila A.R. Complementary and alternative medicine use during early pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014;181:251–255. doi: 10.1016/j.ejogrb.2014.08.017. [DOI] [PubMed] [Google Scholar]
- Peña-Rosas J., De-Regil L., Garcia-Casal M., Dowswell T. Daily oral iron supplementation during pregnancy. Cochrane Database System. Rev. 2015 doi: 10.1002/14651858.CD004736.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posadzki P. Prevalence of use of complementary and alternative medicine (CAM) by patients/consumers in the UK: systematic review of surveys. Clin. Med. 2013;13(2):126–131. doi: 10.7861/clinmedicine.13-2-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassool G. Cultural competence in nursing Muslim patients. Nurs. Times. 2014;111(14):12–15. [PubMed] [Google Scholar]
- Rumbold A., Ota E., Hori H., Miyazaki C., Crowther C. Vitamin E supplementation in pregnancy. Cochrane Database System. Rev. 2015 doi: 10.1002/14651858.CD004069.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumbold A., Ota E., Nagata C., Shahrook S., Crowther C. Vitamin C supplementation in pregnancy. Cochrane Database System. Rev. 2015 doi: 10.1002/14651858.CD004072.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqua T.J. Vitamin B12 supplementation during pregnancy and postpartum improves B12 status of both mothers and infants but vaccine response in mothers only: a randomized clinical trial in Bangladesh. Eur. J. Nutr. 2016;55(1):281–293. doi: 10.1007/s00394-015-0845-x. [DOI] [PubMed] [Google Scholar]
- Strouss L. Complementary and Alternative Medicine use in women during pregnancy: do their healthcare providers know? BMC Complement. Altern. Med. 2014;14(1):1. doi: 10.1186/1472-6882-14-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleiman A.K. Attitudes and beliefs of consumers of herbal medicines in Riyadh, Saudi Arabia. J. Commun. Med. Health Educ. 2014;4(2):269. [Google Scholar]
- Tsui B., Dennehy C.E., Tsourounis C. A survey of dietary supplement use during pregnancy at an academic medical center. Am. J. Obstet. Gynecol. 2001;185(2):433–437. doi: 10.1067/mob.2001.116688. [DOI] [PubMed] [Google Scholar]
- Vutyavanich T., Kraisarin T., Ruangsri R.-A. Ginger for nausea and vomiting in pregnancy: randomized, double-masked, placebo-controlled trial. Obstet. Gynecol. 2001;97(4):577–582. doi: 10.1016/s0029-7844(00)01228-x. [DOI] [PubMed] [Google Scholar]
- Wilkinson J.M. What do we know about herbal morning sickness treatments? A literature survey. Midwifery. 2000;16(3):224–228. doi: 10.1054/midw.1999.0209. [DOI] [PubMed] [Google Scholar]
- Willetts K.E., Ekangaki A., Eden J.A. Effect of a ginger extract on pregnancy-induced nausea: A randomised controlled trial. Aust. N.Z. J. Obstet. Gynaecol. 2003;43(2):139–144. doi: 10.1046/j.0004-8666.2003.00039.x. [DOI] [PubMed] [Google Scholar]
- Yang P.-R. Frequency and co-prescription pattern of Chinese herbal products for hypertension in Taiwan: a Cohort study. BMC Complement. Altern. Med. 2015;15(1):1. doi: 10.1186/s12906-015-0690-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki N.M., Albarraq A.A. Use, attitudes and knowledge of medications among pregnant women: A Saudi study. Saudi Pharmaceut. J. 2014;22(5):419–428. doi: 10.1016/j.jsps.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]