Abstract
Previously, a series of 2-phenoxy-benzo[g]triazoloquinazolines 1–16 were synthesized and fully characterized. The antioxidant activity of the target molecules 1–16 was evaluated using three different assays namely 1,1-diphenyl-2-picryl hydrazyl (DPPH) radical scavenging, ferric reduction antioxidant power (FRAP) and reducing power capability (RPC). The results revealed that some benzotriazoloquinazolines showed good activity and have the capacity to scavenge free radicals. In particular, compounds 1 and 14 have shown the highest activity. The butylated hydroxyl toluene (BHT) used as standard agent.
Density functional theory was carried out to explain the relative importance of C O, C S and NH groups on the radical scavenging activity of the target benzotriazoloquinazolines. The finding in present study shows that the active compounds can be used as template for further development of more potent antioxidant agents.
Keywords: Benzotriazoloquinazolines, DPPH, BHT, DFT, Antioxidant capacity
1. Introduction
Scientific search has shown that there is positive link between degenerative and/or neurodegenerative diseases and the excessive production of free radicals in human body (Thanan et al., 2015, Valko et al., 2007). Over the last twenty years, many literatures have been reported about the free radicals (nature and radix). To epitomize, free radicals are recognized as atoms/molecules with unpaired electrons that are able to create an unstable and highly reactive molecule. This unstable molecule can stabilize itself by taking an electron away from another stable molecule. Consequently, the stable one becomes damaged (a free radical), then a destructive chain reaction will build up, i.e. one radical generating another (Halliwell, 1994, Beckman and Ames, 1998).
Free radicals emit from the environment either from chain reactions of free radicals or from numerous ordinary biological processes, in vivo. Their reactions are started constantly in the body from enzymatic (those involved in cytochrome P450, phagocytosis and biosynthesis of prostaglandin) and non-enzymatic (those started by ionizing radiation) reactions (Halliwell, 1994, Beckman and Ames, 1998, Mermer et al., 2018).
In human system, a fine biological balance between the ordinary physiological production of ROS/RNS and their removal is already existed. However, an excess of oxidative stress can lead to the oxidation of biomolecules, which is accompanied with cell damage and embroil in the pathogenesis of many human diseases (Al-Salahi et al., 2017, Bak et al., 2012).
Antioxidants are very important substances elaborated in the body to counteract the ROS/RNS effects and inhibit the oxidative stress. They can be classified as enzymatic (those included glutaredoxin, glutathione reductase, SOD and catalase) and non-enzymatic (those included vitamin E, beta carotene, ascorbic acid, selenium, zinc and hypotaurine) (Al-Salahi et al., 2017, Bak et al., 2012). Due to oxidative stress multifactorial pathogenesis and its involvement in a large number of lifestyle diseases like cardiovascular, cancer, diabetic and aging, the constant reassessment search was necessitated to adjust an appropriate strategy for designing and developing a potent antioxidant to protect the human body from the causative damage of free radicals (Thanan et al., 2015, Valko et al., 2007, Caliskan-Ergün et al., 2008, Ünver et al., 2016, Elansary, 2014, Kumar et al., 2011).
Recently, many heterocycles bearing triazole and/or quinazoline moiety were described as good antioxidant agents (Caliskan-Ergün et al., 2008, Elansary, 2014, Kumar et al., 2011, Estevão et al., 2010, Sompalle et al., 2016a, Sompalle et al., 2016b). Furthermore, our triazoloquinazolines bearing hydrogen supplying, electron-donating and electron-rich moieties, were found to possess potential antioxidant properties (Al-Salahi et al., 2017). Diversity in triazoloquinazoline and benzoquinazoline biological activities encouraged us to elaborate a new series of benzo[g]triazoloquinazolinones, which have demonstrated significant antimicrobial and anticancer activities (Abuelizz et al., 2018, Al-Salahi et al., 2015). In addition, the promising antioxidant results of our triazoloquinazolines (Al-Salahi et al., 2017) provided us with some useful insights and thoughts about the required characteristics to design more active molecules against free radicals.
In view of the aforementioned facts and taking into account their similarities with our target compounds, we report herein the evaluation of the benzotriazoloquinazolines 1–16 as antioxidants using different assays.
2. Material and methods
2.1. DPPH assay
The antioxidant activity of benzotriazoloquinazolines was performed using DPPH free radical scavenging (Brand-Williams et al., 1995). The prepared methanol solution of DPPH (20 μg/mL) was stored at 10 °C in the dark. The benzotriazoloquinazolines were dissolved in 85% methanol-water (v/v). Benzotriazoloquinazoline sample (0.5 mL) was added to DPPH solution (1.0 mL) and left to stir at RT. After 5 min incubation in the dark room, the absorbance was measured at 517 nm. The DPPH radical absorbance without antioxidant was measured also as control and used 95% methanol as blank. The obtained results were compared with blank control. All the determinations were performed in three replicates and averaged.
Results were also presented as inhibition % of the DPPH radicals.
2.2. FRAP assay
A slight modification was carried out on Benzie and Strain protocol (Benzie and Strain, 1996). Three reagents were prepared; acetate buffer (300 mM, pH = 3.6), 20 mM FeCl3·6H2O solution and 10 mM TPTZ in 40 mM HCl. The FRAP was prepared freshly by mixing FeCl3·6H2O, acetate buffer and TPTZ in ratio of 2.5: 25: 2.5 (v/v/v), respectively. The mixture was warmed at 37◦C. In a dark condition, FRAP solution (2850 μL) was allowed to react with benzotriazoloquinazolines (150 μL) for 30 min. Measurement the absorbance readings of colored product (ferrous tripyridyltriazine complex) at 593 nm. The results are expressed in μmoLTrolox/100 g. The FRAP was calculated and when its measured value exceeded over the linear range of the standard curve, an extra additional dilution was applied.
2.3. RPC assay
In light to Oyaizu procedure, the benzotriazoloquinazolines reducing power was determined (Oyaizu, 1986). Potassium ferricyanide (1%) and phosphate buffer (0.2 M, pH = 6.6) were prepared. A solution of the sample (0.5 mL, 100 μg/mL) was added to the mixture of phosphate buffer (2.5 mL) and K3[Fe(CN)6] (2.5 mL). At a temperature of 50◦C, the mixture was incubated for 20 min, then centrifuged at 1000 r.p.m. for 10 min after aliquots of trichloroacetic acid (2.5 mL, 10%) were added. The mixture was allowed to separate into two layers. A solution of the upper layer (2.5 mL) was mixed with 0.1% FeCl3 (0.5 mL) and distilled water (2.5 mL). At 700 nm, the absorbance of the prepared mixture was measured. Increment in the reducing power indicated by increasing in the absorbance of the reaction mixture.
2.4. Density functional theory (DFT) calculations
Three main mechanisms have been proposed to explain the DPPH scavenging activity (Anouar et al., 2009, Anouar et al., 2013, Anouar et al., 2014) as following:
-
(i)
Proton Coupled-Electron Transfer (PC-ET) versus Hydrogen atom transfer (HAT)
-
(ii)
Electron Transfer-Proton Transfer (ET-PT)
-
(iii)
Sequential Proton Loss Electron Transfer (SPLET)
In the first mechanism, the N-H bond dissociation is hemolytic. Thus, the main parameter used to control such mechanism is called the bond dissociation enthalpy (BDE).
| An-H + DPPH• → An• + DPPH2 | (1) |
In this regards, the BDEs of compounds 1 and 14 are calculated using the following Eq. (2):
| BDE = H(An•, 298 K) + H(H•, 298 K) − H(An-H, 298 K) | (2) |
where H is the enthalpy, H (An-H, 298 K) and H (An•, 298 K) are the enthalpies of parent products and their corresponding radicals, respectively; and H (H•, 298 K) is the enthalpy of hydrogen radical (Al-Salahi et al., 2017).
BDE is an essential parameter that assess the capability of losing a hydrogen atom by molecule. Compound with a lower BDE, is the easier OH bond dissociation (i.e. potent antioxidant).
In the second mechanism, ET from the An-H to the free radical yields a radical cation An-H+•. Then, a heterolytic bond dissociation of the radical cation (i.e., proton loss) forms an An• radical (Eq. (3)). Ionization potential (IP) of the radical cation controls such mechanism. The lower IP value, is the easier electron transfer and the higher antioxidant activity.
| An-H + DPPH• → An-H+• + DPPH− → An• + DPPH2 | (3) |
Three steps are involved in the third mechanism (Eq. (4)). Firstly, a heterolytic bond dissociation of a AnH group yields An− anion and proton. Then, an ET from the An− to the DPPH• radical forms a An• radical and DPPH− anion. Finally, DPPH− protonation forms DPPH2. A higher pH is favorable, which may help in the proton loss or transfer of the first step (Di Meo et al., 2013, Musialik and Litwinienko, 2005).
| An-H → An− + H+ |
| An− + DPPH• → An•+DPPH− | (4) |
| DPPH− + H+ → DPP | H2 |
Herein, the identification of the major mechanism that is involved in scavenging of DPPH radical and to rationalize of the higher antioxidant activity of 1 compared with 14 is carried out by calculation BDEs, IPs and DEs of both compounds.
The detailed DFT methods that described in our previous work (Al-Salahi, et al. 2017), are employed to determine all the quantum chemical as conformational analyses, optimization and frequency calculations.
3. Results and discussion
3.1. Antioxidant activity
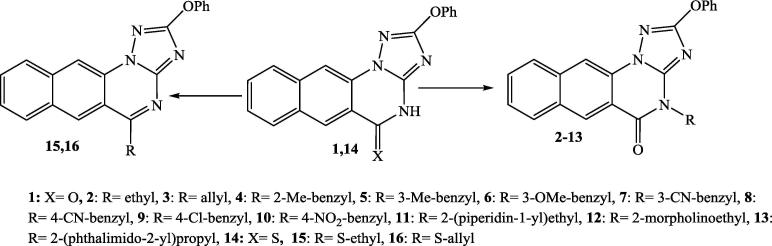
Scheme 1 illustrated the synthetic routes for the target benzotriazoloquinazolines 1–16 (Al-Salahi and Marzouk, 2016). The diphenyl-N-cyanoimidocarbonate was reacted in basic medium with hydrazinonaphthoic acid in DMF and followed by treatment with Conc. HCl to give the parent (1). Reaction of 1 with alkyl(heteroalkyl) halides in DMF produced the targets 2–13. Treatment of 1 with P2S5 in pyridine furnished the target 14, which subsequently transformed into thioethers 15 and 16 by reaction with alkyl halides in basic medium (Al-Salahi and Marzouk, 2016).
Scheme 1.
The synthetic compounds 1–16.
In this study, DPPH, FRAP and RPC assays were used to evaluate the in vitro antioxidant activity of compounds 1–16. The standard synthetic antioxidant BHT was used.
The entire prepared benzotriazoloquinazolines 1–16 demonstrated different antioxidant activity. This activity was ranged from weak to high DPPH scavenging, reducing power and FRAP activities (Table 1, Table 2, Table 3), however, all derivatives were appeared less active than standard BHT. In accordance to the antioxidant results of triazoloquinazolines (Al-Salahi et al., 2017), the targets 1–16 may be behaved the same behavior as redox agents, which can scavenge free radicals by reduction or donation of hydrogen atoms. DPPH is considered as a stable scavenger of free radical. It applied to assess the potential antiradical of antioxidants by quantify measurement the decrease in the absorbance of DPPH radical at 517 nm. DPPH in solution mode was deep violet color and when it gains the hydrogen or electron from the target antioxidant, it becomes pale yellow, which indicates decrease in the absorbance (Al-Salahi et al., 2017). The antioxidant radical scavenging activity of the targets 1–16 was investigated at the same concentrations.
Table 1.
DPPH scavenging activity of benzotriazoloquinazolines 1–16.
| Samples (1 mg/ml) | DPPH % |
|---|---|
| 1 | 50.88 ± 0.15 |
| 2 | 29.88 ± 0.18 |
| 3 | 22.53 ± 0.21 |
| 4 | 16.63 ± 0.20 |
| 5 | 10.69 ± 0.18 |
| 6 | 16.79 ± 0.17 |
| 7 | 19.55 ± 0.13 |
| 8 | 20.12 ± 0.21 |
| 9 | 24.98 ± 0.10 |
| 10 | 14.24 ± 0.23 |
| 11 | 17.47 ± 0.14 |
| 12 | 20.57 ± 0.27 |
| 13 | 18.93 ± 0.15 |
| 14 | 36.01 ± 0.13 |
| 15 | 16.50 ± 0.13 |
| 16 | 27.16 ± 0.17 |
| BHT | 93 |
Each value is expressed as mean ± SD (n = 3).
Table 2.
FRAP of benzotriazoloquinazolines 1–16.
| Samples (1 mg/ml) | Antioxidant activity (µmol Trolox/100 g) |
|---|---|
| 1 | 1376 ± 12.53 |
| 2 | 824 ± 8.19 |
| 3 | 951 ± 9.54 |
| 4 | 201 ± 8.62 |
| 5 | 265 ± 6.00 |
| 6 | 406 ± 6.24 |
| 7 | 203 ± 6.00 |
| 8 | 908 ± 13.01 |
| 9 | 440 ± 10.54 |
| 10 | 661 ± 8.00 |
| 11 | 752 ± 10.02 |
| 12 | 297 ± 6.03 |
| 13 | 946 ± 7.37 |
| 14 | 813 ± 5.86 |
| 15 | 720 ± 10.60 |
| 16 | 515 ± 6.11 |
Each value is expressed as mean ± SD (n = 3).
Table 3.
RPC of benzotriazoloquinazolines 1–16.
| Samples (1 mg/ml) | Absorbance at 700 nm |
|---|---|
| 1 | 0.640 ± 0.014 |
| 2 | 0.361 ± 0.010 |
| 3 | 0.365 ± 0.014 |
| 4 | 0.181 ± 0.009 |
| 5 | 0.214 ± 0.006 |
| 6 | 0.202 ± 0.006 |
| 7 | 0.255 ± 0.011 |
| 8 | 0.344 ± 0.006 |
| 9 | 0.374 ± 0.010 |
| 10 | 0.184 ± 0.006 |
| 11 | 0.204 ± 0.003 |
| 12 | 0.252 ± 0.025 |
| 13 | 0.280 ± 0.011 |
| 14 | 0.396 ± 0.017 |
| 15 | 0.273 ± 0.025 |
| 16 | 0.345 ± 0.010 |
| BHT | 1.56 |
Each value is expressed as mean ± SD (n = 3).
Table 1 presented the scavenging property of benzotriazoloquinazolines in terms of inhibition percent (at the same concentration as DPPH). The DPPH radical scavenging of benzo[g]triazoloquinazolines (1 and 14) showed the highest antioxidant activity in relation to the other derivatives, however, lower than BHT. Moreover, compounds 2, 3, 7–9, 12,13, and 16 exhibited moderate DPPH scavenging effects. Whereas 4–6, 10, 11 and 15 were emerged to have weak DPPH radical scavenging activity in relation to other derivatives and standard BHT at the same concentration (Table 1). The pronounced activity of the targets 1 and 14 could be attributed to the presence of the lactam and thiolactam groups, respectively.
Concerning FRAP method, the targets depend on reducing ferric complex (Fe3+) in TPTZ into (Fe2+) that might be indirectly reflected the antioxidant capability. A very intense navy blue color was created due to binding of ligand with reduced iron (Fe2+). Measurement the absorbance determined the amount of Fe2+ (Al-Salahi et al., 2017) and the FRAP results are summarized in Table 2. In relation to trolox, compounds 1–3, 8, 13 and 14 showed eminent FRAP activity. Their measured values ranged between 813 and 1376 (μmol Trolox/100 g). However, compounds 10, 11, 15, and 16 emerged to have relatively moderate antioxidant activity, while 4-7, 9 and 12 exhibited the lowest activity (Table 2). For interpreting the compiled results in Table 2, it is serious to realize that this method describes the capacity of the tested compound to share in one-electron redox reactions. The greatest in the antioxidant activity is a higher value of FRAP (Al-Salahi et al., 2017)
As presented in Table 3, compounds 1 and 14 exhibited the highest reducing capacities in relation to other derivatives, however, they were less active than BHT. Compounds 2, 3, 8, 9 and 16 showed the moderate effects, while compounds 4–7, 10–13 and 15 demonstrated the lowest activity in regards to 1, 14 and standard BHT. Variation in absorbance values is directly related to the total reducing power capacity of the antioxidant targets. The highest Fe3+ reduction ability of benzotriazoloquinazolines may be attributed to their inherited chemical features as electron donation or supplying hydrogen atom.
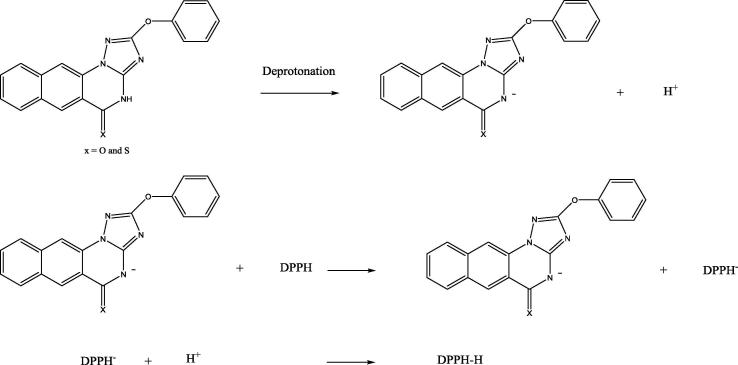
In light to the summarized findings in Table 1, Table 2, Table 3, it can be concluded that the position of substituents and their types are the major determinants of the antioxidant characteristics of the benzotriazoloquinazolines. Moreover, their chemical features as electron donating or withdrawing effected DPPH scavenging activities. Consequently, the modification on the parent structure 1 afforded new derivatives (2–16) with a variety of antioxidant activity, however, they were appeared less active than their parent 1. Thionation of 1 into 14, was accompanied by slightly decrease in the antioxidant activity. However, further transformation of 14 into S-alkylated derivatives doesn’t offer advantageous in the activity profiles. Similarly, alkylation of 1 into 2–13 doesn’t show improvement in the antioxidant activity. Moreover, diversity in N-alkyl substituents resulted in variations of the activity, in which compounds 2 and 3 and 9 were found more active candidates among the N- alkylated compounds. Within this study, we noticed that the most active benzotriazoloquinazolines 1 and 14 are characterized by lactam and thiolactam functional groups, respectively. This may play a remarkable role in the activity profiles (Scheme 2).
Scheme 2.
Plausible mechanism for the antioxidant activity of benzotriazloquinazolines 1 and 14.
3.2. DFT study
On the basis of the percentage inhibition of DPPH free radical by compounds 1 and 14, the former exhibited a strong antioxidant activity compared with latter (Table 1). As can be seen from Scheme 2, the two compounds differ only by C O and C S groups. In order to explain the highest antioxidant activity of 1 (51%) comparable with 14 (36%), their corresponding IPs, BDEs and DEs of NH groups were calculated (see methods and materials section). Both compounds exhibited similar IPs with a difference less than 0.01 eV. Hence, ET-PT is not a favorable mechanism to scavenge DPPH free radical by 1 and 14. Compound 1 exhibited a higher BDE value (96.3 kcal/mol) than 14 with a BDEs value of 90.8 kcal/mol. According to the obtained BDEs, the antioxidant activity of 1 is lower than 14. However, these results are opposed to the experimental ones. In an attempt to understand the experimental results, DEs of 1 and 14 are calculated. Indeed, compound 1 showed a lower DE value of 285 kcal/mol compared with 14 (415 kcal/mol). The lower DE, the high antioxidant activity. This result is in good agreement with the experimental, and also confirms that the main mechanism that is involved in DPPH free radical is SPLET.
A plausible mechanism for the antioxidant activity of the active compounds 1 and 14 was illustrated in Scheme 2 and confirmed by DFT study as sequential proton loss electron transfer (SPLET) mechanism, which included three steps as shown in material and methods part.
4. Conclusion
The obtained results revealed that benzotriazoloquinazolines exhibited good antioxidant activities. The targets 1 and 14 showed the highest antioxidant effects. This can be attributed to the ability of the former to show a lower DE than the later. The SAR study of the tested compounds provided us with useful thought about the characteristic requirements to design more active compounds against free radicals. These requirements will be taken into consideration in the next foreseeable research.
Acknowledgments
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through the research group project No. RG-1439-011.
Conflict of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and publication of this article
Footnotes
Peer review under responsibility of King Saud University.
References
- Abuelizz A.H., El-Dib R.A., Marzouk M., Al-Salahi R. In vitro evaluation of new 2-phenoxy-benzo[g][1,2,4]triazolo[1,5-a]quinazoline derivatives as antimicrobial agents. Microb. Pathog. 2018;117:60–67. doi: 10.1016/j.micpath.2018.02.018. [DOI] [PubMed] [Google Scholar]
- Al-Salahi R., Anouar E.H., Marzouk M., Taie H.A., Abuelizz H.A. Screening and evaluation of antioxidant activity of some 1,2,4-triazolo[1,5-a]quinazoline derivatives. Future Med. Chem. 2017;10:379–390. doi: 10.4155/fmc-2017-0224. [DOI] [PubMed] [Google Scholar]
- Al-Salahi R., Ashour A., Marzouk M., Kumar A. In Vitro cytotoxicity evaluation of a new series of benzo[g][1,2,4]triazolo[1,5-α]quinazolines. Lat. Am. J. Pharm. 2015;34:1926–1930. [Google Scholar]
- Al-Salahi R., Marzouk M. Synthesis of novel 2-phenoxy benzo[g][1,2,4]triazolo[1,5-a]quinazoline and its derivatives starting with diphenyl-N-cyanoimidocarbonate. Russ. J. Gen. Chem. 2016;86:1741–1746. [Google Scholar]
- Anouar E., Calliste C., Kosinova P., Di Meo F., Duroux J., et al. Free radical scavenging properties of guaiacol oligomers: a combined experimental and quantum study of the guaiacyl-moiety role. J. Phys. Chem. A. 2009;113:13881–13891. doi: 10.1021/jp906285b. [DOI] [PubMed] [Google Scholar]
- Anouar E.H., Raweh S., Bayach I., Taha M., Baharudin M.S., et al. Antioxidant properties of phenolic schiff bases: structure–activity relationship and mechanism of action. J. Comput. Aided Mol. Des. 2013;27:951–964. doi: 10.1007/s10822-013-9692-0. [DOI] [PubMed] [Google Scholar]
- Anouar E.H., Shah S.A.A., Hassan N.B., Moussaoui N.E., Ahmad R., et al. Antioxidant activity of hispidin oligomers from medicinal fungi: A DFT study. Molecules. 2014;19:3489–3507. doi: 10.3390/molecules19033489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak M.J., Jun M., Jeong W.S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H2O2-treated HepG2 cells and CCl4-treated mice. Int. J. Mol. Sci. 2012;13:2314–2330. doi: 10.3390/ijms13022314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman K.B., Ames B.N. The free radical theory of ageing matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995;28:25–30. [Google Scholar]
- Caliskan-Ergün B., Süküroğlu M., Coban T., Banoğlu E., Suzen S. Screening and evaluation of antioxidant activity of some pyridazine derivatives. J. Enzyme Inhib. Med. Chem.. 2008;23:225–229. doi: 10.1080/14756360701475167. [DOI] [PubMed] [Google Scholar]
- Di Meo F., Lemaur V., Cornil J.R.M., Lazzaroni R., et al. Free radical scavenging by natural polyphenols: atom versus electron transfer. J. Phys. Chem. A. 2013;117:2082–2092. doi: 10.1021/jp3116319. [DOI] [PubMed] [Google Scholar]
- Elansary H.O. Natural antioxidants and their role against human cancer. J. Plant Biochem. Physiol. 2014;2:1–2. [Google Scholar]
- Estevão M.S., Carvalho L.C., Ribeiro D., Couto D., Freitas M., et al. Antioxidant activity of unexplored indole derivatives: Synthesis and screening. Eur. J. Med. Chem. 2010;45:4869–4878. doi: 10.1016/j.ejmech.2010.07.059. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence. Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- Kumar A., Sharma P., Kumari P., Kalal B.L. Exploration of antimicrobial and antioxidant potential of newly synthesized 2,3-disubstituted quinazoline-4(3H)-ones. Bioorg. Med. Chem. Lett. 2011;21:4353–4357. doi: 10.1016/j.bmcl.2011.05.031. [DOI] [PubMed] [Google Scholar]
- Mermer A., Demirbaş N., Şirin Y., Uslu H., Özdemir Z., Demirbaş A. Conventional and microwave prompted synthesis, antioxidant, anticholinesterase activity screening and molecular docking studies of new quinolone-triazole hybrids. Bioorg. Chem. 2018;78:236–248. doi: 10.1016/j.bioorg.2018.03.017. [DOI] [PubMed] [Google Scholar]
- Musialik M., Litwinienko G. Scavenging of DPPH radicals by vitamin E is accelerated by its partial ionization: the role of sequential proton loss electron transfer. Org. Lett. 2005;7:4951–4954. doi: 10.1021/ol051962j. [DOI] [PubMed] [Google Scholar]
- Oyaizu M. Studies on the product of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986;44:307–315. [Google Scholar]
- Sompalle R., Arunachalam P., Roopan S.M. Conventional spectroscopic identification of N-alkylated triazoloquinazolinones and its antioxidant, solvatochromism studies. J. Mol. Liq. 2016;224:1348–1357. [Google Scholar]
- Sompalle R., Roopan S.M., Al-Dhabi N.A., Suthindhiran K., Sarkar G., Arasu M.V. 1,2,4-triazoloquinazoline-thiones: Non-conventional synthetic approach, study of solvatochromism and antioxidant assessment. J. Photochem. Photobiol. B: Biol. 2016;162:232–239. doi: 10.1016/j.jphotobiol.2016.06.051. [DOI] [PubMed] [Google Scholar]
- Thanan R., Oikawa S., Hiraku Y., Ohnishi S., Ma N., et al. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2015;16:193–217. doi: 10.3390/ijms16010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ünver Y., Deniz S., Çelik F., Akar Z., Küçük M., Sancak K. Synthesis of new 1,2,4-triazole compounds containing Schiff and Mannich bases (morpholine) with antioxidant and antimicrobial activities. J. Enzyme Inhib. Med. Chem. 2016;31:89–95. doi: 10.1080/14756366.2016.1206088. [DOI] [PubMed] [Google Scholar]
- Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–48. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]