Abstract
Asthma is a common chronic inflammatory respiratory disease characterised by airway inflammation and hyperresponsiveness. The present study was designed to clarify the effect of intranasal miR-410 administration in an ovalbumin (OVA)-induced murine model of asthma. It was found that miR-410 expression was significantly decreased in the lungs of OVA-induced asthmatic mice (P<0.05) and miR-410 was overexpressed via intranasal instillation. Bioinformatics indicated that the 3′-untranslated regions of interleukin (IL)-4 and IL-13 messenger RNAs (mRNAs) contain miR-410 binding sites. The IL-4 and IL-13 genes were confirmed to be miR-410-regulated using the dual-luciferase reporter assay. Additionally, intranasal administration of miR-410 markedly attenuated airway inflammation and reduced infiltration of inflammatory cells into bronchoalveolar lavage fluid (P<0.05) as determined by bronchoalveolar lavage fluid analysis. Moreover, miR-410 significantly decreased the lung expression of IL-4 and IL-13 (P<0.05), although the levels of mRNAs encoding IL-4 and IL-13 in lungs did not change significantly as determined by real-time PCR analysis. In conclusion, we found that intranasal administration of miR-410 effectively inhibited airway inflammation in OVA-induced asthmatic mice by targeting IL-4 and IL-13 at the post-transcriptional level. miR-410 is thus a promising treatment for allergic asthma.
Keywords: miR-410, asthma, mice, OVA, IL-13, IL-4
Introduction
Asthma is a heterogeneous, chronic respiratory disease characterised by airway inflammation, increased airway hyperresponsiveness and variable airflow obstruction (1,2). Asthma has the highest rate of morbidity worldwide, and the prevalence has increased significantly over the past decades (3,4). However, the pathophysiology of asthma remains unclear (5).
Interleukin (IL)-4 and IL-13, cytokines expressed by T-helper type 2 (Th2) cells, play key roles in the pathogenesis of atopy and atopic asthma (6,7). Both IL-4 and IL-13 promote acute inflammatory processes of asthma and underlying structural changes in the airways; the receptors are expressed by various cell types (8,9). The inflammatory mediator IL-4 induces eosinophil infiltration in the airway and promotes inflammatory cell chemotaxis (10). IL-13 also induces eosinophil inflammation, airway hyperreactivity (AHR) and mucus hypersecretion (11,12).
MicroRNAs (miRNAs) are small, noncoding RNAs involved in various physiological processes and diseases (13). miRNAs regulate gene expression post-transcriptionally by binding to the 3′-untranslated regions (UTRs) of targeted messenger RNAs (mRNAs) for degradation or translational repression (14,15). Emerging evidence supports a link between miRNAs and bronchial asthma. The levels of miR-148, miR-26a, Let-7a and Let-7d have been found to be altered in sera of asthmatic patients (16,17). miR-410 has not previously been reported to be involved in asthma but affects retinal neovascularisation during oxygen-induced retinopathy in mice, exerting an anti-inflammatory action, although the underlying mechanism has not been described (18). We speculated that miR-410 may also be involved in the pathogenesis of airway inflammation. Our aim was to explore whether intranasal administration of miR-410 attenuates such inflammation in OVA-induced asthmatic mice, and the possible therapeutic mechanism in play.
Materials and methods
Animals and reagents
Female BALB/c mice (6–8 weeks old, 20±2 g) were obtained from the Laboratory Animal Centre of Qingdao University Medical College. The mice were randomly divided into 4 groups, with 6 mice in each group: PBS group (A), OVA-induced asthma group (B), OVA+miR-410 mimic group (C), OVA+miR-410 control group (D). All procedures involving animals were approved by the Laboratory Animal Centre of Qingdao University Medical College Animal Care and Use Committee and conformed to guidelines for the Care and Use of Laboratory Animals of the Ministry of Health, China.
Luciferase experiment
We used dual-luciferase reporter assay to explore whether miR-410 targets IL-4 and/or IL-13 mRNA. The wild-type murine IL-4 and IL-13 mRNA 3′-UTR segments were amplified and cloned into the psiCHECK vector (Sangon Biotech Co., Shanghai, China). 293T cells were transfected with 0.1 µg of psiCHECK-IL-13-mutant, psiCHECK-IL-4-mutant, psiCHECK-IL-13 or psiCHECK-IL-4 along with 40 nM miR-410 mimic or miR-410 control using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The reporter gene assay was performed 48 h after transfection using the Dual luciferase assay kit (Promega Corporation, Madison, WI, USA) according to the manufacturer's instructions.
Establishing the OVA-induced asthmatic murine model and intranasal administration of miR-410 in asthmatic mice
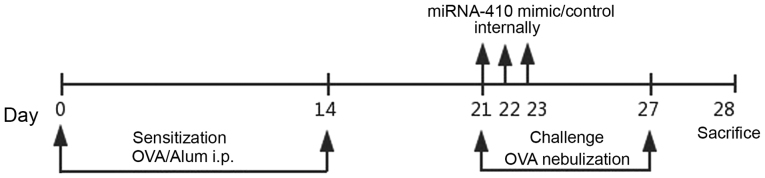
Female BALB/c mice weighing ~20 g were randomly divided into four groups (n=6). Mice were maintained in a controlled environment and fed standard food pellets and water. After 1 week of adaptation, mice were injected intraperitoneally on days 0 and 14 with either 20 µg OVA (Grade V; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 20 mg Al(OH)3 in 0.2 ml phosphate-buffered saline (PBS), or PBS only (control). Following sensitisation, mice were exposed to either aerosolised 1% OVA/PBS or PBS only for 20 min once daily on days 21–27 (19). The miR-410 mimic and a control oligonucleotide were chemically synthesised and specially modified for transfection into animals by Biomics Biotechnologies Co., Ltd. (Shanghai, China). For both the OVA+miR-410 mimic group and OVA+miR-410 control group, miR-410 or its control oligonucleotide was dissolved in endotoxin-free water. Each working dilution was administered intranasally into asthmatic mice at a dose of 10 µg on days 21, 22 and 23. Mice were sacrificed after intraperitoneal administration of 1% pentobarbital sodium (50 mg/kg, P3761; Sigma-Aldrich; Merck KGaA) on day 28 (Fig. 1). Mice did not become severely ill or moribund at any point during the experiment. All mice were raised in a specific pathogen-free facility and sacrificed by cervical dislocation which was following the guidelines for the Care and Use of Laboratory Animals of the Ministry of Health, China.
Figure 1.
Schematic representation of intranasal instillation of miR-410 into ovalbumin (OVA)-induced asthmatic mice. i.p., intraperitoneal.
Bronchoalveolar lavage fluid analysis
BALF was centrifuged at 240 × g for 5 min. The supernatant was collected and stored at −80°C prior to measurement of cytokine levels. Precipitated cells were resuspended in 0.4 ml PBS and 0.1 ml was taken to determine the total cell count. The remaining cells were smeared onto a clean slide and a differential count was performed following Wright-Giemsa staininging. For Wright-Giemsa staininging, two or three drops of resuspension solution of BALF were spread onto the microscope slides, heated, stained with Wright-Giemsa staining for 10 min, and subsequently washed under running tap-water for 2 min (20). IL-4 and IL-13 levels were measured using enzyme-linked immunosorbent assays (ELISAs) (eBioscience; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions.
RNA extraction and quantitative polymerase chain reaction (PCR)
Total lung RNA was extracted from cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Primers amplifying miR-410, IL-4 and IL-13 were purchased from Sangon Biotech Co. (Shanghai, China). mRNA levels were normalised to GAPDH levels and quantitated using the 2−ΔΔCq method (21). PCR was performed on a 7500 Fast Real-Time PCR System (Fermentas; Thermo Fisher Scientific, Inc.).
Histopathology
Mouse lungs were removed 24 h after the final challenge. Left lungs were immediately fixed in 10% (v/v) buffered formalin and embedded in paraffin. Lung sections were stained with hematoxylin and eosin (H&E) (Baso, Zhuhai, China) to detect eosinophil infiltration, as described previously (20).
Statistical analysis
The results were analysed with GraphPad Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA) and are expressed as means ± standard deviation. Correlations among the four groups were calculated using one-way analysis of variance followed by Tukey's multiple comparison post hoc test. A P-value <0.05 was considered statistically significant.
Results
Prediction of miR-410 target genes
To elucidate the potential relationship between miR-410 and asthma, we first screened for potential target genes that contain a highly conserved complementary 3′-UTR sequence using a publicly available bioinformatics tool (http://www.mirna.org/) (22,23). The 3′-UTRs of both IL-4 and IL-13 mRNAs were predicted to contain a binding position for miR-410 (Fig. 2).
Figure 2.
Interleukin (IL)-4 and IL-13 messenger RNAs (mRNAs) are targets of miR-410. Predicted matching target sites of miR-410 in the 3′-UTRs of IL-4 and IL-13 mRNA transcripts. UTR, untranslated region.
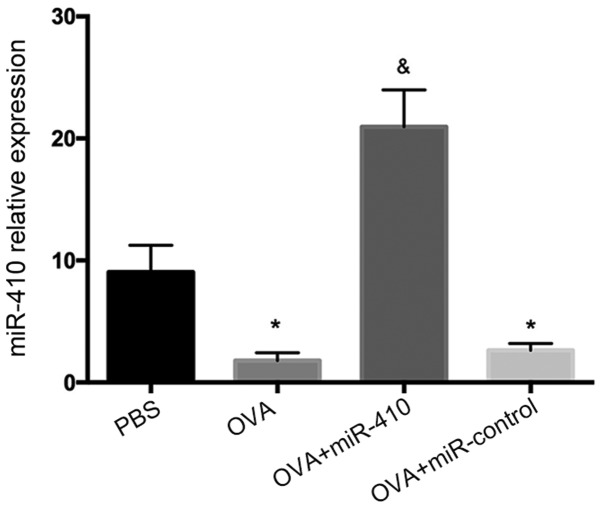
Reduced expression of miR-410 in lungs of OVA-induced asthmatic mice
As shown in Fig. 3, miR-410 expression was significantly decreased in the OVA-induced asthmatic mice, suggesting that miR-410 may negatively regulate OVA-induced asthma. To further evaluate the role played by miR-410, we administered miR-410 intranasally, affording lung gene transduction.
Figure 3.
Expression of miR-410 in lungs of ovalbumin (OVA)-induced asthmatic mice following intranasal administration of miR-410, as determined by quantitative real-time PCR. The results are expressed as means ± standard error of the mean (SEM). *P<0.05 vs. the phosphate-buffered saline (PBS) group; &P<0.05 vs. the OVA and OVA+miR-410 control groups.
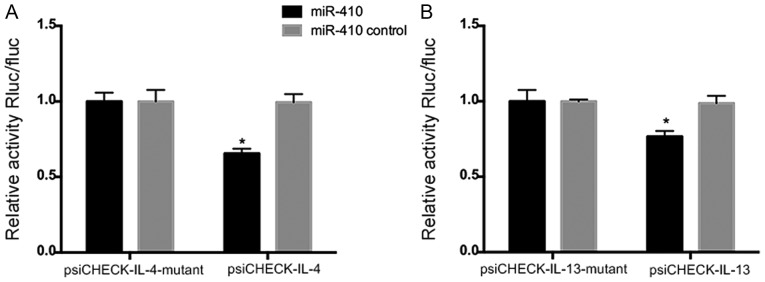
miR-410 targets IL-4 and IL-13 mRNAs
To explore whether miR-410 modulates IL-4 or IL-13 expression directly, we transfected miR-410 and the luciferase-encoding plasmids psiCHECK-IL-4, psiCHECK-IL-13, psiCHECK-IL-4-mutant, and psiCHECK-IL-13-mutant into 293T cells. The luciferase activities of psiCHECK-IL-4 and psiCHECK-IL-13 were significantly decreased in the presence of miR-410 mimics (Fig. 4A and B). Hence, modulation of IL-4 and IL-13 expression by miR-410 was sequence-specific.
Figure 4.
An miR-410 mimic or miR-410 control was co-transfected with luciferase plasmids containing (A) IL-4 3′-UTR (psiCHECK-IL-4) or IL-4 mutant 3′-UTR (psiCHECK-IL-4-mutant), and (B) IL-13 3′-UTR (psiCHECK-IL-13) or IL-13 mutant 3′-UTR (psiCHECK-IL-13-mutant) into 293T cells. Luciferase activity was measured with the aid of the dual-luciferase reporter assay kit. Each bar represents the mean ± SEM, *P<0.05. All experiments were independently repeated three times, with similar results. UTR, untranslated region.
Alleviation of airway inflammation in asthmatic mice via intranasal instillation of miR-410
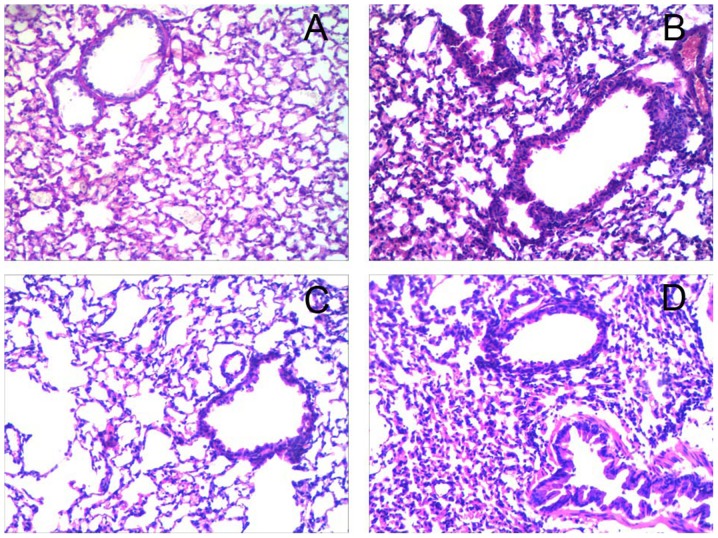
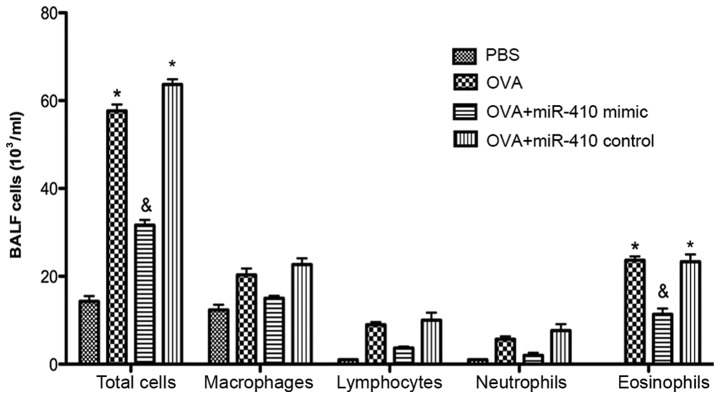
Histological analysis revealed numerous inflammatory cell infiltrations around the bronchiole and a thickened airway epithelium in the OVA and OVA+miR-410 control groups compared with the PBS group. However, OVA-induced asthmatic mice treated with the miR-410 mimic exhibited a marked reduction in bronchiole inflammatory cell infiltration (Fig. 5). We examined whether miR-410 affects inflammatory cells in BALF of OVA-induced asthmatic mice. The numbers of total cells and eosinophils in BALF were significantly increased in the OVA-induced mice after the last challenge, compared with the control mice. These increases were not evident in the miR-410 mimic group (Fig. 6).
Figure 5.
Administration of miR-410 reduces airway inflammation in ovalbumin (OVA)-induced asthmatic mice. (A-D) miR-410 suppressed infiltration of inflammatory airway cells in OVA-induced asthmatic mice. Lung sections from the four groups stained with hematoxylin and eosin are shown (×200 magnification): (A) PBS group, (B) OVA group, (C) OVA+miR-410 mimic group and (D) OVA+miR-410 control group.
Figure 6.
miR-410 decreases the numbers of total cells and eosinophils in BALF compared with both ovalbumin (OVA)-treated and miR-410-control-treated asthmatic mice. *P<0.05 vs. the PBS group and the OVA+miR-410 mimic group; and &P<0.05 vs. the OVA and OVA+miR-410 control groups.
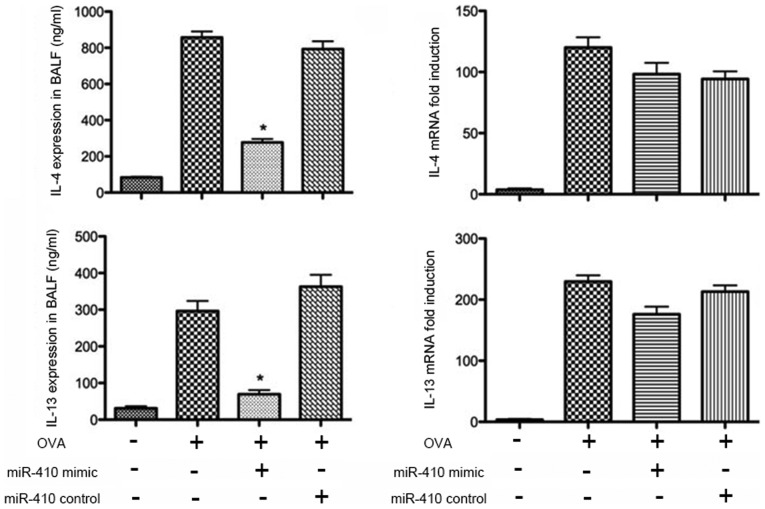
IL-4 and IL-13 expression in lungs of OVA-induced mice is suppressed by intranasal instillation of miR-410
The protein levels of IL-4 and IL-13 in BALF were determined by ELISAs. IL-4 and IL-13 were significantly increased in the OVA group after the last challenge compared with the PBS group. The increased levels of IL-4 and IL-13 were significantly reduced following intranasal administration of the miR-410 mimic (Fig. 7). We further assessed the mRNA levels of IL-4 and IL-13 in the lung tissues; those of IL-4 and IL-13 did not differ significantly among the four groups (Fig. 7).
Figure 7.
Administration of miR-410 attenuates interleukin (IL)-4 and IL-13 expression in ovalbumin (OVA)-induced asthmatic murine lungs. miR-410 did not suppress IL-4 and IL-13 mRNA expression in lungs. *P<0.05 vs. the OVA group and OVA+miR-410 control group.
Discussion
In the present study, the role of intranasal instillation of miR-410 was evaluated in an OVA-induced murine model of asthma. Administration of miR-410 intranasally attenuated inflammatory cell infiltration in bronchioles of OVA-induced asthmatic mice by directly targeting the 3′-UTRs of IL-4 and IL-13 mRNAs.
The comprehensive resource, microrna.org, was utilized for target site analysis; this revealed that miR-410 had binding sites on the 3′-UTRs of both IL-4 and IL −13 mRNAs. miRNA binds specifically to the 3′-UTR of the target mRNA to suppress translation or induce degradation (24–26). Accumulating evidence suggests that miRNAs regulate a variety of biological processes, including inflammation and allergic diseases (25). Abnormal expression of several miRNAs has been observed in the airways, in BALF lymphocytes of asthmatic patients and in several asthmatic murine models (27,28). To date, a~10 miRNAs have been found to play a role in asthma pathogenesis; most have been validated in house dust mite- or OVA-induced asthma mouse models (29,30). Accordingly, our data suggest that miR-410 levels were significantly decreased in the OVA-induced asthmatic mice and miR-410 overexpression reduced the expression levels and stabilities of IL-4 and IL-13 mRNAs. The luciferase reporter assay showed that the miR-410 sequence specifically bound to the 3′-UTRs of IL-4 and IL-13 mRNA. Therefore, we hypothesise that the inhibitory effects of miR-410 on the production of IL-4 and IL-13 may be direct; however, further studies are needed to confirm this using luciferase reporter plasmids containing the 3′-UTR of STAR-6, which plays an important role in Th2 cytokine networking (31).
Asthma is a heterogeneous disease with patients presenting with several distinct clinical phenotypes (2,32). The OVA-induced allergic asthma model is characterised by chronic airway inflammation with infiltration of eosinophils, lymphocytes, macrophages and neutrophils into the bronchial lumen (3,33,34). OVA-induced asthma is associated with pulmonary production of Th2 cytokines (IL-4, IL-5 and IL-13) in BALF (35). Similar changes, such as histopathological alterations in lung tissues and inflammatory cells in BALF, were observed in our present study. As Kumar et al previously reported, intranasal delivery of miR-let-7 to mice with ovalbumin (OVA)-induced allergic airway inflammation triggered overexpression of miR-let-7; others found that intranasal miRNA administration effectively triggered miRNA overexpression in murine models (36,37). We successfully administered miR-410 or a control mimic intranasally to OVA-induced mice.
The aberrant Th2-type response to allergens during asthma initiation and progression is characterised by excessive production of IL-4 and IL-13, which in turn triggers inflammatory airway infiltration by mast cells, eosinophils and basophils (38). IL-4 triggers the type 2 immune response whereas IL-13 is an effector molecule (39). However, pharmacological experiments showed that inhibition of IL-4 or IL-13 alone did not adequately attenuate the allergic inflammation associated with asthma. Thus, further research is needed. Intranasal miR-410 exerted a marked anti-inflammatory effect on airway inflammation in the mouse, attributable to significant reductions in the levels of IL-4, IL-13 and eosinophils in BALF. Through dual-luciferase reporter assay, we found that miR-410 targets the 3′-UTRs of both IL-4 and IL-13 mRNAs. Therefore, miR-410 may directly target lung IL-4/IL-13 mRNAs, reducing lung pathology via a continued decrease in Th2 cytokine levels. Thus, intranasal miR-410 may alleviate asthma; early studies evaluating IL-4- and IL-13-targeting therapies yielded disappointing results (39).
In conclusion, intranasal administration of miR-410 significantly suppressed airway inflammation and exerted therapeutic effects by directly targeting post-transcriptional expression of IL-4 and IL-13. The mechanisms underlying these outcomes are complex, involving both direct and indirect effects on multiple regulatory processes. However, intranasal instillation of miR-410 may protect against asthma.
Acknowledgements
Not applicable.
Funding
The present study was funded by NSFC grant no. 81701587.
Availability of data and materials
The datasets used during the present study are available from the corresponding author upon reasonable request.
Authors' contributions
RL designed the study. RJ and RG carried out the animal experiments. LL and SH carried out the ELISA analysis and data analysis. XL carried out the histopathological analysis. RJ, LL, XL and RG carried out preparation of the manuscript. All authors contributed to the development of interim and final drafts, and read and approved the final manuscript. All authors agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
All procedures involving animals were approved by the Laboratory Animal Centre of Qingdao University Medical College Animal Care and Use Committee and conformed to guidelines for the Care and Use of Laboratory Animals of the Ministry of Health, China.
Patient consent for publication
Not applicable.
Competing interests
The authors state that they have no competing interests.
References
- 1.Hu M, Ou-Yang HF, Han XP, Ti XY, Wu CG. KyoT2 downregulates airway remodeling in asthma. Int J Clin Exp Pathol. 2015;8:14171–14179. [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson DS. Th-2 cytokines in allergic disease. Br Med Bull. 2000;56:956–968. doi: 10.1258/0007142001903625. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen SE, Hurd SS, Lemanske RF, Jr, Becker A, Zar HJ, Sly PD, Soto-Quiroz M, Wong G, Bateman ED. Global Initiative for Asthma: Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46:1–17. doi: 10.1002/ppul.21321. [DOI] [PubMed] [Google Scholar]
- 4.Izuhara K, Ohta S, Shiraishi H, Suzuki S, Taniguchi K, Toda S, Tanabe T, Yasuo M, Kubo K, Hoshino T, et al. The mechanism of mucus production in bronchial asthma. Curr Med Chem. 2009;16:2867–2875. doi: 10.2174/092986709788803196. [DOI] [PubMed] [Google Scholar]
- 5.Huang HY, Chiang BL. siRNA as a therapy for asthma. Curr Opin Mol Ther. 2009;11:652–663. [PubMed] [Google Scholar]
- 6.Liu W, Liu S, Verma M, Zafar I, Good JT, Rollins D, Groshong S, Gorska MM, Martin RJ, Alam R. Mechanism of TH2/TH17-predominant and neutrophilic TH2/TH17-low subtypes of asthma. J Allergy Clin Immunol. 2017;139:1548–1558.e4. doi: 10.1016/j.jaci.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch JP, Ferreira MA, Phipps S. Th2/Th17 reciprocal regulation: Twists and turns in the complexity of asthma phenotypes. Ann Transl Med. 2016;4(Suppl 1):S59. doi: 10.21037/atm.2016.10.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine. 2015;75:68–78. doi: 10.1016/j.cyto.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13:425–437. doi: 10.1080/1744666X.2017.1298443. [DOI] [PubMed] [Google Scholar]
- 10.Tomkinson A, Tepper J, Morton M, Bowden A, Stevens L, Harris P, Lindell D, Fitch N, Gundel R, Getz EB. Inhaled vs subcutaneous effects of a dual IL-4/IL-13 antagonist in a monkey model of asthma. Allergy. 2010;65:69–77. doi: 10.1111/j.1398-9995.2009.02156.x. [DOI] [PubMed] [Google Scholar]
- 11.Moynihan BJ, Tolloczko B, El Bassam S, Ferraro P, Michoud MC, Martin JG, Laberge S. IFN-gamma, IL-4 and IL-13 modulate responsiveness of human airway smooth muscle cells to IL-13. Respir Res. 2008;9:84. doi: 10.1186/1465-9921-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blease K. Therapeutics targeting IL-13 for the treatment of pulmonary inflammation and airway remodeling. Curr Opin Investig Drugs. 2008;9:1180–1184. [PubMed] [Google Scholar]
- 13.Zhou Z, Zhao Y, Gu L, Niu X, Lu S. Inhibiting proliferation and migration of lung cancer using small interfering RNA targeting on Aldo-keto reductase family 1 member B10. Mol Med Rep. 2018;17:2153–2160. doi: 10.3892/mmr.2017.8173. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Qin HB, Xu B, Mei JJ, Li D, Liu JJ, Zhao DY, Liu F. Inhibition of miRNA-221 suppresses the airway inflammation in asthma. Inflammation. 2012;35:1595–1599. doi: 10.1007/s10753-012-9474-1. [DOI] [PubMed] [Google Scholar]
- 15.Li JJ, Tay HL, Maltby S, Xiang Y, Eyers F, Hatchwell L, Zhou H, Toop HD, Morris JC, Nair P, et al. MicroRNA-9 regulates steroid-resistant airway hyperresponsiveness by reducing protein phosphatase 2A activity. J Allergy Clin Immunol. 2015;136:462–473. doi: 10.1016/j.jaci.2014.11.044. [DOI] [PubMed] [Google Scholar]
- 16.Dissanayake E, Inoue Y. MicroRNAs in allergic disease. Curr Allergy Asthma Rep. 2016;16:67. doi: 10.1007/s11882-016-0648-z. [DOI] [PubMed] [Google Scholar]
- 17.Feng MJ, Shi F, Qiu C, Peng WK. MicroRNA-181a, −146a and −146b in spleen CD4+ T lymphocytes play proinflammatory roles in a murine model of asthma. Int Immunopharmacol. 2012;13:347–353. doi: 10.1016/j.intimp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Chen N, Wang J, Hu Y, Cui B, Li W, Xu G, Liu L, Liu S. MicroRNA-410 reduces the expression of vascular endothelial growth factor and inhibits oxygen-induced retinal neovascularization. PLoS One. 2014;9:e95665. doi: 10.1371/journal.pone.0095665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin R, Guo S, Wang MY, Li YH, Wu LX, Ma H, Lowrie DB, Fan XY, Zhang JH. Administration of mycobacterial Ag85A and IL-17A fusion protein attenuates airway inflammation in a murine model of asthma. Int Immunopharmacol. 2013;17:1067–1074. doi: 10.1016/j.intimp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, Lin R, Zhao B, Guan R, Li T, Jin R. Correlation between oxidative stress and the NF-κB signaling pathway in the pulmonary tissues of obese asthmatic mice. Mol Med Rep. 2016;13:1127–1134. doi: 10.3892/mmr.2015.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Xue Y, Liu Y, Song G, Lv G, Wang Y, Wang Y, Li X, Yang L. MicroRNA-146a expression inhibits the proliferation and promotes the apoptosis of bronchial smooth muscle cells in asthma by directly targeting the epidermal growth factor receptor. Exp Ther Med. 2016;12:854–858. doi: 10.3892/etm.2016.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Y, Yang K, Shi H, Xu J, Zhang D, Wu Y, Zhou S, Sun X. MiR-21 modulates human airway smooth muscle cell proliferation and migration in asthma through regulation of PTEN expression. Exp Lung Res. 2015;41:535–545. doi: 10.3109/01902148.2015.1090501. [DOI] [PubMed] [Google Scholar]
- 24.Ariel D, Upadhyay D. The role and regulation of microRNAs in asthma. Curr Opin Allergy Clin Immunol. 2012;12:49–52. doi: 10.1097/ACI.0b013e32834ecb7f. [DOI] [PubMed] [Google Scholar]
- 25.Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, Nguyen C, Solon M, Nguyen C, Barczak AJ, et al. Airway epithelial miRNA expression is altered in asthma. Am J Respir Crit Care Med. 2012;186:965–974. doi: 10.1164/rccm.201201-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H, Li J, Gao P, Wang Q, Zhang J. miR-155: A novel target in allergic asthma. Int J Mol Sci. 2016;17(pii):E1773. doi: 10.3390/ijms17101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deshpande DA, Dileepan M, Walseth TF, Subramanian S, Kannan MS. MicroRNA regulation of airway inflammation and airway smooth muscle function: Relevance to asthma. Drug Dev Res. 2015;76:286–295. doi: 10.1002/ddr.21267. [DOI] [PubMed] [Google Scholar]
- 28.Al-Quraishy S, Dkhil MA, Delic D, Abdel-Baki AA, Wunderlich F. Organ-specific testosterone-insensitive response of miRNA expression of C57BL/6 mice to Plasmodium chabaudi malaria. Parasitol Res. 2012;111:1093–1101. doi: 10.1007/s00436-012-2937-3. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Sun E, Li X, Zhang M, Tang Z, He L, Lv K. miR-155 contributes to Df1-induced asthma by increasing the proliferative response of Th cells via CTLA-4 downregulation. Cell Immunol. 2017;314:1–9. doi: 10.1016/j.cellimm.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Sharma A, Kumar M, Ahmad T, Mabalirajan U, Aich J, Agrawal A, Ghosh B. Antagonism of mmu-mir-106a attenuates asthma features in allergic murine model. J Appl Physiol. 2012;113:459–464. doi: 10.1152/japplphysiol.00001.2012. [DOI] [PubMed] [Google Scholar]
- 31.Nelms K, Keegan AD, Zamorano J, Ryan JJ, Paul WE. The IL-4 receptor: Signaling mechanisms and biologic functions. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 32.Stirling RG, Chung KF. Future treatments of allergic diseases and asthma. Br Med Bull. 2000;56:1037–1053. doi: 10.1258/0007142001903526. [DOI] [PubMed] [Google Scholar]
- 33.Jia Y, Fang X, Zhu X, Bai C, Zhu L, Jin M, Wang X, Hu M, Tang R, Chen Z. IL-13+ type 2 innate lymphoid cells correlate with asthma control status and treatment response. Am J Respir Cell Mol Biol. 2016;55:675–683. doi: 10.1165/rcmb.2016-0099OC. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Oh SY, Wu X, Oh MH, Wu F, Schroeder JT, Takemoto CM, Zheng T, Zhu Z. SHP-1 deficient mast cells are hyperresponsive to stimulation and critical in initiating allergic inflammation in the lung. J Immunol. 2010;184:1180–1190. doi: 10.4049/jimmunol.0901972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veremeyko T, Siddiqui S, Sotnikov I, Yung A, Ponomarev ED. IL-4/IL-13-dependent and independent expression of miR-124 and its contribution to M2 phenotype of monocytic cells in normal conditions and during allergic inflammation. PLoS One. 2013;8:e81774. doi: 10.1371/journal.pone.0081774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar M, Ahmad T, Sharma A, Mabalirajan U, Kulshreshtha A, Agrawal A, Ghosh B. Let-7 microRNA-mediated regulation of IL-13 and allergic airway inflammation. J Allergy Clin Immunol. 2011;128:1077–1085. doi: 10.1016/j.jaci.2011.04.034. e1-e10. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Chen X, Wu Q, Song J, Wang L, Li G. miR-125b inhibits goblet cell differentiation in allergic airway inflammation by targeting SPDEF. Eur J Pharmacol. 2016;782:14–20. doi: 10.1016/j.ejphar.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 38.Cortes JR, Rivas MD, Molina-Infante J, Gonzalez-Nuñez MA, Perez-G M, Masa JF, Sanchez JF, Zamorano J. Omeprazole inhibits IL-4 and IL-13 signaling signal transducer and activator of transcription 6 activation and reduces lung inflammation in murine asthma. J Allergy Clin Immunol. 2009;124:607–610. doi: 10.1016/j.jaci.2009.06.023. 610.e1. [DOI] [PubMed] [Google Scholar]
- 39.Karo-Atar D, Bitton A, Benhar I, Munitz A. Therapeutic targeting of the interleukin-4/interleukin-13 signaling pathway: In allergy and beyond. BioDrugs. 2018 May 7; doi: 10.1007/s40259-018-0280-7. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used during the present study are available from the corresponding author upon reasonable request.