Abstract
Diabetic retinopathy (DR) is a retinal disease caused by metabolic disorders of glucose tolerance that can lead to irreversible blindness if not adequately treated. Retinal pigment epithelial cell (RPEC) dysfunction contributes to the pathogenesis of DR. In the present study the anti-inflammatory effect of curcumin (CUR) was investigated in RPECs damaged by high glucose levels. RPEC treated with 30 mmol/l glucose was regarded as high glucose group, and cells treated with 24.4 mmol/l mannitol was set as equivalent osmolarity group. Cell Counting Kit-8 assay was used to measure RPEC viability, the expression of phosphorylated (p)-AKT and p-mammalian target of rapamycin (mTOR) were assessed by western blot, and secretion of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-1β in the culture medium was measured by ELISA. Intracellular reactive oxygen species (ROS) levels were measured by laser scanning confocal microscope. The present data indicated that, compared with mannitol treatment, high glucose treatment reduced RPEC viability, increased TNF-α, IL-6 and IL-1β secretion, increased ROS formation and promoted phosphorylation of AKT and mTOR. The antioxidant N-acetylcysteine, the phosphoinositide 3-kinase (PI3K)/AKT inhibitor LY294002 and the mTOR inhibitor rapamycin ameliorated the effects of high glucose. In addition, pretreatment with 10 µmol/l CUR reduced secretion levels of TNF-α, IL-6 and IL-1β, ROS formation and phosphorylation of AKT and mTOR. In conclusion, CUR inhibited high glucose-induced inflammatory injury in RPECs by interfering with the ROS/PI3K/AKT/mTOR signaling pathway. The present study may reveal the molecular mechanism of CUR inhibition effects to high glucose-induced inflammatory injury in RPEC.
Keywords: curcumin, diabetic retinopathy, inflammation, phosphoinositide 3 kinase/AKT signaling pathway, mammalian target of rapamycin signaling pathway
Introduction
Diabetes occurs in ~8.5% of adults aged 18 years or older, and resulted in 1.6 million mortalities in 2014 (1). Hyperglycemia may cause injury to the peripheral nerves, and the renal and vascular systems (2). Diabetic retinopathy (DR), a microvascular complication of diabetes, is cause of adult blindness (3–5); visual impairment in DR is associated with increased apoptosis of retinal cells, including pigment epithelial cells, pericytes and endothelial cells (6–8). Although DR can be treated by vitrectomy or laser photocoagulation, these approaches are not satisfactory (9,10), and novel and effective interventions are required to reduce retinal injury in patients with DR.
High levels of glucose serve a key role in retinal cell death. Several lines of evidence indicate that high glucose induces overproduction of pro-inflammatory cytokines, such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β, which act as a positive feedback mechanism to induce retinal cell apoptosis (11–14). High concentrations of glucose within retinal cells can induce oxidative stress by increasing intracellular reactive oxygen species (ROS) production through the mitochondrial electron transport chain (15–17). Oxidative stress activates a cascade of several biochemical and molecular events, which may ultimately lead to DR (18); therefore, anti-oxidants may represent a promising therapy for the treatment of DR.
Curcumin (CUR), a natural phytochemical compound in turmeric is reported to possess a variety of pharmacological properties, including anti-oxidative (19–21), anti-inflammatory (22–24) and anti-carcinogenic activities (25–27). CUR has been proposed to prevent DR by modulating antioxidant activities and several signaling pathways (28,29). It remains unclear whether CUR exerts its therapeutic effects in DR through its anti-inflammatory properties, and to determine whether CUR was able to prevent inflammatory injury in DR, its effect on retinal pigment epithelial cells (RPECs) cultured in high levels of glucose were investigated. Therefore, the present study aimed to investigate the potential effects of CUR on RPECs, including the secretion of pro-inflammatory cytokines, ROS production and the underlying molecular mechanism, which may provide a theoretical basis for the use of CUR as treatment strategy for DR.
Materials and methods
Regents
CUR (see Fig. 1 for chemical structure) was obtained from BioBioPha Co., Ltd. (Kunming, China). Glucose and mannitol were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). RPMI-1640 medium with glucose (5.6 mmol/l) was purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Fetal bovine serum (FBS) was purchased from HyClone (GE Healthcare, Chicago, IL, USA). Penicillin and streptomycin were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). 2′,7′-dichlorodihydrofluororescein diacetate (DCFH-DA), DAPI, LY294002, rapamycin and N-acetylcysteine (NAC) were obtained from Beyotime Institute of Biotechnology (Jiangsu, China). Cell Counting Kit (CCK)-8 was purchased from Invitrogen (Thermo Fisher Scientific, Inc.). AKT (catalog no. 9272), phosphorylated (p)-AKT (catalog no. 9611) and p-mTOR (catalog no. 2971) antibodies were purchased from Cell Signaling Technology Inc. (Danvers, MA, USA). mTOR (catalog no. 66888-1-Ig) and β-actin (catalog no. 60008-1-Ig) antibodies were obtained from ProteinTech Group, Inc. (Chicago, IL, USA). Horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary antibodies (catalog no. TA130004) and HRP-conjugated goat anti-rabbit secondary antibodies (catalog no. TA130023) were purchased from the OriGene Technologies, Inc. (Beijing, China).
Figure 1.
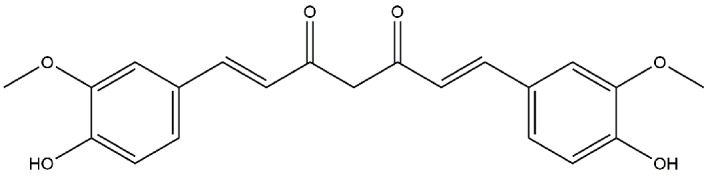
The chemical structure of curcumin; 1,7-Bis(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione.
Cell culture
ARPE-19 human RPECs were obtained from the Type Culture Collection of the Chinese Academy of Sciences (catalog no. CRL-4000; Shanghai, China). RPECs were cultured in RPMI-1640 medium supplemented with 10% FBS and 100 U/ml penicillin/streptomycin, and maintained at 37°C in a saturated humidified atmosphere with 5% CO2. Prior to experiments, RPECs were cultured in RPMI-1640 medium with 1% FBS at 37°C for 12 h, and glucose was added to the culture medium (30 mmol/l) for 0, 6, 12 and 24 h. To exclude the effects of high osmolarity on RPECs, 24.4 mmol/l mannitol was used as an equivalent osmolarity control to 30 mmol/l glucose. For specific inhibitors or antioxidant treatments, RPECs were incubated with LY294002 (1 µmol/l), rapamycin (10 µmol/l) or NAC (1 mmol/l) for 1 h at 37°C before high glucose treatment; and in CUR treatment experiments, RPECs were incubated with CUR for 1 h at 37°C prior to high glucose treatment. The concentration of LY294002 was selected based on our previous study (30), whereas the concentrations of rapamycin and NAC were determined according to previously reported methods (31).
CCK-8 assay
RPEC viability was measured using a CCK-8 method. Briefly, RPECs were seeded in 96-well plates at a density of 1–1.5×104 cells/ml. After culturing at 37°C for 0, 6, 12 or 24 h, 10 µl CCK-8 solution was added and the cells were incubated at 37°C for 4 h. Optical density (OD) was measured at 450 nm using a microplate reader (Molecular Devices, LLC, Sunnyvale, CA, USA). This experiment was replicated three times.
ELISA
RPECs were seeded in 96-well plates at a density of 1–1.5×104 cells/ml and incubated for 12 h at 37°C. Following incubation, the medium was collected and TNF-α (catalog no. ab181421), IL-1β (catalog no. ab100562) and IL-6 (catalog no. ab46027) content were measured by ELISA kits (Abcam, Cambridge, UK), according to the manufacturer's protocols. This experiment was replicated three times.
Detection of intracellular ROS
The intracellular ROS content of RPECs was examined following incubation with cell-permeable DCFH-DA, which is converted to fluorescent DCF in the presence of ROS. Briefly, cells were seeded in a 6-well plate at a density of 1–1.5×107 cells/ml. Following treatment with 30 mmol/l glucose or 24.4 mmol/l mannitol for 12 h, 10 µmol/l DCFH-DA and 1 µg/l DAPI in FBS-free RPMI-1640 were added and the plates were incubated for 20 min at 37°C. Cells were washed using RPMI-1640 with 10% FBS, and images were captured using a laser scanning confocal microscope (Olympus Corporation, Tokyo, Japan). The fluorescence intensity was measured in six random fields and analyzed using ImageJ software (version 1.4l; National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
RPECs in the various treatments cultured on 6-well plates were collected with pancreatin and lysed on ice for 30 min in Western and IP Cell Lysis buffer (catalog no. P0013J; Beyotime Institute of Biotechnology) supplemented with protease inhibitor cocktail and phosphatase inhibitors (150 µl). Protein levels were quantified using a Bicinchoninic Acid Protein Assay kit (catalog no. P0009; Beyotime Institute of Biotechnology). Protein extracts (50 µg) were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Following blocking with 10% skim milk overnight at 4°C, the membranes were incubated with primary antibodies against p-AKT (1:500), p-mTOR (1:600) or control β-actin (1:50,000) at 4°C for 12 h, washed three times with PBS, followed by incubation with HRP-conjugated secondary antibodies (1:2,000) for 2.5 h at room temperature. Each membrane was stripped and re-probed for its corresponding total protein, AKT (1:1,000) or mTOR (1:1,200). Protein bands were visualized using a chemiluminescence detection system (Pierce; Thermo Fisher Scientific, Inc.). Images were captured and analyzed using ImageJ software (version 1.41; National Institutes of Health, Bethesda, MD, USA); β-actin was used for normalization.
Statistical analysis
Statistical analysis was performed on GraphPad Prism 6.0 (GraphPad Software, Inc. La Jolla, CA, USA). All data are presented as the mean ± standard error of the mean of three independent experiments. Statistical analysis was performed by one-way analysis of variance, followed by the Tukey-Kramer post-test to determine any significant differences. P<0.05 was considered to indicate a statistically significant difference.
Results
CUR ameliorates glucose-induced toxicity in RPECs
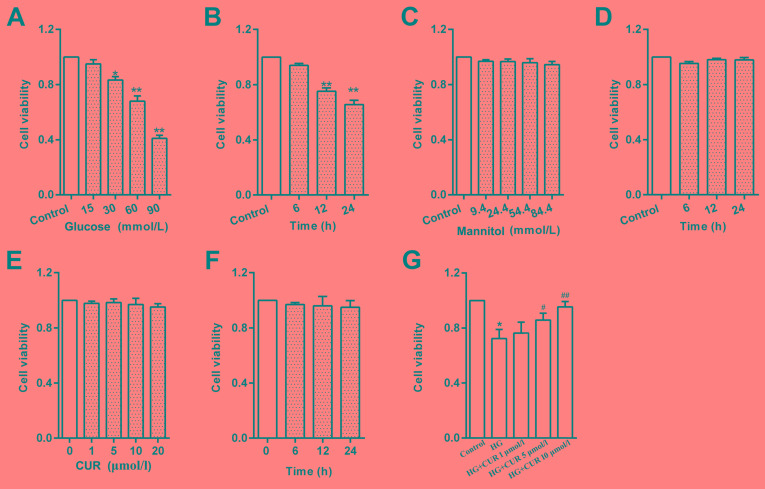
Cells treated with glucose for 12 h exhibited a significant reduction in viability in a concentration-dependent manner, compared with untreated Control cells (Fig. 2A). Treatment with 30 mmol/l glucose was considered high glucose group in subsequent experiments. To examine the effects of high glucose on RPECs, cells treated with 30 mmol/l glucose was set as high glucose group (HG), and the control equivalent osmolarity group was 24.4 mmol/l mannitol. Cells treated high glucose for 24 h exhibited a reduction in viability in a time-dependent manner (Fig. 2B). However, cells treated with various concentrations of mannitol (9.4–84.4 mmol/l) did not exhibit any effects on RPEC viability (Fig. 2C). Cells treated with 24.4 mmol/l mannitol for 2 h 4, which has considered the equivalent osmolarity to 30 mmol/l glucose, did not exhibit any effects on viability (Fig. 2D). These results suggested that the effect of high glucose on viability may not be attributed to high osmolarity. Cells treated with CUR (0–20 µmol/l) for 12 h did not exhibit any effects on RPECs viability (Fig. 2E); CUR also did not exhibit effects on viability in cells treated for 0–24 h with CUR at a concentration of 10 µmol/l (Fig. 2F). However, pretreatment with CUR (5 or 10 µmol/l) for 1 h increased the cell viability of RPECs cultured with high glucose (Fig. 2G). These data indicated that CUR may serve a pro-survival role under the high glucose condition by interfering with related signaling pathways, whereas without high glucose stimulation the Control group exhibited no obvious effects following CUR treatment.
Figure 2.
HG treatment reduces RPEC viability. (A) Effects of various glucose concentrations (0–90 mmol/l) on RPECs viability following 12 h incubation. (B) Effects of HG (30 mmol/l) on RPECs at varying incubation times (0–24 h). (C) Viability of RPECs incubated with different concentrations of mannitol (0–84.4 mmol/l) for 12 h. (D) Viability of RPECs treated with 24.4 mmol/l mannitol for 0–24 h. (E) Viability of RPECs incubated with different concentrations of CUR (0–20 µmol/l) for 12 h. (F) Viability of RPECs incubated with 10 µmol/l CUR for 0–24 h. (G) Viability of RPECs incubated with HG for 12 h, with or without 1 h pretreatment with 1, 5 or 10 µmol/l CUR. Data are presented as the mean ± standard error of the mean; n=6 independent experiments; *P<0.05 and **P<0.01 vs. Control; #P<0.05 and ##P<0.01 vs. HG. CUR, curcumin; HG, high glucose; RPEC, retinal pigment epithelial cell.
High glucose treatment induces RPEC secretion of IL-1β, IL-6 and TNF-α via the ROS/PI3K/AKT/mTOR signaling pathway
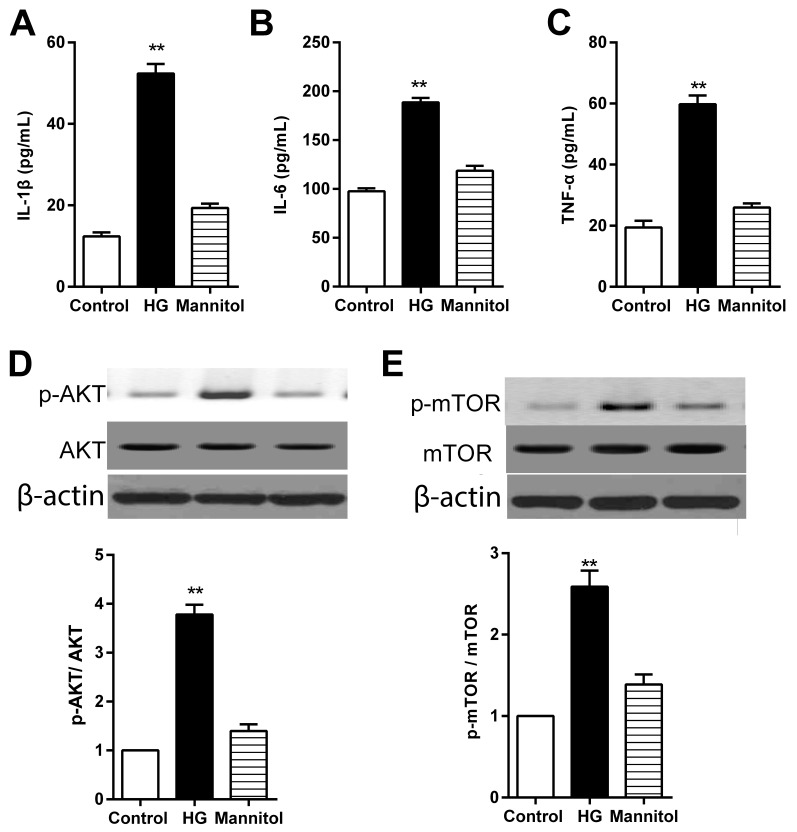
Expression levels of IL-1β, IL-6 and TNF-α in the culture medium were significantly higher in RPECs incubated in high glucose conditions compared with the respective secretion levels in the untreated Control cells (Fig. 3A-C, respectively); no significant differences in secretion levels were observed in cells treated with mannitol, which suggested that the high glucose-induced increase in IL-1β, IL-6 and TNF-α secretion of may not mediated by high osmolarity. The results from western blotting indicated that the protein expression levels of p-AKT and p-mTOR were significantly higher in RPECs treated with high glucose compared with the Control group (Fig. 3D and E, respectively), whereas mannitol treatment exhibited no significant effects.
Figure 3.
High glucose treatment increases the secretion levels of IL-1β, IL-6 and TNF-α in RPECs. (A-C) Expression levels of (A) IL-1β, (B) IL-6 and (C) TNF-α in the culture medium of cells treated with either 30 mmol/l glucose or 24.4 mmol/l mannitol for 12 h were measured by ELISA. (D and E) Expression levels of p-AKT, total AKT, p-mTOR and total mTOR in cells treated with either 30 mmol/l glucose or 24.4 mmol/l mannitol for 12 h as measured by western blot analysis. Data are presented as the mean ± standard error of the mean; n=3; **P<0.01 vs. control. CUR, curcumin; HG, high glucose; IL, interleukin; mTOR, mammalian target of rapamycin; p, phosphorylated; PI3K, phosphoinositide 3-kinase; RPEC, retinal pigment epithelial cell; TNF, tumor necrosis factor.
Cells treated with high glucose, but not mannitol, exhibited significantly increased intracellular ROS formation in RPECs (Fig. 4A). Pretreatment of RPECs with the antioxidant NAC (1 mmol/l) for 1 h inhibited the phosphorylation of AKT and mTOR (Fig. 4B and C). Furthermore, high glucose-induced increases in secretion of IL-1β, IL-6 and TNF-α were significantly decreased by pretreatment with NAC, PI3K inhibitor LY294002 (1 µmol/l) or the mTOR inhibitor rapamycin (10 µmol/l) (Fig. 4D-F). Taken together, these data suggested that high glucose exposure may have induced the secretion of TNF-α, IL-6 and IL-1β via the ROS/PI3K/AKT/mTOR signaling pathway in RPECs.
Figure 4.
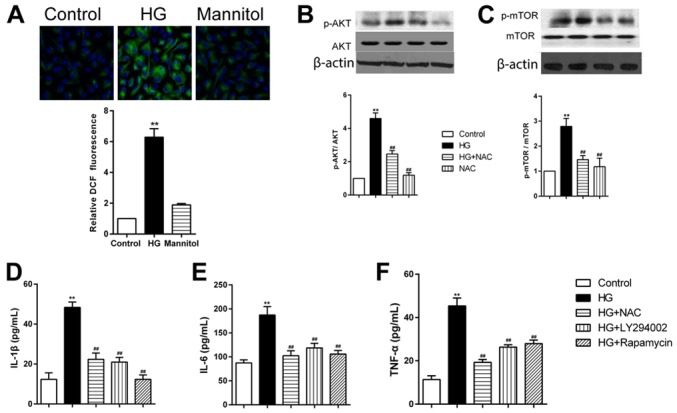
HG treatment increases cytokine secretion RPECs via the ROS/PI3K/AKT/mTOR signaling pathway. (A) ROS formation in cells treated with high glucose or mannitol for 12 h was measured by 2′,7′-dichlorodihydrofluororescein diacetate staining (green); nuclei were stained with DAPI (blue); magnification, ×600. (B and C) Expression levels of (B) p-AKT and total AKT, and (C) p-mTOR and total mTOR in cells treated with 30 mmol/l glucose and 1 mmol/l NAC, either alone or in combination, were measured by western blot analysis. (D-F) Expression levels of (D) IL-1β, (E) IL-6 and (F) TNF-α in the culture medium of cells treated with 30 mmol/l glucose alone or in combination with 1 mmol/l NAC, 1 µmol/l LY294002 or 10 µmol/l rapamycin were measured by ELISA. Data are presented as the mean ± standard error of the mean; **P<0.01 vs. Control; ##P<0.01 vs. HG. HG, high glucose; IL, interleukin; mTOR, mammalian target of rapamycin; NAC, N-acetylcysteine; p, phosphorylated; PI3K, phosphoinositide 3-kinase; ROS, reactive oxygen species; RPEC, retinal pigment epithelial cell; TNF, tumor necrosis factor.
CUR inhibits the high glucose-induced secretion of TNF-α, IL-6 and IL-1β through the ROS-AKT/mTOR cascade
High glucose treatment increased secretion of TNF-α, IL-6 and IL-1β in RPECs, which were significantly reduced in cells pretreated with 10 µmol/l CUR for 12 h (Fig. 5A-C). In addition, pretreatment with CUR significantly reduced the formation of intracellular ROS, in high glucose co-treated RPECs (Fig. 5D). High glucose-induced upregulation of p-AKT and p-mTOR expression levels were significantly reduces by pretreatment with 10 µmol/l CUR for 12 h (Fig. 5E and F). These results suggested that the CUR may inhibit the high glucose-induced secretion of TNF-α, IL-6 and IL-1β by interfering with the ROS-AKT/mTOR cascade in RPECs.
Figure 5.
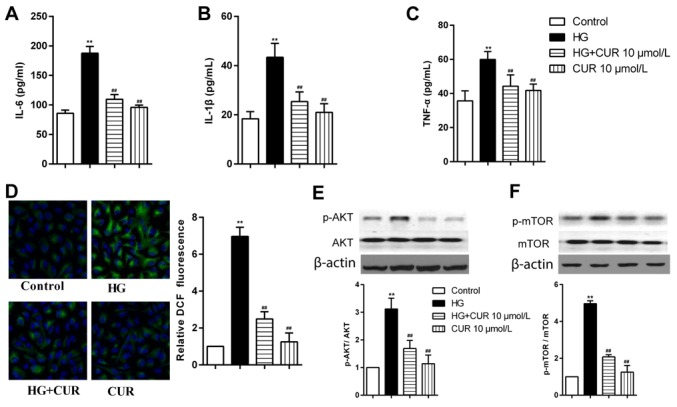
CUR treatment reduces the high glucose-induced secretion of IL-1β, IL-6 and TNF-α via the ROS/PI3K/AKT/mTOR signaling pathway in RPECs. (A-C) Expression levels of (A) IL-6, (B) IL-1β and (C) TNF-α in cells treated with 30 mmol/l glucose, 10 µmol/l CUR or their combination were measured by ELISA. (D) ROS formation in cells treated with high glucose, 10 µmol/l CUR or their combination for 12 h was measured by 2′,7′-dichlorodihydrofluororescein diacetate staining (green); nuclei were stained with DAPI (blue); magnification, ×600. (E and F) Expression levels of (E) p-AKT and AKT, and (F) p-mTOR and mTOR in cells treated with 30 mmol/l glucose, 10 µmol/l CUR or their combination were measured by western blot analysis. **P<0.01 vs. Control; ##P<0.01 vs. HG. CUR, curcumin; HG, high glucose; IL, interleukin; mTOR, mammalian target of rapamycin; p, phosphorylated; PI3K, phosphoinositide-3 kinase; ROS, reactive oxygen species; RPEC, retinal pigment epithelial cell; TNF, tumor necrosis factor.
Discussion
Hyperglycemia is a primary factor that contributes to retinal injury in the development of DR (32). RPECs serve many important functions in the retina, including phagocytosis of photoreceptor outer segments, isomerization of retinoids and various metabolic and neurotrophic support functions (33). RPEC dysfunction contributes to the pathogenesis of DR (34,35). Hyperglycemia induces inflammation and oxidative stress in the retina, eventually leading to apoptosis of RPECs (36,37). In addition, increased levels of inflammatory cytokines serve an important role in the pathogenic development of DR (38,39). It has been reported that high concentrations of glucose increase production of inflammatory cytokines in RPECs (40–42). Consistent with these reports, results from the present study indicated that high glucose treatment increased the secretion levels of TNF-α, IL-1β and IL-6 in RPECs. In addition, CUR co-treatment ameliorated these high glucose-induced secretion levels via the ROS/PI3K/AKT/mTOR signaling pathway. These results suggest that CUR may represent a promising therapeutic agent for the treatment of DR.
In the present study, high glucose exposure induced ROS production in RPECs. Previous studies reported that high levels of ROS are observed in chronic human diseases, such as atherosclerosis and other cardiovascular diseases (43–45). The unbalanced ROS generation or ROS elimination results in the presence of oxidative stress, which eventually leads to inflammatory responses (46). In hyperglycemic conditions, mitochondrial damage and endoplasmic reticulum stress have been reported to trigger injury of many retinal cells in the retina, and the activation of multiple inflammatory pathways and oxidative damage to RPECs contributes to the pathogenesis of DR (16,47). In the present study, high glucose concentrations induced intracellular ROS formation, whereas pretreatment with the antioxidant NAC significantly inhibited high glucose-induced secretion of IL-1β, IL-6 and TNF-α in RPECs. The results suggested that ROS contributed to glucose-induced secretion of inflammatory cytokines. The PI3K/AKT/mTOR signaling pathway is important in inflammation, and PI3K/AKT/mTOR signaling activation promotes expression of many pro-inflammatory cytokines (48,49). In the present study, high glucose treatment activated the PI3K/AKT/mTOR signaling pathway, which may have resulted in increased secretion of TNF-α, IL-1β and IL-6 in RPECs, but this requires further validation.
CUR, is a natural product of the rhizomes of Curcuma longa, and is widely used as an anti-oxidant and anti-inflammatory agent (50,51). CUR is reported to delay development of DR by inhibiting vascular endothelial growth factor and nuclear transcription factors (28). In addition, CUR can scavenge ROS, reduce degradation of anti-oxidant enzymes and reduce lipid peroxidation (52). In the present study, CUR treatment ameliorated the high glucose-induced ROS formation and the secretion of pro-inflammatory cytokines in RPECs. CUR was reported to exert its anticancer effects through the PI3K/AKT/mTOR signaling pathway (53,54). It was demonstrated in the present study that high glucose exposure increased the secretion of pro-inflammatory cytokines via the AKT/mTOR signaling pathway in RPECs, and CUR ameliorated these effects via the PI3K/AKT/mTOR cascade. However, the in vivo effects of CUR on DR remains to be further investigated.
In summary, high glucose exposure induced the secretion of TNF-α, IL-1β and IL-6, in RPECs via the ROS/PI3K/AKT/mTOR cascade. CUR inhibited the glucose-induced inflammation via interfering with the ROS/PI3K/AKT/mTOR cascade in RPECs. These findings confirmed the mechanism underlying the inflammatory effect of glucose in RPECs and suggested that CUR may represent a potential therapeutic strategy for glucose-induced inflammation.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National Science Foundation of China (grant no. 30973252).
Availability of data and materials
All data generated or analyzed during the present study are included in this published article. The data sets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
ZR and JM designed the present study. ZR analyzed and interpreted the results, and drafted the manuscript. YZ and XW performed experiments. JM edited the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chang LY, Lee AC, Sue W. Prevalence of diabetic retinopathy at first presentation to the retinal screening service in the greater Wellington region of New Zealand 2006–2015, and implications for models of retinal screening. N Z Med J. 2017;130:78–88. [PubMed] [Google Scholar]
- 2.Maugh TH., II Diabetic retinopathy: New ways to prevent blindness. Science. 1976;192:539–540. doi: 10.1126/science.192.4239.539. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed MS, Tahrani A. Diabetic retinopathy in cystic fibrosis related diabetes. Diabetologia. 2017;60:S472–S473. [Google Scholar]
- 4.Byberg S, Jorgensen ME, Larsen M, Lundandersen H, Vistisen D. Risk score for diabetic retinopathy. Diabetes. 2017;66:A161. [Google Scholar]
- 5.Bajestani NS, Kamyad AV, Esfahani EN, Zare A. Prediction of retinopathy in diabetic patients using type-2 fuzzy regression model. Eur J Oper Res. 2018;264:859–869. doi: 10.1016/j.ejor.2017.07.046. [DOI] [Google Scholar]
- 6.Zvornicanin J, Zvorničanin E. Imaging in diabetic retinopathy. Middle East Afr J Ophthalmol. 2015;22:531. doi: 10.4103/0974-9233.167809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasquel FJ, Hendrick AM, Ryan M, Cason E, Ali MK, Narayan KM. Cost-effectiveness of different diabetic retinopathy screening modalities. J Diabetes Sci Technol. 2015;10:301–307. doi: 10.1177/1932296815624109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jenkins AJ, Joglekar MV, Hardikar AA, Keech AC, O'Neal DN, Januszewski AS. Biomarkers in diabetic retinopathy. Rev Diabet Stud. 2015;12:159–195. doi: 10.1900/RDS.2015.12.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurley B. Therapeutic revolution in the management of diabetic retinopathy. Can J Ophthalmol. 2017;52(Suppl 1):S1–S2. doi: 10.1016/j.jcjo.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Vaziri K, Schwartz SG, Relhan N, Kishor KS, Flynn HW., Jr New therapeutic approaches in diabetic retinopathy. Rev Diabet Stud. 2015;12:196–210. doi: 10.1900/RDS.2015.12.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rangasamy S, McGuire PG, Das A. Diabetic retinopathy and inflammation: Novel therapeutic targets. Middle East Afr J Ophthalmol. 2012;19:52–59. doi: 10.4103/0974-9233.92116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, Schraermeyer U, Kociok N, Fauser S, Kirchhof B, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 14.Abcouwer SF, Antonetti DA. A role for systemic inflammation in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54:2384. doi: 10.1167/iovs.13-11977. [DOI] [PubMed] [Google Scholar]
- 15.Behl T, Kaur I, Kotwani A. Implication of oxidative stress in progression of diabetic retinopathy. Surv Ophthalmol. 2016;61:187–196. doi: 10.1016/j.survophthal.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Kowluru RA, Mishra M. Oxidative stress, mitochondrial damage and diabetic retinopathy. Biochim Biophys Acta. 2015;1852:2474–2483. doi: 10.1016/j.bbadis.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Kowluru RA, Kowluru A, Mishra M, Kumar B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res. 2015;48:40–61. doi: 10.1016/j.preteyeres.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calderon GD, Juarez OH, Hernandez GE, Punzo SM, De la Cruz ZD. Oxidative stress and diabetic retinopathy: Development and treatment. Eye (Lond) 2017;31:1122–1130. doi: 10.1038/eye.2017.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choudhury AK, Raja S, Mahapatra S, Nagabhushanam K, Majeed M. Synthesis and evaluation of the anti-oxidant capacity of curcumin glucuronides, the major curcumin metabolites. Antioxidants (Basel) 2015;4:750–767. doi: 10.3390/antiox4040750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandal MN, Patlolla JM, Zheng L, Agbaga MP, Tran JT, Wicker L, Kasus-Jacobi A, Elliott MH, Rao CV, Anderson RE. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic Biol Med. 2009;46:672–679. doi: 10.1016/j.freeradbiomed.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xie M, Fan D, Zhao Z, Li Z, Li G, Chen Y, He X, Chen A, Li J, Lin X, et al. Nano-curcumin prepared via supercritical: Improved anti-bacterial, anti-oxidant and anti-cancer efficacy. Int J Pharm. 2015;496:732–740. doi: 10.1016/j.ijpharm.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Baghdasaryan A, Claudel T, Kosters A, Gumhold J, Silbert D, Thüringer A, Leski K, Fickert P, Karpen SJ, Trauner M. Curcumin improves sclerosing cholangitis in Mdr2-/- mice by inhibition of cholangiocyte inflammatory response and portal myofibroblast proliferation. Gut. 2010;59:521–530. doi: 10.1136/gut.2009.186528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bisht S, Khan MA, Bekhit M, Bai H, Cornish T, Mizuma M, Rudek MA, Zhao M, Maitra A, Ray B, et al. A polymeric nanoparticle formulation of curcumin (NanoCurc™) ameliorates CCl4-induced hepatic injury and fibrosis through reduction of pro-inflammatory cytokines and stellate cell activation. Lab Invest. 2011;91:1383–1395. doi: 10.1038/labinvest.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Y, Gao R, Cao Y, Guo M, Wei Z, Zhou E, Li Y, Yao M, Yang Z, Zhang N. Curcumin attenuates inflammatory responses by suppressing TLR4-mediated NF-κB signaling pathway in lipopolysaccharide-induced mastitis in mice. Int Immunopharmacol. 2014;20:54–58. doi: 10.1016/j.intimp.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 25.Das L, Vinayak M. Anti-carcinogenic action of curcumin by activation of antioxidant defence system and inhibition of NF-κB signalling in lymphoma-bearing mice. Biosci Rep. 2012;32:161–170. doi: 10.1042/BSR20110043. [DOI] [PubMed] [Google Scholar]
- 26.Heng MC. Curcumin targeted signaling pathways: Basis for anti-photoaging and anti-carcinogenic therapy. Int J Dermatol. 2010;49:608–622. doi: 10.1111/j.1365-4632.2010.04468.x. [DOI] [PubMed] [Google Scholar]
- 27.Samuels TL, Pearson AC, Wells CW, Stoner GD, Johnston N. Curcumin and anthocyanin inhibit pepsin-mediated cell damage and carcinogenic changes in airway epithelial cells. Ann Otol Rhinol Laryngol. 2013;122:632–641. [PubMed] [Google Scholar]
- 28.Aldebasi YH, Aly SM, Rahmani AH. Therapeutic implications of curcumin in the prevention of diabetic retinopathy via modulation of anti-oxidant activity and genetic pathways. Int J Physiol Pathophysiol Pharmacol. 2013;5:194–202. [PMC free article] [PubMed] [Google Scholar]
- 29.Steigerwalt R, Nebbioso M, Appendino G, Belcaro G, Ciammaichella G, Cornelli U, Luzzi R, Togni S, Dugall M, Cesarone MR, et al. Meriva®, a lecithinized curcumin delivery system, in diabetic microangiopathy and retinopathy. Panminerva Med. 2012;54:11–16. [PubMed] [Google Scholar]
- 30.Su Q, Wang Y, Yang X, Li XD, Qi YF, He XJ, Wang YJ. Inhibition of endoplasmic reticulum stress apoptosis by estrogen protects human umbilical vein endothelial cells through the PI3 Kinase-Akt signaling pathway. J Cell Biochem. 2017;118:4568–4574. doi: 10.1002/jcb.26120. [DOI] [PubMed] [Google Scholar]
- 31.Mao N, Cheng Y, Shi XL, Wang L, Wen J, Zhang Q, Hu QD, Fan JM. Ginsenoside Rg1 protects mouse podocytes from aldosterone-induced injury in vitro. Acta Pharmacol Sin. 2014;35:513–522. doi: 10.1038/aps.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahsan H. Diabetic retinopathy-biomolecules and multiple pathophysiology. Diabetes Metab Syndr. 2015;9:51–54. doi: 10.1016/j.dsx.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 33.White C, DiStefano T, Olabisi R. The influence of substrate modulus on retinal pigment epithelial cells. J Biomed Mater Res A. 2017;105:1260–1266. doi: 10.1002/jbm.a.35992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okamura N, Ito Y, Shibata MA, Ikeda T, Otsuki Y. Fas-mediated apoptosis in human lens epithelial cells of cataracts associated with diabetic retinopathy. Med Electron Microsc. 2002;35:234–241. doi: 10.1007/s007950200027. [DOI] [PubMed] [Google Scholar]
- 35.Zhou W, Yu W, Xie W, Huang L, Xu Y, Li X. The role of SLIT-ROBO signaling in proliferative diabetic retinopathy and retinal pigment epithelial cells. Mol Vis. 2011;17:1526–1536. [PMC free article] [PubMed] [Google Scholar]
- 36.Kim DI, Park MJ, Lim SK, Choi JH, Kim JC, Han HJ, Kundu TK, Park JI, Yoon KC, Park SW, et al. High-glucose-induced CARM1 expression regulates apoptosis of human retinal pigment epithelial cells via histone 3 arginine 17 dimethylation: Role in diabetic retinopathy. Arch Biochem Biophys. 2014;560:36–43. doi: 10.1016/j.abb.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 37.Song MK, Roufogalis BD, Huang TH. Reversal of the caspase-dependent apoptotic cytotoxicity pathway by taurine from Lycium barbarum (Goji Berry) in human retinal pigment epithelial cells: Potential benefit in diabetic retinopathy. Evid Based Complement Alternat Med. 2012;2012:323784. doi: 10.1155/2012/323784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaštelan S, Tomić M, Gverović Antunica A, Salopek Rabatić J, Ljubić S. Inflammation and pharmacological treatment in diabetic retinopathy. Mediators Inflamm. 2013;2013:213130. doi: 10.1155/2013/213130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.El-Asrar AM. Role of inflammation in the pathogenesis of diabetic retinopathy. Middle East Afr J Ophthalmol. 2012;19:70–74. doi: 10.4103/0974-9233.92118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun J, Huang P, Liang J, Li J, Shen M, She X, Feng Y, Luo X, Liu T, Sun X. Cooperation of Rel family members in regulating Aβ1-40-mediated pro-inflammatory cytokine secretion by retinal pigment epithelial cells. Cell Death Dis. 2017;8:e3115. doi: 10.1038/cddis.2017.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh M, Tyagi SC. Homocysteine mediates transcriptional changes of the inflammatory pathway signature genes in human retinal pigment epithelial cells. Int J Ophthalmol. 2017;10:696–704. doi: 10.18240/ijo.2017.05.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kutty RK, Samuel W, Duncan T, Postnikova O, Jaworski C, Nagineni CN, Redmond TM. Proinflammatory cytokine interferon-γ increases the expression of BANCR a long non-coding RNA, in retinal pigment epithelial cells. Cytokine. 2018;104:147–150. doi: 10.1016/j.cyto.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118:1808–1829. doi: 10.1161/CIRCRESAHA.116.306923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu T, Peng Y, Yan S, Li N, Chen Y, Lan T. Andrographolide ameliorates atherosclerosis by suppressing pro-inflammation and ROS generation-mediated foam cell formation. Inflammation. 2018;41:1681–1689. doi: 10.1007/s10753-018-0812-9. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Tabas I. Emerging roles of mitochondria ROS in atherosclerotic lesions: Causation or association? J Atheroscler Thromb. 2014;21:381–390. doi: 10.5551/jat.23929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kanellakis P, Pomilio G, Walker C, Husband A, Huang JL, Nestel P, Agrotis A, Bobik A. A novel antioxidant 3,7-dihydroxy-isoflav-3-ene (DHIF) inhibits neointimal hyperplasia after vessel injury attenuating reactive oxygen species and nuclear factor-kappaB signaling. Atherosclerosis. 2009;204:66–72. doi: 10.1016/j.atherosclerosis.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Chen W, Zhao M, Zhao S, Lu Q, Ni L, Zou C, Lu L, Xu X, Guan H, Zheng Z, Qiu Q. Activation of the TXNIP/NLRP3 inflammasome pathway contributes to inflammation in diabetic retinopathy: A novel inhibitory effect of minocycline. Inflamm Res. 2017;66:157–166. doi: 10.1007/s00011-016-1002-6. [DOI] [PubMed] [Google Scholar]
- 48.Wu X, Long L, Liu J, Zhang J, Wu T, Chen X, Zhou B, Lv TZ. Gambogic acid suppresses inflammation in rheumatoid arthritis rats via PI3K/Akt/mTOR signaling pathway. Mol Med Rep. 2017;16:7112–7118. doi: 10.3892/mmr.2017.7459. [DOI] [PubMed] [Google Scholar]
- 49.Choi YH, Jin GY, Li LC, Yan GH. Inhibition of protein kinase C delta attenuates allergic airway inflammation through suppression of PI3K/Akt/mTOR/HIF-1 alpha/VEGF pathway. PLoS One. 2013;8:e81773. doi: 10.1371/journal.pone.0081773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber WM, Hunsaker LA, Abcouwer SF, Deck LM, Vander Jagt DL. Anti-oxidant activities of curcumin and related enones. Bioorg Med Chem. 2005;13:3811–3820. doi: 10.1016/j.bmc.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 51.Edwards RL, Luis PB, Varuzza PV, Joseph AI, Presley SH, Chaturvedi R, Schneider C. The anti-inflammatory activity of curcumin is mediated by its oxidative metabolites. J Biol Chem. 2017;292:21243–21252. doi: 10.1074/jbc.RA117.000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fazal Y, Fatima SN, Shahid SM, Mahboob T. Effects of curcumin on angiotensin-converting enzyme gene expression, oxidative stress and anti-oxidant status in thioacetamide-induced hepatotoxicity. J Renin Angiotensin Aldosterone Syst. 2015;16:1046–1051. doi: 10.1177/1470320314545777. [DOI] [PubMed] [Google Scholar]
- 53.Tian B, Zhao Y, Liang T, Ye X, Li Z, Yan D, Fu Q, Li Y. Curcumin inhibits urothelial tumor development by suppressing IGF2 and IGF2-mediated PI3K/AKT/mTOR signaling pathway. J Drug Target. 2017;25:626–636. doi: 10.1080/1061186X.2017.1306535. [DOI] [PubMed] [Google Scholar]
- 54.Cianciulli A, Calvello R, Porro C, Trotta T, Salvatore R, Panaro MA. PI3k/Akt signalling pathway plays a crucial role in the anti-inflammatory effects of curcumin in LPS-activated microglia. Int Immunopharmacol. 2016;36:282–290. doi: 10.1016/j.intimp.2016.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the present study are included in this published article. The data sets used and/or analyzed during the present study are available from the corresponding author on reasonable request.