Abstract
Background
Oxidized low-density lipoprotein (ox-LDL) causes vascular endothelial cell inflammatory response and apoptosis and plays an important role in the development and progression of atherosclerosis. Ginsenoside compound K (CK), a metabolite produced by the hydrolysis of ginsenoside Rb1, possesses strong anti-inflammatory effects. However, whether or not CK protects ox-LDL-damaged endothelial cells and the potential mechanisms have not been elucidated.
Methods
In our study, cell viability was tested using a 3-(4, 5-dimethylthiazol-2yl-)-2,5-diphenyl tetrazolium bromide (MTT) assay. Expression levels of interleukin-6, monocyte chemoattractant protein-1, tumor necrosis factor-α, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 were determined by enzyme-linked immunosorbent assay and Western blotting. Mitochondrial membrane potential (ΔΨm) was detected using JC-1. The cell apoptotic percentage was measured by the Annexin V/ propidium iodide (PI) assay, lactate dehydrogenase, and caspase-3 expression. Apoptosis-related proteins, nuclear factor (NF)-κB, and mitogen-activated protein kinases (MAPK) signaling pathways protein expression were quantified by Western blotting.
Results
Our results demonstrated that CK could ameliorate ox-LDL-induced human umbilical vein endothelial cells (HUVECs) inflammation and apoptosis, NF-κB nuclear translocation, and the phosphorylation of p38 and c-Jun N-terminal kinase (JNK). Moreover, anisomycin, an activator of p38 and JNK, significantly abolished the anti-apoptotic effects of CK.
Conclusion
These results demonstrate that CK prevents ox-LDL-induced HUVECs inflammation and apoptosis through inhibiting the NF-κB, p38, and JNK MAPK signaling pathways. Thus, CK is a candidate drug for atherosclerosis treatment.
Keywords: apoptosis, ginsenoside compound K, human umbilical vein endothelial cells, inflammation, oxidized low-density lipoprotein
1. Introduction
Atherosclerosis, a chronic inflammatory disease, is one of the most widespread and dangerous cardiovascular diseases [1]. Endothelial dysfunction is an early pro-atherogenic process associated with various risk factors [2]. Oxidized low-density lipoprotein (ox-LDL) is a key inducer in endothelial injury, which stimulates endothelial cells inflammation, oxidative stress, and apoptosis [3]. Lectin-like ox-LDL receptor-1 (LOX-1), an ox-LDL-specific receptor in endothelial cells, is a major endothelial dysfunction marker [4], [5]. Moreover, ox-LDL damages endothelial cells through various complex mechanisms, which are involved in mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) signaling pathways [5], [6].
Ginseng, the root of Panax ginseng Meyer, an important traditional Chinese medicine, has been used for millennia in Asia. Ginsenosides, the main active components isolated from ginseng, possess multiple pharmacological activities. Ginsenoside compound K [CK; 20-O-D-glucopyranosyl-20(S)-protopanaxadiol; Fig. 1A] is a metabolite of ginsenoside Rb1 [7]. Recent in vitro and in vivo studies have shown that CK exerts various pharmacological properties [7], [8], [9], [10], [11], [12], [13], [14]. Moreover, it blocks tumor necrosis factor alpha (TNF-α) -induced monocyte–endothelia interaction and transmigration and thereby possesses anti-atherogenic activity [15]. Nevertheless, whether or not CK prevents human umbilical vein endothelial cells (HUVECs) from ox-LDL-induced inflammatory injury and the potential mechanisms have not been studied.
Fig. 1.
CK reduces ox-LDL-induced cytotoxicity. (A) Molecular structure of CK. (B) HUVECs were incubated with CK alone (0.625μM, 1.25μM, and 2.5 μM) for 12 h, and cell viability was assayed by the MTT assay. (C) HUVECs were pretreated with various concentrations of CK (0.625μM, 1.25μM, and 2.5μM) for 12 h and incubated with ox-LDL (80 μg/mL) for an additional 24 h. Then, cell viability was assessed by the MTT assay. Values are expressed as mean ± SD, n = 3. ##p < 0.01 versus control group; ∗∗p < 0.01 versus ox-LDL group. CK, compound K; HUVECs, human umbilical vein endothelial cells; MTT, 3-(4, 5-dimethylthiazol-2yl-)-2,5-diphenyl tetrazolium bromide; NS, no significance; ox-LDL, oxidized low-density lipoprotein; SD, standard deviation.
The current study explored the protective effects and mechanisms of CK against HUVECs inflammatory responses and apoptosis induced by ox-LDL. Results showed that CK significantly ameliorated the inflammation and apoptosis of endothelial cells. Moreover, CK pretreatment downregulated the expression of LOX-1 and reduced the activation of p38, c-Jun N-terminal kinase (JNK) MAPK, and NF-κB signaling pathways. Thus, our study demonstrates that CK protects HUVECs against ox-LDL-induced injury partly via LOX-1-dependent NF-κB, p38, and JNK MAPK signaling pathways.
2. Materials and methods
2.1. Ethics statement
The research method was permitted by the Ethics Committee of Peking Union Medical College (SYXK-2013-0023, Beijing, China) and was administrated in accordance with the Declaration of Helsinki.
2.2. Materials
CK (molecular weight, 622.87; purity, 99%) was acquired from Shanghai Winherb Medical Science Co., Ltd (Shanghai, China). Ox-LDL (by copper ion-induced LDL oxidation, malondialdehyde > 40 nmoL/mL) was acquired from Peking Union-Biology Co., Ltd. (Beijing, China). VascuLife Basal Medium was acquired from Lifeline Cell Technology (Carlsbad, CA, USA). Dimethylsulfoxide (DMSO), and 3-(4, 5-dimethylthiazol-2yl-)-2,5-diphenyl tetrazolium bromide (MTT) were acquired from Sigma-Aldrich (St. Louis, MO, USA). The annexin V/ propidium iodide (PI) staining kit was acquired from Invitrogen (Eugene, OR, USA). JC-1 fluorescent dye was acquired from Invitrogen (Eugene, OR, USA). Lactate dehydrogenase (LDH) detection kit was acquired from Nanjing Jiancheng Institute of Biological Engineering (Nanjing, China). Caspase-3 activity kit was acquired from BioVision (Milpitas, CA, USA). Human interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), and TNF-α enzyme-linked immunosorbent assay (ELISA) kits were obtained from BioLegend (CA, USA). Primary antibodies against p-IκBα, p-JNK, p-P38, p-ERK, p-IKKβ, IκBα, JNK, P38, and ERK were obtained from Cell Signaling Technology (Beverly, MA, USA). Vascular cell adhesion molecule-1 (VCAM-1), NF-κB, intercellular adhesion molecule-1 (ICAM-1), Bax, and Bcl2 were derived from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Caspase 3, cyt C, histone H3, and Lox-1 were acquired from Abcam (Cambridge, England). Anisomycin was acquired from Selleckchem (Houston, TX, USA).
2.3. HUVEC culture and treatment
The donated neonate cords were derived from the Maternal and Child Care Service Centre in Beijing, China. The study protocol was explained, and written informed consents were obtained from all the participating donors. Collagenase I (0.1%) was used to isolate HUVECs from fresh human umbilical veins according to a previous study [16]. Briefly, the isolated cells were cultured using the Vasculife complete medium combined with streptomycin (100 μg/mL) and penicillin (100 U/mL). After 24 h, the adherent cells were washed with phosphate-buffered saline (PBS), and fresh medium was added. Then, the cells were continuously incubated at 37°C and 5% CO2. HUVECs from passages 2 to 5 were used for the experiments. CK was dissolved in DMSO as the stock solution. HUVECs were treated with CK (0.625μM, 1.25μM, and 2.5μM) for 12 h, and 80 μg/mL ox-LDL for additional 24 h.
2.4. Cell viability measurement
MTT assay was used to detect cell viability. Briefly, the cells were cultured on 96-well cell culture plates for 24 h and then incubated with different concentrations of CK for 12 h before exposure to 80 μg/mL ox-LDL. To measure the cell viability, 1 mg/mL MTT assay solution was added before incubation for another 4 h. After removing the supernatant, 150 μL DMSO was added to dissolve the formazan crystals. Next, the absorbance was determined by a microplate reader (Tecan, Switzerland) at 570 nm. The ratio of living cells was analyzed by the ratio of optical density compared with that of normal cells.
2.5. Mitochondrial membrane potential determination
Cells were pretreated with or without different concentrations of CK (0.625μM, 1.25μM, 2.5μM) for 12 h and then exposed to 80 μg/mL ox-LDL for another 24 h. After the treatments, the cells were cleaned twice with PBS, and 2μM JC-1 was loaded. The cells were incubated for 30 min in the dark at 37°C, cleaned twice with PBS, and then photographed under a fluorescence microscope (Carlsbad, CA, EVOS FL Color, Life Technologies).
2.6. FITC-Annexin V/PI assay
HUVEC apoptosis was determined using an FITC-Annexin V/PI kit in compliance with the manufacturer's instructions. Cells were treated with or without different concentrations of CK (0.625μM, 1.25μM, 2.5μM) for 12 h, followed by 80 μg/mL ox-LDL for another 24 h. The cells were acquired and cleaned thrice with PBS; then, 5 μL of FITC-Annexin V was dyed for 30 min, and 1 μL of PI working solution (100 μg/mL) was added for 5 min in the dark at room temperature. Apoptotic cells were measured using a flow cytometer (FACSCalibur, BD Biosciences, CA, USA).
2.7. LDH release detection
HUVECs were pre-incubated with different concentrations of CK (0.625μM, 1.25μM, and 2.5μM) for 12 h before exposing to ox-LDL. After incubation for 24 h, the supernatant was collected to test LDH release using the LDH assay kit based on the manufacturer's brochures.
2.8. Caspase-3 activation analysis
The caspase-3 activity was detected by a fluorometric assay kit in accordance with the manufacturer's descriptions. In brief, the cells were pretreated with different concentrations of CK (0.625μM, 1.25μM, 2.5μM) for 12 h, followed by 80 μg/mL ox-LDL for additional 24 h. The cells were incubated with 50 μL iced cell lysate buffer for 10 min. Then, the cells were incubated with caspase-3 substrate (DEVD-AFC, 1μM) at 37°C for 2 h. The fluorescence intensity was read at wavelengths of 400 nm excitation and 505 nm emission using a microplate reader (Tecan, Switzerland). The results are calculated as fold changes compared with the control group.
2.9. Immunofluorescence assay
NF-κB translocation was determined using immunofluorescence assay. The cells were incubated in 24-well plates for 24 h and fixed with 4% paraformaldehyde (Sigma, St. Louis, MO, USA) for 10 min. After all treatments, the cells were cleaned twice with PBS, incubated in 0.1% Triton X-100 for 30 min and then washed twice. The goat serum was used to block the reaction for 1 h and subsequently incubated with mouse anti-NF-κB p65 primary antibody (1:100 dilution) overnight at 4°C. Subsequently, the cells were cleaned and incubated for 2 h with the FITC-conjugated anti-mouse IgG at a 1:100 dilution at room temperature. To visualize the nuclei, the cells were incubated with DAPI for 3 min. Images were photographed under a fluorescence microscope (EVOS FL Color, Life Technologies).
2.10. ELISA assay
HUVECs were seeded in 6-well plates at 2 × 105 cells/well. The cells were incubated with different concentrations of CK for 12 h. After incubation with ox-LDL for 24 h, the supernatant was collected to measure IL-6, MCP-1, and TNF-α levels with ELISA kits in accordance with the manufacturer's descriptions.
2.11. Western blot analysis
Protein expression levels were measured by Western blot assay. When the HUVECs reached 80% confluence of culture plates, the cells were incubated with CK (2.5μM) for 12 h, with or without anisomycin (1μM) for another 1 h. Then, cells were exposed to 80 μg/mL ox-LDL for additional 24 h. Total cellular, cytoplasmic, and nuclear proteins were extracted. The protein concentrations were measured by a BCA kit (Pierce Corporation, Rockford, IL, USA). Furthermore, 10% or 12% sodium dodecyl sulfate polyacrylamide gel was used to separate the protein sample through electrophoresis and then electrotransferred onto nitrocellulose membranes (Millipore Corporation, Bedford, MD, USA). Membranes were blocked for at least 2 h in 5% skim milk at room temperature and then incubated overnight with the primary antibodies at 4°C. After washing with Tris-buffered saline and Tween 20 for 30 min, the membranes were incubated with the horseradish peroxidase-conjugated secondary antibodies for 2 h at room temperature. The membranes were washed and developed with an enhanced chemiluminescence solution. The protein expression levels were calculated using the Gel Pro software (Media Cybernetics, Rockville, MD, USA).
2.12. Statistical analysis
Data were calculated as mean ± SD of at least three independent experiments. Statistical comparisons between various groups were performed using a one-way analysis of variance or the Tukey test with GraphPad Prism 5.0 (SPAA, Inc., Chicago, Illinois, USA). A value of p < 0.05 was considered to be statistically significant.
3. Results
3.1. CK prevented ox-LDL-induced HUVECs injury
First, we explored the ameliorative effects of CK against ox-LDL-damaged endothelial cells using MTT assay. As shown in Fig. 1B, the results revealed that treatments with various concentrations (0.625μM, 1.25μM, and 2.5μM) of CK alone for 12 h did not influence cell viability, indicating the nontoxicity of CK. Furthermore, the endothelial cell protective effect was assessed. As shown in Fig. 1C, pretreatment with CK for 12 h obviously increased cell viability in a concentration-dependent manner. These data suggest that CK can prevent ox-LDL-induced HUVECs injury. Also, 2.5μM CK concentration was used in the subsequent experiments.
3.2. CK inhibited inflammatory cytokine production in ox-LDL-treated HUVECs
Excessive inflammatory factors, such as MCP-1, and adhesion molecule, such as ICAM-1 are the features of early AS. Thus, the levels of inflammatory cytokines were determined by ELISA and Western blot analysis. The levels of IL-6, MCP-1, and TNF-α were significantly higher in the ox-LDL-treated cell culture supernatants than in the control group. CK pretreatment inhibited the expression of these cytokines by a dose-dependent manner (Fig. 2A–C). Moreover, as shown in Fig. 2D and E, CK inhibited the expression levels of VCAM-1 and ICAM-1, indicating that CK may directly reduce the adhesion molecule expression.
Fig. 2.
CK attenuates ox-LDL-induced HUVEC inflammation. (A–C) HUVECs were pretreated with various concentrations of CK (0.625μM, 1.25μM, and 2.5μM) for 12 h and incubated with or without ox-LDL (80 μg/mL) for an additional 24 h. The levels of IL-6, MCP-1 and TNF-α in the culture supernatant were assayed with enzyme-linked immunosorbent assay. (D) HUVECs were pretreated with CK (2.5μM) for 12 h, followed by treatment with ox-LDL (80 μg/mL) for another 24 h. VCAM-1, ICAM-1, and β-actin were evaluated by Western blot analysis. (E) Densitometric analysis was used to quantify the levels of VCAM-1 and ICAM-1. Values are expressed as mean ± SD, n = 3. #p < 0.05, ##p < 0.01 versus control group; ∗p < 0.05, ∗∗p < 0.01 versus ox-LDL group. CK, compound K; HUVECs, human umbilical vein endothelial cells; ICAM-1, intercellular adhesion molecule-1; IL-6, interleukin-6; MCP-1, monocyte chemoattractant protein-1; ox-LDL, oxidized low-density lipoprotein; SD, standard deviation; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cell adhesion molecule-1.
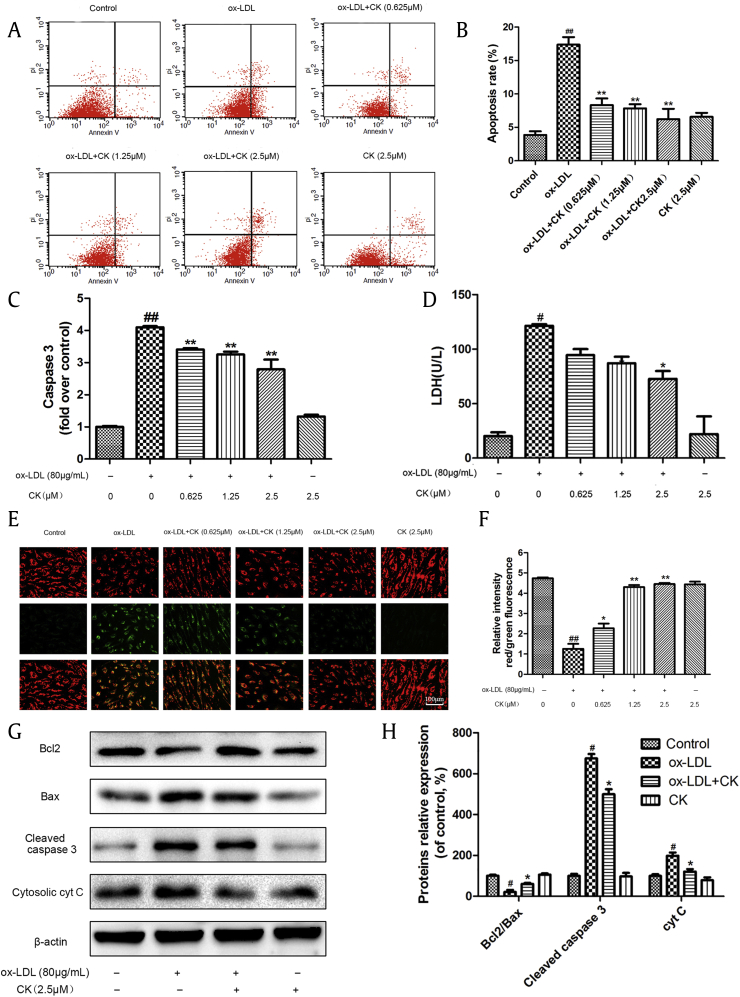
3.3. CK decreased ox-LDL-induced HUVECs apoptosis
Based on the above results, we next assessed the anti-apoptotic effects of CK. Phosphatidylserine externalization is a characteristic of cells suffering apoptosis. Annexin V/PI double staining was applied to detect the cell apoptosis. After 24 h stimulation with ox-LDL (80 μg/mL), the ratio of Annexin V/PI-labeled cells remarkably increased. Incubation with different CK concentrations sharply decreased the number of early apoptotic cells, whereas CK alone had no effects on HUVECs apoptosis compared with the control group (Fig. 3A and B).
Fig. 3.
CK inhibits ox-LDL-induced HUVECs apoptosis. HUVECs were treated with ox-LDL (80μg/mL) in the presence or absence of various concentrations of CK (0.625μM, 1.25μM, and 2.5μM) for 12h, and incubated with or without ox-LDL (80 μg/mL) for additional 24 h. (A) A scatter diagram of apoptotic HUVECs was detected through annexin V/PI double staining by flow cytometry. (B) Quantity analysis of the percentages of apoptotic cells. (C) Caspase-3 activity was measured using a fluorometric assay. (D) The effect of CK on LDH level in HUVECs was measured using an LDH assay kit. (E) Cells were dyed with JC-1 and then detected using a fluorescence microscope. (F) Quantitative analysis of JC-1 red/green rates. (G) HUVECs were treated with ox-LDL (80μg/mL) in the presence or absence of CK (2.5μM) for 12h, and incubated with or without ox-LDL (80 μg/mL) for additional 24 h. Bcl-2, Bax, cleaved caspase-3, cyt C, and β-actin were evaluated by Western blot analysis. (H) Densitometric analysis was used to quantify the levels of bcl-2, bax, cleaved caspase-3, and cyt C. Values are expressed as the mean ± SD, n = 3. #p < 0.05, ##p < 0.01 ox-LDL group versus control group; ∗p < 0.05, ∗∗p < 0.01 versus ox-LDL group. CK, compound K; HUVECs, human umbilical vein endothelial cells; LDH, lactate dehydrogenase; ox-LDL, oxidized low-density lipoprotein; SD, standard deviation; V/PI, Annexin V/ propidium iodide.
Caspase-3, an effect molecule, mediates cell apoptosis. As indicated in caspase-3 fluorometric assay (Fig. 3C), CK treatment sharply decreased the caspase-3 expression. LDH release indicates endothelial injury. As shown in Fig. 3D, in the ox-LDL-treated group, LDH release was increased compared with the control group, whereas CK treatment markedly decreased. The protective effects of CK against ox-LDL-induced cytotoxicity were similar to those determined by the MTT assay.
The mitochondrial membrane potential (MMP; ΔΨm) disruption is an early event in the apoptotic cascade. To further investigate the effects of CK on MTP, a JC-1 assay was used to evaluate MMP depolarization. We found that MMP was depolarized in the cells treated with ox-LDL through decreasing the ratio of red and green fluorescence compared with the control group. However, different CK concentrations pretreatment significantly reversed these effects (Fig. 3E and F).
Next, we determined the expression levels of apoptosis-related proteins. As shown in Fig. 3G, CK pre-incubation decreased Bcl-2 downregulation and Bax upregulation in the ox-LDL-exposed HUVECs. Caspase-3 is an essential execution of apoptosis in ox-LDL-exposed HUVECs. As shown in Fig. 3G and H, ox-LDL remarkably increased the cleaved caspase-3 expression in HUVECs; however, this effect was restrained by CK incubation. Furthermore, CK pretreatment inhibited the cytosolic cyt C expression when exposed to ox-LDL.
3.4. CK mitigated ox-LDL-induced HUVECs NF-κB translocation
The NF-κB translocation in HUVECs is activated after endothelial dysfunction during atherosclerosis. First, the immunofluorescence assay showed that CK could significantly inhibit ox-LDL-augmented p65 translocation from the cytoplasm to the nucleus (Fig. 4A). As shown in Fig. 4B, NF-κB p65 increased in the nucleus and decreased in the cytoplasm after ox-LDL treatment. However, CK treatment significantly decreased the p65 translocation to the nucleus and reduced IκBα and IKKβ phosphorylation as opposed to ox-LDL. Moreover, CK obviously ameliorated LOX-1 expression.
Fig. 4.
CK reduces ox-LDL-induced HUVECs inflammation through inhibiting the NF-κB pathway. (A) HUVECs were pretreated with CK (2.5μM) for 12 h, followed by treatment with ox-LDL (80 μg/mL) for another 24 h. NF-κB p65 immunoreactivity was observed by immunofluorescence assay. (B) HUVECs were treated as described in (A). LOX-1, p-IKKβ, p-IκBα, IκBα, NF-κB p65 (nuclear), NF-κB p65 (cytoplasm), NF-κB p65, Histone H3, and β-actin were evaluated by Western blot analysis. (C) Densitometric analysis was used to quantify the levels of LOX-1, p-IKKα/β, p-IκB and IκB. (D) Densitometric analysis was used to quantify the levels of NF-κB p65. Values are expressed as the mean ± SD, n = 3. #p < 0.05, ##p < 0.01 versus control group; ∗p < 0.05, ∗∗p < 0.01 versus ox-LDL group. CK, compound K; DAPI, 4′,6-diamidino-2-phenylindole; HUVECs, human umbilical vein endothelial cells; LOX-1, lectin-like ox-LDL receptor-1; NF-κB, nuclear factor-κB; ox-LDL, oxidized low-density lipoprotein; SD, standard deviation.
3.5. CK modulated the MAPK pathway in ox-LDL-treated HUVECs
Next, we investigated the protective mechanism of CK against ox-LDL-induced endothelial cell apoptosis. The MAPK signaling pathway is important in endothelial cell inflammation and apoptosis; therefore, we tested the expression levels of p38, JNK, and extracellular signal-regulated protein kinases 1 and 2 (ERK1/2) activation. As shown in Fig. 5A and B, CK pretreatment notably ameliorated ox-LDL-induced p38 and JNK phosphorylation. Interestingly, CK exerted no effect on ERK1/2 phosphorylation. To assess whether the protective effect of CK is related to p38 and JNK inhibition, we pretreated HUVECs with anisomycin, a p38 and JNK activator. Results showed that the inhibitive effect of CK was remarkably abolished by anisomycin pretreatment (Fig. 5C and D). Moreover, we also measured cell viability upon anisomycin pre-incubation. As shown in Fig. 6A, the cell viability was reduced by anisomycin compared with the CK group. We further detected the apoptosis-related protein expression; anisomycin pretreatment significantly downregulated the anti-apoptosis protein Bcl-2 and upregulated the pro-apoptosis proteins Bax, Caspase 3, and cyt C expression levels compared with the CK group (Fig. 6B and C). Taken together, CK protects ox-LDL-induced endothelial cells apoptosis via the inhibition of NF-κB, p38, and JNK MAPK pathways.
Fig. 5.
CK prevents ox-LDL-induced HUVECs apoptosis through inhibiting the MAPK pathway. (A) HUVECs were pretreated with CK (2.5μM) for 12 h, followed by treatment with ox-LDL (80 μg/mL) for another 24 h. The expression levels of phosphorylated and total ERK1/2, p38, and JNK were detected by Western blot analysis. (B) Densitometric analysis was used to quantify the ratios of phospho-p38 to total p38, p-JNK to total JNK, p-ERK1/2 to total ERK1/2. (C) HUVECs were treated with CK (2.5μM) in the presence or absence of anisomycin (1μM) for 1 h, followed by treatment with ox-LDL (80 μg/mL) for another 24 h. Representative Western blot analysis of phosphorylated and total p38, and JNK in HUVECs was performed. (D) Densitometric analysis was used to quantify the levels of p-p38, p-JNK, and p-ERK1/2. Values are expressed as mean ± SD, n = 3. #p < 0.05, ##p < 0.01 versus control group; ∗p < 0.05, ∗∗p < 0.01 versus ox-LDL group; $p < 0.05, $$p < 0.01 versus ox-LDL and CK treatment group. AM, anisomycin; CK, compound K; ERK1/2, extracellular signal-regulated protein kinases 1 and 2; HUVECs, human umbilical vein endothelial cells; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-κB; ox-LDL, oxidized low-density lipoprotein; SD, standard deviation.
Fig. 6.
Anisomycin reverses the anti-apoptotic activity of CK. (A) HUVECs were treated with CK (2.5μM) in the presence or absence of anisomycin (1μM) for 1 h, followed by treatment with ox-LDL (80 μg/mL) for another 24 h. Cell viability was detected by the MTT assay. (B) HUVECs were treated as described in (A), and the expression levels of Bax, Bcl-2, cleaved caspase-3, and cyt C were detected by Western blot analysis. (C) Densitometric analysis was used to quantify the levels of Bax, Bcl-2, cleaved caspase-3, and cyt C. Values are expressed as the mean ± SD, n = 3. #p < 0.05, ##p < 0.01 versus control group; ∗p < 0.05, ∗∗p < 0.01 versus ox-LDL group; $p < 0.05, $$p < 0.01 versus ox-LDL and CK treatment group. AM, anisomycin; CK, compound K; HUVECs, human umbilical vein endothelial cells; MTT, (4, 5-dimethylthiazol-2yl-)-2,5-diphenyl tetrazolium bromide; ox-LDL, oxidized low-density lipoprotein; SD, standard deviation.
4. Discussion
Atherosclerosis is a major risk factor for coronary heart disease events. It is generally accepted that the atherosclerosis progression is associated with chronic inflammation in the vessel wall [17]. Ox-LDL-injured endothelial cell is an initial step in atherosclerosis [18]. LOX-1, the specific receptor for ox-LDL in endothelial cells, contributes to the induction of endothelial dysfunction via several mechanisms, including MAPKs [19] and NF-κB [19], [20], [21] pathways. To the best of our knowledge, this study is the first to demonstrate the anti-inflammatory and anti-apoptotic roles of CK in ox-LDL-treated HUVECs. CK inhibited ox-LDL-induced HUVECs damage by decreasing pro-inflammatory protein expression and increasing anti-apoptotic protein expression through LOX-1-mediated NF-κB, p38, and JNK pathways.
When endothelial cells are damaged by ox-LDL, the secretion of various cytokines, such as ICAM-1, VCAM-1, and MCP-1 are elevated [22]. In the present study, CK decreased IL-6, MCP-1, TNF-α, VCAM-1, and ICAM-1 expression levels in the stimulated HUVECs. Our findings suggest that alleviation of the ox-LDL-stimulated dysfunction of HUVECs is attributable to the anti-inflammatory effects of CK.
Many studies have shown that LOX-1 mediates the pro-inflammatory effect of ox-LDL. Ox-LDL increases LOX-1 expression, which causes endothelial injuries [23], [24], [25]. In the current study, our results showed that CK opposed ox-LDL-induced LOX-1 expression. Moreover, the NF-κB signaling pathway is implicated in endothelial dysfunction, which regulates the expression of various chemokines and adhesion molecules [26]. Ox-LDL could aggravate IκBα degradation and phosphorylation as well as accelerate p65 translocation to the nucleus [20], [24]. Therefore, the anti-inflammatory effects of CK prompted us to further investigate the potential role on NF-κB activation. In our research, CK downregulated the ox-LDL-stimulated degradation and phosphorylation of IκBα, thereby inhibiting the NF-κB p65 activation. The phosphorylation of IκBα is regulated by the IKK complex, and the IKK/IκB/NF-κB pathway is involved in the palmitic acid-induced dysfunction of HUVECs [27]. In the present study, the p-IKKβ expression significantly increased in the stimulated HUVECs, whereas CK pre-treatment could reduce the expression of p-IKKβ. The above result further indicates that the alleviative effect of CK on the ox-LDL-stimulated dysfunction of HUVECs may be mediated via the LOX-1-dependent IKK/IκB/NF-κB signaling pathway.
Cell apoptosis plays a considerable role in the pathogenesis of atherosclerosis. Vascular endothelial cell apoptosis is critical for the development of atherosclerosis. Moreover, ox-LDL is a vital risk factor that can induce endothelial cell apoptosis [28], [29], [30]. Previous studies have demonstrated that ox-LDL causes endothelial apoptosis mainly through Bcl-2 and caspase-9 dependent way [28]. In addition, LOX-1 antibody can block the pro-apoptotic effects of ox-LDL [28]. These data demonstrate that LOX-1 plays an important role in ox-LDL-induced endothelial cell apoptosis. In the present study, CK significantly ameliorated ΔΨm and caspase-3 activity, reduced LDH release, and modulated the expression of apoptosis-related proteins. We first demonstrated that CK could ameliorate ox-LDL-induced HUVECs apoptosis.
MAPK signaling pathway, including p38, JNK, and ERK, serve various biological functions, including apoptosis. Previous studies have proved that ox-LDL could activate p38 [31] and JNK phosphorylation in endothelial cells [32]. Furthermore, JNK and p38 activations participated in ox-LDL-induced HUVECs apoptosis [28], [33]. Consequently, we hypothesized that the protective effects of CK against ox-LDL-induced apoptosis in HUVECs are related to MAPK signaling cascades. As expected, ox-LDL induced p38 and JNK phosphorylation which was inhibited by CK pretreatment. Interestingly, CK has no effect on ERK1/2 phosphorylation. To assess whether the protective effect of CK is related to p38 and JNK inhibition, we pretreated HUVECs with anisomycin, a p38 and JNK activator. Results showed that the protective effects of CK were abrogated by anisomycin pretreatment. Moreover, anisomycin pretreatment significantly increased the apoptosis-related proteins expression compared with the CK treatment group. The data showed that CK possessed the anti-apoptosis activity by inhibiting the p38 and JNK MAPK pathways in ox-LDL-induced HUVECs injuries (Fig. 7).
Fig. 7.
Hypothetical mechanism by which CK prevents ox-LDL-induced HUVECs injury. CK protects ox-LDL-induced HUVECs injury through LOX-1-mediated NF-κB, p-38, and JNK MAPK pathways. CK, compound K; ERK1/2, extracellular signal-regulated protein kinases 1 and 2; HUVECs, human umbilical vein endothelial cells; ICAM-1, intercellular adhesion molecule-1; IL-6, interleukin-6; JNK, c-Jun N-terminal kinase; LOX-1, lectin-like ox-LDL receptor-1; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein-1; NF-κB, nuclear factor-κB; ox-LDL, oxidized low-density lipoprotein; SD, standard deviation; TNF-α, tumor necrosis factor-α; VCAM-1, vascular cell adhesion molecule-1.
In summary, this study first demonstrated that CK exerts cytoprotective effects on endothelial cells stimulated by ox-LDL and elucidated the potential molecular mechanism. Results showed that CK can reduce the release of inflammatory cytokines and decrease the cell apoptosis induced by ox-LDL partly via inhibiting the NF-κB, p38, and JNK pathways.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant no. 81374011), the Major Scientific and Technological Special Project for “Significant New Drug Formulations” (Grant no. 2012ZX09501001-004), the Special Project for the national Traditional Chinese Medicine Industry of China (Grant no. 201507004).
Contributor Information
Guibo Sun, Email: sunguibo@126.com.
Xiaobo Sun, Email: sunpapersubmit@sina.com.
References
- 1.Siegel D., Devaraj S., Mitra A., Raychaudhuri S.P., Raychaudhuri S.K., Jialal I. Inflammation, atherosclerosis, and psoriasis. Clin Rev Allergy Immunol. 2013;44:194–204. doi: 10.1007/s12016-012-8308-0. [DOI] [PubMed] [Google Scholar]
- 2.Gutierrez E., Flammer A.J., Lerman L.O., Elizaga J., Lerman A., Fernandez-Aviles F. Endothelial dysfunction over the course of coronary artery disease. Eur Heart J. 2013;34:3175–3181. doi: 10.1093/eurheartj/eht351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matsuura E., Atzeni F., Sarzi-Puttini P., Turiel M., Lopez L.R., Nurmohamed M.T. Is atherosclerosis an autoimmune disease? BMC Med. 2014;12:47. doi: 10.1186/1741-7015-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chistiakov D.A., Orekhov A.N., Bobryshev Y.V. LOX-1-mediated effects on vascular cells in atherosclerosis. Cell Physiol Biochem. 2016;38:1851–1859. doi: 10.1159/000443123. [DOI] [PubMed] [Google Scholar]
- 5.Ogura S., Kakino A., Sato Y., Fujita Y., Iwamoto S., Otsui K., Yoshimoto R., Sawamura T. Lox-1: the multifunctional receptor underlying cardiovascular dysfunction. Circ J. 2009;73:1993–1999. doi: 10.1253/circj.cj-09-0587. [DOI] [PubMed] [Google Scholar]
- 6.Pirillo A., Norata G.D., Catapano A.L. LOX-1, OxLDL, and atherosclerosis. Mediators Inflamm. 2013;2013:152786. doi: 10.1155/2013/152786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chae S., Kang K.A., Chang W.Y., Kim M.J., Lee S.J., Lee Y.S., Kim H.S., Kim D.H., Hyun J.W. Effect of compound K, a metabolite of ginseng saponin, combined with gamma-ray radiation in human lung cancer cells in vitro and in vivo. J Agric Food Chem. 2009;57:5777–5782. doi: 10.1021/jf900331g. [DOI] [PubMed] [Google Scholar]
- 8.Shin Y.W., Kim D.H. Antipruritic effect of ginsenoside rb1 and compound k in scratching behavior mouse models. J Pharmacol Sci. 2005;99:83–88. doi: 10.1254/jphs.fp0050260. [DOI] [PubMed] [Google Scholar]
- 9.Kim K., Park M., Lee Y.M., Rhyu M.R., Kim H.Y. Ginsenoside metabolite compound K stimulates glucagon-like peptide-1 secretion in NCI-H716 cells via bile acid receptor activation. Arch Pharm Res. 2014;37:1193–1200. doi: 10.1007/s12272-014-0362-0. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z., Du G.J., Wang C.Z., Wen X.D., Calway T., Li Z., He T.C., Du W., Bissonnette M., Musch M.W. Compound K, a ginsenoside metabolite, inhibits colon cancer growth via multiple pathways including p53-p21 interactions. Int J Mol Sci. 2013;14:2980–2995. doi: 10.3390/ijms14022980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joh E.H., Lee I.A., Jung I.H., Kim D.H. Ginsenoside Rb1 and its metabolite compound K inhibit IRAK-1 activation–the key step of inflammation. Biochem Pharmacol. 2011;82:278–286. doi: 10.1016/j.bcp.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y.L., Liu Z.F., Li C.M., Shen J.Y., Yin H.X., Li G.S. Subchronic toxicity studies with ginsenoside compound K delivered to dogs via intravenous administration. Food Chem Toxicol. 2011;49:1857–1862. doi: 10.1016/j.fct.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Tsutsumi Y.M., Tsutsumi R., Mawatari K., Nakaya Y., Kinoshita M., Tanaka K., Oshita S. Compound K, a metabolite of ginsenosides, induces cardiac protection mediated nitric oxide via Akt/PI3K pathway. Life Sci. 2011;88:725–729. doi: 10.1016/j.lfs.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Lee H.U., Bae E.A., Han M.J., Kim N.J., Kim D.H. Hepatoprotective effect of ginsenoside Rb1 and compound K on tert-butyl hydroperoxide-induced liver injury. Liver Int. 2005;25:1069–1073. doi: 10.1111/j.1478-3231.2005.01068.x. [DOI] [PubMed] [Google Scholar]
- 15.Lee E., Choi J., Kim M.S., You H.J., Ji G.E., Kang Y. Ginsenoside metabolite compound K differentially antagonizing tumor necrosis factor-α-induced monocyte–endothelial trafficking. Chem-Biol Interact. 2011;194:13–22. doi: 10.1016/j.cbi.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Qin M., Luo Y., Meng X.B., Wang M., Wang H.W., Song S.Y., Ye J.X., Yao F., Wu P., Sun G.B. Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: an insight into PI3K/Akt activation and STAT3 signaling pathways. Vascul Pharmacol. 2015;70:23–34. doi: 10.1016/j.vph.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 18.Fan J., Watanabe T. Inflammatory reactions in the pathogenesis of atherosclerosis. J Atheroscler Thromb. 2003;10:63–71. doi: 10.5551/jat.10.63. [DOI] [PubMed] [Google Scholar]
- 19.Li D., Chen H., Romeo F., Sawamura T., Saldeen T., Mehta J.L. Statins modulate oxidized low-density lipoprotein-mediated adhesion molecule expression in human coronary artery endothelial cells: role of LOX-1. J Pharmacol Exp Ther. 2002;302:601–605. doi: 10.1124/jpet.102.034959. [DOI] [PubMed] [Google Scholar]
- 20.Zheng J., Liu B., Lun Q., Gu X., Pan B., Zhao Y., Xiao W., Li J., Tu P. Longxuetongluo capsule inhibits atherosclerosis progression in high-fat diet-induced ApoE-/- mice by improving endothelial dysfunction. Atherosclerosis. 2016;255:156–163. doi: 10.1016/j.atherosclerosis.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 21.Mehta J.L., Chen J., Hermonat P.L., Romeo F., Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res. 2006;69:36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Hansson G.K. Inflammatory mechanisms in atherosclerosis. J Thromb Haemost. 2009;7:328–331. doi: 10.1111/j.1538-7836.2009.03416.x. [DOI] [PubMed] [Google Scholar]
- 23.Bao M.H., Zhang Y.W., Zhou H.H. Paeonol suppresses oxidized low-density lipoprotein induced endothelial cell apoptosis via activation of LOX-1/p38MAPK/NF-kappaB pathway. J Ethnopharmacol. 2013;146:543–551. doi: 10.1016/j.jep.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Jiang J.X., Zhang S.J., Liu Y.N., Lin X.X., Sun Y.H., Shen H.J., Yan X.F., Xie Q.M. EETs alleviate ox-LDL-induced inflammation by inhibiting LOX-1 receptor expression in rat pulmonary arterial endothelial cells. Eur J Pharmacol. 2014;727:43–51. doi: 10.1016/j.ejphar.2014.01.045. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H., Ma G., Yao Y., Qian H., Li W., Chen X., Jiang W., Zheng R. Olmesartan attenuates the impairment of endothelial cells induced by oxidized low density lipoprotein through downregulating expression of LOX-1. Int J Mol Sci. 2012;13:1512–1523. doi: 10.3390/ijms13021512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., Mu Q., Zhou Z., Song H., Zhang Y., Wu F., Jiang M., Wang F., Zhang W., Li L. Protective effect of irisin on atherosclerosis via suppressing oxidized low density lipoprotein induced vascular inflammation and endothelial dysfunction. PLoS One. 2016;11 doi: 10.1371/journal.pone.0158038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu L., Xu R., Wang S., Li S., Sheng H., Wu J., Qu Y. Honokiol ameliorates endothelial dysfunction through suppression of PTX3 expression, a key mediator of IKK/IkappaB/NF-kappaB, in atherosclerotic cell model. Exp Mol Med. 2015;47 doi: 10.1038/emm.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen J., Mehta J.L., Haider N., Zhang X., Narula J., Li D. Role of caspases in Ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circ Res. 2004;94:370–376. doi: 10.1161/01.RES.0000113782.07824.BE. [DOI] [PubMed] [Google Scholar]
- 29.Stoneman V.E., Bennett M.R. Role of apoptosis in atherosclerosis and its therapeutic implications. Clin Sci (Lond) 2004;107:343–354. doi: 10.1042/CS20040086. [DOI] [PubMed] [Google Scholar]
- 30.Li D., Mehta J.L. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler Thromb Vasc Biol. 2000;20:1116–1122. doi: 10.1161/01.atv.20.4.1116. [DOI] [PubMed] [Google Scholar]
- 31.Su X., Ao L., Shi Y., Johnson T.R., Fullerton D.A., Meng X. Oxidized low density lipoprotein induces bone morphogenetic protein-2 in coronary artery endothelial cells via Toll-like receptors 2 and 4. J Biol Chem. 2011;286:12213–12220. doi: 10.1074/jbc.M110.214619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi J.S., Choi Y.J., Shin S.Y., Li J., Kang S.W., Bae J.Y., Kim D.S., Ji G.E., Kang J.S., Kang Y.H. Dietary flavonoids differentially reduce oxidized LDL-induced apoptosis in human endothelial cells: role of MAPK- and JAK/STAT-signaling. J Nutr. 2008;138:983–990. doi: 10.1093/jn/138.6.983. [DOI] [PubMed] [Google Scholar]
- 33.Yin Y., Liu W., Ji G., Dai Y. The essential role of p38 MAPK in mediating the interplay of oxLDL and IL-10 in regulating endothelial cell apoptosis. Eur J Cell Biol. 2013;92:150–159. doi: 10.1016/j.ejcb.2013.01.001. [DOI] [PubMed] [Google Scholar]