Abstract
The purpose of this review is to summarize the research progress of PI3K/Akt signaling pathway in erythropoiesis and glycolysis. Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K) is activated by numerous genes and leads to protein kinase B (Akt) binding to the cell membrane, with the help of phosphoinositide-dependent kinase, in the PI3K/Akt signal transduction pathway. Threonine and serine phosphorylation contribute to Akt translocation from the cytoplasm to the nucleus and further mediates enzymatic biological effects, including those involved in cell proliferation, apoptosis inhibition, cell migration, vesicle transport and cell cancerous transformation. As a key downstream protein of the PI3K/Akt signaling pathway, hypoxia-inducible factor (HIF)-1 is closely associated with the concentration of oxygen in the environment. Maintaining stable levels of HIF-1 protein is critical under normoxic conditions; however, HIF-1 levels quickly increase under hypoxic conditions. HIF-1α is involved in the acute hypoxic response associated with erythropoietin, whereas HIF-2α is associated with the response to chronic hypoxia. Furthermore, PI3K/Akt can reduce the synthesis of glycogen and increase glycolysis. Inhibition of glycogen synthase kinase 3β activity by phosphorylation of its N-terminal serine increases accumulation of cyclin D1, which promotes the cell cycle and improves cell proliferation through the PI3K/Akt signaling pathway. The PI3K/Akt signaling pathway is closely associated with a variety of enzymatic biological effects and glucose metabolism.
Keywords: PI3K/Akt, erythropoiesis, glycolysis, hypoxia
1. PI3K/Akt/mTOR signaling pathway
Phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/protein kinase B (Akt) pathway
The PI3K/Akt pathway is an intracellular signaling pathway of great importance in the cell cycle process. It is associated with cellular quiescence, proliferation, cancer and longevity. PI3K activation phosphorylates and further activates Akt, which localizes to the cellular membrane (1). Activated Akt fulfills various biological functions, including activating cAMP-response element binding protein (2), inhibiting p27 (3), localizing forkhead box protein O (FOXO) in the cytoplasm (3), activating phosphatidylinositol 3-phosphate (PtdIns3 ps or PI3P) (4) and activating mammalian target of rapamycin (mTOR) (3). It has been identified that the PI3K/Akt pathway can be enhanced by several biological molecules, including epidermal growth factor (EGF) (5), sonic hedgehog (2), insulin-like growth factor (IGF)-1 (2), insulin (3) and calmodulin (4). Conversely, this pathway is antagonized by other molecules, including phosphatase and tensin homolog (PTEN) (6), glycogen synthase kinase 3β (2) and transcription factor HB9 (5).
Structure and function of PI3Ks
The PI3Ks are a family of lipid kinases, which generate second messengers by specific catalytic 3-hydroxy phosphorylation of phosphatidylinositol (PI) (7). The PI3K family is divided into three classes, I–III; these classes share four homologous regions, among which, the kinase domain is the most conserved.
The substrate for Class I PI3Ks includes PI, phosphatidylinositol 4-phosphate and phosphatidylinositol (4,5) bisphosphate [PI(4,5)P2]. Class I PI3Ks are heterodimeric molecules composed of a regulatory and a catalytic subunit, which are further divided into IA and IB subsets. Class IA PI3Ks are composed of a heterodimer between a p110 catalytic subunit and a p85 regulatory subunit (8,9). The p85 subunit consists of α, β and γ, which are encoded in mammals by PI3K regulatory subunit (PIK3R)1, PIK3R2 and PIK3R3, respectively. The p85 subunit serves different roles in receptor binding, enzyme activation and localization (10). The p110 subunit consists of α, β and δ, which are encoded by PI3K catalytic subunit (PIK3C)A, PIK3CB and PIK3CD, respectively (11). p110α and p110β mainly influence cellular proliferation and insulin signaling in various tissues, whereas p110δ is found only in leukocytes and is involved in immune function and inflammation (12). Class IB PI3Ks consist of regulatory p101 and catalytic p110γ, and are encoded by a single gene each (8). p110γ is mainly expressed in leukocytes, with reduced expression in the heart, pancreas, liver and skeletal muscle. It combines with the Gβγ subunit of the G protein-coupled receptor and activates PI3K (13). The catalytic p110 subunit of Class IA PI3Ks consists of an N-terminal p85-binding domain (p110γ does not contain this domain), Ras binding domain, a proline-rich region [which reacts with proteins containing the SRC Homology (SH)3 domain], C-terminal homologous region (HR)3 (containing a basic leucine zipper-like domain bZIP), HR2 region (also termed PIK domain) and a C-terminal HR1 region (catalytic effect domain). HR3, HR2 and HR1 regions are PI3K HRs, which are responsible for membrane binding, substrate presentation and kinase domain, and catalytic inositol lipid 3-hydroxy-phosphorylated (14). The p85 regulatory subunit of Class IA PI3Ks consists of an SH3 domain, RHO-binding domain/breakpoint cluster region homology region, C-terminal SH2 domain and a connecting region (15).
Class II PI3Ks have monomeric catalytic isoforms, and contain α, β and γ subtypes. They contain a proline-rich region, Ras binding domain, HR3 region, HR2 region, HR1 region, PX domain and the C2 domain (16). Class II PI3Ks catalyze the production of PI(3)P from PI and phosphatidylinositol (3,4)-bisphosphate [PI(3,4)P2] from phosphoinositide (PIP). However, little is currently known about the mechanism.
The sole member of Class III PI3K is Vps34, which is the human homolog of a yeast gene product. Vps34 is a heterodimer formed by a p150 regulatory subunit and a p100 myristoyl-flower catalytic subunit, which phosphorylates PI to PI(3)P. Human Class III PI3K is a threonine/serine kinase also named as Vps34, which is the only PI3-kinase expressed in all eukaryotic cells. It was first identified in a Saccharomyces cerevisiae (budding yeast) screen for proteins involved in vesicle-mediated vacuolar protein sorting. It is tightly bound to regulatory subunit p150 and its function is not only phosphorylation, but also the recruitment of catalytic subunits to the cell membrane (17). Although PI(3)P is widespread, its level does not change when cells are stimulated. Therefore, Class III PI3K may be a housekeeping kinase that does not serve a role in signal transduction (18).
Akt
Akt (~60 kDa) has 68% homology with protein kinase A (PKA) and 73% homology with protein kinase C (PKC); therefore, Akt is also termed PKA and PKC-associated kinase. PKB is a product of oncogene v-akt encoding the retrovirus Akt-8, which is also known as Akt (19). Akt is a serine/threonine kinase, which is the central mediator of the PI3K pathway that serves a key role in numerous cellular processes, including glucose metabolism, apoptosis, cell proliferation, transcription and cell migration. There are three Akt subtypes: PKBα (Akt1), PKBβ (Akt2) and PKBγ (Akt3). Akt1 is widely expressed in several tissues, whereas Akt2 is mainly expressed in insulin-sensitive tissues, with a lower expression in other tissues. Akt3 is specific to the brain, lung, heart, kidney, testis and skeletal muscle (20). The three Akt isoforms share homologous amino acid sequences, including the N-terminal regulatory region, kinase catalytic domain and C-terminal regulatory domain. The N-terminal regulatory region is also termed the Akt homology domains/pleckstrin homology domains (PHD) domain. The kinase catalytic domain is highly homologous to enzymatically active regions in PKA and PKC, and its Thr308 site is necessary for Akt activation. The Ser473 site of C-terminal regulatory domain is important for complete activation of Akt (21).
mTOR
mTOR-targeted proteins belong to the phosphatidylinositol 3-kinase-related kinase family, which are serine/threonine protein kinases. This family serves a key role in identifying nutrition signals, cell growth and proliferation. mTOR is composed of mTOR complex (mTORC)1 and mTORC2, which contain five domains: TOR1, focal adhesion kinase (FAT), FKBP-rapamycin-binding (FRB) domain, kinase domain, negative regulatory domain, and FRAP, ATM, TRRAP C-terminal (FATC) domain (22). It has been confirmed that there is an amino acid residues kinase domain near the C-terminus of mTOR, which has a similar structure to the catalytic domain of PI3K. Upstream of the kinase domain is an FRB domain, which is the binding site of the FKBP12-rapamycin complex (23). Upstream of the FRB kinase domain is the FAT domain, which lies in the C-terminal of mTOR. This is also termed the FATC domain and serves a key role in mTOR stability. Deletion of one amino acid within its structure can lead to loss of mTOR activity (24). mTORC1, which comprises regulatory-associated protein of mTOR and mammalian ortholog of LST8 (mLST8), mainly regulates cell growth and energy metabolism, and is sensitive to rapamycin (25–28). mTORC2, which comprises rapamycin insensitive companion of mTOR, mitogen-activated protein kinase-associated protein 1 and mLST8, is mainly involved in cytoskeletal remodeling and cell survival, and is not sensitive to rapamycin (29,30).
2. Regulation of the PI3K/Akt signaling pathway
PI3K activation
PI3KIA kinase is activated while the growth factor receptor such as insulin receptor is bound to the p85 regulatory subunit of the SH2 domain of PI3KIA kinase. PI3K is also activated by other members of the non-receptor tyrosine kinase Src family. It can be activated by thrombin through focal adhesion kinase (FAK), which interacts with the SH3 domain of p85 subunits. Phosphorylation of FAK itself promotes the interaction with the SH2 domain of p85 subunits. PI3K is also activated by small GTP enzyme Ras through interaction with p110α (31). In general, PI3K is mainly activated directly and indirectly through the FAK pathway. It can be activated i) through receptor tyrosine protein kinase (RTK) dimerization, phosphorylation and interaction with the SH2 domain of p85 subunit by various growth factors, including platelet-derived growth factor (PDGF), IGF and EGF (32–34). ii) By RTK recruiting related proteins including Shc, Grb2 and Grb1 and Ras-related small molecules G proteins (Rho, Rac and Cdc42) (35). iii) By non-receptor protein tyrosine kinases, including Janus kinase, integrin linked kinase, FAK, Fyn, Lck, Lyn and Src through p85/p110 (36). iv) By the Gβγ subunit of G protein-coupled receptors (37).
Akt activation and PI3K/Akt negative regulation
PI(3,4,5)P3 has been identified as an Akt activator following the discovery of Akt. It serves an important role in the process of Akt activation. The head group of the lipid is directly attached to the N-end PH domain of Akt and is indirectly regulated through the PH domain of lysis protein kinase-3-phosphoinositide-dependent kinase 1 (PDK1). PI(3,4,5)P3 recruits Akt from the cytoplasm to the cell membrane, which causes Akt conformational alterations and ring-phosphorylation through a combination of the PH domain of PDK1 (8,38). With the aid of PDK2, the Akt C hydrophobic terminal is phosphorylated and double-phosphorylated Akt separates from the membrane, thus resulting in the cellular reaction with the substrate including phosphoinositide-dependent kinase 2 (PDK2), integrin-linked kinase (ILK), mechanistic target of rapamycin complex (mTORC) and DNA-dependent protein kinase (DNA-PK). It has been confirmed that the PI3K/Akt pathway is activated by the following steps: i) PI(3,4,5)P3 generation, ii) Akt conformational alterations and iii) double-phosphorylation of Akt (20,39,40). The PI3K/Akt signaling pathway regulates cell proliferation, migration, differentiation and apoptosis through activation or inhibition of downstream proteins (40). Phosphatase and tensin homologue (PTEN) transform PI-3,4,5-P3 into PI-4,5-P2 (41); therefore, Akt and the downstream signaling pathway is activated when PTEN activity is inhibited (42). PIP3 expression levels are higher in various tissue and cells, such as hypothalamus and pancreatic beta cells in PTEN gene knockout mice compared with in wild-type mice (43,44). In addition, carboxyl terminal modulator protein C can block the PI3K/Akt signaling pathway, acting as a negative regulator by inhibiting Akt phosphorylation (45).
3. PI3K/Akt signaling transduction pathway and HIF-1α
Hypoxia-inducible factor (HIF)-1 structure and regulation
HIF-1 structure
HIF-1 is a heterodimer, which is composed of HIF-1α and HIF-1β (also known as the aryl hydrocarbon receptor nuclear transporter, ARNT) (46). The HIF-1α and HIF-1β subunits (120 kDa and 91–94 kDa, respectively) belong to the basic helix-loop-helix (bHLH) protein family and contain the period circadian protein-ARNT-single-minded protein (PAS) domain (47). The HIF-1α gene is located at the q21-24 region of human chromosome 14 and is regulated by hypoxic signaling. The HIF-1β gene is located at the q21 region of human chromosome 1 and is stably expressed (48,49). The two subunits contain an N-terminal bHLH/PAS homologous region, which is essential for dimerization and binding of target genes. The middle of the HIF-1α subunit is an oxygen-dependent degradation domain (ODDD), which is rich in proline-serine-threonine. The C-terminal region contains two transactivation domains (TAD)-N and TAD-C and an inhibitory domain located between two TADs, which inhibits transcriptional activation of TAD (50). HIF-1 serves its important role in the regulation of transcription only when the two subunits form a dimer (51).
HIF-1 regulation
HIF-1α maintains the balance between synthesis and decomposition under normoxic conditions. The stability and transcription of HIF-1α are significantly increased under hypoxia conditions. HIF-1α is regulated by two oxygen-dependent factors; factor-inhibiting HIF-1 and prolyl hydroxylase (PH).
Under normoxic conditions, HIF-1 transcription is inhibited by C-terminal transactivation domain through CBP/p300 (52). However, under hypoxic conditions, the increase in HIF-1α expression induces transcription of downstream target genes (53,54). Under normoxic conditions, HIF-1α is degraded by ubiquitin-proteasome, which is formed via prolyl hydroxylase and causes ubiquitination of the HIF-1α subunit (55,56). Conversely, under hypoxic conditions, HIF-1α degradation is reduced by von Hippel-Lindau tumor suppressor (57). HIF-1α combines with nuclear pore protein under the nuclear localization signal and enters the nucleus to form a dimer with HIF-1β. Subsequently, the dimer combines with CBP/p300 and initiates the transcription of HIF-1 target genes.
HIF-1α is regulated through the PI3K/Akt pathway, which is activated by growth factors including PDGF, IGF and EGF, transforming growth factor (TGF), tumor necrosis factor-α and interleukin-1β (58). Expression of HIF-1α is enhanced when the PI3K/Akt pathway is activated through RTK.
Arrest-defective-1 (ARD1) acetylates the middle ODDD 532 lysine of the HIF-1α subunit. Since the mRNA and protein expression levels of ARD1 are reduced under hypoxic conditions, expression of HIF-1α increases due to decreased HIF-1α acetylation (59). HIF-1α, as a phosphoric acid protein, can regulate the synthesis and degradation through the phosphoric process by itself. However, hypoxia-induced HIF-1α phosphorylation enhances HIF-1 transcriptional activity (60).
PI3K/Akt signaling pathway and HIF-1α under normoxic conditions
A number of factors such as growth factors and cytokines indirectly regulate HIF-1α stabilization under normoxic conditions. Growth factors and cytokines regulate the PI3K/Akt and mitogen-activated protein kinase (MAPK) signaling pathways. Phosphorylation of HIF-1α causes activation of its transcription by the extracellular signal-regulated kinase/MAPK pathway, whereas HIF-1α protein levels are regulated by PI3K/Akt.
Nitric oxide (NO) increases HIF-1α stability under normoxic whereas the opposite occurs under hypoxia (61–64). Co-culture of mouse astrocytes and endothelial cells demonstrated that vascular endothelial cells increase the stability of HIF-1α in astrocytes by producing endothelial NO synthase. It also increases the production of glucose transporter-1 (GLUT1), hexokinase-2, monocarboxylate transporter-4 and lactate (65). HIF-1α is associated with the activation of cyclooxygenase-2 via the PI3K/Akt pathway in lung cancer cells (66).
IGF-1 increases the expression of HIF-1α protein in UCT116 cells, whereas the inhibitor of PI3K, LY294002, inhibits induction of HIF-1α (67). It is unclear whether the PI3K/Akt pathway induces HIF-1α expression or inhibits HIF-1α degradation under normoxic conditions. The study have demonstrated that in response to PI3K/Akt/mTOR induction in the normoxic environment, the synthesis of HIF-1α is increased, whereas its degradation is not inhibited (68).
The PI3K inhibitors wortmannin and LY294002, or the mTOR inhibitor rapamycin, suppress expression of HIF-1α and transcription of HIF-1 target genes which are induced and activated by EGF and angiotensinII via the PI3K/Akt pathway (69,70). Isakoff et al (71) revealed that EGFR can combine with the p85 regulatory subunit of PI3K through its C-terminal amino acid sequence. EGF binding to EGFR can indirectly activate expression of HIF-1α protein via the PI3K/Akt pathway (72).
PI3K/Akt signaling pathway and HIF-1α under hypoxic conditions
The controversy over the association between the PI3K/Akt signaling pathway and HIF-1α may be associated with disease and cell type under hypoxic. Stability of HIF-1α is associated with the PI3K/Akt signaling pathway, and HIF-1α expression is reduced by PI3K inhibitors (68,69,73–78). Under hypoxia (8% O2), hepatocytes exhibit increased HIF-1α levels (~6-fold), HIF-2α levels (~5-fold) and HIF-3α levels (~3-fold) compared with under normoxia. The insulin-dependent increase of the HIF 1α protein is mediated via PI3K, inasmuch as the PI3K inhibitor, wortmannin, eradicates the insulin-dependent enhancement of HIF-1α (79). FOXO4 not only reduces HIF-1α protein expression in HeLa cells under hypoxia or deferoxamine-simulated chemical hypoxia via the PI3K/Akt pathway, but also downregulate the stability of HIF-1α which cannot be caused by hydroxylation of proline (80). Blocking the PI3K/Akt/mTOR pathway inhibits serum-induced HIF-1, but not hypoxia-induced HIF-1 expression, hypoxia-inducible transcription gene activation of phosphoglycerate kinase (PGK) and GLUT1 (81). It has also been suggested that hypoxia can not only activate the PI3K/Akt signaling pathway in HeLa cells but can also promote the expression of HIF-1α. However, it appears that hypoxia-induced HIF-1α precedes PI3K/Akt activation (82). Nevertheless, hypoxia does not cause Akt phosphorylation in HEK293T, PC-3, COS-7, U373 and 3T3 cells. These data demonstrated that PI3K/Akt activity is only induced in response to hypoxia in certain cell types (82).
4. Hypoxia and erythropoiesis
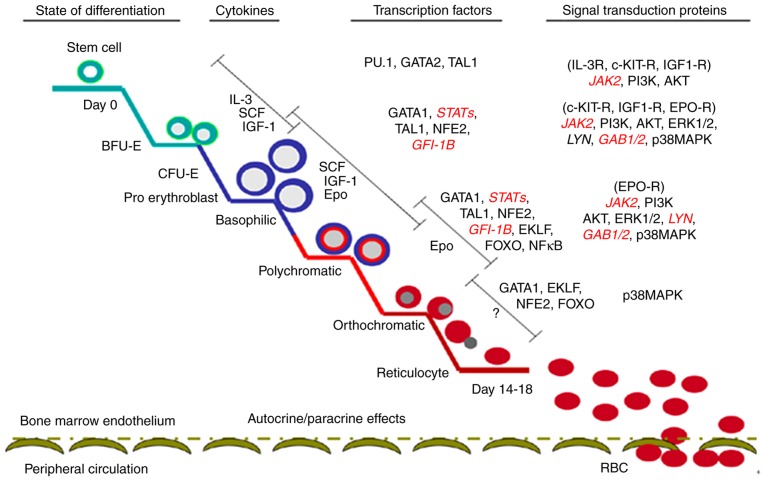
Erythropoiesis is a complex and sophisticated process, which originates in hematopoietic stem cells (HSCs); during this process, HSCs sequentially form burst-forming units of erythroid (BFU-E), colony-forming units of erythroid (CFU-E), proerythroblast and erythroblast, finally resulting in the formation of mature erythrocytes in the bone marrow (83–85). The main regulatory mechanism underlying erythropoiesis includes external hematopoietic cytokines, hematopoietic cytokine receptors, transcription factors and signaling molecules (Fig. 1) (86).
Figure 1.
Different stages of erythropoiesis and regulatory processes (including cytokines, transcription factors and related signal transduction proteins).
HIF-1 regulates numerous downstream genes, including angiogenesis genes [vascular endothelial growth factor (VEGF) and endothelin 1], erythropoiesis and energy metabolism genes (GLUT1, ALDOA, enolase 1, lactate dehydrogenase A, phosphofructokinase, liver type, PGK1 and HK), cell proliferation and differentiation genes (fibroblast growth factor, TGF and IGF), and apoptosis-associated genes (caspase-3 and cytochrome c) (87,88). HIF-α is divided into three subtypes (HIF-1α, HIF-2α and HIF-3α), each with specific functions. HIF-1α is involved in the acute hypoxic response, whereas HIF-2α is associated with the response to chronic hypoxia (89). Hematopoietic organs improve the oxygen-carrying capacity of the blood by increasing the number of red blood cells during oxygen plateau or strenuous exercise. This process is achieved via the erythropoietin (EPO) gene, which is mediated by HIF-α in the acute hypoxic response (89,90). León-Velarde et al (91) reported that EPO levels in the normal population at oxygen plateau and in patients with high altitude polycythemia (HAPC) were higher compared with in the normal population. However, no differences were observed between in normal population at oxygen plateau and patients with HAPC. The above findings indicate that EPO is an important regulatory factor of erythropoiesis in acute hypoxia but not in a chronic hypoxic environment.
Excess iron causes oxidative stress via the Fenton reaction and inhibits the interaction of HIF-2α with the EPO promoter in the kidneys of mice (92), however, an antioxidant compound, such as tempol, can restore this process. It has also been demonstrated that iron supplementation reduces EPO gene expression via an oxidative stress-HIF-2α-dependent signaling pathway (92). HIF prolyl hydroxylase enzyme inhibitors serve their roles by stabilizing the HIF complex and stimulating endogenous EPO production. They also improve iron mobilization to the bone marrow in patients with end-stage kidney disease (93). Classically, erythrocytosis is classified as either primary, caused by intrinsic defects in erythroid progenitor cells in the presence of normal or low serum EPO levels, or secondary. The causes of primary erythrocytosis include mutations in the Janus kinase 2 (JAK2) and EPOR genes, which can lead to EPO-independent proliferation of erythroid precursors or hypersensitivity to EPO (94). Secondary erythrocytosis is due to defects in the oxygen-sensing pathway, including mutations in the genes for HIF prolyl 4-hydroxylase 2 (HIF-P4H-2), HIF-2α, and von Hippel Lindau (VHL) protein, and impaired oxygen delivery or tissue hypoxia, all associated with the activation of the EPO pathway and elevated serum EPO levels (95,96). Large-spectrum conditional inactivation of HIF-P4H-2 in mice leads to severe erythrocytosis (97). HIF stabilization can thus mediate non-erythropoietin-driven splenic erythropoiesis via altered Notch signaling pathway (97). Genes associated with hypoxic environments have been identified in Tibetan populations living at high altitude; these populations are adapted to the plateau environment, in order to protect from polycythemia. The encoded PHD2-specific EGLN1 haplotypes can reduce HIF accumulation under hypoxic conditions. The effects of the EGLN1 haplotype on hemoglobin at low altitude are age-dependent, whereas EPAS1 rs142764723 C/C alleles exist to maintain low levels of hemoglobin at high altitude (98).
5. PI3K/Akt signaling pathway and glycolysis
Glycogen synthase kinase 3β (GSK-3) is an important downstream molecule regulated by Akt. It consists of Axis inhibition protein, β-catenin and adenomatous polyposis coli protein, and belongs to the serine/threonine protein kinase family. There are two subtypes: GSK-3α and GSK-3β, and 97% of amino acid sequences in the catalytic region of these two subtypes is homologous. They are both expressed widely in numerous cells and tissues, and possess similar biological properties. Previous studies have identified that GSK-3β can phosphorylate various endogenous substrates, including proteins associated with metabolism and transcription factors. GSK-3β serves an important role in cell growth, development, tumorigenesis and the regulation of glucose homeostasis (99–102). PI3K-dependent Akt is activated by insulin and growth factors that cause GSK-3β N terminal serine phosphorylation to inhibit GSK-3β activity. The decreased glycogen synthesis and increased accumulation of cyclin D1 result in cell cycle progression and proliferation (103). HIF-1α can activate the expression of associated glycolytic enzymes (87,88). HIF-1α is regulated by growth factors and cytokines via the PI3K/Akt/mTOR pathway and through this pathway HIF-1α can activate the expression of VEGF (104,105). Furthermore, PI3K/Akt regulates fructose 2,6-bisphosphatase (PFKFB2) expression and strengthens glycolysis. The activity of PFKFB2 is regulated by phosphorylation of C-terminal Ser466 and Ser483 residues (106–109). By pretreating LNCaP cells with R1881 (methyltrienolone) for 72 h, followed by LY294002 PI3K inhibition, Moon et al (110) revealed that phosphorylation of Ser466 and Ser483 was reduced and reversed by R1881. The association between glycolysis target genes regulated by HIF-1α and PI3K/Akt remains to be elucidated. HIF-1α is regulated by downstream mTOR of the PI3K/Akt signaling pathway (111). A previous study suggested that HIF-1α regulates glycolysis via the PI3K/Akt pathway (111). PI3K/Akt can regulate the reduction of glycogen synthesis and strengthen glycolysis. N-terminal serine phosphorylation of GSK-3β initiates cell cycle progression and proliferation by inhibiting GSK-3β activity and increasing the accumulation of cyclin D1. Therefore, reduction of glycogen synthesis and glycolysis may be a key point in cell proliferation regulation.
PDGF activates HIF-1α and c-Myc to reduce mitochondrial complex IV activity by activating the PI3K/Akt pathway in vitro. Furthermore, PDGF stimulation can reverse the decrease in glucose uptake and lactate production resulting from GLUT1 inhibitors in colon cancer cells (112). These findings suggested that PDGF may lead to reduced mitochondrial activity and increased glycolysis (112). Silencing cluster of differentiation 147 by specific small interfering RNA can downregulate GLUT1 levels by inhibiting PI3K/Akt signaling and decreasing glucose uptake in A375 cells (113). Under hypoxic conditions, the expression of HIF-1α and associated glycolytic enzymes, including GLUT1, hexokinase II, phosphofructokinase 2 and lactate dehydrogenase A, are increased, and extracellular lactate concentration is increased in the esophageal carcinoma cell lines Eca109 and TE13. However, the use of the PI3K/Akt inhibitor Wortmannin can reverse the alterations in glycolytic enzymes and the secretion of lactic acid (114). Primary exudative lymphoma (PEL) is a rare subtype of B-cell non-Hodgkin lymphoma, in which the dual PI3K and mTOR inhibitor PF-04691502 and Akt inhibitor (Akti-½) can reduce lactate production and extracellular acidification rate. PF-04691502 and Akti-½ can alter the metabolism of PEL cells from aerobic glycolysis towards oxidative respiration in combination with the glycolysis inhibitor 2-deoxyglucose (115). Serum stimulation can induce a slight accumulation of HIF-1α protein in a PI3K/Akt pathway-dependent manner (81). However, hypoxia induces much higher levels of HIF-1α protein and HIF-1 DNA binding activity independent of PI3K and mTOR activity. In addition, it has been identified that the effects of active Akt on HIF-1 activity are cell-type specific. High levels of Akt signaling can modestly increase HIF-1α protein, but not affect HIF-1 target gene expression (81). Therefore, the PI3K/Akt pathway may not be necessary for hypoxic induction of HIF-1 subunits or activity, and constitutively active Akt is not sufficient to induce HIF-1 activity by itself (81).
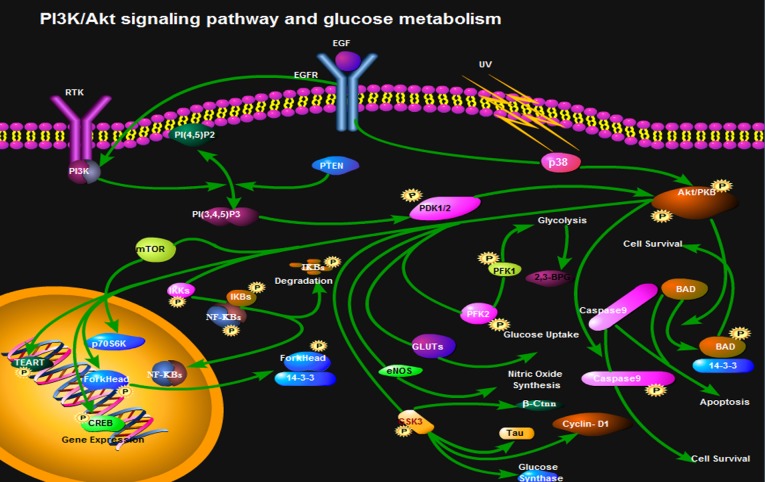
In cell glycolysis, l, 3-bisphosphoglycerate (BPG) catalyzed by PGK generates 3-phosphoglyceric acid. However, 1,3-BPG catalyzed by BPG mutase (BPGM) generates 2,3-BPG in the red blood cells. Under normal circumstances, the 2,3-BPG branch is only 19% of the glycolytic pathway (116); however, 2,3-BPG accounts for 50% of the glycolytic pathway under hypoxic conditions. Benesch et al (117) hypothesized and confirmed that 2,3-BPG and hemoglobin mainly regulate the affinity of hemoglobin and O2. The mRNA expression of BPGM is distributed mainly during development from bone marrow erythroid cells to mature erythrocytes. Low levels of BPGM mRNA can be detected in non-red blood cells, whereas the synthesis of 2,3-BPG only occurs in red blood cells (118). The Creteil I mutation, which results in inactivation of BPGM, or the Creteil II mutation, which is a reading frame shift, results in the inability of cells to express BPGM correctly, which then leads to a low content of 2,3-BPG in human peripheral blood. As a result, the bone marrow produces more red blood cells to transport more O2 into tissues (119). Nevertheless, it remains to be elucidated as to whether the PI3K/Akt signaling pathway regulates synthesis of BPGM (Fig. 2).
Figure 2.
PI3K/Akt signaling pathway and glucose metabolism (glycolysis, glucose uptake, nitric oxide glucose synthase, apoptosis and cell survival).
6. Summary and perspective
The PI3K/Akt signaling pathway is involved in numerous biological processes including cell cycle, cell apoptosis, angiogenesis and glucose metabolism. The PI3K/Akt pathway is indispensable to cell proliferation and apoptosis, and serves an important role in the occurrence and development of tumors (120). It has been confirmed that inhibition of PI3K/Akt can suppress tumor cell proliferation and induce apoptosis in vitro and in vivo. Certain inhibitors of PI3K/Akt have been applied in clinical trials (121). The efficacy of PI3K inhibition can also derive from interfering with the ability of cancer cells to respond to stromal signals, as illustrated by the approved PI3Kδ inhibitor Idelalisib in B-cell malignancies (121). Inhibition of the leukocyte-enriched PI3Kδ or PI3Kγ may unleash more potent antitumor T-cell responses by inhibiting regulatory T cells and immune-suppressive myeloid cells. In addition, tumor angiogenesis may be targeted by PI3K inhibitors to enhance cancer therapy. Chronic mountain sickness is characterized by erythrocytosis with or without pulmonary hypertension (122). Whether the PI3K/Akt signaling pathway is involved in erythrocytosis and chronic hypoxia-induced glucose metabolism requires further study, in order to provide novel directions for the diagnosis or prevention of chronic mountain sickness. Inhibitors of the PI3K/Akt signaling pathway may prevent overproduction of red blood cells (123,124).
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural Science Foundation of China (grant no. 81360084), the project of 2014 Qinghai Talent ‘Xiao Gao Di’ and the National Key Disciplines (hematology) of Qinghai Provincial People's Hospital (grant no. Ministry of Finance and Public Health 2011-170), the guiding project of Qinghai Provincial Health and Family Planning Commission (2018-wjzdx-21).
Availability of data and materials
Not applicable.
Authors' contributions
JL and YG designed the study. YX, XS, KS, GH, WL, QZ, BJ and JF wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing interests.
References
- 1.King D, Yeomanson D, Bryant HE. PI3King the lock: Targeting the PI3K/Akt/mTOR pathway as a novel therapeutic strategy in neuroblastoma. J Pediatr Hematol Oncol. 2015;37:245–251. doi: 10.1097/MPH.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 2.Peltier J, O'Neill A, Schaffer DV. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol. 2007;67:1348–1361. doi: 10.1002/dneu.20506. [DOI] [PubMed] [Google Scholar]
- 3.Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Prog Neurobiol. 2011;93:182–203. doi: 10.1016/j.pneurobio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D'Souza S, Wong TP, Taghibiglou C, Lu J, et al. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/S0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- 5.Ojeda L, Gao J, Hooten KG, Wang E, Thonhoff JR, Dunn TJ, Gao T, Wu P. Critical role of PI3K/Akt/GSK3β in motoneuron specification from human neural stem cells in response to FGF2 and EGF. PLoS One. 2011;6:e23414. doi: 10.1371/journal.pone.0023414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyatt LA, Filbin MT, Keirstead HS. PTEN inhibition enhances neurite outgrowth in human embryonic stem cell-derived neuronal progenitor cells. J Comp Neurol. 2014;522:2741–2755. doi: 10.1002/cne.23580. [DOI] [PubMed] [Google Scholar]
- 7.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 8.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 9.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breitkopf SB, Yang X, Begley MJ, Kulkarni M, Chiu YH, Turke AB, Lauriol J, Yuan M, Qi J, Engelman JA, et al. A cross-species study of PI3K protein-protein interactions reveals the direct interaction of P85 and SHP2. Sci Rep. 2016;6:20471. doi: 10.1038/srep20471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: Variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 13.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: Implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 14.Amzel LM, Huang CH, Mandelker D, Lengauer C, Gabelli SB, Vogelstein B. Structural comparisons of class I phosphoinositide 3-kinases. Nat Rev Cancer. 2008;8:665–669. doi: 10.1038/nrc2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schauder C, Ma LC, Krug RM, Montelione GT, Guan R. Structure of the iSH2 domain of human phosphatidylinositol 3-kinase p85β subunit reveals conformational plasticity in the interhelical turn region. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66:1567–1571. doi: 10.1107/S1744309110041333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falasca M, Maffucci T. Role of class II phosphoinositide 3-kinase in cell signalling. Biochem Soc Trans. 2007;35:211–214. doi: 10.1042/BST0350211. [DOI] [PubMed] [Google Scholar]
- 17.Backer JM. The regulation and function of Class III PI3Ks: Novel roles for Vps34. Biochem J. 2008;410:1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- 18.Bohdanowicz M, Cosío G, Backer JM, Grinstein S. Class I and class III phosphoinositide 3-kinases are required for actin polymerization that propels phagosomes. J Cell Biol. 2010;191:999–1012. doi: 10.1083/jcb.201004005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staal SP, Hartley JW. Thymic lymphoma induction by the AKT8 murine retrovirus. J Exp Med. 1988;167:1259–1264. doi: 10.1084/jem.167.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coffer PJ, Jin J, Woodgett JR. Protein kinase B (c-Akt): A multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Andrade MA, Bork P. HEAT repeats in the Huntington's disease protein. Nat Genet. 1995;11:115–116. doi: 10.1038/ng1095-115. [DOI] [PubMed] [Google Scholar]
- 23.Jacinto E, Hall MN. Tor signalling in bugs, brain and brawn. Nat Rev Mol Cell Biol. 2003;4:117–126. doi: 10.1038/nrm1071. [DOI] [PubMed] [Google Scholar]
- 24.Peterson RT, Beal PA, Comb MJ, Schreiber SL. FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem. 2000;275:7416–7423. doi: 10.1074/jbc.275.10.7416. [DOI] [PubMed] [Google Scholar]
- 25.Du K, Tsichlis PN. Regulation of the Akt kinase by interacting proteins. Oncogene. 2005;24:7401–7409. doi: 10.1038/sj.onc.1209099. [DOI] [PubMed] [Google Scholar]
- 26.Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- 27.Tokunaga E, Oki E, Egashira A, Sadanaga N, Morita M, Kakeji Y, Maehara Y. Deregulation of the Akt pathway in human cancer. Curr Cancer Drug Targets. 2008;8:27–36. doi: 10.2174/156800908783497140. [DOI] [PubMed] [Google Scholar]
- 28.Manning BD, Toker A. AKT/PKB Signaling: Navigating the network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yip CK, Murata K, Walz T, Sabatini DM, Kang SA. Structure of the human mTOR complex I and its implications for rapamycin inhibition. Mol Cell. 2010;38:768–774. doi: 10.1016/j.molcel.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 31.Ward SG, Finan P. Isoform-specific phosphoinositide 3-kinase inhibitors as therapeutic agents. Curr Opin Pharmacol. 2003;3:426–434. doi: 10.1016/S1471-4892(03)00078-X. [DOI] [PubMed] [Google Scholar]
- 32.Yokota J, Chosa N, Sawada S, Okubo N, Takahashi N, Hasegawa T, Kondo H, Ishisaki A. PDGF-induced PI3K-mediated signaling enhances the TGF-β-induced osteogenic differentiation of human mesenchymal stem cells in a TGF-β-activated MEK-dependent manner. Int J Mol Med. 2014;33:534–542. doi: 10.3892/ijmm.2013.1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma X, Bai Y. IGF-1 activates the P13K/AKT signaling pathway via upregulation of secretory clusterin. Mol Med Rep. 2012;6:1433–1437. doi: 10.3892/mmr.2012.1110. [DOI] [PubMed] [Google Scholar]
- 34.Dudu V, Able RA, Jr, Rotari V, Kong Q, Vazquez M. Role of epidermal growth factor-triggered PI3K/Akt signaling in the migration of medulloblastoma-derived cells. Cell Mol Bioeng. 2012;5:413–502. doi: 10.1007/s12195-012-0253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 36.Geltz NR, Augustine JA. The p85 and p110 subunits of phosphatidylinositol 3-kinase-alpha are substrates, in vitro, for a constitutively associated protein tyrosine kinase in platelets. Blood. 1998;91:930–939. [PubMed] [Google Scholar]
- 37.Kang BH, Shim YJ, Tae YK, Song JA, Choi BK, Park IS, Min BH. Clusterin stimulates the chemotactic migration of macrophages through a pertussis toxin sensitive G-protein-coupled receptor and Gβγ-dependent pathways. Biochem Biophys Res Commun. 2014;445:645–650. doi: 10.1016/j.bbrc.2014.02.071. [DOI] [PubMed] [Google Scholar]
- 38.Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 39.Hresko RC, Mueckler M. mTOR RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280:40406–40416. doi: 10.1074/jbc.M508361200. [DOI] [PubMed] [Google Scholar]
- 40.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 41.Tang JM, He QY, Guo RX, Chang XJ. Phosphorylated Akt overexpression and loss of PTEN expression in non-small cell lung cancer confers poor prognosis. Lung Cancer. 2006;51:181–191. doi: 10.1016/j.lungcan.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Wishart MJ, Dixon JE. PTEN and myotubularin phosphatases: From 3-phosphoinositide dephosphorylation to disease. Trends Cell Biol. 2002;12:579–585. doi: 10.1016/S0962-8924(02)02412-1. [DOI] [PubMed] [Google Scholar]
- 43.Stiles BL, Kuralwalla-Martinez C, Guo W, Gregorian C, Wang Y, Tian J, Magnuson MA, Wu H. Selective deletion of Pten in pancreatic beta cells leads to increased islet mass and resistance to STZ-induced diabetes. Mol Cell Biol. 2006;26:2772–2781. doi: 10.1128/MCB.26.7.2772-2781.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nguyen KT, Tajmir P, Lin CH, Liadis N, Zhu XD, Eweida M, Tolasa-Karaman G, Cai F, Wang R, Kitamura T, et al. Essential role of Pten in body size determination and pancreatic beta-cell homeostasis in vivo. Mol Cell Biol. 2006;26:4511–4518. doi: 10.1128/MCB.00238-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao S, Fu J, Liu F, Rastogi R, Zhang J, Zhao Y. Small interfering RNA directed against CTMP reduces acute traumatic brain injury in a mouse model by activating Akt. Neurol Res. 2014;36:483–490. doi: 10.1179/1743132814Y.0000000353. [DOI] [PubMed] [Google Scholar]
- 46.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 47.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Semenza GL. Hypoxia-inducible factor 1 and cancer pathogenesis. IUBMB Life. 2008;60:591–597. doi: 10.1002/iub.93. [DOI] [PubMed] [Google Scholar]
- 49.Loor G, Schumacker PT. Role of hypoxia-inducible factor in cell survival during myocardial ischemia-reperfusion. Cell Death Differ. 2008;15:686–690. doi: 10.1038/cdd.2008.13. [DOI] [PubMed] [Google Scholar]
- 50.Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272:19253–19260. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- 51.Adams JM, Difazio LT, Rolandelli RH, Luján JJ, Haskó G, Csóka B, Selmeczy Z, Németh ZH. HIF-1: A key mediator in hypoxia. Acta Physiol Hung. 2009;96:19–28. doi: 10.1556/APhysiol.96.2009.1.2. [DOI] [PubMed] [Google Scholar]
- 52.Lendahl U, Lee KL, Yang H, Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet. 2009;10:821–832. doi: 10.1038/nrg2665. [DOI] [PubMed] [Google Scholar]
- 53.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 54.Peet DJ, Lando D, Whelan DA, Whitelaw ML, Gorman JJ. Oxygen-dependent asparagine hydroxylation. Methods Enzymol. 2004;381:467–487. doi: 10.1016/S0076-6879(04)81031-0. [DOI] [PubMed] [Google Scholar]
- 55.Kaelin WG. Proline hydroxylation and gene expression. Annu Rev Biochem. 2005;74:115–128. doi: 10.1146/annurev.biochem.74.082803.133142. [DOI] [PubMed] [Google Scholar]
- 56.Kondo K, Kaelin WG., Jr The von Hippel-Lindau tumor suppressor gene. Exp Cell Res. 2001;264:117–125. doi: 10.1006/excr.2000.5139. [DOI] [PubMed] [Google Scholar]
- 57.Arjumand W, Sultana S. Role of VHL gene mutation in human renal cell carcinoma. Tumour Biol. 2012;33:9–16. doi: 10.1007/s13277-011-0257-3. [DOI] [PubMed] [Google Scholar]
- 58.Niu G, Briggs J, Deng J, Ma Y, Lee H, Kortylewski M, Kujawski M, Kay H, Cress WD, Jove R, Yu H. Signal transducer and activator of transcription 3 is required for hypoxia-inducible factor-1alpha RNA expression in both tumor cells and tumor-associated myeloid cells. Mol Cancer Res. 2008;6:1099–1105. doi: 10.1158/1541-7786.MCR-07-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fisher TS, Etages SD, Hayes L, Crimin K, Li B. Analysis of ARD1 function in hypoxia response using retroviral RNA interference. J Biol Chem. 2005;280:17749–17757. doi: 10.1074/jbc.M412055200. [DOI] [PubMed] [Google Scholar]
- 60.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 61.Sandau KB, Fandrey J, Brüne B. Accumulation of HIF-1alpha under the influence of nitric oxide. Blood. 2001;97:1009–1015. doi: 10.1182/blood.V97.4.1009. [DOI] [PubMed] [Google Scholar]
- 62.Kasuno K, Takabuchi S, Fukuda K, Kizaka-Kondoh S, Yodoi J, Adachi T, Semenza GL, Hirota K. Nitric oxide induces hypoxia-inducible factor 1 activation that is dependent on MAPK and phosphatidylinositol 3-kinase signaling. J Biol Chem. 2004;279:2550–2558. doi: 10.1074/jbc.M308197200. [DOI] [PubMed] [Google Scholar]
- 63.Park YK, Ahn DR, Oh M, Lee T, Yang EG, Son M, Park H. Nitric oxide donor, (+/-)-S-nitroso-N-acetylpenicillamine, stabilizes transactive hypoxia-inducible factor-1alpha by inhibiting von Hippel-Lindau recruitment and asparagine hydroxylation. Mol Pharmacol. 2008;74:236–245. doi: 10.1124/mol.108.045278. [DOI] [PubMed] [Google Scholar]
- 64.Sogawa K, Numayama-Tsuruta K, Ema M, Abe M, Abe H, Fujii-Kuriyama Y. Inhibition of hypoxia-inducible factor 1 activity by nitric oxide donors in hypoxia. Proc Natl Acad Sci USA. 1998;95:7368–7373. doi: 10.1073/pnas.95.13.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brix B, Mesters JR, Pellerin L, Jöhren O. Endothelial cell-derived nitric oxide enhances aerobic glycolysis in astrocytes via HIF-1α-mediated target gene activation. J Neurosci. 2012;32:9727–9735. doi: 10.1523/JNEUROSCI.0879-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115–2117. doi: 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 67.Bárdos JI, Chau NM, Ashcroft M. Growth factor-mediated induction of HDM2 positively regulates hypoxia-inducible factor 1alpha expression. Mol Cell Biol. 2004;24:2905–2914. doi: 10.1128/MCB.24.7.2905-2914.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Laughner E, Taghavi P, Chiles K, Mahon PC, Semenza GL. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: Novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol. 2001;21:3995–4004. doi: 10.1128/MCB.21.12.3995-4004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541–1545. [PubMed] [Google Scholar]
- 70.Pagé EL, Robitaille GA, Pouysségur J, Richard DE. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J Biol Chem. 2002;277:48403–48409. doi: 10.1074/jbc.M209114200. [DOI] [PubMed] [Google Scholar]
- 71.Isakoff SJ, Cardozo T, Andreev J, Li Z, Ferguson KM, Abagyan R, Lemmon MA, Aronheim A, Skolnik EY. Identification and analysis of PH domain-containing targets of phosphatidylinositol 3-kinase using a novel in vivo assay in yeast. EMBO J. 1998;17:5374–5387. doi: 10.1093/emboj/17.18.5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Karar J, Maity A. Modulating the tumor microenvironment to increase radiation responsiveness. Cancer Biol Ther. 2009;8:1994–2001. doi: 10.4161/cbt.8.21.9988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zundel W, Schindler C, Haas-Kogan D, Koong A, Kaper F, Chen E, Gottschalk AR, Ryan HE, Johnson RS, Jefferson AB, et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000;14:391–396. [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12:363–369. [PubMed] [Google Scholar]
- 76.Mazure NM, Chen EY, Laderoute KR, Giaccia AJ. Induction of vascular endothelial growth factor by hypoxia is modulated by a phosphatidylinositol 3-kinase/Akt signaling pathway in Ha-ras-transformed cells through a hypoxia inducible factor-1 transcriptional element. Blood. 1997;90:3322–3331. [PubMed] [Google Scholar]
- 77.Blancher C, Moore JW, Robertson N, Harris AL. Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3′-kinase/Akt signaling pathway. Cancer Res. 2001;61:7349–7355. [PubMed] [Google Scholar]
- 78.Chen EY, Mazure NM, Cooper JA, Giaccia AJ. Hypoxia activates a platelet-derived growth factor receptor/phosphatidylinositol 3-kinase/Akt pathway that results in glycogen synthase kinase-3 inactivation. Cancer Res. 2001;61:2429–2433. [PubMed] [Google Scholar]
- 79.Kietzmann T, Samoylenko A, Roth U, Jungermann K. Hypoxia-inducible factor-1 and hypoxia response elements mediate the induction of plasminogen activator inhibitor-1 gene expression by insulin in primary rat hepatocytes. Blood. 2003;101:907–914. doi: 10.1182/blood-2002-06-1693. [DOI] [PubMed] [Google Scholar]
- 80.Tang TT, Lasky LA. The forkhead transcription factor FOXO4 induces the down-regulation of hypoxia-inducible factor 1 alpha by a von Hippel-Lindau protein-independent mechanism. J Biol Chem. 2003;278:30125–30135. doi: 10.1074/jbc.M302042200. [DOI] [PubMed] [Google Scholar]
- 81.Arsham AM, Plas DR, Thompson CB, Simon MC. Phosphatidylinositol 3-kinase/Akt signaling is neither required for hypoxic stabilization of HIF-1 alpha nor sufficient for HIF-1-dependent target gene transcription. J Biol Chem. 2002;277:15162–15170. doi: 10.1074/jbc.M111162200. [DOI] [PubMed] [Google Scholar]
- 82.Alvarez-Tejado M, Alfranca A, Aragonés J, Vara A, Landázuri MO, del Peso L. Lack of evidence for the involvement of the phosphoinositide 3-kinase/Akt pathway in the activation of hypoxia-inducible factors by low oxygen tension. J Biol Chem. 2002;277:13508–13517. doi: 10.1074/jbc.M200017200. [DOI] [PubMed] [Google Scholar]
- 83.Heath DS, Axelrad AA, McLeod DL, Shreeve MM. Separation of the erythropoietin-responsive progenitors BFU-E and CFU-E in mouse bone marrow by unit gravity sedimentation. Blood. 1976;47:777–792. [PubMed] [Google Scholar]
- 84.Fader CM, Colombo MI. Multivesicular bodies and autophagy in erythrocyte maturation. Autophagy. 2006;2:122–125. doi: 10.4161/auto.2.2.2350. [DOI] [PubMed] [Google Scholar]
- 85.Swiers G, Patient R, Loose M. Genetic regulatory networks programming hematopoietic stem cells and erythroid lineage specification. Dev Biol. 2006;294:525–540. doi: 10.1016/j.ydbio.2006.02.051. [DOI] [PubMed] [Google Scholar]
- 86.Wickrema A, Crispino JD. Erythroid and megakaryocytic transformation. Oncogene. 2007;26:6803–6815. doi: 10.1038/sj.onc.1210763. [DOI] [PubMed] [Google Scholar]
- 87.Brahimi-Horn C, Pouysségur J. The role of the hypoxia-inducible factor in tumor metabolism growth and invasion. Bull Cancer. 2006;93:E73–E80. [PubMed] [Google Scholar]
- 88.Lee JW, Bae SH, Jeong JW, Kim SH, Kim KW. Hypoxia-inducible factor (HIF-1)alpha: Its protein stability and biological functions. Exp Mol Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 89.Holmquist-Mengelbier L, Fredlund E, Löfstedt T, Noguera R, Navarro S, Nilsson H, Pietras A, Vallon-Christersson J, Borg A, Gradin K, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 90.Lee FS. Genetic causes of erythrocytosis and the oxygen-sensing pathway. Blood Rev. 2008;22:321–332. doi: 10.1016/j.blre.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.León-Velarde F, Monge CC, Vidal A, Carcagno M, Criscuolo M, Bozzini CE. Serum immunoreactive erythropoietin in high altitude natives with and without excessive erythrocytosis. Exp Hematol. 1991;19:257–260. [PubMed] [Google Scholar]
- 92.Oshima K, Ikeda Y, Horinouchi Y, Watanabe H, Hamano H, Kihira Y, Kishi S, Izawa-Ishizawa Y, Miyamoto L, Hirayama T, et al. Iron suppresses erythropoietin expression via oxidative stress-dependent hypoxia-inducible factor-2 alpha inactivation. Lab Invest. 2017;97:555–566. doi: 10.1038/labinvest.2017.11. [DOI] [PubMed] [Google Scholar]
- 93.Gupta N, Wish JB. Hypoxia-inducible factor prolyl hydroxylase inhibitors: A potential new treatment for anemia in patients with CKD. Am J Kidney Dis. 2017;69:815–826. doi: 10.1053/j.ajkd.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 94.Lee FS, Percy MJ. The HIF pathway and erythrocytosis. Annu Rev Pathol. 2011;6:165–192. doi: 10.1146/annurev-pathol-011110-130321. [DOI] [PubMed] [Google Scholar]
- 95.Prchal JT, Sokol L. ‘Benign erythrocytosis’ and other familial and congenital polycythemias. Eur J Haematol. 1996;57:263–268. doi: 10.1111/j.1600-0609.1996.tb01376.x. [DOI] [PubMed] [Google Scholar]
- 96.Patnaik MM, Tefferi A. The complete evaluation of erythrocytosis: Congenital and acquired. Leukemia. 2009;23:834–844. doi: 10.1038/leu.2009.54. [DOI] [PubMed] [Google Scholar]
- 97.Myllymäki MN, Määttä J, Dimova EY, Izzi V, Väisänen T, Myllyharju J, Koivunen P, Serpi R. Notch downregulation and extramedullary erythrocytosis in hypoxia-inducible factor prolyl 4-hydroxylase 2-deficient mice. Mol Cell Biol. 2017;37(pii):e00529–16. doi: 10.1128/MCB.00529-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tashi T, Scott Reading N, Wuren T, Zhang X, Moore LG, Hu H, Tang F, Shestakova A, Lorenzo F, Burjanivova T, et al. Gain-of-function EGLN1 prolyl hydroxylase (PHD2 D4E:C127S) in combination with EPAS1 (HIF-2α) polymorphism lowers hemoglobin concentration in Tibetan highlanders. J Mol Med (Berl) 2017;95:665–670. doi: 10.1007/s00109-017-1519-3. [DOI] [PubMed] [Google Scholar]
- 99.Inkster B, Zai G, Lewis G, Miskowiak KW. GSK3β: A plausible mechanism of cognitive and hippocampal changes induced by erythropoietin treatment in mood disorders. Transl Psychiatry. 2018;8:216. doi: 10.1038/s41398-018-0270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van der Vaart A, Meng X, Bowers MS, Batman AM, Aliev F, Farris SP, Hill JS, Green TA, Dick D, COGA Consortium et al. Glycogen synthase kinase 3 beta regulates ethanol consumption and is a risk factor for alcohol dependence. Neuropsychopharmacology. 2018 doi: 10.1038/s41386-018-0202-x. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sopjani M, Millaku L, Nebija D, Emini M, Dermaku-Sopjani M, Rifati-Nixha A. The glycogen synthase kinase-3 in the regulation of ion channels and cellular carriers. Curr Med Chem. 2018 Oct 9; doi: 10.2174/0929867325666181009122452. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 102.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dokken BB, Sloniger JA, Henriksen EJ. Acute selective glycogen synthase kinase-3 inhibition enhances insulin signaling in prediabetic insulin-resistant rat skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E1188–E1194. doi: 10.1152/ajpendo.00547.2004. [DOI] [PubMed] [Google Scholar]
- 104.Secades P, de Santa-María IS, Merlo A, Suarez C, Chiara MD. In vitro study of normoxic epidermal growth factor receptor-induced hypoxia-inducible factor-1-alpha, vascular endothelial growth factor, and BNIP3 expression in head and neck squamous cell carcinoma cell lines: Implications for anti-epidermal growth factor receptor therapy. Head Neck. 2015;37:1150–1162. doi: 10.1002/hed.23733. [DOI] [PubMed] [Google Scholar]
- 105.Park ST, Kim BR, Park SH, Lee JH, Lee EJ, Lee SH, Rho SB. Suppression of VEGF expression through interruption of the HIF-1α and Akt signaling cascade modulates the anti-angiogenic activity of DAPK in ovarian carcinoma cells. Oncol Rep. 2014;31:1021–1029. doi: 10.3892/or.2013.2928. [DOI] [PubMed] [Google Scholar]
- 106.Kitamura K, Kangawa K, Matsuo H, Uyeda K. Phosphorylation of myocardial fructose-6-phosphate,2-kinase: fructose-2,6-bisphosphatase by cAMP-dependent protein kinase and protein kinase C. Activation by phosphorylation and amino acid sequences of the phosphorylation sites. J Biol Chem. 1988;263:16796–16801. [PubMed] [Google Scholar]
- 107.Deprez J, Vertommen D, Alessi DR, Hue L, Rider MH. Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J Biol Chem. 1997;272:17269–17275. doi: 10.1074/jbc.272.28.17269. [DOI] [PubMed] [Google Scholar]
- 108.Bertrand L, Alessi DR, Deprez J, Deak M, Viaene E, Rider MH, Hue L. Heart 6-phosphofructo-2-kinase activation by insulin results from Ser-466 and Ser-483 phosphorylation and requires 3-phosphoinositide-dependent kinase-1, but not protein kinase B. J Biol Chem. 1999;274:30927–30933. doi: 10.1074/jbc.274.43.30927. [DOI] [PubMed] [Google Scholar]
- 109.Depre C, Rider MH, Veitch K, Hue L. Role of fructose 2,6-bisphosphate in the control of heart glycolysis. J Biol Chem. 1993;268:13274–13279. [PubMed] [Google Scholar]
- 110.Moon JS, Jin WJ, Kwak JH, Kim HJ, Yun MJ, Kim JW, Park SW, Kim KS. Androgen stimulates glycolysis for de novo lipid synthesis by increasing the activities of hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2 in prostate cancer cells. Biochem J. 2011;433:225–233. doi: 10.1042/BJ20101104. [DOI] [PubMed] [Google Scholar]
- 111.Agani F, Jiang BH. Oxygen-independent regulation of HIF-1: Novel involvement of PI3K/AKT/mTOR pathway in cancer. Curr Cancer Drug Targets. 2013;13:245–251. doi: 10.2174/1568009611313030003. [DOI] [PubMed] [Google Scholar]
- 112.Moench R, Grimmig T, Kannen V, Tripathi S, Faber M, Moll EM, Chandraker A, Lissner R, Germer CT, Waaga-Gasser AM, Gasser M. Exclusive inhibition of PI3K/Akt/mTOR signaling is not sufficient to prevent PDGF-mediated effects on glycolysis and proliferation in colorectal cancer. Oncotarget. 2016;7:68749–68767. doi: 10.18632/oncotarget.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Su J, Gao T, Jiang M, Wu L, Zeng W, Zhao S, Peng C, Chen X. CD147 silencing inhibits tumor growth by suppressing glucose transport in melanoma. Oncotarget. 2016;7:64778–64784. doi: 10.18632/oncotarget.11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zeng L, Zhou HY, Tang NN, Zhang WF, He GJ, Hao B, Feng YD, Zhu H. Wortmannin influences hypoxia-inducible factor-1 alpha expression and glycolysis in esophageal carcinoma cells. World J Gastroenterol. 2016;22:4868–4880. doi: 10.3748/wjg.v22.i20.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mediani L, Gibellini F, Bertacchini J, Frasson C, Bosco R, Accordi B, Basso G, Bonora M, Calabrò ML, Mattiolo A, et al. Reversal of the glycolytic phenotype of primary effusion lymphoma cells by combined targeting of cellular metabolism and PI3K/Akt/ mTOR signaling. Oncotarget. 2016;7:5521–5537. doi: 10.18632/oncotarget.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mulquiney PJ, Bubb WA, Kuchel PW. Model of 2,3-bisphosphoglycerate metabolism in the human erythrocyte based on detailed enzyme kinetic equations: In vivo kinetic characterization of 2,3-bisphosphoglycerate synthase/phosphatase using 13C and 31P NMR. Biochem J 342 Pt. 1999;3:567–580. doi: 10.1042/bj3420567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Benesch R, Benesch RE, Yu CI. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci USA. 1968;59:526–532. doi: 10.1073/pnas.59.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Narita H, Yanagawa S, Sasaki R, Chiba H. Synthesis of 2,3-bisphosphoglycerate synthase in erythroid cells. J Biol Chem. 1981;256:7059–7063. [PubMed] [Google Scholar]
- 119.Lemarchandel V, Joulin V, Valentin C, Rosa R, Galactéros F, Rosa J, Cohen-Solal M. Compound heterozygosity in a complete erythrocyte bisphosphoglycerate mutase deficiency. Blood. 1992;80:2643–2649. [PubMed] [Google Scholar]
- 120.Spangle JM, Roberts TM, Zhao JJ. The emerging role of PI3K/AKT-mediated epigenetic regulation in cancer. Biochim Biophys Acta Rev Cancer. 2017;1868:123–131. doi: 10.1016/j.bbcan.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Okkenhaug K, Graupera M, Vanhaesebroeck B. Targeting PI3K in cancer: Impact on tumor cells, their protective stroma, angiogenesis, and immunotherapy. Cancer Discov. 2016;6:1090–1105. doi: 10.1158/2159-8290.CD-16-0716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Villafuerte FC, Corante N. Chronic mountain sickness: Clinical aspects, etiology, management, and treatment. High Alt Med Biol. 2016;17:61–69. doi: 10.1089/ham.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hermida MA, Dinesh Kumar J, Leslie NR. GSK3 and its interactions with the PI3K/AKT/mTOR signalling network. Adv Biol Regul. 2017;65:5–15. doi: 10.1016/j.jbior.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 124.Li N, Zhou H, Tang Q. miR-133: A suppressor of cardiac remodeling? Front Pharmacol. 2018;9:903. doi: 10.3389/fphar.2018.00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.