Abstract
Background
Ginsenosides with less sugar moieties may exhibit the better adsorptive capacity and more pharmacological activities.
Methods
An efficient method for the separation of four minor saponins, including gypenoside XVII, notoginsenoside Fe, ginsenoside Rd2, and notoginsenoside Fd, from Panax notoginseng leaves (PNL) was established using biotransformation, macroporous resins, and subsequent preparative high-performance liquid chromatography.
Results
The dried PNL powder was immersed in the distilled water at 50°C for 30 min for converting the major saponins, ginsenosides Rb1, Rc, Rb2, and Rb3, to minor saponins, gypenoside XVII, notoginsenoside Fe, ginsenoside Rd2, and notoginsenoside Fd, respectively, by the enzymes present in PNL. The adsorption characteristics of these minor saponins on five types of macroporous resins, D-101, DA-201, DM-301, X-5, and S-8, were evaluated and compared. Among them, D-101 was selected due to the best adsorption and desorption properties. Under the optimized conditions, the fraction containing the four target saponins was separated by D-101 resin. Subsequently, the target minor saponins were individually separated and purified by preparative high-performance liquid chromatography with a reversed-phase column.
Conclusion
Our study provides a simple and efficient method for the preparation of these four minor saponins from PNL, which will be potential for industrial applications.
Keywords: ginsenoside Rd2, gypenoside XVII, notoginsenoside Fd, notoginsenoside Fe, Panax notoginseng leaves
1. Introduction
The toots of Panax notoginseng (Burk) FH. Chen (Sanqi in Chinese) has been regarded as one of the most valuable herbal medicines in China due to its wide range of therapeutic effects, including blood circulation promotion, blood stasis removal, and pain relief, as recorded in ancient Chinese documents [1]. Dammarane-type triterpenoid saponins, including the protopanaxadiol and the protopanaxatriol groups, have been considered to be the main bioactive components [2]; they can be found in different parts of P. notoginseng, including the roots, rhizomes, leaves, and flowers [3], [4]. The roots of P. notoginseng have been more commonly used than other parts, and their supply can hardly meet market demand due to their increased usage and the strict environmental conditions for their growth in recent years. Fortunately, P. notoginseng leaves (PNL) might be a feasible alternative source of saponins because of their annual recovery.
PNL has been used as a therapeutic agent in the treatment of insomnia, and its saponins have been shown to exert neuroprotective and antidepressant effects [5], [6] as well as protective effects against alcoholic liver injury [7]. Protopanaxadiol-type saponins, including ginsenosides Rb1, Rc, Rb2, and Rb3, have been found to be the main components in PNL [8], [9]. Our pilot study demonstrated that high-performance liquid chromatography (HPLC)-UV profiles of aqueous and alcohol extracts of PNL were remarkably different, indicating that severe transformations of ginsenosides might occur when PNL is extracted with water [10]. Specifically, ginsenosides Rb1 (1), Rc (3), Rb2 (4), and Rb3 (5), the major saponins of PNL, were transformed to the minor saponins of PNL, including gypenoside XVII (6), notoginsenoside Fe (ginsenoside compound Mc1, C-Mc1, 7), ginsenoside Rd2 (ginsenoside compound O, C-O, 8), and notoginsenoside Fd (ginsenoside compound Mx1, C-Mx1, 9), respectively, by removing a glucose residue from position C-3, which might be driven by the enzyme present in the original plant.
Little attention has been paid to the pharmacological activities of these minor saponins due to their rarity in PNL or other Panax plants. Moreover, a large body of studies has demonstrated that ginsenosides with less sugar moieties may exhibit better adsorptive capacity and more pharmacological activities, including anticancer, immunomodulatory, antiinflammatory, and antidiabetic effects [11], [12], [13], [14]. Thus, in recent decades, enzymatic and microbiological biotransformation, and also cloned ginsenosidase, have attracted more and more attention to obtain the minor ginsenosides with higher bioactivities [8], [15], [16], [17], [18]. For example, notoginsenoside Fe (C-Mc1), ginsenoside Rd2 (C-O), and notoginsenoside Fd (C-Mx1) can be produced from ginsenosides Rc, Rb2, and Rb3, respectively, by the glucosidases from Aspergillus niger g.848 strain [8], [15] and Sphingomonas sp. 2F2 [16]. Ginsenoside Rc also could be hydrolyzed to notoginsenoside Fe by glucosidases from fungus, such as Fusarium sacchari [17] and Armillaria mellea mycelia [18]. These studies have focused on ginsenoside biotransformation by the β-glucosidases isolated from diverse bacterial and fungus. Furthermore, in these biotransformation methods, notoginsenoside Fe, ginsenoside Rd2, and notoginsenoside Fd were the intermediate products that were further hydrolyzed to compound K or ginsenoside Mc [8], [15], [17], [18]. Therefore, in the present study, we aimed to characterize the biotransformation of ginsenosides Rb1, Rc, Rb2, and Rb3, the major saponins of PNL, to gypenoside XVII, notoginsenoside Fe, ginsenoside Rd2, and notoginsenoside Fd, respectively, by the enzyme in PNL itself, and further separate and purify the transformed saponins using macroporous resins and preparative HPLC (pre-HPLC).
2. Materials and methods
2.1. Sample, chemicals, and reagents
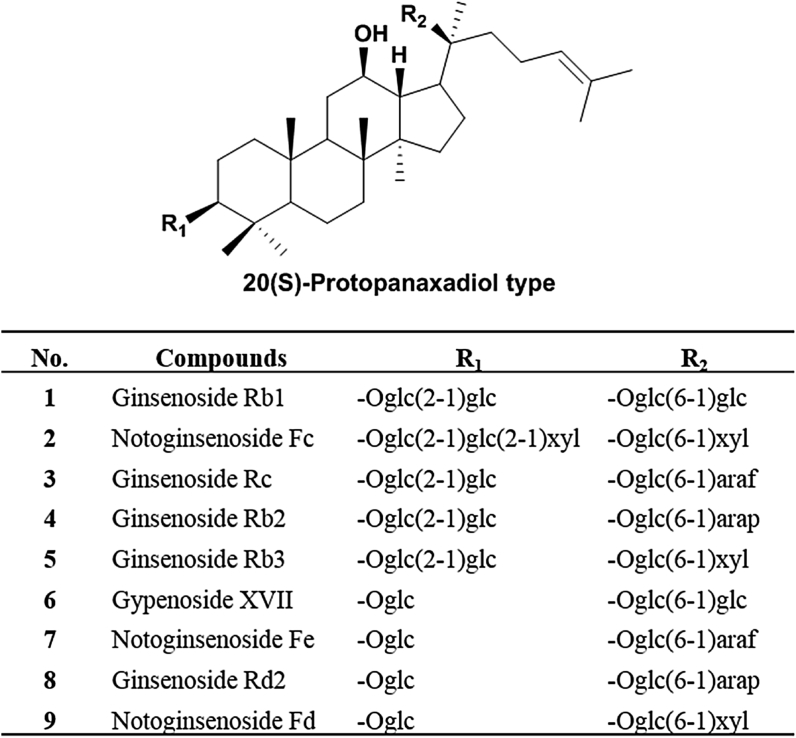
PNL was collected from Yanshan County, Wenshan region, Yunnan Province, China in October 2015. The botanical origin of the material was authenticated by Professor Ni Ma, Wenshan Sanqi Institute of Science and Technology, Wenshan University, Wenshan, Yunnan, China. The voucher specimen was deposited in the Institute of Chinese Medical Sciences, University of Macau, Macao, China. Reference standards, including ginsenoside Rb1 and notoginsenoside Fe, were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Ginsenosides Rb2, Rb3, and Rc were supplied by Must Bio-technology Co., Ltd. (Chengdu, P.R. China), ginsenoside Rd2 and notoginsenoside Fd were purchased from Herb Source Bio-technology Co., Ltd. (Nanjing, China), and gypenoside XVII and notoginsenoside Fc were obtained from Chenguang Bio-technology Co., Ltd. (Baoji, China). The purity of all reference standards was determined to be greater than 98% by HPLC-UV analysis. Their chemical structures are shown in Fig. 1. HPLC-grade acetonitrile, methanol, and formic acid were purchased from Merck (Darmstadt, Germany); deionized water was prepared by a Milli-Q purification system (Millipore, Bedford, MA, USA). Other reagents, including absolute ethanol, were supplied by Sam Yao Hong (Macao, China).
Fig. 1.
Chemical structures of the nine saponins in Panax notoginseng leaves (PNL). Araf, α-L-arabinose (furanose); Arap, α-L-arabinose (pyranose); Glc, β-D-glucose; Xyl, β-D-xylose.
2.2. Preparation of standard solutions and PNL crude extracts after ginsenoside transformation
For quantitative determination, standard stock solutions of nine pure saponins, including ginsenoside Rb1 (1), notoginsenoside Fc (2), ginsenoside Rc (3), ginsenoside Rb2 (4), ginsenoside Rb3 (5), gypenoside XVII (6), notoginsenoside Fe (7), ginsenoside Rd2 (8), and notoginsenoside Fd (9), were individually prepared in methanol. The solutions were then mixed and diluted to six different concentrations for the standard curves and method validation. The stock solutions were stored at 4°C until HPLC-UV analysis.
The air-dried PNL (200 g) was powdered in a mill and then immersed in 600 mL of distilled water at 50°C for 30 min in a water bath. After the addition of 4000 mL of absolute ethanol, the PNL was ultrasonically extracted for 1 h, twice. The temperature of the ultrasonic bath was kept constant (25 ± 2°C) with running water. After filtration, the combined ethanol extracts were concentrated to dryness by removing ethanol solvent under the reduced pressure in a rotary evaporator at 55°C. The residue was reconstructed in 100 mL of distilled water.
2.3. HPLC-UV analysis
HPLC-UV analysis was performed on a Waters Alliance e2695 HPLC system (Waters, Milford, MA, USA) composed of a vacuum degasser, a binary pump, a column oven, an autosampler, and a Waters 2996 photodiode array detector. The chromatographic separation was achieved on a reversed-phase Zorbax C8 column [250 mm × 4.6 mm (internal diameter, i.d.), 5μm] at a temperature of 35°C. The mobile phase consisted of acetonitrile (solvent A) and water (solvent B), eluted with a gradient program as follows: 0–5 min, 15–30% A; 5–15 min, 30–32% A; 15–35 min, 32–32% A; 35–45 min, 32–38% A; 45–60 min, 38–50% A. The flow rate was maintained at 0.8 mL/min. The detection wavelength was set at 203 nm. All samples were filtered through a 0.45μm membrane filter prior to HPLC-UV analysis.
2.4. Commercial adsorbents
Macroporous adsorption resins ranging from nonpolar to polar, including D-101, X-5, DM-301, S-8, and DA-201, were purchased from Tianjin Haiguang Chemical Co., Ltd. (Tianjin, P.R. China). Their chemical and physical characteristics are summarized in Table S1. The macroporous resins were pretreated according to the manufacturer protocol prior to use.
2.5. Static absorption and desorption tests
To select the best resins to separate the target ginsenosides from PNL crude extract, the adsorption and desorption properties of the different resins were characterized. Static adsorption and desorption tests of saponins were performed using the following procedures: dry adsorbent (1.0 g) was placed into a 50 mL Erlenmeyer flask with a lid, and 20 mL of the sample solution prepared above was added. Then, to reach adsorption equilibrium with the resins, the flasks were sealed tightly and shaken at 25°C for 12 h in a WNB-14 Memmert water bath with a shaking device (Memmert GmbH + Co. KG, Memmert, Schwabach, Germany) with the shaking control set to 40. The supernatant after adsorption was removed and analyzed by HPLC-UV. Subsequently, the resins were washed three times with distilled water, and then, 20 mL of 80% aqueous ethanol was added for the desorption test. The flasks were shaken for 12 h at 25°C, and the desorption solutions were also analyzed by HPLC-UV.
2.6. Adsorption isotherms
The adsorption isotherms of the minor transformed ginsenosides on the preliminarily selected D-101 resin were determined by adding 20 mL of PNL extract at different concentrations (0.2 g/mL, 0.4 g/mL, 0.6 g/mL, 0.8 g/mL, 1.2 g/mL, and 1.6 g/mL) in a shaker bath at a constant temperature of 25°C. The initial and equilibrium concentrations were determined by HPLC-UV. The Langmuir and Freundlich theoretical equations were used to evaluate the linearity fitness and to describe the interactions between the target saponins from the PNL extract and the adsorbed resins.
2.7. Dynamic adsorption and desorption tests
Dynamic adsorption and desorption experiments were conducted in a glass column (30 cm × 1.6 cm i.d.) wet-packed with the selected D-101 resin. The bed volume (BV) was 18 mL. The adsorption process was performed by loading PNL extracts into the pretreated glass column. Then, the concentration of each target component in the eluents was monitored by HPLC-UV to evaluate the breakthrough volume of the dynamic adsorption experiment. After reaching adsorptive equilibrium, the column was eluted by a gradient system with water and ethanol at different ratios. The volume of each eluent was 20 mL. The contents of the impurities and the four target compounds were analyzed by HPLC-UV.
2.8. Small-scale enrichment of the four target compounds from the crude PNL extracts
Small-scale preparation of the fraction containing the four target saponins was conducted in a glass column (45 cm × 6 cm i.d.). The dried PNL powder (300 g) was immersed in 1,000 mL of distilled water at 50°C for 30 min in a water bath for ginsenoside biotransformation and was subsequently extracted by 6,000 mL of ethanol using ultrasonication. After filtration, the PNL extract after biotransformation was subjected to a glass column filled with D-101 resin (700 g) with a BV of 850 mL. Ten BVs of 30% aqueous ethanol and four BVs of 60% aqueous ethanol were used to elute the column. The flow rate was set as 10 mL/min. The 60% aqueous ethanol eluates were combined and evaporated.
2.9. Preparative separation of the four target compounds by pre-HPLC
After separation by macroporous resins, the individual target saponins were further separated and purified by one-step pre-HPLC. A Shimadzu LC-20AP pre-HPLC apparatus (Shimadzu, Tokyo, Japan) coupled with an SPD-M20A photodiode array detector (Waters, Milford, MA, USA) was used. Chromatographic separation was performed on a Grace Alltech Alltima C18 column (20 mm × 250 mm, 10μm, Grace, Milford, MA, USA). Preparative separation of the four target saponins was achieved by a two-phase solvent system composed of acetonitrile-water (33:67, v/v) with a flow-rate of 10 mL/min. The monitoring wavelength was set at 203 nm. The peaks corresponding to gypenoside XVII (6), notoginsenoside Fe (7), ginsenoside Rd2 (8), and notoginsenoside Fd (9) were collected by an automatic fraction collector. After that, the fractions were combined according to the results of HPLC analysis.
3. Results and discussion
3.1. HPLC-UV analysis
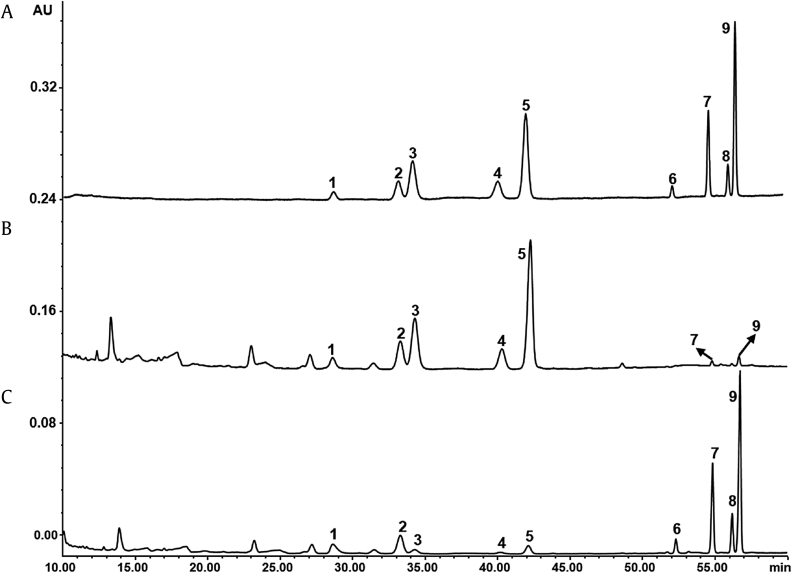
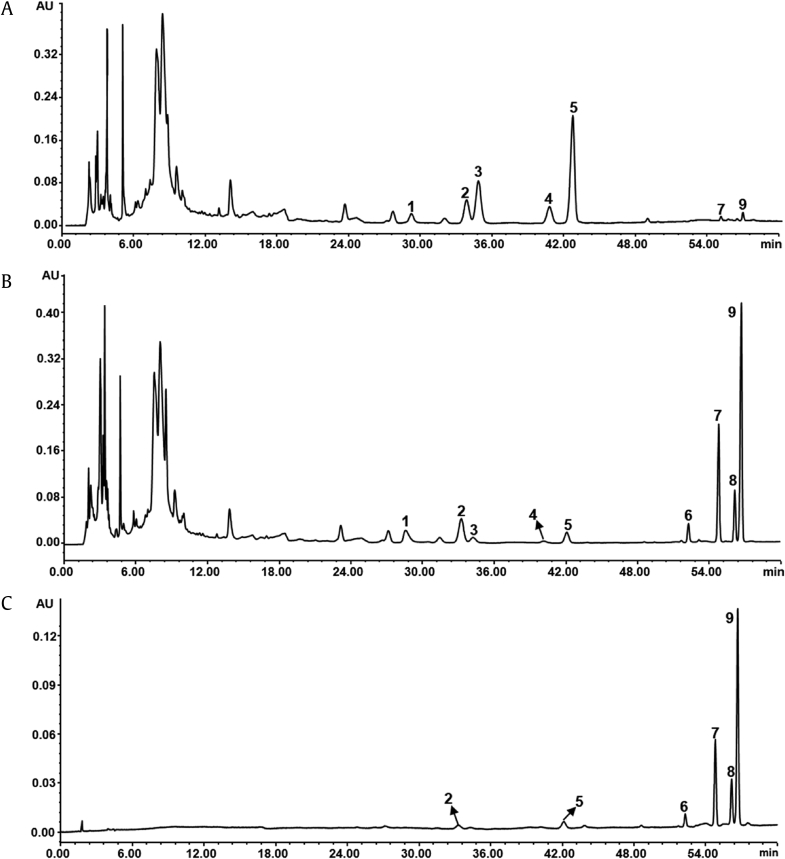
An HPLC-UV method was developed to determine the nine saponins in PNL extracts and fractions. In a large number of studies, notoginsenoside Fc (2) and ginsenoside Rc (3), two main components of PNL, could not be well separated [19], [20]. In the present study, the good chromatographic separation of all saponins investigated was achieved on a C8 column with a total analytical duration of 60 min (Fig. 2A). The linearity, regression, and linear ranges of the nine saponins in the developed HPLC-UV method were measured and are shown in Table 1. The correlation coefficient values (R2 > 0.9994) indicated good linearity between the concentrations of the investigated saponins and their peak areas within the test ranges. Meanwhile, the established method shown good repeatability for quantification of the nine saponins with an overall precision of less than 3.2% relative standard deviation.
Fig. 2.
HPLC-UV chromatograms of (A) mixed standards containing nine saponins; (B) ethanol extract of Panax notoginseng leaves (PNL), and (C) ethanol extract of PNL after water immersion. 1, ginsenoside Rb1; 2, notoginsenoside Fc; 3–5, ginsenosides Rc, Rb2, Rb3; 6, gypenoside XVII; 7, notoginsenoside Fe; 8, ginsenoside Rd2; and 9, notoginsenoside Fd. HPLC, high-performance liquid chromatography.
Table 1.
Standard curves for the nine saponins investigated
| Saponins | RT (min) | Standard curves 1) | R2 | Test range (mg/mL) |
|---|---|---|---|---|
| G-Rb1 | 29.02 | y = 3 × 106x + 6181 | 0.9999 | 0.004–0.288 |
| NG-Fc | 33.56 | y = 3 × 106x + 21,489 | 0.9998 | 0.010–1.230 |
| G-Rc | 34.52 | y = 3 × 106x + 1,390 | 1 | 0.011–0.700 |
| G-Rb2 | 40.36 | y = 3 × 106x – 5,182 | 0.9996 | 0.009–0.300 |
| G- Rb3 | 42.29 | y = 3 × 106x + 22,689 | 0.9995 | 0.021–1.350 |
| GY-XVII | 52.33 | y = 3 × 106x + 7,461 | 0.9995 | 0.004–0.513 |
| NG Fe | 54.83 | y = 2 × 106x + 13,941 | 0.9996 | 0.009–1.100 |
| G- Rd2 | 56.19 | y = 2 × 106x + 6,370 | 1 | 0.001–0.625 |
| NG-Fd | 56.69 | y = 4 × 106x + 34,454 | 0.9994 | 0.010–1.300 |
G, ginsenoside; GY, gypenoside; NG, notoginsenoside; RT, retention time.
y: peak area; x: concentration (mg/mL).
As shown in Fig. 2B and 2C, the HPLC profiles of the two extracts were remarkably different. Ginsenoside Rb1 (1), notoginsenoside Fc (2), and ginsenosides Rc (3), Rb2 (4), and Rb3 (5) were found as major saponins, while gypenoside XVII (6), notoginsenoside Fe (7), ginsenoside Rd2 (8), and notoginsenoside Fd (9) were the minor components in ethanol extract, which reflects the real chemical profile of PNL. Compared to ethanol extract, the contents of Compounds 1 and 3–5 were decreased and those of Compounds 6–9 were greatly increased in ethanol extract after water immersion. These findings suggested that ginsenoside transformations might occur during water immersion.
3.2. Biotransformations of ginsenosides in PNL
The comparative study of the HPLC profiles of the ethanol extract and the ethanol extract after water immersion indicated that severe transformations of ginsenosides might occur when PNL was immersed in the distilled water. Specifically, ginsenosides Rb1 (1), Rc (3), Rb2 (4), and Rb3 (5), the major saponins in PNL, might be transformed to gypenoside XVII (6), notoginsenoside Fe (7), ginsenoside Rd2 (8), and notoginsenoside Fd (9), respectively, by removing a glucose residue from position C-3 via mild acid/base hydrolysis or enzymatic hydrolysis (Fig. 1). Neither acid nor base was added in immersion solvent, it was presumed that these transformations were driven by a ginsenoside-hydrolyzing glycosidase that could selectively cleave the β (1→2)-glucosidic linkage at position C-3 of ginsenosides and this enyzme might be present in the PNL itself.
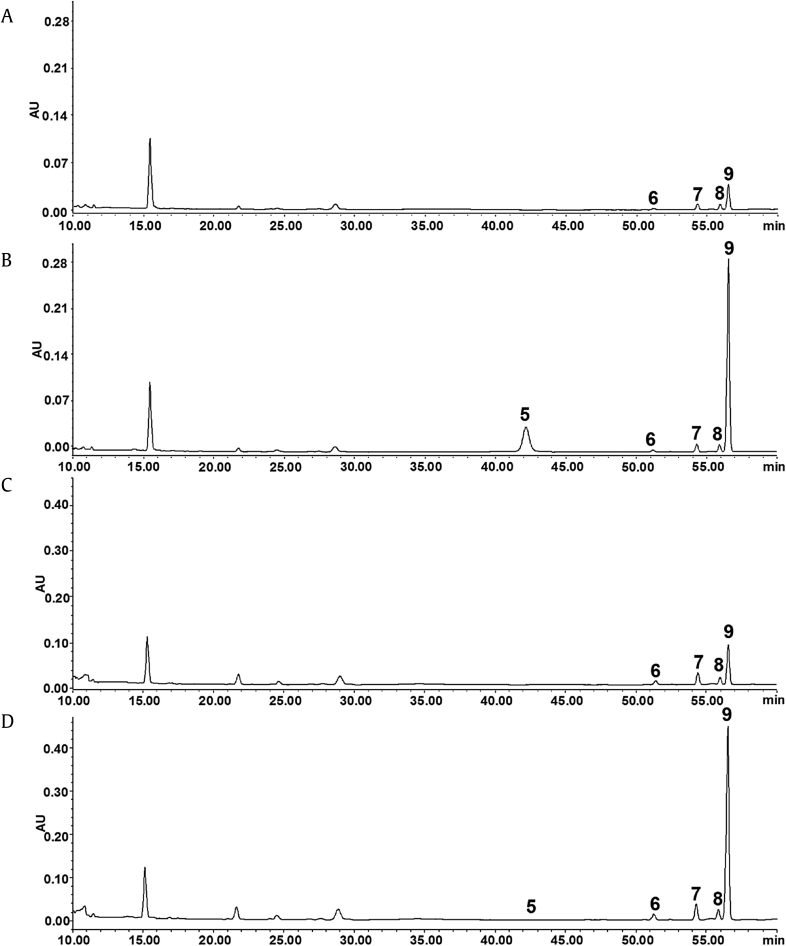
To investigate whether the ginsenoside biotransformation was associated with a protein of PNL, the total protein was extracted from both the dried and fresh PNL with a one-step plant active protein extraction kit (Sangon Biotech Co., Ltd., Shanghai, P.R. China) according to the manufacturer's directions. Ginsenoside Rb3 (1 mg/mL) was added into the total protein solution and incubated in a water bath at 50°C for 30 min. After incubation, ethanol was added to the solution to terminate the biotransformation process. The mixture was centrifuged at 12,000 rpm for 10 min to remove any solid debris, and the supernatant was subjected to HPLC-UV analysis to examine the possible biotransformation. As shown in Fig. 3, the content of notoginsenoside Fd (9) in the protein solution spiked with ginsenoside Rb3 was the higher than that in control protein solution, which indicated that ginsenoside Rb3 was converted to notoginsenoside Fd after the incubation with the total protein extracted from either the fresh or the dried PNL. These data indicate that total protein isolated from PNL is responsible for ginsenoside biotransformation. However, whether the bioactive protein is derived from the PNL plant, or epiphytic or endophytic bacteria, remains to be investigated in further study.
Fig. 3.
HPLC-UV chromatograms of total protein extracted from the dried Panax notoginseng leaves (PNL) (A) before and (B) after the incubation of ginsenoside Rb3. HPLC-UV chromatograms of total protein extracted from the fresh PNL (C) before and (D) after the incubation of ginsenoside Rb3. 5, ginsenoside Rb3; 6, gypenoside XVII; 7, notoginsenoside Fe; 8, ginsenoside Rd2, and 9, notoginsenoside Fd.
HPLC, high-performance liquid chromatography.
3.3. Optimization of the biotransformation conditions
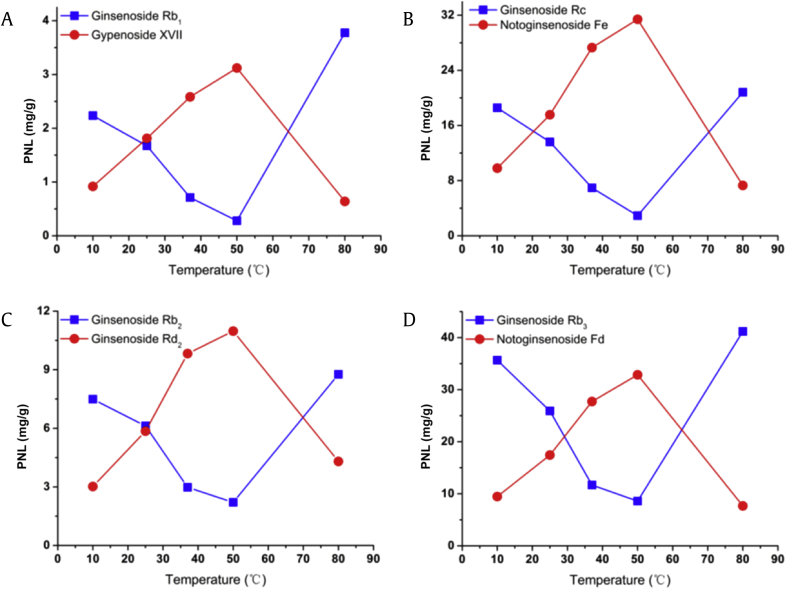
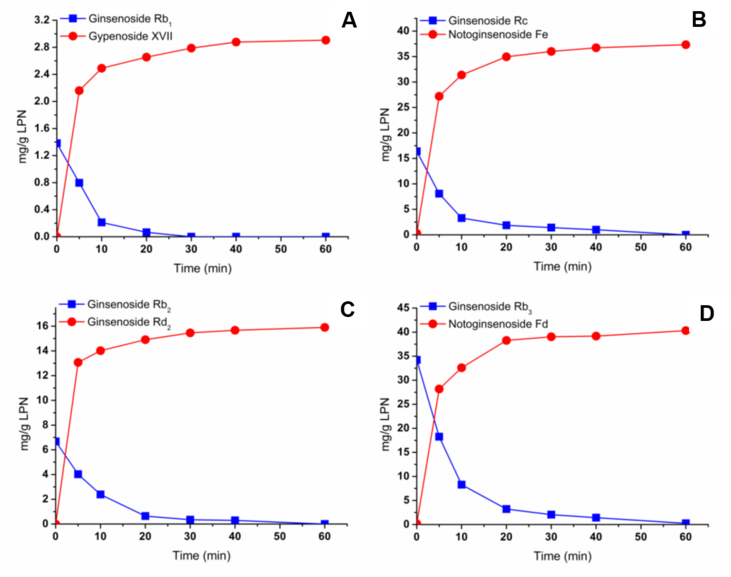
The biotransformation conditions, including immersion temperature and immersion time, were optimized to obtain the maximum yield of four target ginsenosides in PNL. The effects of reaction temperature ranging from 10°C to 80°C in water immersion on the ginsenoside transformation were examined and are shown in Fig. 4. The contents of gypenoside XVII (6), notoginsenoside Fe (7), ginsenoside Rd2 (8), and notoginsenoside Fd (9) gradually increased with the increasing temperature from 10°C to 50°C along with the decreasing contents of ginsenosides Rb1 (1), Rc (3), Rb2 (4), and Rb3 (5). However, the production of the four target saponins dramatically decreased at 80°C, suggesting the possible inactivation of the ginsenoside-hydrolyzing glycosidase at high temperature (Fig. 4). The optimum temperature for maximal biotransformation of the four target saponins was 50°C, but not the 37°C expected [21], [22].
Fig. 4.
Effects of the immersion temperature on the transformations from ginsenosides (A) Rb1 to gypenoside XVII; (B) Rc to notoginsenoside Fe; (C) Rb2 to ginsenoside Rd2; and (D) Rb3 to notoginsenoside Fd in Panax notoginseng leaves (PNL).
At the optimal temperature of 50°C, the effects of reaction time ranging from 5 min to 60 min on the transformation of the four target saponins in PNL were also examined. As shown in Fig. S1, the contents of the four target saponins dramatically increased in the initial 10 min and reached the reaction equilibrium at 30 min, which indicated that the activity of the ginsenoside-hydrolyzing glycosidase present in PNL was greatly efficient.
3.4. Selection of optimal resin
The resins were initially evaluated by adsorption capacity, desorption capacity, and desorption ratios to the four target saponins, which were calculated according to the following Eqs. (1), (2), (3), respectively:
| (1) |
| (2) |
| (3) |
Here, Qe (mg/g dry resins) is the adsorption capacity at the adsorption equilibrium; C0 and Ce (mg/mL) are the initial and equilibrium concentrations of target saponins in the solutions, respectively; Qd (mg/g dry resins) and D (%) are the desorption capacity and the desorption ratio, respectively; Cd (mg/mL) is the concentration of the solutes in the desorption solutions; Vi and Vd (mL) are the volumes of the initial sample solutions and desorption solutions, respectively; and W (g) is the weight of the dried adsorbent.
The four target saponins have a relatively low polarity, and the resins with low polarity possessed the better adsorption capacity. As shown in Table S2, among these resins, D-101 resin exhibited the highest adsorption and desorption capacities towards the four target saponins as well as the best average desorption ratio. Therefore, D-101 resin was selected and used for further study.
3.5. Adsorption isotherms
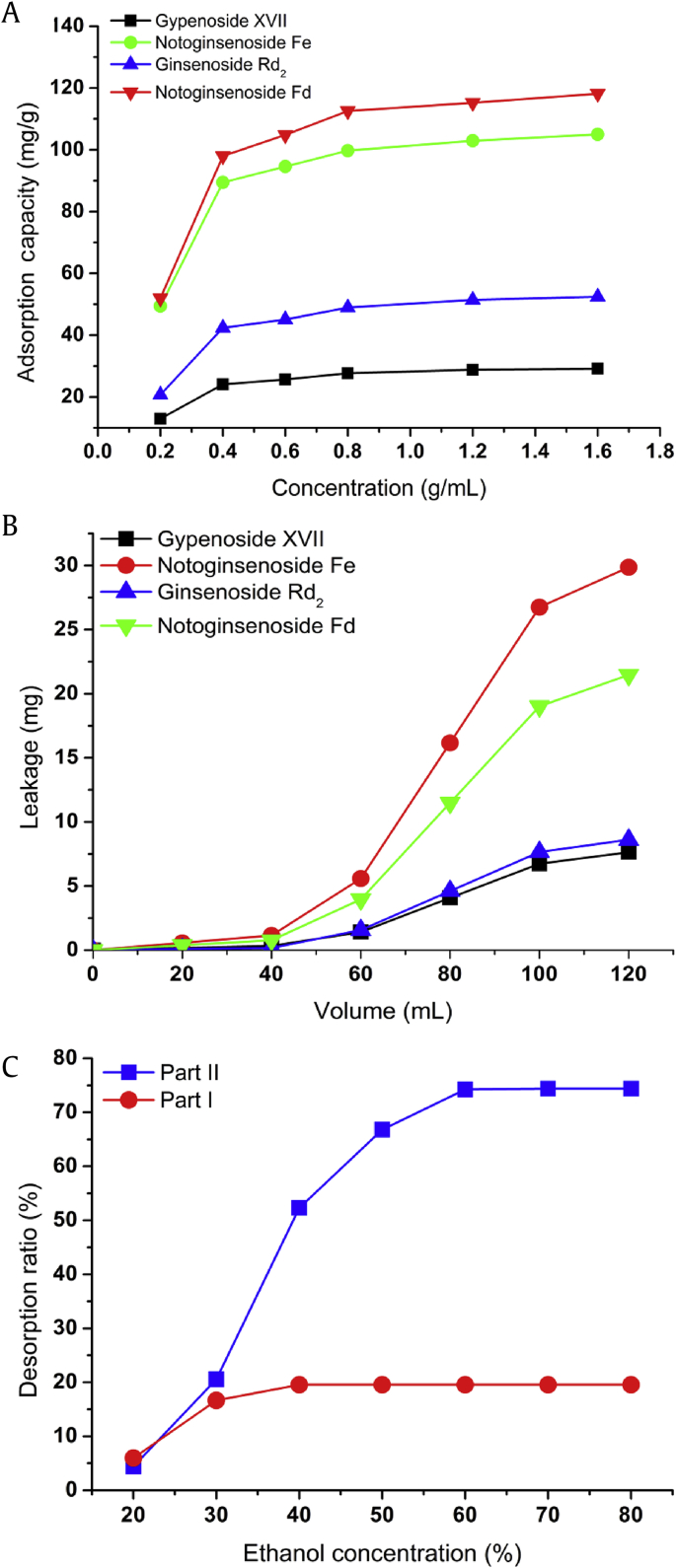
To further illuminate the adsorption property of D-101 resin, the equilibrium adsorption isotherms on D-101 resin were investigated with six different concentrations of PNL extracts at 25°C. As shown in Fig. 5A, the adsorption capacities of the four target saponins in the PNL extract on D-101 resin increased when the initial concentration of the PNL extract increased and reached saturation status when the concentration of the PNL extract was 0.8 g/mL. Two common theoretical models for modeling adsorption equilibrium data, the Langmuir and Freundlich equations, are extensively used to reveal the adsorption behavior of a monomolecular layer and the adsorption behavior of a combination of a multimolecular layer and monomolecular layer, respectively [23], [24]. The Langmuir and Freundlich equations are shown as the following Eqs. (4), (5), (6).
Fig. 5.
Adsorption isotherms (A) of the four target saponins, gypenoside XVII, notoginsenoside Fe, ginsenoside Rd2, and notoginsenoside Fd, on D-101 resin at 25°C. Dynamic leakage curves of the four target saponins from the Panax notoginseng leaves (PNL) extract (B) on D-101 resin; and (C) the static desorption ratios of impurity (Part I) and target saponins (Part II) from the PNL extract on D-101 resin by using different concentrations of aqueous ethanol.
Freundlich equation:
| (4) |
| (5) |
where KF is a Freundlich constant that reflects the adsorption capacity and 1/n is a value calculated from the slope in the linear regression results and is associated with the adsorption intensity of resins. A linearized form of Eq. (4) can be described as Eq. (5), and the linear regression is constructed by plotting log Qe versus log Ce.
Langmuir equation:
| (6) |
where KL is the adsorption equilibrium constant, Qm is the theoretical maximum adsorption capacity (mg/g resin), and Qe and Ce are the same as mentioned above. The linear regression is constructed by calculating from the slope and intercept of a linear plot of Ce/Qe versus Ce.
As shown in Table 2, the correlation coefficients (R2) of the four target saponins were higher than 0.95, suggesting that the isothermal adsorption equation of D-101 resin fits well with both the Langmuir and Freundlich models. Furthermore, the calculated R2 values of the Langmuir equation for the target saponins (0.9986–0.9997) were slightly higher than those of the Freundlich equation (0.9584–0.9969), which implied that the Langmuir model could explain the adsorption process reasonably well. In addition, adsorption and desorption can occur easily when the 1/n value of the Freundlich model is between 0.1 and 0.5. In the present study, the 1/n values varied from 0.1 to 0.2, indicating that D-101 resin exhibits good adsorption and desorption ability of the four target compounds.
Table 2.
Langmuir and Freundlich adsorption parameters of the four target saponins on D-101 resin at 2°C
| Compounds | Langmuir equation | R2 | Freundlich equation | 1/n | R2 |
|---|---|---|---|---|---|
| GY-XVII | Qe = 29.x6Ce/(Ce + 0.0888) | 0.9997 | Qe = 25.3Ce0.1122 | 0.1122 | 0.9821 |
| NG-Fe | Qe = 114.9Ce/(Ce + 0.4942) | 0.9989 | Qe = 83.1Ce0.1076 | 0.1076 | 0.9935 |
| G-Rd2 | Qe = 57.5Ce/(Ce + 0.2414) | 0.9986 | Qe = 42.3Ce0.1609 | 0.1609 | 0.9584 |
| NG-Fd | Qe = 128.2Ce/(Ce + 0.4487) | 0.9991 | Qe = 94.0Ce0.1026 | 0.1026 | 0.9969 |
G, ginsenoside; GY, gypenoside; NG, notoginsenoside.
3.6. Breakthrough volume
The breakthrough volume represents the sample volume that can be preconcentrated without loss of analytes during the sample loading. The breakthrough point is generally defined as the exit adsorbate concentration reaching 1% of the inlet concentration [25]. The leakage curves of the four target compounds are shown in Fig. 5B. The breakthrough volume of the target components on D-101 resin was determined and calculated as 40 mL (approximately 2 BVs).
3.7. Optimization of ethanol concentration for the desorption process
To optimize the appropriate ethanol concentration to separate impurities (Part I, mainly consists of ginsenoside Fc and unconverted ginsenosides Rb1, Rc, Rb2, and Rb3) and the four target saponins (Part II), different concentrations of aqueous ethanol ranging from 20% to 80% (v/v) were used to perform the desorption test after adsorption equilibrium. The total contents of Compounds 1–5 were calculated as the impurity in the final product and named as Part I, and the masses of Compounds 6–9 were considered to be the target minor saponins of PNL and named as Part II. As shown in Fig. 5C, the desorption ratios of both Part I and Part II on D-101 resin gradually increased with the increasing ethanol concentration. At a concentration of 30% aqueous ethanol, the desorption of Part I nearly reached the maximum; however, the desorption ratio of Part II was relatively low. When the ethanol concentration was over 30%, the desorption of Part II increased dramatically and reached a peak value at 60% ethanol. Thus, 30% and 60% aqueous ethanol solutions were selected as the appropriate desorption solutions for the impurities and target saponins, respectively.
3.8. Preparation of the refined fraction containing the four target saponins
Under the above-determined optimal conditions, small-scale preparation of the fraction containing the four target saponins was conducted using a glass column (45 cm × 6 cm i.d.). After biotransformation, ginsenosides Rc (3), Rb2 (4), and Rb3 (5) in PNL were largely transformed to notoginsenoside Fe (7), ginsenoside Rd2 (8), and notoginsenoside Fd (9), respectively (Fig. 6A and B). After the D-101 resin treatment, a large number of polar compounds as well as notoginsenoside Fc were successfully removed, and the four target saponins (6–9) were enriched in the refined fraction (Fig. 6C). A final product of the fraction containing the four target saponins (9.3 g) was obtained with a recovery of 88.5%, in which the contents of gypenoside XVII (6), notoginsenoside Fe (7), ginsenoside Rd2 (8), and notoginsenoside Fd (9) were 3.2%, 20.6%, 11.6%, and 50.5%, respectively.
Fig. 6.
HPLC chromatograms of (A) ethanol extract of Panax notoginseng leaves (PNL); (B) ethanol extract after ginsenoside biotransformation; and (C) and the refined fraction by D101 resins. 1, ginsenoside Rb1; 2, notoginsenoside Fc; 3–5, ginsenosides Rc, Rb2, Rb3; 6, gypenoside XVII; 7, notoginsenoside Fe; 8, ginsenoside Rd2; and 9, notoginsenoside Fd. HPLC, high-performance liquid chromatography.
3.9. Purification of individual target saponins by pre-HPLC
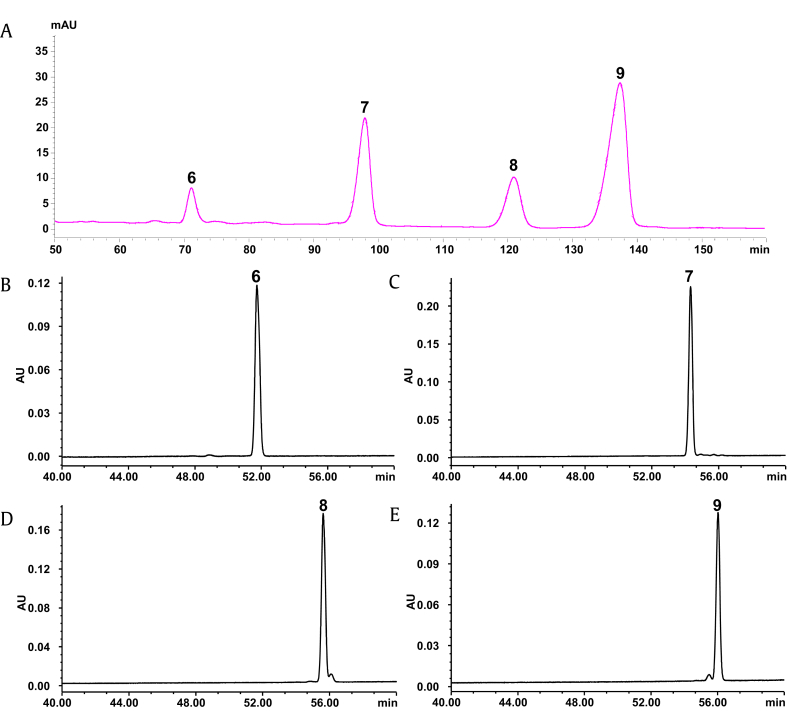
Under the optimized conditions, gypenoside XVII (6), notoginsenoside Fe (7), ginsenoside Rd2 (8), and notoginsenoside Fd (9) were separated with satisfactory resolution in pre-HPLC (Fig. 7A). The peaks corresponding to these target saponins were individually collected and combined. The purities of these four saponins were 99.1%, 99.4%, 95.7%, and 96.7%, respectively, as examined by HPLC-UV (Fig. 7B and D). The purified target saponins were also identified by their mass spectra and a comparison of the retention times of the peaks with those of the reference compounds eluted under the same conditions in HPLC-UV.
Fig. 7.
The chromatograms of: (A) the refined extract by preparative HPLC; (B) individually purified gypenoside XVII (6); (C) notoginsenoside Fe (7); (D) ginsenoside Rd2 (8); and (E) notoginsenoside Fd (9). HPLC, high-performance liquid chromatography.
In conclusion, we developed an efficient method for the simultaneous preparative separation of minor saponins from PNL, including gypenoside XVII (6), notoginsenoside Fe (7), ginsenoside Rd2 (8), and notoginsenoside Fd (9), using biotransformation, macroporous resins, and subsequent pre-HPLC. In the biotransformation step, water immersion was necessary, and the optimal immersion temperature and duration were 50°C and 30 min, respectively. Macroporous resins were used to remove impurities and to enrich the target saponins. Among five macroporous resins tested, D-101, a nonpolar resin, showed the best separation behavior. The adsorption isotherms of the four target compounds on D-101 resin at 25°C were fitted to Langmuir and Freundlich equations. Several parameters, including the volume of feed sample and the ethanol concentration for elution, were optimized for the most effective separation of the fraction containing the target saponins. Subsequently, the individual saponins, including gypenoside XVII (6), notoginsenoside Fe (7), ginsenoside Rd2 (8), and notoginsenoside Fd (9), were successfully separated and purified by pre-HPLC with a reversed-phase column. The preparative separation of these minor saponins from PNL will provide an ample quantity of highly purified references for their quality control and pharmacological studies.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Acknowledgments
We are grateful to Fancheng Meng and Hongwei Gao from our institute for their expert technical assistance and for having provided essential reagents. This work was financially supported by the Research Committee of the University of Macau under Grant MYRG2016-00042-ICMS-QRCM, the National Natural Science Foundation of China (NSFC, Number 81503288) and opening fund of the State Key Laboratory of Quality Research in Chinese Medicine, University of Macau (Number 007).
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jgr.2017.09.003.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Fig. S1.
Effects of the immersion duration on the transformations from ginsenosides Rb1 (A), Rc (B), Rb2 (C) and ginsenoside Rb3 (D) to gypenoside XVII, notoginsenoside Fe, ginsenoside Rd2 and notoginsenoside Fd, respectively, in PNL.
References
- 1.Ma W.G., Mizutani M., Malterud K.E., Lu S.L., Ducrey B., Tahara S. Saponins from the roots of Panax notoginseng. Phytochemistry. 1999;52:1133–1139. [Google Scholar]
- 2.Qi L.W., Wang C.Z., Yuan C.S. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;80:947–954. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 3.Wan J., Yang F., Li S., Wang Y., Cui X. Chemical characteristics for different parts of Panax notoginseng using pressurized liquid extraction and HPLC-ELSD. J Pharm Biomed Anal. 2006;41:1596–1601. doi: 10.1016/j.jpba.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 4.Wan J.B., Zhang Q.W., Hong S.J., Li P., Li S.P., Wang Y.T. Chemical investigation of saponins in different parts of Panax notoginseng by pressurized liquid extraction and liquid chromatography-electrospray ionization-tandem mass spectrometry. Molecules. 2012;17:5836–5853. doi: 10.3390/molecules17055836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu Y.X., Shi J.S., Jiang Z.L. Inhibitory influence of ginsenoside Rb3 on activation of strychnine-sensitive glycine receptors in hippocampal neurons of rat. Brain Res. 2005;1037:99–106. doi: 10.1016/j.brainres.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 6.Xiang H., Liu Y., Zhang B., Huang J., Li Y., Yang B., Huang Z., Xiang F., Zhang H. The antidepressant effects and mechanism of action of total saponins from the caudexes and leaves of Panax notoginseng in animal models of depression. Phytomedicine. 2011;18:731–738. doi: 10.1016/j.phymed.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 7.Wang M., Zhang X.J., Liu F., Hu Y.J., He C.W., Li P., Su H.X., Wan J.B. Saponins isolated from the leaves of Panax notoginseng protect against alcoholic liver injury via inhibiting ethanol-induced oxidative stress and gut-derived endotoxin-mediated inflammation. J Funct Foods. 2015;19:214–224. [Google Scholar]
- 8.Mao Q., Yi L., L S.L., Ye J., Zhang P.H., Wang Q. Chemical profiles and anticancer effects of saponin fractions of different polarity from the leaves of Panax notoginseng. Chin J Nat Med. 2014;12:30–37. doi: 10.1016/S1875-5364(14)60006-6. [DOI] [PubMed] [Google Scholar]
- 9.Mao Q., Yang J., Cui X.M., Li J.J., Qi Y.T., Zhang P.H., Wang Q. Target separation of a new anti-tumor saponin and metabolic profiling of leaves of Panax notoginseng by liquid chromatography with eletrospray ionization quadrupole time-of-flight mass spectrometry. J Pharma Biomed Anal. 2012;59:67–77. doi: 10.1016/j.jpba.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Liu F., Ma N., He C., Hu Y., Li P., Chen M., Su H., Wan J.B. Qualitative and quantitative analysis of the saponins in Panax notoginseng leaves using ultra-performance liquid chromatography coupled with time-of-flight tandem mass spectrometry and high performance liquid chromatography coupled with UV detector. J Ginseng Res. 2018;42:149–157. doi: 10.1016/j.jgr.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi S.H., Shin T.J., Hwang S.H., Lee B.H., Kang J.Y., Kim H.J., Oh J.W., Bae C.S., Lee S.H., Nah S.Y. Differential effects of ginsenoside metabolites on HERG K+ channel currents. J Ginseng Res. 2011;35:191–199. doi: 10.5142/jgr.2011.35.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim M., Ahn B.Y., Lee J.S., Chung S.S., Lim S., Park S.G., Jung H.S., Lee H.K., Park K.S. The ginsenoside Rg3 has a stimulatory effect on insulin signaling in L6 myotubes. Biochem Biophys Res Commun. 2009;389:70–73. doi: 10.1016/j.bbrc.2009.08.088. [DOI] [PubMed] [Google Scholar]
- 13.Quan K., Liu Q., Wan J.Y., Zhao Y.J., Guo R.Z., Alolga R.N., Li P., Qi L.W. Rapid preparation of rare ginsenosides by acid transformation and their structure-activity relationships against cancer cells. Sci Rep. 2015;5:8598. doi: 10.1038/srep08598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Z., Zhao T., Liu H., Zhang L. Ginsenoside Rh2 inhibits hepatocellular carcinoma through beta-catenin and autophagy. Sci Rep. 2016;6 doi: 10.1038/srep19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu C.Z., Yu K.H., Sun C., Zhang T., Xu L., Jin Y., Im W.T., Jin F. Preparation of minor ginsenosides C-Mx and C-K from notoginseng leaf ginsenosides by a special ginsenosidase type-I. Process Biochem. 2015;50:2158–2167. [Google Scholar]
- 16.Liu C.Y., Zhou R.X., Sun C.K., Jin Y.H., Yu H.S., Zhang T.Y., Xu L.Q., Jin F.X. Preparation of minor ginsenosides C-Mc, C-Y, F2, and C-K from American ginseng PPD-ginsenoside using special ginsenosidase type-I from Aspergillus niger g.848. J Ginseng Res. 2015;39:221–229. doi: 10.1016/j.jgr.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L., Liu Q.M., Sung B.H., An D.S., Lee H.G., Kim S.G., Kim S.C., Lee S.T., Im W.T. Bioconversion of ginsenosides Rb(1), Rb(2), Rc and Rd by novel beta-glucosidase hydrolyzing outer 3-O glycoside from Sphingomonas sp. 2F2: cloning, expression, and enzyme characterization. J Biotechnol. 2011;156:125–133. doi: 10.1016/j.jbiotec.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 18.Han Y., Sun B., Jiang B., Hu X., Spranger M.I., Zhang Y., Zhao Y. Microbial transformation of ginsenosides Rb1, Rb3 and Rc by Fusarium sacchari. J Appl Microbiol. 2010;109:792–798. doi: 10.1111/j.1365-2672.2010.04707.x. [DOI] [PubMed] [Google Scholar]
- 19.Upadhyaya J., Yoon M.S., Kim M.J., Ryu N.S., Song Y.E., Kim Y.H., Kim M.K. Purification and characterization of a novel ginsenoside Rc-hydrolyzing beta-glucosidase from Armillaria mellea mycelia. AMB Express. 2016;6:112. doi: 10.1186/s13568-016-0277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pang Z., Gao Y.L., Wang Y., Huang S.F. HPLC-UV analysis of main saponins from Panax notoginseng leaves after absorption of 5 macroporous resins. Zhong Cheng Yao. 2009;31:1130–1132. [Google Scholar]
- 21.Zhang C., Yu H., Bao Y., An L., Jin F. Purification and characterization of ginsenoside-beta-glucosidase from ginseng. Chem Pharm Bull. 2001;49:795–798. doi: 10.1248/cpb.49.795. [DOI] [PubMed] [Google Scholar]
- 22.Sunwoo H.H., Gujral N., Huebl A.C., Kim C.T. Application of high hydrostatic pressure and enzymatic hydrolysis for the extraction of ginsenosides from fresh ginseng root (Panax ginseng C.A. Myer) Food Bioprocess Tech. 2014;7:1246–1254. [Google Scholar]
- 23.Li H., Liu Y., Yi Y., Miao Q., Liu S., Zhao F., Cong W., Wang C., Xia C. Purification of quercetin-3-O-sophoroside and isoquercitrin from Poacynum hendersonii leaves using macroporous resins followed by Sephadex LH-20 column chromatography. J Chromatogr B. 2017;1048:56–63. doi: 10.1016/j.jchromb.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 24.Bai C.L., Zhao G.R. Separation of salvianic acid A from the fermentation broth of engineered Escherichia coli using macroporous resins. J Sep Sci. 2015;38:2833–2840. doi: 10.1002/jssc.201500416. [DOI] [PubMed] [Google Scholar]
- 25.Wan J.B., Zhang Q.W., Ye W.C., Wang Y.T. Quantification and separation of protopanaxatriol and protopanaxadiol type saponins from Panax notoginseng with macroporous resins. Sep Purif Technol. 2008;60:198–205. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.