Abstract
Hepatitis C virus (HCV) infection remains a major public health issue despite the introduction of several direct-acting antiviral agents (DAAs), with some 185 million individuals infected with HCV worldwide. There is an urgent need for an effective prophylactic HCV vaccine. In the present study, we constructed genetic vaccines based on novel recombinant adeno-associated viral (rAAV) vectors (AAV2/8 or AAV2/rh32.33) that express the envelope glycoprotein E2 from the HCV genotype 1b. Expression of HCV E2 protein in 293 cells was confirmed by western blot analysis. rAAV2/8.HCV E2 vaccine or rAAV2/rh32.33.HCV E2 vaccine was intramuscularly injected into C57BL/6 mice. HCV E2-specific antigen was produced, and long-lasting specific antibody responses remained detectable XVI weeks following immunization. In addition, the rAAV2/rh32.33 vaccine induced higher antigen-specific antibody levels than the rAAV2/8 vaccine or AAV plasmid. Moreover, both AAV vaccines induced neutralizing antibodies against HCV genotypes 1a and 1b. Finally, it is worth mentioning that neutralizing antibody levels directed against AAV2/rh32.33 were lower than those against AAV2/8 in both mouse and human serum. These results demonstrate that AAV vectors, especially the AAVrh32.33, have particularly favorable immunogenicity for development into an effective HCV vaccine.
Keywords: hepatitis C virus, glycoprotein E2, adeno-associated virus, prophylactic vaccine
Introduction
Hepatitis C virus (HCV) infects approximately 185 million people worldwide and is a leading cause of liver cirrhosis and hepatocellular carcinoma (1). Yet, no effective HCV vaccine is available. Although the introduction of new direct-acting antiviral agents (DAAs) is highly effective in the treatment of all HCV genotype infections, their use is limited by high cost, treatment accessibility and potential drug resistance (2). Furthermore, patients cured following DAA use are susceptible to reinfection (3). Therefore, an effective prophylactic vaccine is necessary to protect against HCV infection.
HCV, a positive-stranded RNA virus, exists as seven major genotypes and numerous subtypes. However, the high genetic variability of the HCV genome presents many challenges for vaccine development (4). Previous studies have shown that the T-cell immune response plays a crucial role in HCV clearance (5,6). It is now known that timely production of cross-neutralizing antibodies (NAbs) are associated with viral resolution (7).
HCV envelope glycoproteins E1 and E2 form a heterodimer on the surface of HCV. Various types of E1E2-based vaccine candidates have been tested. However, a phase I study with recombinant HCV E1/E2 envelope glycoprotein as a candidate vaccine did not induce a strong immune response in volunteers (8,9). E2, the larger one of the two envelope proteins, interacts directly with cellular receptors CD81 and scavenger receptor class B member 1 (SR-B1) to mediate viral entry (10). E2 is an optimal antigen candidate for HCV vaccination because it possesses most NAb-recognized epitopes (11–13). To date, a number of HCV vaccine candidates based on E2 have been explored. A DNA vaccine encoding HCV E2 has been shown to induce specific antibody responses in mice (14). Prime-boost immunization with the virus-like particles (VLPs) containing E2, E1, or both elicited NAbs in non-human primates (15). A subunit vaccine based on soluble E2 (sE2) of the Con1 strain (GT1b) induces NAbs in mice and rhesus monkeys (16,17). A recent study also demonstrated that a trivalent HCV vaccine containing sE2 from genotypes 1a, 1b and 3a elicited a broad, synergistic polyclonal antibody response in mice and rhesus monkeys (18).
Viral vector vaccines have been demonstrated to induce strong cellular and humoral immune responses. A variety of viral vectors, such as adenovirus, poxvirus and measles, have been tested as platforms for HCV vaccination (19,20). In recent years, the adeno-associated virus (AAV) vector, a single-stranded DNA virus from the Parvoviridae family, has emerged as an attractive agent for vaccine development owing to its long-term persistence, high efficiency, low immunogenicity and lack of pathogenicity in gene delivery studies. Moreover, AAVs are also able to infect both non-dividing and dividing cells in the liver, muscle and brain (21–23). A remarkable feature of AAV vector vaccines is their capacity to induce strong and long-lasting antibody responses. Several studies have documented that the induction of humoral responses could last for many months (24–27). Such prominent antibody response may be relevant to the high and sustained expression of the immunogen by most AAV serotypes (27–29). AAV8 has shown remarkable potential as a gene delivery vector in vivo (30). AAVrh32.33, a novel vector isolated from rhesus macaques, has relatively low seroprevalence in humans compared to AAV2 and AAV8 (31,32). Genetic vaccines based on AAV8 and AAVrh32.33 vectors encoding truncated dengue virus envelope proteins have been shown to elicit a long-lasting humoral responses in mice (33).
Previously, we constructed an HCV vaccine based on AAVrh32.33 expressing NS3/4 protein, which exhibits immunogenic properties superior to those of an NS3-protein-based vaccine in C57BL/6 mice (34). In the present study, we continued to focus on AAV vectors and generated AAV2/8 and AAV2/rh32.33 vectors expressing HCV E2 protein. After purification and titration of the two recombinant vectors, we evaluated their humoral immunity induced in C57BL/6 mice.
Materials and methods
Plasmid construction
Serum samples of HCV GT1b were collected from six patients (four females and two males, aged 30–50 years) diagnosed at the Affiliated Hospital of Jining Medical University (Shandong, China) between April and August 2017 after obtaining written informed consent from the HCV-infected patients. The study was approved by the Ethics Committee of Jining Medical University. Total RNA was obtained using a viral RNA Mini kit (Qiagen, Duesseldorf, Germany) according to the manufacturer's protocol. cDNA was synthesized using the PrimeScript II First-Strand cDNA Synthesis kit (Takara Bio Inc., Tokyo, Japan). Next, the HCV E2 gene was amplified by PCR with Pyrobest DNA polymerase (Takara Bio Inc.) and specific primer. Two primer sequences were used: forward, 5′-GGAAGATCTCGCCGCCACCATGGTGGGGAACTGGGC-3′ and reverse, 5′-GTCTAGCGGCCATTAAACTCACGCCTCCGCTTGGGAT-3′. NotI and BglII sites were used to clone the amplicons into the AAV cis-plasmid (a kind gift from Dr Wilson, Gene Therapy Program, Department of Pathology and Laboratory Medicine, University of Pennsylvania, Philadelphia, PA, USA). The identification of AAV recombinants was confirmed by restriction enzyme digestion and sequencing. The recombinant plasmid was named pAAV.CMV.HCV.E2.
Detection of HCV E2 expression in 293 cells
293 cells were plated at a density of 1×106 cells in a 6-well plate with Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and then incubated at 37°C in a 5% CO2 incubator. After 24 h, they were transfected with 4 mg AAV plasmids encoding eGFP (pAAV.CMV.eGFP) or HCV E2 (pAAV.CMV.HCV E2) by Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer's instructions. Seventy-two hours after the transfection, eGFP gene expression was confirmed using a direct fluorescence microscopy (Micro Publisher 3.3 RTV; Olympus Corp., Tokyo, Japan) and HCV E2 gene expression was detected by western blot analysis. The transfected cells were harvested and lysed as samples. After separation using 10% sodium dodecyl-sulfate polyacrylamide gel electrophoresis gels (SDS-PAGE), samples were transferred onto a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked for 2 h with 5% non-fat milk, incubated overnight at 4°C with an anti-HCV E2 monoclonal antibody (cat. no. 1876-E2; 1:100; Virostat; Bio-Lab Laboratories Ltd., Beijing, China), washed three times in Tris-buffered saline with Tween-20 (TBST), and then incubated with the horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary antibody (cat. no. ab6789; 1:10,000; Abcam, Cambridge, MA, USA) for 1 h at room temperature. Finally, protein expression was visualized using Pierce ECL Western Blotting substrate (Pierce Biotechnology, Inc.; Thermo Fisher Scientific, Inc.), quantified using densitometry, and analyzed using Gel-Pro software version 3.2 (Media Cybernetics, Inc., Rockville, MD, USA). Glyceraldehyde 3-phosphate ehydrogenase (GAPDH) was used an internal control in western blot analyses.
Production of rAAV vectors
All rAAV vectors were packaged by triple plasmid transfection in 293 cells, as previously described (35,36). The triple plasmid system is comprised of an AAV cis-plasmid containing HCV E2 cDNA (pAAV.CMV.HCV E2), an adenovirus helper plasmid (pAd.F6), and a chimeric packaging plasmid that contains the AAV2 rep gene and the AAV8 (or AAVrh32.33) cap gene (pAAV2/8 or pAAV2/rh32.33). All plasmids were extracted using a Plasmid Maxi kit (Qiagen) following the manufacturer's instructions. Briefly, 2 h before transfection, at the point when the 293 cells were cultured in 15-cm culture dishes reaching high confluence (70–80%), they were treated with 20 ml DMEM supplemented with 10% FBS without antibiotics. Equimolar plasmids were dissolved in 650 µl of CaCl2 (2.5 M) and 5.9 ml of Milli-Q water, then mixed rapidly with 12.5 ml of 2X 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-buffered saline (pH 7.05) to prepare the transfection solution. A 2.5 ml aliquot of the above transfection solution was gently added to each dish. After slowly swirling the contents to mix, the cells were incubated at 37°C in a 5% CO2 incubator continuously. At 16 h post-transfection, the medium was replaced with fresh DMEM containing 10% FBS and 100 mg/ml streptomycin and penicillin. Another 72 h after medium replacement [at which time eGFP signals were visualized as distinctly shaped foci on fluorescence microscopy (Thermo Fisher Scientific, Inc.)], cells were collected and resuspended with 10 ml of NaCl (150 mM) and Tris (20 mM, pH 8.0). Next, benzonase nuclease (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was added to a final concentration of 50 U/ml to remove nucleic acid contamination. rAAV was obtained from lysed 293 cells by means of three consecutive freeze-thaw-cycles (−80°C and 37°C). The rAAV vectors were purified by three rounds of cesium chloride gradient centrifugation, and then concentrated using Amicon Ultra-15 centrifugal filter devices (100K; Merck Millipore, Billerica, MA, USA). The AAV genome titers [genome copies (GC) per ml] were detected by real-time PCR using Premix Ex Taq (Takara Bio Inc.) corresponding to the polyA region of the AAV vector.
Immunization of mice
All animal procedures were performed in accordance with the protocols approved by the Ethics Committee of Jining Medical University. Female C57BL/6 mice (4–6 weeks of age, weight 15.00±0.31 g) were purchased from SLAC Laboratory Animal Company (Shanghai, China) and housed at a constant temperature with a 12 h dark/light cycle. Food and water were available ad libitum. The mice were randomly divided into 4 groups of 10 mice each. After being anesthetized by inhalation of 1.5% isoflurane in oxygen, two groups of mice were immunized intramuscularly (tibialis anterior muscle of the hind leg) with rAAV2/8.HCV E2 vaccine or rAAV2/rh32.33.HCV E2 vaccine (1×1011 GC per mouse). The other two groups were immunized with pAAV.CMV.HCV.E2 plasmid (100 µg per mouse) or phosphate-buffered saline (PBS) (100 ml per mouse) as controls. At weeks 12 and 16 post-immunization, two mice in each group were sacrificed by inhalant isoflurane overdose (1.5%), for skeletal muscle harvesting from the vaccine injection area. The tissue samples were stored at −80°C for subsequent western blot analysis. Blood samples of mice were collected via retro-orbital puncture under anesthesia as above at weeks 0, 4, 8, 12 and 16 post-immunization and stored at −20°C.
Evaluation of HCV E2-specific antibody reactivity
To measure HCV E2-specific antibody reactivity, mouse sera were evaluated by enzyme-linked immunosorbent assay (ELISA). Briefly, 96-well plates were coated with GST-E2 peptide and incubated overnight at 4°C. Plates were then washed with phosphate-buffered solution (PBST) and blocked with 1% bovine serum albumin (BSA) for 2 h at room temperature. A 1:1,000 dilution anti-mouse IgG HRP-conjugated antibody (cat. no. A5278; Sigma-Aldrich; Merck KGaA) was used as a secondary antibody. Mouse serum samples were diluted to 1:2 and 1:20 for ELISA analysis. After washing with PBST, 3,3′,5,5′-tetramethylbenzidine (Invitrogen; Thermo Fisher Scientific, Shanghai, China) was added to each well. Finally, the plates were read at a wavelength of 450 nm (OD450 nm; ELx800; BioTek Instruments, Inc., Winooski, VT, USA). IgG titers are presented as the reciprocal of the highest serum dilution at which the OD450 nm was higher than twice that of the control.
Evaluation of neutralizing antibody response to hepatitis C virus
To evaluate the neutralizing ability of HCV-specific antibodies, infectious pseudo-particles expressing HCV envelope glycoproteins (HCVpp) with E2 protein of five genotypes [1a (H77), 1b (Hebei), 2a (JFH1), 3a (S52) and 5a (SA13)] were produced to evaluate NAb induction (37,38). After purification in a protein G column, serum samples were serially diluted and incubated with HCVpp for 1 h at 37°C, at which time the mixtures were used to infect Huh7.5 cells. After 48 h, luciferase activity in the infected Huh7.5 cells was detected with a Bright-Glo luciferase assay (Promega, Madison, WI, USA). Data were reported as relative luminescence units. The positive control, AP33, is a monoclonal anti-E2 antibody with broad cross-neutralization ability. Neutralizing capacity was evaluated by comparing the luciferase activity in cells infected with HCVpp that had been incubated with serum from vaccinated animals to those that had been incubated with pre-vaccinated serum from the same mice.
Evaluation of neutralizing antibody response against the AAV vector
Serum samples collected from 20 naive mice and 20 humans were diluted to determine anti-AAV2/rh32.33 and -AAV2/8 vector NAb levels. Briefly, serum samples were inactivated at 56°C for 35 min. The AAV2/8.CMV.eGFP and AAV2/rh32.33.CMV.eGFP vectors were diluted in serum-free DMEM and incubated with serial dilutions (1:20 and 1:80) of serum samples at 37°C for 1 h. Next, 100 µl of the serum-vector mixture was added to the 293 cells that had been infected 2 h earlier with wild-type adenovirus 5. After incubation at 37°C under 5% CO2 for 24 h, eGFP protein expression was evaluated for each group with fluorescence microscopy (Thermo Fisher Scientific, Inc.). NAb titer was determined as the highest serum dilution that inhibited AAV transduction (eGFP expression) by 50%, relative to the mouse serum control.
Statistical analysis
Mean values are reported with standard deviations (SDs). GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA) and SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA) were used for statistical analyses. Differences between groups were examined by Student's t-test for two groups and one-way analysis of variance (ANOVA) followed by a least significant difference test for multiple comparisons. A P-value of <0.05 was considered statistically significant.
Results
Expression of the AAV plasmid encoding the HCV E2 gene in vitro
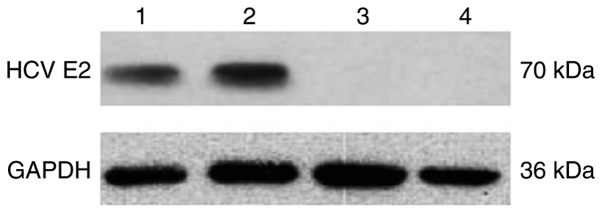
In vitro expression of HCV E2 protein was evaluated by western blot analysis in recombinant AAV plasmid-transfected 293 cell lysates 72 h after transfection. 293 cells transfected with pAAV.CMV.HCV E2 plasmid had a band of ~70 kDa, whereas those transfected with pAAV.CMV.eGFP had no detectable band (Fig. 1).
Figure 1.
Expression of a recombinant HCV E2 gene. Western blot detection of HCV E2 expression in 293 cells after transfection with pAAV.CMV.HCV E2 (lanes 1 and 2) or pAAV.CMV.eGFP (lanes 3 and 4). The HCV E2 protein is approximately 70 kDa. The protein GAPDH (36 kDa) was used as an internal control. The result shown is representative of three experiments.
Comparison of HCV E2 expression among the different vaccines
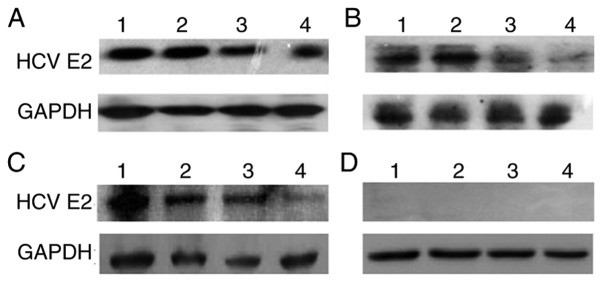
The rAAV final yield ranged from 5.53×1011 GC to 1.02×1012 GC. HCV E2 expression was compared between HepG2 cells infected with rAAV2/8.HCV E2 vaccine or rAAV2/rh32.33.HCV E2 vaccine, respectively (MOI=3×104 GC). HepG2 cells transfected with pAAV.CMV.HCV.E2 plasmid or pAAV.CMV.eGFP plasmid served as positive and negative controls, respectively. After 72 h, the 70-kDa antigen was effectively expressed in cell lysates (data not shown). Western blot analysis of E2 expression in injection-area skeletal muscle (2 mice/group) showed that AAV plasmid (control group) and the two rAAV vaccines (AAV2/rh32.33 and AAV2/8) expressed HCV E2 protein in immunized mice for at least 16 weeks post-immunization (Fig. 2). In addition, the HCV E2 expression level did not differ between the rAAV and the AAV plasmid.
Figure 2.
Comparison of HCV E2 expression across vaccine groups in mouse skeletal muscle. Western blot analysis of injection-area skeletal muscle from 2 mice/group 12 weeks (lanes 1 and 2) and 16 weeks (lanes 3 and 4) after immunization. (A) rAAV2/rh32.33.HCV E2; (B) rAAV2/8. HCV E2; (C) pAAV.CMV.HCV.E2; (D) non-immunized control. The protein GAPDH (36 kDa) was used as an internal control. The result shown is a representative of three experiments.
HCV E2-specific antibody induced by AAV vaccine
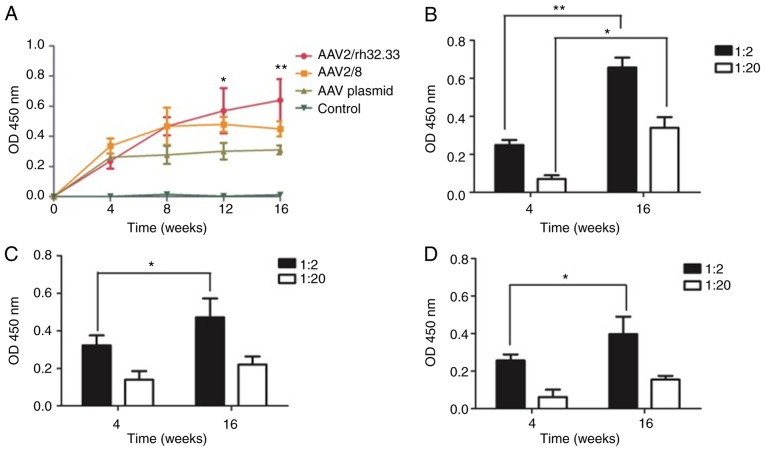
To assess AAV immunogenicity, HCV E2-specific IgG antibody in sera from mice vaccinated with AAV was measured by ELISA. Four groups of mice were immunized with rAAV2/rh32.33.HCV E2 vaccine, rAAV2/8.HCV E2 vaccine, pAAV.CMV.HCV E2 plasmid, or PBS (control). The result showed that antigen expressed by the AAV vaccine elicited HCV E2-specific antibody production that persisted for more than 16 weeks post-vaccination (time course shown in Fig. 3A). All animals immunized with rAAV vaccines or AAV plasmid produced anti-HCV E2 antibody. Furthermore, the antibody level in the rAAV2/rh32.33 vaccinated group was significantly higher than that of the PBS control group at week 12 (P=0.012) and 16 post-immunization (P=0.006). For each group, the finding that antibody levels were higher at week 16 compared to week 4 post-immunization (Fig. 3B-D) indicates that the immunologic effect of each vaccine is time-dependent over a specified time frame. When the serum was diluted out, it was also found that the HCV E2-specific antibody level induced by rAAV2/rh32.33 vaccination was higher than that induced by rAAV2/8 vaccination or AAV plasmid administration, while the HCV E2-specific antibody response induced by rAAV2/8 vaccine was similar to that induced by the AAV plasmid (Fig. 3B-D).
Figure 3.
HCV E2-specific antibody elicited by different AAV vaccines. (A) ELISA-determined time course of mean (± SD) serum anti-HCV E2 IgG levels (n=8). The antibody level induced by the rAAV2/rh32.33 vaccine was higher than that induced by the rAAV2/8 vaccine or AAV plasmid. (B-D) Mean (± SD) antibody level for each group with 1:2 and 1:20 serum dilution (n=8). (B, rAAV2/rh32.33.HCV E2; C, rAAV2/8.HCV E2; D, pAAV.CMV.HCV E2). *P<0.05; **P<0.01.
Neutralizing antibody against HCV induced by AAV vaccine
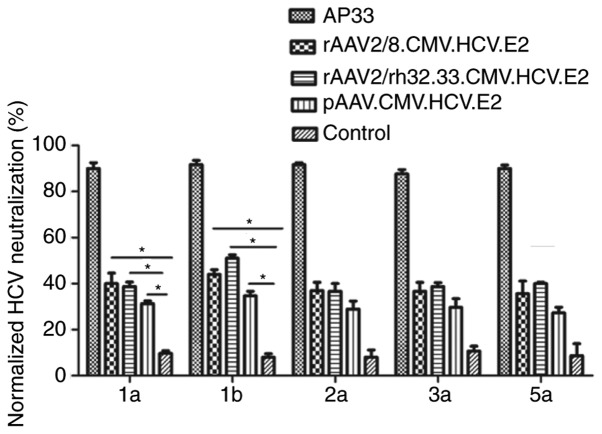
To assess the cross-neutralization ability of HCV-specific antibodies, a luciferase reporter HCVpp with E2 protein of five genotypes (1a, 1b, 2a, 3a and 5a) was generated. Serum samples were collected at weeks 12 and 1:50 diluted antisera were tested for neutralization against HCVpp of five genotypes. As shown in Fig. 4, both AAV vaccines demonstrated significant neutralization of HCVpp derived from HCV genotypes 1a and 1b at week 12.
Figure 4.
Detection of AAV vaccine-induced neutralizing antibody in mouse serum. Cross-neutralization was tested at a dilution of 1:50 for serum sample capacity to neutralize infection with HCVpp of various genotypes. Huh-7.5 cells were infected with 100 TCID50. Mean (± SD) percentages of HCVpp neutralization are shown; *P<0.05.
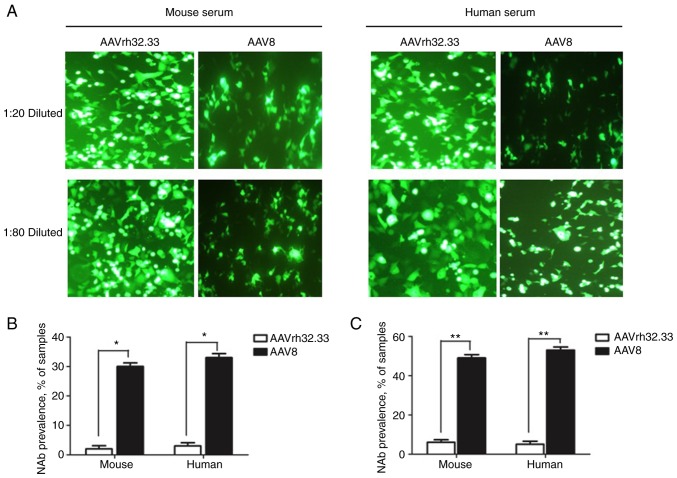
Neutralizing antibody against AAV in human and mouse sera
To evaluate the NAbs to AAV vectors, serum samples from human and naive mice were collected and diluted to detect the levels of NAbs directed against AAV2/rh32.33 and AAV2/8 vectors. The NAb titer was determined as the highest serum dilution that inhibited AAV transduction (eGFP expression) by 50% compared to mouse control serum. As shown in Fig. 5, the eGFP protein expression levels in the AAV2/8 group were inhibited >50%, which is more than that observed for the AAV2/rh32.33 group both at serum dilutions of 1:20 and 1:80. Thus, NAb titers to AAV2/rh32.3 were lower than NAb titers to AAV2/8.
Figure 5.
Neutralizing antibody against AAV vector in human and mice sera. Serum samples from human and naive mice were diluted (1:20 and 1:80) and tested against AAV8 or AAVrh32.33. (A) The eGFP protein expression levels for each group indicated the NAb levels against AAV vectors (magnification, ×200). Samples were considered positive at a serum dilution of 1:20 (B) or 1:80 (C) when vector transduction was inhibited by 50%. *P<0.05; **P<0.01.
Discussion
There are many obstacles to develop a prophylactic hepatitis C virus (HCV) vaccine, such as the extensive variability of the HCV genome and the absence of a suitable small animal model. However, several candidate HCV vaccines have been created, including DNA vaccines, recombinant viral vectors, proteins and virus-like particles (VLPs) (39,40). Vector comparison is valuable to illustrate factors influencing vaccine immunogenicity and to improve genetic vaccination.
HCV vaccines based on recombinant viral vectors have generated promising results in preclinical experiments. To date, four vector vaccines have been able to induce T-cell and B-cell responses in rhesus macaques in prime-boost regimens: DNA, SFV, human serotype 5 adenovirus (HuAd5) and modified vaccinia ankara (MVA) poxvirus expressing HCV core, E1, E2 and NS3 (41). Simian adenoviral vaccine vectors (ChAdOx) encoding genetically conserved gene segments from HCV primed broad, cross-reactive T-cell responses successfully in C57BL/6 mice (42). Relative to these commonly used viral vectors, rAAV vectors have potential advantages for vaccine and gene transfer applications owing to their lower inflammatory potential, availability of viral serotypes with different tissue tropisms, and possible long-lasting gene expression. Additionally, AAV-based genetic vaccines encoding the antigen genes of interest can elicit both humoral and cellular immune responses to the transgene. A genetic HIV vaccine based on AAV vectors induced anti-HIV T-cell responses in mice (43). The use of AAV vectors for delivery of broadly neutralizing HIV antibodies could stimulate long-term, systemic broadly neutralizing antibody in the absence of immunization to prevent HIV infection (28). However, AAV vaccines have been limited by factors such as high sero-prevalence in humans. To date, more than 120 AAV serotypes and variants have been identified; several serotypes, such as AAV8 and AAVrh32.33, show high transduction efficiency but low sero-prevalence (44). Genetic vaccines based on AAV8 and AAVrh32.33 vectors encoding truncated dengue virus envelope proteins have been shown to elicit long-lasting humoral responses in mice (33). Hence, we chose AAV8 and AAVrh32.33 vectors to create HCV vaccines and compared their immunogenicity in C57BL/6 mice by intramuscular injection.
The present study is the first study to show that AAV vectors can express HCV E2 antigen and induce humoral immune responses in mice. In this study, both AAV vaccines produced high absolute levels of HCV E2 antigen in mice. Antigen synthesis lasted for several weeks, and the synthesized antigen provided continuous stimulation to the mouse immune system. However, the HCV E2 expression level did not differ between the rAAV and the DNA. There are a few possible explanations for this finding. Firstly, our AAV vaccine was of low purity and quality. Secondly, the small sample size of immunized mice may have underpowered the study to detect a true difference. We will conduct further experiments to clarify whether a difference does exist. Furthermore, the HCV E2-specific antibody assay showed that the AAV2/rh32.33-based HCV vaccine induced higher levels of E2-specific antibodies than did the AAV2/8 vaccine. Thus, the AAV2/rh32.33-based HCV vaccine appears to possess superior immunogenic properties compared to the AAV2/8-based vaccine. Moreover, there were fewer NAbs to AAVrh32.33 than to AAV8 in mice and human sera, indicating that AAVrh32.33 may be safer than AAV8.
Previously, we developed HCV vaccines based on an AAVrh32.33 vector expressing HCV NS3 or NS3/4b that could elicit strong and persistent T-cell immunity in mice (34). Moreover, there have been studies indicating that an AAVrh32.33 vector encoding antigens of HIV-1 would stimulate stronger immune responses than an AAV8 vector in non-human primates (31). Above all, we speculate that, beyond safety, AAVrh32.33 could stimulate potent transgene production more efficiently. Thus, AAV vectors, especially AAVrh32.33, are attractive potential vectors for HCV vaccine design.
Since humoral immunity and NAbs play a pivotal role in HCV clearance, the identification of conserved epitopes associated with viral neutralization is a crucial factor in the development of an effective prophylactic HCV vaccine. The envelop glycoprotein E2 is the primary target of the anti-HCV NAb response. In past decades, several studies have helped to elucidate the structural features of HCV glycoprotein E2, showing that key epitopes targeted by NAbs on the ‘front layer’ of E2 exhibit structural heterogeneity. Thus, E2 appears to be an ideal antigen candidate for HCV vaccine design (45,46). In this study, we developed HCV vaccines based on AAV vectors expressing E2 protein from GT1b, which is the predominant genotype in the Chinese population, accounting for 56.8% (47). Firstly, we investigated and compared HCV E2 protein expression levels for three different designs. Western blot analysis showed that all constructs produced the 70-kDa E2 protein. We then determined that AAV vaccines encoding HCV E2 could induce high-level production of HCV E2-specific antibodies and NAbs in C57BL/6 mice. Additionally, cross protection against genotypes 1a and 1b were superior to that directed against genotypes 2a, 3a and 5a HCVpp. Our finding is consistent with that of previous research showing that genotype 1 antigen induced weaker neutralization against HCV genotypes 2, 3 and 5 than genotype 1 (48). However, efficient T-cell responses were not detected in immunized mice in this study (data not shown). One possible explanation is that T-cell-specific epitopes are not properly exposed in whole HCV E2 protein. Therefore, further exploration of truncated E2 proteins possessing T-cell-specific epitopes is needed.
In conclusion, we constructed novel AAV-based vaccines encoding E2 protein directed against HCV and confirmed their ability to elicit E2-specific antibody and NAb responses in C57BL/6 mice. We plan to further evaluate the immunity generated by AAV vaccines in immune-competent humanized mice. Moreover, we will explore other optimal antigens to induce both humoral and cell responses against HCV, with the goal of developing an efficient prophylactic HCV vaccine.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science Foundation of Shandong Province, China (grant no. ZR2017LH009) and the PhD Research Startup Foundation of the Affiliated Hospital of Jining Medical University (grant no. 2016-BS-015).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
HH and FZ designed the study. FZ, YW, ZX, HQ, HZ, LN, HX and DJ performed the experiments. YW, ZX, HQ, HZ, LN, HX and DJ analyzed the data. FZ prepared the manuscript. HH revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
All patients provided written informed consent to participate. All experimental procedures performed with human and mice were approved by the Ethics Committee of Jining Medical University (Shandong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Li D, Huang Z, Zhong J. Hepatitis C virus vaccine development: Old challenges and new opportunities. Natl Sci Rev. 2015;2:285–295. doi: 10.1093/nsr/nwv040. [DOI] [Google Scholar]
- 2.Pawlotsky JM. Hepatitis C virus resistance to direct-acting antiviral drugs in interferonfree regimens. Gastroenterology. 2016;151:70–86. doi: 10.1053/j.gastro.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Gardiner DF, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath PJ, Schwartz H, et al. Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med. 2014;370:211–221. doi: 10.1056/NEJMoa1306218. [DOI] [PubMed] [Google Scholar]
- 4.Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77–87. doi: 10.1002/hep.27259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassett SE, Guerra B, Brasky K, Miskovsky E, Houghton M, Klimpel GR, Lanford RE. Protective immune response to hepatitis C virus in chimpanzees rechallenged following clearance of primary infection. Hepatology. 2001;33:1479–1487. doi: 10.1053/jhep.2001.24371. [DOI] [PubMed] [Google Scholar]
- 6.Rollier C, Depla E, Drexhage JA, Verschoor EJ, Verstrepen BE, Fatmi A, Brinster C, Fournillier A, Whelan JA, Whelan M, et al. Control of heterologous hepatitis C virus infection in chimpanzees is associated with the quality of vaccine-induced peripheral T-helper immune response. J Virol. 2004;78:187–196. doi: 10.1128/JVI.78.1.187-196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osburn WO, Snider AE, Wells BL, Latanich R, Bailey JR, Thomas DL, Cox AL, Ray SC. Clearance of hepatitis C infection is associated with the early appearance of broad neutralizing antibody responses. Hepatology. 2014;59:2140–2151. doi: 10.1002/hep.27013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frey SE, Houghton M, Coates S, Abrignani S, Chien D, Rosa D, Pileri P, Ray R, Di Bisceglie AM, Rinella P, et al. Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. Vaccine. 2010;28:6367–6373. doi: 10.1016/j.vaccine.2010.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray R, Meyer K, Banerjee A, Basu A, Coates S, Abrignani S, Houghton M, Frey SE, Belshe RB. Characterization of antibodies induced by vaccination with hepatitis C virus envelope glycoproteins. J Infect Dis. 2010;202:862–866. doi: 10.1086/655902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong L, Giang E, Nieusma T, Kadam RU, Cogburn KE, Hua Y, Dai X, Stanfield RL, Burton DR, Ward AB, et al. Hepatitis C virus E2 envelope glycoprotein core structure. Science. 2013;342:1090–1094. doi: 10.1126/science.1243876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng L, Zhong L, Struble E, Duan H, Ma L, Harman C, Yan H, Virata-Theimer ML, Zhao Z, Feinstone S, et al. Structural evidence for a bifurcated mode of action in the antibody-mediated neutralization of hepatitis C virus. Proc Natl Acad Sci USA. 2010;110:7418–7422. doi: 10.1073/pnas.1305306110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kong L, Lee DE, Kadam RU, Liu T, Giang E, Nieusma T, Garces F, Tzarum N, Woods VL, Jr, Ward AB, et al. Structural flexibility at a major conserved antibody target on hepatitis C virus E2 antigen. Proc Natl Acad Sci USA. 2016;113:12768–12773. doi: 10.1073/pnas.1609780113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das S, Mullick R, Kumar A, Tandon H, Bose M, Gouthamchandra K, Chandra M, Ravishankar B, Khaja MN, Srinivasan N, et al. Identification of a novel epitope in the C terminus of hepatitis C virus-E2 protein that induces potent and cross-reactive neutralizing antibodies. J Gen Virol. 2017;98:962–976. doi: 10.1099/jgv.0.000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tedeschi V, Akatsuka T, Shih JW, Battegay M, Feinstone SM. A specific antibody response to HCV E2 elicited in mice by intramuscular inoculation of plasmid DNA containing coding sequences for E2. Hepatology. 1997;25:459–462. doi: 10.1002/hep.510250234. [DOI] [PubMed] [Google Scholar]
- 15.Garrone P, Fluckiger AC, Mangeot PE, Gauthier E, Dupeyrot-Lacas P, Mancip J, Cangialosi A, Du Chéné I, LeGrand R, Mangeot I, et al. A prime-boost strategy using virus-like particles pseudotyped for HCV proteins triggers broadly neutralizing antibodies in macaques. Sci Transl Med. 2011;3:94ra71. doi: 10.1126/scitranslmed.3002330. [DOI] [PubMed] [Google Scholar]
- 16.Li D, von Schaewen M, Wang X, Tao W, Zhang Y, Li L, Heller B, Hrebikova G, Deng Q, Ploss A, et al. Altered glycosylation patterns increase immunogenicity of a subunit hepatitis C virus vaccine, inducing neutralizing antibodies which confer protection in mice. J Virol. 2016;90:10486–10498. doi: 10.1128/JVI.01462-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li D, Wang X, von Schaewen M, Tao W, Zhang Y, Heller B, Hrebikova G, Deng Q, Sun Q, Ploss A, et al. Immunization with a subunit hepatitis C virus vaccine elicits pan-genotypic neutralizing antibodies and intrahepatic T-cell responses in nonhuman primates. J Infect Dis. 2017;215:1824–1831. doi: 10.1093/infdis/jix180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Yan Y, Gan T, Yang X, Li D, Zhou D, Sun Q, Huang Z, Zhong J. A trivalent HCV vaccine elicits broad and synergistic polyclonal antibody response in mice and rhesus monkey. Gut. 2017 Nov 27; doi: 10.1136/gutjnl-2017-314870. (Epub ahead of print). doi: 10.1136/gutjnl-2017-314870. [DOI] [PubMed] [Google Scholar]
- 19.Draper SJ, Heeney JL. Viruses as vaccine vectors for infectious diseases and cancer. Nat Rev Microbiol. 2010;8:62–73. doi: 10.1038/nrmicro2240. [DOI] [PubMed] [Google Scholar]
- 20.Guo J, Mondal M, Zhou D. Development of novel vaccine vectors: Chimpanzee adenoviral vectors. Hum Vaccin Immunother. 2018;14:1679–1685. doi: 10.1080/21645515.2017.1419108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mak KY, Rajapaksha IG, Angus PW, Herath CB. The adeno-associated virus-A safe and promising vehicle for liverspecific gene therapy of inherited and non-inherited disorders. Curr Gene Ther. 2017;17:4–16. doi: 10.2174/1566523217666170314141931. [DOI] [PubMed] [Google Scholar]
- 22.Chen Q, Zhai H, Li X, Ma Y, Chen B, Liu F, Lai H, Xie J, He C, Luo J, et al. Recombinant adeno-associated virus serotype 9 in a mouse model of atherosclerosis: Determination of the optimal expression time in vivo. Mol Med Rep. 2017;15:2090–2096. doi: 10.3892/mmr.2017.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabral-Miranda F, Nicoloso-Simões E, Adão-Novaes J, Chiodo V, Hauswirth WW, Linden R, Chiarini LB, Petrs-Silva H. rAAV8-733-mediated gene transfer of CHIP/Stub-1 prevents hippocampal neuronal death in experimental brain ischemia. Mol Ther. 2017;25:392–400. doi: 10.1016/j.ymthe.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manning WC, Paliard X, Zhou S, Pat Bland M, Lee AY, Hong K, Walker CM, Escobedo JA, Dwarki V. Genetic immunization with adeno-associated virus vectors expressing herpes simplex virus type 2 glycoproteins B and D. J Virol. 1997;71:7960–7962. doi: 10.1128/jvi.71.10.7960-7962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuck D, Lau T, Leuchs B, Kern A, Müller M, Gissmann L, Kleinschmidt JA. Intranasal vaccination with recombinant adeno-associated virus type 5 against human papillomavirus type 16 L1. J Virol. 2006;80:2621–2630. doi: 10.1128/JVI.80.6.2621-2630.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nieto K, Kern A, Leuchs B, Gissmann L, Müller M, Kleinschmidt JA. Combined prophylactic and therapeutic intranasal vaccination against human papillomavirus type-16 using different adeno-associated virus serotype vectors. Antivir Ther. 2009;14:1125–1137. doi: 10.3851/IMP1469. [DOI] [PubMed] [Google Scholar]
- 27.Ploquin A, Szécsi J, Mathieu C, Guillaume V, Barateau V, Ong KC, Wong KT, Cosset FL, Horvat B, Salvetti A. Protection against henipavirus infection by use of recombinant adeno-associated virus-vector vaccines. J Infect Dis. 2013;207:469–478. doi: 10.1093/infdis/jis699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin J, Zhi Y, Mays L, Wilson JM. Vaccines based on novel adeno-associated virus vectors elicit aberrant CD8+ T-cell responses in mice. J Virol. 2007;8:11840–11849. doi: 10.1128/JVI.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin SW, Hensley SE, Tatsis N, Lasaro MO, Ertl HC. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J Clin Invest. 2007;117:3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu Y, Tao L, Zheng S, Lin R, Fu X, Chen Z, Lei C, Wang J, Li H, Li Q, Lei B. AAV8-mediated angiotensin-converting enzyme 2 gene delivery prevents experimental autoimmune uveitis by regulating MAPK, NF-κB and STAT3 pathways. Sci Rep. 2016;6:31912. doi: 10.1038/srep31912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J, Calcedo R, Vandenberghe LH, Bell P, Somanathan, Wilson JM. A new genetic vaccine platform based on an adeno-associated virus isolated from a rhesus macaque. J Virol. 2009;83:12738–12750. doi: 10.1128/JVI.01441-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mays LE, Vandenberghe LH, Xiao R, Bell P, Nam HJ, Agbandje-McKenna M, Wilson JM. Adeno-associated virus capsid structure drives CD4-dependent CD8+ T cell response to vector encoded proteins. J Immunol. 2009;182:6051–6060. doi: 10.4049/jimmunol.0803965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X, Cao H, Wang Q, Di B, Wang M, Lu J, Pan L, Yang L, Mei M, Pan X, et al. Novel AAV-based genetic vaccines encoding truncated dengue virus envelope proteins elicit humoral immune responses in mice. Microbes Infect. 2012;14:1000–1007. doi: 10.1016/j.micinf.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Zhu F, Chen T, Zhang Y, Sun H, Cao H, Lu J, Zhao L, Li G. A novel adeno-associated virus-based genetic vaccine encoding the hepatitis C virus NS3/4 protein exhibits immunogenic properties in mice superior to those of an NS3-protein-based vaccine. PLoS One. 2015;10:e0142349. doi: 10.1371/journal.pone.0142349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Calcedo R, Nichols TC, Bellinger DA, Dillow A, Verma IM, Wilson JM. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005;105:3079–3786. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- 37.Dreux M, Cosset FL. Detection of neutralizing antibodies with HCV pseudoparticles (HCVpp) Methods Mol Biol. 2009;510:427–438. doi: 10.1007/978-1-59745-394-3_32. [DOI] [PubMed] [Google Scholar]
- 38.Guan J, Wen B, Deng Y, Zhang K, Chen H, Wu X, Ruan L, Tan W. Effect of route of delivery on heterologous protection against HCV induced by an adenovirus vector carrying HCV structural genes. Virol J. 2011;8:506. doi: 10.1186/1743-422X-8-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roohvand F, Kossari N. Advances in hepatitis C virus vaccines, part two: Advances in hepatitis C virus vaccine formulations and modalities. Expert Opin Ther Pat. 2012;22:391–415. doi: 10.1517/13543776.2012.673589. [DOI] [PubMed] [Google Scholar]
- 40.Liang TJ. Current progress in development of hepatitis C virus vaccines. Nat Med. 2013;19:869–878. doi: 10.1038/nm.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rollier CS, Verschoor EJ, Verstrepen BE, Drexhage JA, Paranhos-Baccala G, Liljeström P, Sutter G, Arribillaga L, Lasarte JJ, Bartosch B, et al. T- and B-cell responses to multivalent prime-boost DNA and viral vectored vaccine combinations against hepatitis C virus in non-human primates. Gene Ther. 2016;23:753–759. doi: 10.1038/gt.2016.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Delft A, Donnison TA, Lourenço J, Hutchings C, Mullarkey CE, Brown A, Pybus OG, Klenerman P, Chinnakannan S, Barnes E. The generation of a simian adenoviral vectored HCV vaccine encoding genetically conserved gene segments to target multiple HCV genotypes. Vaccine. 2018;36:313–321. doi: 10.1016/j.vaccine.2017.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brady JM, Baltimore D, Balazs AB. Antibody gene transfer with adeno-associated viral vectors as a method for HIV prevention. Immunol Rev. 2017;275:324–333. doi: 10.1111/imr.12478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meola A, Tarr AW, England P, Meredith LW, McClure CP, Foung SK, McKeating JA, Ball JK, Rey FA, Krey T. Structural flexibility of a conserved antigenic region in hepatitis C virus glycoprotein E2 recognized by broadly neutralizing antibodies. J Virol. 2015;89:2170–2181. doi: 10.1128/JVI.02190-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasiliauskaite I, Owsianka A, England P, Khan AG, Cole S, Bankwitz D, Foung SKH, Pietschmann T, Marcotrigiano J, Rey FA, et al. Conformational flexibility in the immunoglobulin-like domain of the hepatitis C virus glycoprotein E2. MBio. 2017;8:e00382–17. doi: 10.1128/mBio.00382-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan G, Liu J, Hu C, Huang H, Qi M, Wu T, Liang W, Li YP, Zhang YY, Zhou Y. Genotype distribution and molecular epidemiology of hepatitis C virus in Guangzhou, China: Predominance of genotype 1b and increasing incidence of genotype 6a. Cell Physiol Biochem. 2017;43:775–787. doi: 10.1159/000481561. [DOI] [PubMed] [Google Scholar]
- 48.Law JL, Chen C, Wong J, Hockman D, Santer DM, Frey SE, Belshe RB, Wakita T, Bukh J, Jones CT, et al. A hepatitis C virus (HCV) vaccine comprising envelope glycoproteins gpE1/gpE2 derived from a single isolate elicits broad cross-genotype neutralizing antibodies in humans. PLoS One. 2013;8:e59776. doi: 10.1371/journal.pone.0059776. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.