Abstract
Age-related skeletal muscle changes may impact respiratory muscle function, and low muscle mass is associated with low pulmonary function in older adults. Stroke not only causes weakness in the muscles of the upper and lower limbs, but it can also affect the respiratory system. This study aimed to investigate the relationships between grip strength and pulmonary function and respiratory muscle strength in stroke patients over 50 years of age. Fifty-one patients (68.69±10.40 years) who had been clinically diagnosed with ischemic or hemorrhagic stroke were included in this study. We measured these patients’ forced vital capacity (FVC), forced expiratory volume in 1 sec (FEV1), peak expiratory flow (PEF), maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), and hand grip strength. The data were analyzed using descriptive statistics and Pearson correlation analysis. Grip strength showed significant correlations with FVC (r=0.686, P=0.000), FEV1 (r=0.607, P=0.000), PEF (r=0.494, P=0.000), MIP (r=0.239, P=0.091), and MEP (r=0.348, P=0.012). This study demonstrated that grip strength is associated with pulmonary function and MEP in stroke patients over 50 years of age.
Keywords: Sarcopenia, Stroke, Grip strength, Pulmonary function, Respiratory muscle strength
INTRODUCTION
The loss of muscle mass, strength, and function due to aging is defined as sarcopenia (Holmes et al., 2017). Sarcopenia is a reduction of skeletal muscle mass that is associated with aging in older adults (Jeon et al., 2015). Sarcopenia can not only lead to diminished skeletal muscles, but also to reduced mobility, an increased risk of falls and fractures, a decrease in activity related to daily living, increased medical costs, increased mortality risk, and a decrease in one’s quality of life (Cruz-Jentoft et al., 2010; Rolland et al., 2008).
Because respiratory muscle strength is related to skeletal muscle mass (Jeon et al., 2015), aging-associated alterations in the skeletal muscles can also affect the function of the respiratory muscles (Bahat et al., 2014); as a result, sarcopenia can influence one’s pulmonary function (Jeon et al., 2018). The low pulmonary function of community-dwelling older adults is typically associated with low muscle mass (Jeon et al., 2015). Moreover, an aging-related sedentary lifestyle can also accelerate the reduction of one’s inspiratory and expiratory muscle strength (Bahat et al., 2014).
The impairment of pulmonary function due to the weakening of respiratory muscles can cause atelectasis, ineffective cough, and other respiratory complications (Bahat et al., 2014; Park et al., 2010). These respiratory complications can lead to cardiovascular disease and all-cause mortality, as well as to bronchitis and pneumonia (Sin et al., 2005; Son et al., 2018).
Stroke is a form of brain injury that impairs the coordination of respiratory muscles as a result of damaged motor control function (Jandt et al., 2011). Such muscle weakness and impaired coordination lead to the reduction of respiratory function (Jo and Kim, 2017; Jung and Kim, 2013). Compared with healthy age-matched subjects, stroke patients show lower forced vital capacity (FVC), forced expiratory volume in 1 sec (FEV1), peak expiratory flow (PEF), maximal inspiratory pressure (MIP), and maximal expiratory pressure (MEP) (Jo and Kim, 2016; Jo and Kim, 2017; Teixeira-Salmela et al., 2005). Therefore, chronic stroke patients over 60 years of age may not be able to cough effectively due to the weakening of their respiratory muscles as a result of neurological damage and aging; consequently, the prevalence of aspiration and chest infections may be heightened for this population (Jo and Kim, 2016; Jo and Kim, 2017; Martino et al., 2005).
The measurement of hand grip strength is a widely used method to easily measure muscle strength with a high level of reliability (Bahat et al., 2014). Grip strength is also recommended as a simple, standardized clinical measure that can be used to diagnose sarcopenia (Cruz-Jentoft et al., 2010). In addition, hand grip strength has been shown to correlate with pulmonary function and inspiratory and expiratory muscle strength (Efstathiou et al., 2016; Holmes et al., 2017; Jeon et al., 2015; Son et al., 2018). However, due to the fact that the pulmonary function test should be performed by a specialist using a medical device, it is relatively expensive.
As the age of stroke patients who spend most of their time sitting increases, their risk of developing sarcopenia increases to an even greater extent, and their pulmonary function may deteriorate to a greater extent than that of a typical older adults. As a result, this patient group requires special monitoring. Although the relationship between hand grip strength and pulmonary function has been studied in older adults and in patients with specific diseases (Efstathiou et al., 2016; Holmes et al., 2017; Jeon et al., 2015; Son et al., 2018), research on the relationship between sarcopenia and pulmonary function in aging stroke patients with a higher risk of respiratory complications is currently lacking.
The aim of this study is to investigate the relationships between grip strength and pulmonary function and respiratory muscle strength in stroke patients over 50 years of age.
MATERIALS AND METHODS
Participants
This study’s subjects were comprised of 51 stroke patients who had the disease for at least 6 months. The recruitment criteria for this study’s subjects included the following: (a) they had experienced their first episode of unilateral stroke with hemiparesis during the previous 12 months, (b) they had sufficient unilateral upper torso and extremity nerve function and strength to accomplish arm crank ergometry, (c) they had the ability to understand and follow simple verbal instructions, and (d) they were not on any medications that would influence their metabolic or cardiorespiratory responses to exercise (Sutbeyaz et al., 2010). The exclusion criteria included chronic pulmonary and/or cardiac disease, clinical signs of cardiac and/or respiratory disease, an impaired level of consciousness, and evidence of gross cognitive impairment (Sutbeyaz et al., 2010).
All participants in this study received an explanation of the purpose of this study and voluntarily provided their consent to participate in this study. All protocols were approved by the Ethics Committee of the Catholic University of Pusan (CUPIRB- 2013-021).
Measurements
The patients’ hand grip strength was measured using a hydraulic hand dynamometer (Saehan Hydraulic Hand Dynamometer, Saehan Corp., Masan, Korea) according to the standard procedures. The hand on the nonaffected side was measured three times, and the mean value was used for the analysis. During the test, the subjects were asked to adopt a posture with the shoulder adducted and neutrally rotated, the elbow flexed to 90°, and the forearm and wrist in a neutral position. The opening of the handle was adjusted prior to testing, so that the crease of the proximal interphalangeal joints rested on the top of the adjustable handle, allowing 90° of flexion. A 30-sec break was given after every measurement (Efstathiou et al., 2016; Nascimento et al., 2012).
Pulmonary function was measured using spirometry (Pony FX; COSMED Inc., Rome, Italy) according to the American Thoracic Society’s guidelines (American Thoracic Society, 1995). The FVC, FEV1, and PEF were measured as descriptions of pulmonary function. Respiratory muscle strength was measured using respiratory muscle strength measurement equipment (Pony FX MIP/MEP; COSMED Inc., Rome, Italy) according to the standards of the American Thoracic Society/European Respiratory Society (American Thoracic Society/European Respiratory Society, 2002). In order to produce accurate measurements, trained medical technicians provided sufficient explanations and demonstrated the method to the participants so that they could fully understand the procedure before the measurements were performed. The test was performed while each subject maintained a sitting position with a 90° flexion of the hip joint (Jo and Kim, 2016). Each subject was tested 3 times, and each subject’s highest value was used as the data for the analysis.
Statistical analysis
The collected data were analyzed using IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). The general characteristics, means, and standard deviations of the measured variables were analyzed via descriptive analysis. The subjects were divided into two groups according to age (50–64 years of age and over 65 years of age), and the differences in each variable between the two groups were analyzed using an independent t-test. The correlation between the subjects’ grip strength and pulmonary function was analyzed using the Pearson correlation method. The statistical significance was set at P<0.05.
RESULTS
Fifty-one subjects were divided into a middle-aged group (50–64 years old) and an older adults group (65 years or older), and their general characteristics and the measured variables were compared (Table 1). There were no significant differences between the two groups—with the exception of age—with respect to the participants’ sex, weight, and height.
Table 1.
Participant characteristics and their pulmonary function and grip strength
| Characteristic | Total (n=51) | Middle age (n=18) | Older adults (n=33) | t/χ2 | P-value |
|---|---|---|---|---|---|
| Sex, male:female | 18±27 | 9:9 | 12:21 | 0.894 | 0.344 |
| Age (yr) | 68.69±10.40 | 58.11±3.94 | 75.36±6.36 | −10.430 | 0.000* |
| Weight (kg) | 60.88±10.60 | 61.75±9.54 | 61.00±10.99 | 0.244 | 0.809 |
| Height (cm) | 159.51±8.53 | 161.78±9.38 | 158.69±7.77 | 1.257 | 0.215 |
| Grip strength (kg) | 22.34±8.55 | 23.35±9.62 | 20.56±7.61 | 1.138 | 0.261 |
| FVC (L) | 1.96±0.49 | 2.21±0.46 | 1.77±0.42 | 3.470 | 0.001* |
| FEV1 (L) | 1.57±0.48 | 1.90±0.47 | 1.34±0.33 | 4.933 | 0.000* |
| FEV1/FVC | 80.52±13.46 | 86.53±10.42 | 76.57±12.90 | 2.807 | 0.007* |
| PEF (L/min) | 2.87±1.40 | 3.58±1.71 | 2.34±0.85 | 2.871 | 0.009* |
| MIP (cmH2O) | 28.56±10.51 | 31.17±12.68 | 26.90±8.83 | 1.406 | 0.166 |
| MEP (cmH2O) | 30.98±9.81 | 33.83±8.62 | 29.03±10.17 | 1.696 | 0.096 |
Values are presented as mean±standard deviation.
FVC, forced vital capacity; FEV1, forced expiratory volume in 1 sec; PEF, peak expiratory flow; MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure.
P<0.05, significantly different between middle age and older adults groups.
The grip strength of the middle-aged group was significantly higher than it was for older adults group, but there was no significant difference between these values. The mean values of pulmonary function (FVC, FEV1, and PEF) were significantly higher in the middle-aged group than they were in older adults group (P< 0.05) (Table 1). The mean values of MIP and MEP were higher in the middle-aged group than they were in older adults group, but there was no significant difference between these values.
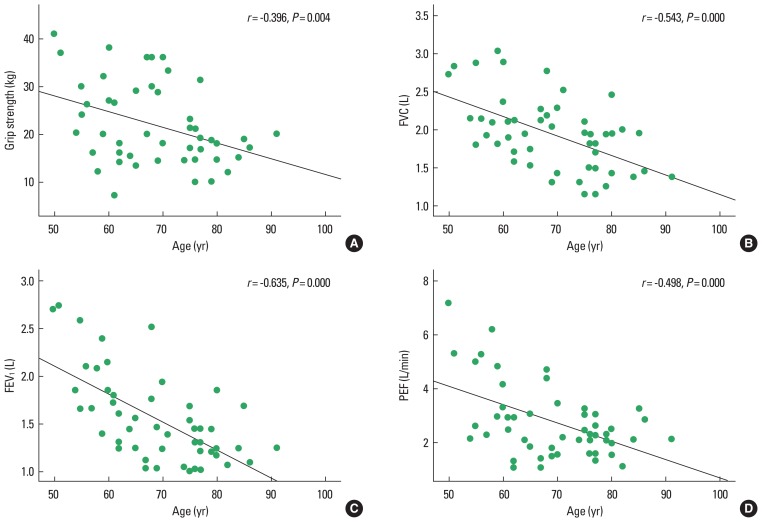
Furthermore, the relationships between age and grip strength, pulmonary function, and respiratory muscle strength were analyzed. This analysis revealed that age showed significant negative correlations with grip strength, FVC, FEV1, and PEF (P<0.05) (Fig. 1), but no significant correlations between age and MIP and MEP were apparent.
Fig. 1.
The correlation between age and grip strength and pulmonary function: grip strength (A), FVC (B), FEV1 (C), PEF (D). FVC, forced vital capacity; FEV1, forced expiratory volume in 1 sec; PEF, peak expiratory flow.
In addition, the correlations between grip strength and pulmonary function and respiratory muscle strength were analyzed. Grip strength was shown to have significant positive correlations with FVC, FEV1, and PEF for all subjects, including subjects from both the middle-aged group and older adults group (P<0.05) (Table 2). Between these two groups, the middle-aged group showed greater positive correlations with respect to FVC, FEV1, and PEF than older adults group.
Table 2.
The correlation between grip strength and pulmonary function and respiratory muscle strength
| Group | FVC | FEV1 | PEF | MIP | MEP |
|---|---|---|---|---|---|
| Total (n=51) | 0.686** | 0.607** | 0.494** | 0.239 | 0.348* |
| Middle age (n=18) | 0.812** | 0.764** | 0.598** | 0.150 | 0.071 |
| Older adults (n=33) | 0.613** | 0.521** | 0.345* | 0.277 | 0.477** |
FVC, forced vital capacity; FEV1, forced expiratory volume in 1 sec; PEF, peak expiratory flow; MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure.
P<0.05.
P<0.01.
With respect to respiratory muscle strength, there was no correlation between grip strength and MIP for all subjects, for the middle-aged group, or for older adults group; however, there was a significant positive correlation between grip strength and MEP for all subjects and for older adults group (P<0.05) (Table 2).
DISCUSSION
When stroke patient’s age, their skeletal muscle strength tends to reduce and sarcopenia is likely to follow, which, in turn, tends to influence their respiratory muscle strength as well. In this study, the subjects were divided into two age groups in order to investigate the correlation between the patients’ pulmonary function and age-related sarcopenia in stroke patients with respiratory muscle weakness due to hemiplegia.
As muscle strength of older adults is related not only to age but also to sex, height, and weight (Holmes et al., 2017), a homogeneity test was performed to determine whether there were any intergroup differences—irrespective of age—in the sex, height, weight, and body mass index of the two groups; no significant difference was discovered.
The present study aimed to investigate the relationships between grip strength and pulmonary function and respiratory muscle strength in older adults. Consistent with a previous report that the lung function of disabled, older women was reduced due to their decreased physical performance (Simpson et al., 2005), the pulmonary function of the subjects in this study, who were stroke patients with reduced physical activity due to hemiplegia, was also lower than that of the normal control group (Jeon et al., 2015). When the pulmonary function of community-dwelling older men and women aged 71–73 years on average was compared according to their sarcopenia status, it was found that FVC was significantly higher in the group without sarcopenia, but no significant difference in FEV1 was found between the two groups (Jeon et al., 2015). Similarly, when community-dwelling older women with sarcopenia (aged 75.4±5.3 years on average) and community-dwelling older women without sarcopenia (aged 73.0±6.2 years on average) were compared, it was found that the left and right grip strength and FVC were significantly higher in the group without sarcopenia; however, no significant difference was found with respect to FEV1, PEF, MIP, and MEP (Jeon et al., 2018). Also, in the present study, the middle-aged group whose grip strength was higher than that of older adults group—although there was no significant difference—displayed significantly higher FEV1 and PEF, as well as significantly higher FVC, in comparison to that of older adults group. This finding—that there were significant differences in FEV1 and PEF between the two groups—suggests that there is a significant correlation between age and pulmonary function (Jeon et al., 2015).
It has been shown that the height-adjusted strength of community-dwelling older men was positively correlated with FVC (r=0.206, P=0.001) and FEV1 (r=0.315, P=0.001) (Jeon et al., 2015). Similarly, the hand grip strength of the general population (at an average age of 56.4 years) was reported to have positive correlations with FVC (r=0.76, P=0.001) and FEV1 (r=0.69, P=0.001) (Jeong et al., 2017). In addition, it was shown that the higher the FVC and FEV1 of apparently healthy community-dwelling older women over 65 years of age, the greater their hand grip strength, suggesting that hand grip strength is positively correlated with FVC and FEV1 (Son et al., 2018). In the present study conducted with stroke patients, grip strength was found to have positive correlations with FVC, FEV1, and PEF for both the middle-aged group and older adults group. This result indicates that aging-related reduction in the subjects’ skeletal muscle mass resulted in the reduction of these patients’ respiratory muscle mass and strength (Greising et al., 2013), ultimately leading to reduce pulmonary function.
In particular, FVC showed a higher level of correlation than FEV1, which can be explained by the fact that, while FVC is mainly influenced by respiratory muscle strength, FEV1 is influenced not only by respiratory muscle strength, but also by the patient’s airway status (Son et al., 2018). Consistent with this finding, FVC showed a higher correlation with grip strength than FEV1 in the present study conducted with stroke patients. This can be attributed to the fact that restrictive pulmonary disease caused by decreased respiratory muscle function as a result of neurological damage is more common in stroke patients than obstructive pulmonary disease in which the airway becomes narrow.
When the relationship between grip strength and lung function was investigated in older in-patients aged over 85 years on average, there was a significant positive correlation between grip strength and FEV1 in older men, and the grip strength of older women had significant positive correlations with slow vital capacity and PEF (Holmes et al., 2017). In the same study, respiratory muscle strength was not measured; however, both the older men and women showed correlations with FEV1 and PEF, which are closely associated with expiratory muscle strength. Another study reported that the grip strength of male nursing home residents was only positively correlated with peak cough flow among other pulmonary function items (r=0.34, P<0.01) (Bahat et al., 2014). The grip strength of older patients and older adults living in nursing homes, who have a more sedentary lifestyle than typical older adults, showed relatively low correlations with FVC. Similarly, the results of the present study also showed that the correlation between grip strength and FVC was slightly lower for older adults group in comparison to that of the middle-aged group.
This relationship between grip strength and pulmonary function suggests that an aging-related decrease in pulmonary function is manifested as a loss of muscle mass and that sarcopenia can be predicted by the extent of a patient’s relative decrease in pulmonary function (Jeon et al., 2015). In the present study conducted with stroke patients, the level of pulmonary function was significant as an indicator in the prediction of the extent of sarcopenia, as the correlation levels of grip strength with FVC or FEV1 were 0.5 or higher.
The relationship between age and maximal static respiratory pressures was unclear in a cross-sectional study, but many studies have suggested that MEP and MIP are associated with peripheric muscle strength indicating sarcopenia along with age in reduced respiratory muscle performance (Enright et al., 1994). However, in the present study, no significant relationships were found between age and MIP and MEP.
Sarcopenia of respiratory muscles such as the diaphragm has not yet been discovered, but an aging-related decrease in respiratory muscle strength is referred to as sarcopenia of the respiratory muscles (Greising et al., 2013). Since physical activity plays an important role in maintaining inspiratory and expiratory muscle strength, a sedentary lifestyle, in addition to aging, can reduce inspiratory and expiratory muscle strength (Kim et al., 2009). In addition to aging, sarcopenia of respiratory muscles can contribute to respiratory complications (Greising et al., 2013). The stroke patients who participated in the present study had a more sedentary lifestyle than the typical older adults, and the MIP and MEP of both older adults group and the middle-aged group were lower than the MIP (52.9±15.4) and MEP (48.4±13.5) obtained from typical older women without sarcopenia and the MIP (50.3± 17.1) and MEP (50.3±18.8) obtained from typical older women with sarcopenia (Jeon et al., 2018). Although there was no significant difference between these levels, the older adults group had even lower MIP and MEP levels than the middle-aged group. These results suggest that stroke patients tend to spend more time sitting than the general older adults, due to difficulties in physical activity as a result of hemiplegia, and that their respiratory muscle function was also decreased due to damage to their nervous system. These results also suggest that they are likely to be at greater risk for respiratory complications than the general older adults.
Low MIP and normal MEP levels indicate a weakening of the diaphragmatic muscle—a main inspiratory muscle—whereas low MIP and MEP levels indicate a weakening of the overall skeletal muscles (Bahat et al., 2014). Therefore, MEP can act as a sensitive indicator of deterioration of the respiratory muscle as patients with neuromuscular disease age (Schramm, 2000). In a study conducted in a subject group of apparently healthy older women, MIP and MEP were positively correlated with hand grip strength (Son et al., 2018). However, in the present study conducted with stroke patients who had experienced nervous system damage, their MIP and MEP levels were lower than those of the general older adults; this is indicative of overall muscle reduction, and a significant correlation was found only between hand grip strength and MEP.
The limitations of this study are that the homogeneity of sex, which could affect the results of the study, was statistically obtained, as the analysis could not be performed with respect to sex due to the small number of subjects. In order to generalize the results of the present study, it is necessary to conduct a similar study using a larger number of subjects in consideration of sex.
This study showed that stroke patients over 50 years of age had decreased grip strength, pulmonary function, MIP, and MEP, in comparison with the general older adults, and that grip strength is correlated with pulmonary function and MEP. Therefore, it is necessary to apply muscular strengthening exercise to prevent sarcopenia and to monitor pulmonary function using grip strength test in older stroke patients.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society/European Respiratory Society ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- Bahat G, Tufan A, Ozkaya H, Tufan F, Akpinar TS, Akin S, Bahat Z, Kaya Z, Kiyan E, Erten N, Karan MA. Relation between hand grip strength, respiratory muscle strength and spirometric measures in male nursing home residents. Aging Male. 2014;17:136–140. doi: 10.3109/13685538.2014.936001. [DOI] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstathiou ID, Mavrou IP, Grigoriadis KE. Correlation between maximum inspiratory pressure and hand-grip force in healthy young and middle-age individuals. Respir Care. 2016;61:925–929. doi: 10.4187/respcare.04319. [DOI] [PubMed] [Google Scholar]
- Enright PL, Kronmal RA, Manolio TA, Schenker MB, Hyatt RE. Respiratory muscle strength in the elderly. Correlates and reference values. Cardiovascular Health Study Research Group. Am J Respir Crit Care Med. 1994;149(2 Pt 1):430–438. doi: 10.1164/ajrccm.149.2.8306041. [DOI] [PubMed] [Google Scholar]
- Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Exp Gerontol. 2013;48:881–887. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes SJ, Allen SC, Roberts HC. Relationship between lung function and grip strength in older hospitalized patients: a pilot study. Int J Chron Obstruct Pulmon Dis. 2017;12:1207–1212. doi: 10.2147/COPD.S120721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandt SR, Caballero RM, Junior LA, Dias AS. Correlation between trunk control, respiratory muscle strength and spirometry in patients with stroke: an observational study. Physiother Res Int. 2011;16:218–224. doi: 10.1002/pri.495. [DOI] [PubMed] [Google Scholar]
- Jeon YK, Shin MJ, Kim CM, Lee BJ, Kim SH, Chae DS, Park JH, So YS, Park H, Lee CH, Kim BC, Chang JH, Shin YB, Kim IJ. Effect of squat exercises on lung function in elderly women with sarcopenia. J Clin Med. 2018;7:167. doi: 10.3390/jcm7070167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YK, Shin MJ, Kim MH, Mok JH, Kim SS, Kim BH, Kim SJ, Kim YK, Chang JH, Shin YB, Kim IJ. Low pulmonary function is related with a high risk of sarcopenia in community-dwelling older adults: the Korea National Health and Nutrition Examination Survey (KNHANES) 2008–2011. Osteoporos Int. 2015;26:2423–2429. doi: 10.1007/s00198-015-3152-8. [DOI] [PubMed] [Google Scholar]
- Jeong M, Kang HK, Song P, Park HK, Jung H, Lee SS, Koo HK. Hand grip strength in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2017;12:2385–2390. doi: 10.2147/COPD.S140915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo MR, Kim NS. Combined respiratory muscle training facilitates expiratory muscle activity in stroke patients. J Phys Ther Sci. 2017;29:1970–1973. doi: 10.1589/jpts.29.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo MR, Kim NS. The correlation of respiratory muscle strength and cough capacity in stroke patients. J Phys Ther Sci. 2016;28:2803–2805. doi: 10.1589/jpts.28.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JH, Kim NS. Effects of inspiratory muscle training on diaphragm thickness, pulmonary function, and chest expansion in chronic stroke patients. J Korean Soc Phys Med. 2013;8:59–69. [Google Scholar]
- Kim J, Davenport P, Sapienza C. Effect of expiratory muscle strength training on elderly cough function. Arch Gerontol Geriatr. 2009;48:361–366. doi: 10.1016/j.archger.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- Nascimento LR, Polese JC, Faria CDCM, Teixeira-Salmela LF. Isometric hand grip strength correlated with isokinetic data of the shoulder stabilizers in individuals with chronic stroke. J Bodyw Mov Ther. 2012;16:275–280. doi: 10.1016/j.jbmt.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Park JH, Kang SW, Lee SC, Choi WA, Kim DH. How respiratory muscle strength correlates with cough capacity in patients with respiratory muscle weakness. Yonsei Med J. 2010;51:392–397. doi: 10.3349/ymj.2010.51.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland Y, Czerwinski S, Abellan Van Kan G, Morley JE, Cesari M, Onder G, Woo J, Baumgartner R, Pillard F, Boirie Y, Chumlea WM, Vellas B. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm CM. Current concepts of respiratory complications of neuromuscular disease in children. Curr Opin Pediatr. 2000;12:203–207. doi: 10.1097/00008480-200006000-00004. [DOI] [PubMed] [Google Scholar]
- Simpson CF, Punjabi NM, Wolfenden L, Shardell M, Shade DM, Fried LP. Relationship between lung function and physical performance in disabled older women. J Gerontol A Biol Sci Med Sci. 2005;60:350–354. doi: 10.1093/gerona/60.3.350. [DOI] [PubMed] [Google Scholar]
- Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127:1952–1959. doi: 10.1378/chest.127.6.1952. [DOI] [PubMed] [Google Scholar]
- Son DH, Yoo JW, Cho MR, Lee YJ. Relationship between handgrip strength and pulmonary function in apparently healthy older women. J Am Geriatr Soc. 2018;66:1367–1371. doi: 10.1111/jgs.15410. [DOI] [PubMed] [Google Scholar]
- Sutbeyaz ST, Koseoglu F, Inan L, Coskun O. Respiratory muscle training improves cardiopulmonary function and exercise tolerance in subjects with subacute stroke: a randomized controlled trial. Clin Rehabil. 2010;24:240–250. doi: 10.1177/0269215509358932. [DOI] [PubMed] [Google Scholar]
- Teixeira-Salmela LF, Parreira VF, Britto RR, Brant TC, Inácio EP, Alcântara TO, Carvalho IF. Respiratory pressures and thoracoabdominal motion in community-dwelling chronic stroke survivors. Arch Phys Med Rehabil. 2005;86:1974–1978. doi: 10.1016/j.apmr.2005.03.035. [DOI] [PubMed] [Google Scholar]