Abstract
Clear cell carcinoma is the most common form of renal cell carcinoma (RCC). Metastatic RCC is poorly responsive to treatment and has a bleak prognosis. Newer systemic agents have improved outcomes. Furthermore, their interaction with radiation treatment (RT) may provide further therapeutic options. RCC is considered to be radioresistant, however we report the case of a patient with progression on targeted therapy and immunotherapy who achieved a substantial and sustained local, and possibly abscopal, response to low dose palliative radiation therapy.
Keywords: Immunotherapy, Radiation therapy, Renal cell carcinoma, Targeted therapy
Introduction
In Australia, RCC is one of the ten most common cancer diagnoses; the incidence has doubled between 1982 and 2007. Clear cell carcinoma is the most common form of RCC. One in three cases will present at an advanced stage. Metastatic RCC are poorly responsive to treatment and have a poor prognosis.
There are a number of targeted agents now approved for clinical use in the treatment of advanced RCC, including tyrosine kinase inhibitors (TKI, e.g. sunitinib, axitinib), multi-kinase inhibitors (e.g. sorafenib), anti-vascular endothelial growth factor monoclonal antibody (e.g. bevacizumab) and the mammalian target of rapamycin (mTOR, e.g. everolimus). In addition, immunotherapy with nivolumab is approved for advanced disease following failure of one or two lines of anti-angiogenic treatments.
Unfortunately, almost all patients with advanced disease will progress despite these treatments. RCC has been considered to be radioresistant [1] and many clinicians believe high biologic doses are necessary to achieve meaningful palliation, which may not always be feasible or appropriate. Many patients, therefore, do not have a trial of palliative RT, especially when volumes are large and potential toxicity may be thought to be high.
Case
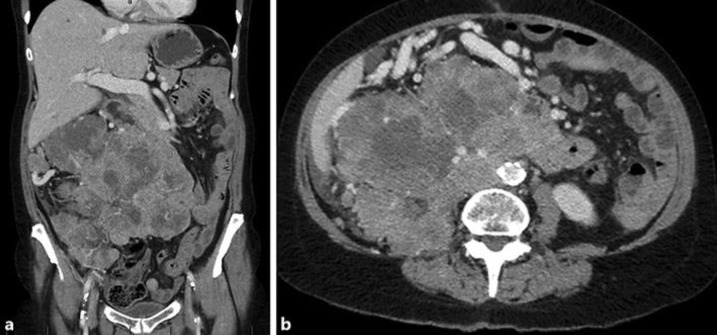
A 66 year old female presented with a large abdominal mass on a background of metastatic clear cell RCC, which had progressed despite a first line TKI and subsequent immunotherapy. The large mass was recurrent tumor, arising from the surgical bed as well as confluent retroperitoneal and peritoneal nodules. It had dramatically increased in size, measuring 110 mm (anterior/posterior) × 149 mm (transverse) × 196 mm (superior/inferior), and extending from the subhepatic space, into the right iliac fossa and across the midline (Fig. 1a and b).
Fig. 1.
a and b: Coronal and axial views on diagnostic CT of mass before palliative RT.
She had been diagnosed 1 year previously following resection of a large right sided renal mass. Histopathology was a pathological T3, grade 4 clear cell RCC, 250 mm in maximal dimension, with vascular and lymphatic invasion. A computed tomography (CT) scan 3 months following nephrectomy demonstrated retroperitoneal soft tissue deposits 34 mm in size and two suspicious 4 mm lung lesions. Treatment with a TKI (sunitinib) was initiated at the time of relapse. After 4 months the sunitinib was ceased after a restaging CT scan confirmed disease progression, with an increase in size of the retroperitoneal deposits and new deposits in the nephrectomy bed and peritoneum. After a month break, she was switched to second line therapy with nivolumab. During the 2 months of immunotherapy, she continued to progress in the abdomen to the size described above (see Fig. 1), a large confluent mass of local recurrence, peritoneal and retroperitoneal nodules. There were also multiple new lung metastases. The nivolumab was ceased and a week later a second line TKI, axitinib, was commenced and she was referred to Radiation Oncology for consideration of palliative RT to the large mass.
At the time she reported symptoms of malaise and lethargy, with abdominal bloating and some intermittent abdominal discomfort. Her abdomen was visibly distended with a firm palpable mass extending from the right upper quadrant to the right iliac fossa. She was very tender on examination.
After discussion, she consented to RT treatment, with the understanding that the treatment intent was palliative. The radiation treatment fields were extensive, and were designed for symptomatic stabilization whilst sparing the remaining kidney. The prescribed dose was 36 Gy in 12 fractions (a lower than ideal dose for RCC, dictated by the volume of the treatment field) with a low threshold for stopping the RT if she experienced unacceptable side effects, such as nausea or diarrhea. The RT commenced 3 weeks after her last dose of nivolumab, the axitinib was continued during the RT treatment. The patient had just received her first fraction of 3 Gy, when she received news about the sudden death of a family member in another state. Treatment was delayed while she attended the funeral.
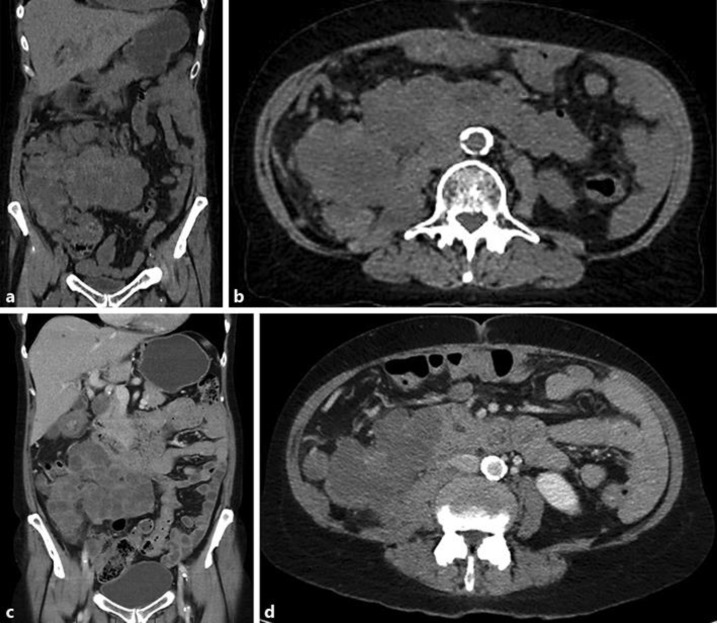
On her return 2 weeks later, a re-simulation CT scan demonstrated that the mass had reduced in size by approximately 25% following the single fraction of 3 Gy (Fig. 2a and b). The treatment was re-planned and she completed the remaining 33Gy in 11 fractions to a smaller treatment volume. She tolerated the remainder of the treatment well with the only side effect being a slight increase in bowel frequency.
Fig. 2.
a and b: Coronal and axial view on re-simulation CT of mass 2 weeks after 3Gy of RT. c and d: Coronal and axial CT views at 1 month post completion of 36Gy in 12 fractions of RT.
A restaging CT scan performed 1 month post the completion of her RT confirmed stable disease, with the new dimensions of the mass measuring 91 mm × 113 mm × 148 mm (Fig. 2c and d). There was ongoing low density change, likely reflecting tumor response. In addition, there had been a reduction in the size of the lung metastases which were well outside the radiotherapy field. She remained on the axitinib, which she tolerated well, despite some fluctuation in her blood pressure.
With subsequent imaging, 5 months post RT, the large abdominal mass had further reduced in size, now measuring 52 mm × 71 mm × 118 mm. There was evidence of disease progression at other sites, with a new adrenal metastasis and multiple liver metastases. By seven months post RT, the mass was demonstrating further regression, with a 50–70% reduction in size from pre-treatment measurements.
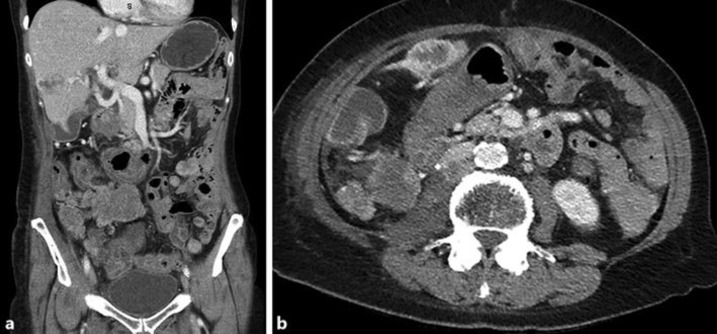
At 15 months post RT, there remained excellent local control of the abdominal mass (Fig. 3a and b), despite disease progression in other sites. During this time, due to progressive disease, the patient's axitinib dose was initially increased to a dose of 5 mg mane and 2 mg nocte, and subsequently changed to Everolimus 10 mg daily due to poor tolerance. Unfortunately she continued to progress in the right adrenal gland, liver and lungs and was referred for further radiotherapy treatment to the large liver metastasis. She received 14Gy/2F to the large left sided liver lesions which she tolerated well. There was no subsequent abdominal imaging performed to comment on response to treatment.
Fig. 3.
a and b: Coronal and axial CT views at 15 months post completion of RT.
At 17 months after the initial radiotherapy treatment, the patient was admitted with balance and gait disturbance with new weakness of her right hip flexors. An MRI brain and spine confirmed no intracranial or spinal pathology; however the right retroperitoneal mass was invading the psoas and iliacus muscles with compression of the right lumbar plexus and right femoral nerve, contributing to the right hip and leg weakness. The patient was referred for further radiotherapy but her condition declined significantly at this time and RT was cancelled. She died peacefully at a palliative care facility with her family present.
Discussion
Patients with metastatic RCC have a poor prognosis with demonstrated resistance to cytotoxic chemotherapy. With the discovery of genetic alterations in RCC, there are now a number of targeted therapeutic agents available which have improved progression free and overall survival for these patients [2]. In addition immunotherapy has been shown to improve progression free and overall survival [3]. But despite these new agents, many patients continue to have progressive disease.
There is a preconception of radioresistance in RCC due to the von Hippel-Lindau (VHL) tumour-suppressor gene mutation and hypoxia-inducible factor-1 (HIF-1) [4]. Recently there has been increased attention given to ablative techniques, delivering doses greater than 10Gy per fraction safely. This is possible due to the improved capabilities of modern radiotherapy to accurately deliver precise treatment under image guidance. It is thought that with these techniques, the inherent radioresistance of RCC may be overcome [5]. Unfortunately, these high doses are not always feasible due to large volumes and potential treatment toxicity, as was the case in this patient. But despite a conventional palliative dose regime, this patient achieved an excellent response to her initial 3Gy of RT treatment.
Concurrent with the palliative RT, the patient received axitinib, an antiangiogenic drug (a small molecule TKI) which has been demonstrated in preclinical studies to radiosensitise tumour endothelial cells, increase apoptosis of endothelial cells and improve tumour response when used in conjunction with stereotactic body radiotherapy (SBRT) [6]. While studies suggest a benefit with axitinib and stereotactic radiotherapy doses, a further preclinical study confirmed an advantage when combining axitinib with fractionated radiotherapy in standard 2 Gy per fraction doses [7]. Therefore, one possible explanation for the dramatic reduction in the size of the abdominal mass following 3 Gy of radiotherapy could be attributed to the combined effect of the radiotherapy and concurrent axitinib.
Prior to starting the axitinib, the patient had received 2 months of immunotherapy with nivolumab, which was ceased only 3 weeks prior to the radiotherapy. Nivolumab is an anti-programmed death-1 receptor monoclonal antibody that exhibits checkpoint mediated immune response against tumour cells. When used in conjunction with RT, the RT can increase quantity and variety of tumoral antigen presentation, enhancing the anti-tumor immune response achieved with these drugs. While this remains investigational and the subject of many clinical trials, retrospective evidence suggests that nivolumab, given within 2 weeks of RT treatment, is well tolerated with excellent radiological and symptomatic responses [8]. In this patient, despite progressing on her immunotherapy, when treated with only 3 Gy of RT 3 weeks after her last dose, she experienced a dramatic response in the size of her abdominal mass, leading us to suspect that another possible reason for such a response was that she had been primed with the nivolumab.
A recent phase 1 study looking at the combination of axitinib and pembrolizumab in treatment naïve patients with advanced RCC reported a 73% objective response (complete or partial) [9]. It remains unknown, and the subject of a phase 3 trial, whether this combination is superior to sunitinib monotherapy. Our patient was not treatment naïve, having previously received sunitinib, and the axitinib and anti PD-1 therapy were given sequentially rather than concurrently, however it is possible that an interaction between these treatments may have contributed to her treatment response, both in the radiation field and in the lung metastases.
With the use of immunotherapy, radiotherapy is able to induce tumor regression at distant sites which have not been irradiated. The “abscopal effect” is poorly understood, but the immune system is hypothesised to be a key component after the use of radiotherapy [10]. The abscopal effect has been reported following treatment with SBRT, however a number of case reports have similarly reported abscopal effects following standard fractionation doses of 2Gy per fraction [11]. Our patient demonstrated a response in her lung metastases on subsequent imaging, following the RT to the abdominal mass, suggesting either a response to the TKI or perhaps an abscopal effect from the immunotherapy and RT.
In patients with advanced RCC and progression on immunotherapy, who are not suitable candidates for high dose SBRT due to potential toxicity, our experience suggests that even low doses of RT, which are safe and non-toxic, could provide meaningful and prolonged benefit to patients and should be strongly considered.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received.
Author Contributions
Each author acknowledges that he or she participated sufficiently in the work to take public responsibility for its content and agree with its submission to Case reports in Oncology.
References
- 1.Deschavanne PJ, Fertil B. A review of human cell radiosensitivity in vitro. Int J Radiat Oncol Biol Phys. 1996 Jan;34((1)):251–66. doi: 10.1016/0360-3016(95)02029-2. [DOI] [PubMed] [Google Scholar]
- 2.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011 Dec;378((9807)):1931–9. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 3.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. CheckMate 025 Investigators Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015 Nov;373((19)):1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005 Aug;8((2)):99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Ishiyama H, Blanco A, Lo S, Butler EB, Teh BS. Stereotactic body radiotherapy (SBRT)/stereotactic ablative body radiotherapy (SABR) for “radioresistant” renal cell carcinoma (RCC) J Radiat Oncol. 2014;3((4)):339–46. [Google Scholar]
- 6.Rao SS, Thompson C, Cheng J, Haimovitz-Friedman A, Powell SN, Fuks Z, et al. Axitinib sensitization of high Single Dose Radiotherapy. Radiother Oncol. 2014 Apr;111((1)):88–93. doi: 10.1016/j.radonc.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenton BM, Paoni SF. Alterations in daily sequencing of axitinib and fractionated radiotherapy do not affect tumor growth inhibition or pathophysiological response. Radiat Res. 2009 May;171((5)):606–14. doi: 10.1667/RR1595.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ansari J, Ali M, Alomair A, Mohammed Ali A, Murshid E, Shaukat A, et al. Concurrent immune-radiotherapy in lung and renal cancer - a new treatment paradigm. Ann Oncol. 2017;28(suppl_5):s5. [Google Scholar]
- 9.Atkins MB, Plimack ER, Puzanov I, Fishman MN, McDermott DF, Cho DC, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018 Mar;19((3)):405–15. doi: 10.1016/S1470-2045(18)30081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009 Jul;10((7)):718–26. doi: 10.1016/S1470-2045(09)70082-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev. 2015 Jun;41((6)):503–10. doi: 10.1016/j.ctrv.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]