Abstract
Genomic sequencing provides many opportunities in newborn clinical care, but the challenges of interpreting and reporting newborn genomic sequencing (nGS) results need to be addressed for its broader and effective application. The BabySeq Project is a pilot randomized clinical trial that explores the medical, behavioral, and economic impacts of nGS in well newborns and those admitted to a neonatal intensive care unit (NICU). Here we present childhood-onset and actionable adult-onset disease risk, carrier status, and pharmacogenomics findings from nGS of 159 newborns in the BabySeq Project. nGS revealed a risk of childhood-onset disease in 15/159 (9.4%) newborns; none of the disease risks were anticipated based on the infants’ known clinical or family histories. nGS also revealed actionable adult-onset disease risk in 3/85 (3.5%) newborns whose parents consented to receive this information. Carrier status for recessive diseases and pharmacogenomics variants were reported in 88% and 5% of newborns, respectively. Additional indication-based analyses were performed in 29/32 (91%) NICU newborns and 6/127 (5%) healthy newborns who later had presentations that prompted a diagnostic analysis. No variants that sufficiently explained the reason for the indications were identified; however, suspicious but uncertain results were reported in five newborns. Testing parental samples contributed to the interpretation and reporting of results in 13/159 (8%) newborns. Our results suggest that nGS can effectively detect risk and carrier status for a wide range of disorders that are not detectable by current newborn screening assays or predicted based on the infant’s known clinical or family history, and the interpretation of results can substantially benefit from parental testing.
Keywords: whole-exome sequencing, genomic sequencing, newborn, newborn screening, newborn sequencing
Introduction
Recent advances in genomic sequencing (GS) technologies have raised the possibility of its routine implementation in newborn care.1, 2 Newborn GS (nGS) provides many potential opportunities in the clinical management of a newborn. First, it might identify risk for a broad range of disorders in babies who are asymptomatic at birth and thereby expand the spectrum of conditions for which screening is possible. This would avoid constraints, such as the availability of a biochemical screening method, or confounding factors, such as the baby’s gestational age at birth, transfusion status, age at sample collection, or metabolic and feeding states. Second, nGS could reduce the diagnostic odyssey for ill newborns by allowing for the timely application of appropriate treatments. The success of nGS in providing a rapid diagnosis for critically ill newborns suspected of having single-gene disorders has been demonstrated in recent studies.3, 4 Third, pharmacogenomics (PGx) information from nGS has the potential to inform the selection and dosing of drugs for optimal treatment strategies in childhood and throughout life. Finally, nGS can reveal carrier-status information that could help in future reproductive planning at a time when families are having children. In addition to its utility in the newborn period, nGS can provide a genomic dataset that can be reanalyzed throughout the individual’s life whenever new indications arise. All of these potential benefits can be achieved with a single test, which also provides the opportunity to inexpensively and conveniently re-interrogate the sequence over time as needed when new healthcare issues arise.
Despite its anticipated benefits, some of the major challenges in the use of nGS are the analysis, interpretation, and appropriate reporting of healthcare-related information from genomic data in a timely manner. Variant interpretation, as well as the prediction of likelihood, severity, and timing of a phenotype from a specific variant, are especially difficult in the newborn population because of the absence or obscurity of a phenotype for many disorders at birth. Yet estimating the penetrance and age-of-onset of variants is particularly critical for newborns because of concerns about returning low-risk or adult-onset findings. Predicting the inheritance pattern of variants might not always be straightforward either because both dominant and recessive variants have been reported for many genes. Data derived from studies addressing the technical and interpretive aspects of nGS are needed if researchers are to develop best practices for its responsible and effective implementation. Newborn Sequencing in Genomic Medicine and Public Health (NSIGHT) is an NIH-funded consortium of four research programs designed to address some of these questions.5 Within NSIGHT, the BabySeq Project is a pilot randomized clinical trial that explores the application of nGS in healthy and ill newborns without selecting for those suspected to have a genetic disorder, and it assesses the medical, behavioral, and economic impacts of nGS.6 Here we present the analysis and reporting of nGS results in 159 newborns enrolled in the BabySeq Project; the report includes (1) risk and carrier status for childhood-onset disease, (2) risk for medically actionable adult-onset disease, (3) selected PGx findings relevant to medications used in pediatrics, and (4) variants related to a specific indication that either was present at birth or arose during the course of the study.
Subjects and Methods
The BabySeq Project Study Design
A description of the BabySeq Project, the enrollment process, and the demographic characteristics of the participants has been published elsewhere.6, 7 In brief, two cohorts of newborns and their parents were enrolled in the BabySeq Project: (1) healthy newborns from the well-baby nursery at Brigham and Women’s Hospital (BWH) and (2) ill newborns from the neonatal and pediatric intensive care units (NICUs) at Boston Children’s Hospital (BCH), BWH, and Massachusetts General Hospital (MGH). Enrollees from the NICUs were not preselected on the basis of having a suspected genetic disorder. Three-generation pedigrees were obtained for each family during the consent and enrollment sessions with a genetic counselor. Half of the newborns in each cohort were randomized to receive standard care, including state-mandated newborn screening, plus genetic counseling based on their family histories; the others received nGS in addition to standard care and genetic counseling based on their family histories. The nGS group consisted of 56% white, 23% multi-racial, 2.5% Hispanic or Latino, 1.3% black or African American, 1.3% Asian, and 1.3% Native Hawaiian, other Pacific Islander, or other, although 16% did not specify their ethnicity. The nGS reports of those who were randomized to receive sequencing were disclosed during genetic counseling and were entered into the newborn’s medical record, as well as delivered directly to the newborn’s clinicians. The impacts of nGS on the infant’s clinical care, parent and clinician behaviors, and economic outcomes were evaluated via deep phenotyping of a subset of infants, as well as implementation of baseline, 3-month, and 10-month post-disclosure surveys in parents and baseline, post-disclosure, and end-of-study surveys in clinicians. The impact on clinicians’ behavior was ascertained via baseline, post-disclosure, and end-of-study surveys. This study was approved by the BCH and Partners Healthcare institutional review boards. When the identity of both biological parents was known, both provided informed consent for themselves as well as their infant; if applicable, consent was obtained from any non-biological legal guardians.
nGS Analysis
Whole-exome sequencing (WES) was performed at the CLIA-accredited Clinical Research Sequencing Platform of the Broad Institute, and Sanger confirmation was performed at the CLIA-accredited Partners Healthcare Laboratory for Molecular Medicine as previously described.8 Variants were assessed and classified as described.9, 10 All nGS results were returned in a Newborn Genomic Sequencing Report (NGSR), which included an indication-based analysis (IBA) for any additional diagnostic assessment related to a clinical indication. The first page of the NGSRs summarized the analysis approach and the results in order to concisely communicate key findings from the nGS; subsequent pages provided more detailed information about each reported variant; such information included gene coverage, interpretation of the variant’s clinical significance, a summary of related disease(s), and associated reproductive risks. The criteria used for return of results were as previously described.6 In brief, three groups of results were returned in the NGSR: (1) monogenic disease risks (MDR, defined as pathogenic or likely pathogenic [P/LP] variants in genes associated with dominantly inherited diseases or as bi-allelic P/LP variants in genes associated with recessively inherited diseases) that present or are manageable during childhood (i.e., the earliest reported onset is before the age of 18); (2) carrier status for any gene meeting the MDR reporting criteria; and (3) PGx-associated genes, which were captured by our WES method, that are related to atypical reactions to medications used in the pediatric population. Later in the study, information about risk for a limited number of actionable adult-onset conditions (for which screening, treatment, and preventative actions that would significantly reduce morbidity and mortality are available during adulthood) was also offered for return, and this information was included in the NGSRs for newborns whose parents consented to receive this information for their infant. The actionable adult-onset disease-associated genes included five additional genes (BRCA1 [MIM: 113705], BRCA2 [MIM: 600185], MLH1 [MIM: 120436], MSH2 [MIM: 609309], and MSH6 [MIM: 600678]), which are all found on ACMG 59, a list of clinically important genes that are recommended for reporting incidental findings by the American College of Medical Genetics and Genomics,11 and are associated with hereditary breast and ovarian cancer or Lynch syndrome. It should be noted that PMS2 (MIM: 600259) was excluded from the analysis because the majority of its pathogenic variation could not be reliably assessed by standard WES. The remaining 53 genes on the ACMG 59 list were already being returned on the basis of our baseline criteria for returning childhood-onset and childhood-actionable conditions. For the NICU cohort and the newborns who were enrolled from the well-baby nursery and who later had an indication revealed through record review or clinical follow-up by referring study physicians, an IBA was performed so that all variants in genes with potential relevance to the presenting phenotype could be assessed. Only P/LP variants were returned in the NGSRs; however, all variants with evidence supporting a contribution to the infant’s indication, including variants of uncertain significance (VUSs) in genes related to the existing phenotype and genes with moderate or limited evidence of causing the specific indication, were returned in IBAs. This allowed for studies such as segregation analysis or further clinical evaluation that could help clarify their clinical significance. When needed, parental samples were also collected and tested so that the clinical significance could be clarified and/or the familial risk of variants detected in the newborn could be described. All reported variants were confirmed by Sanger sequencing. Because of the limitations of next-generation sequencing and standard Sanger sequencing in analyzing the CYP21A2 (MIM: 613815) gene, the identified c.844G>T (p.Val282Leu) and c.1447C>T (p.Pro483Ser) variants were confirmed to occur on the authentic gene via a long-range PCR assay at an outside reference laboratory.
Results
127 healthy newborns from the well-newborn nursery and 32 ill newborns who were in a NICU and were enrolled in the BabySeq Project were randomized to receive nGS as previously described.8
Monogenic Disease Risk
We interpreted nGS results in 159 subjects to identify P/LP variants in (1) genes with at least strong evidence of causing highly penetrant (>80% penetrance based on cases reported in the literature) disorders that present or are clinically manageable during childhood and (2) genes with moderate evidence and/or penetrance associated with conditions for which intervention during childhood might prevent a devastating outcome later in life.8 Variants that conferred disease risk, met these criteria, and were unrelated to any known existing phenotype in the newborn were identified in 15 of 159 (9.4%) newborns; 10 of the newborns were healthy and were enrolled from the well-baby nursery (Table 1), and the 5 remaining newborns were from the NICUs. In 3 of 85 (3.5%) newborns whose parents consented to receive information about actionable adult-onset disease risk, pathogenic variants conferring risk for hereditary breast and ovarian cancer or Lynch syndrome were identified (Table 1).
Table 1.
Findings Pertaining to Monogenic-Disease Risk
| Phenotype at Enrollment | Sex | Ethnicity or Race | Gene (Transcript) | Variant(s), (Classification) | Zygosity | Disease | Inh | Parent of Origin | Penetrancea |
|---|---|---|---|---|---|---|---|---|---|
| Well-Baby Cohort | |||||||||
| healthy | m | white | BRCA2b (GenBank: NM_000059.3) | c.8297delC (p.Thr2766Asnfs∗11), (P) | het | hereditary breast and ovarian cancer | AD | mat | high |
| healthy | f | white | BTD (GenBank: NM_000060.2) | c.[44+1G>A;1612C>T] (p.[?;Arg538Cys]), (LP;P) | comp het | biotinidase deficiency | AR | mat & pat | high |
| healthy | f | unspecified | CD46 (GenBank: NM_002389.4) | c.286+2T>G (p.?), (LP) | het | atypical hemolytic-uremic syndrome | AD | mat | moderate |
| healthy | m | white | ELN (GenBank: NM_000501.3) | c.1957G>T (p.Gly653∗), (P) | het | supravalvular aortic stenosis | AD | pat | moderate |
| healthy | f | unspecified | KCNQ4 (GenBank: NM_004700.3) | c.1671_1672insACGAC (p.Val558Thrfs∗3), (LP) | het | non-syndromic hearing loss | AD | pat | high |
| healthy | m | white | MYBPC3 (GenBank: NM_000256.3) | c.1624G>C (p.Glu542Gln), (P) | het | hypertrophic cardiomyopathy | AD | mat | moderate |
| healthy | m | white | TTN (GenBank: NM_133378.4) | c.34894_34895insG (p.Met11632Serfs∗8), (LP) | het | dilated cardiomyopathy | AD | mat | moderate |
| healthy | f | multi-racial | TTN (GenBank: NM_133432.3) | c.12344delC (p.Pro4115Glnfs∗14), (LP) | het | dilated cardiomyopathy | AD | mat | moderate |
| healthy | m | unspecified | TTN (GenBank: NM_133378.4) | c.54172C>T (p.Arg18058∗), (LP) | het | dilated cardiomyopathy | AD | pat | moderate |
| healthy | f | white | TTN (GenBank: NM_133378.4) | c.64276_64282delinsTA (p.Ala21426∗), (P) | het | dilated cardiomyopathy | AD | pat | moderate |
| healthy | f | white | VCL (GenBank: NM_014000.2) | c.1713delA (p.Ala573Hisfs∗8), (LP) | het | dilated cardiomyopathy | AD | mat | moderate |
| NICU Cohort | |||||||||
| anteriorly displaced and imperforate anus | f | white | ANKRD11 (GenBank: NM_001256182.1) | c.2409_2412del (p.Glu805Argfs∗57), (LP) | het | KBG syndrome | AD | de novo | high |
| hypoplastic left heart | m | white | BRCA2b (GenBank: NM_000059.3) | c.3545_3546del (p.Phe1182∗), (P) | het | hereditary breast and ovarian cancer | AD | mat | high |
| congenital severe chronic lung disease | f | unspecified | CYP21A2 (GenBank: NM_000500.7) | c.[844G>T;1447C>T] (p.[Val282Leu;Pro483Ser]), (P;P) | comp hetc | congenital adrenal hyperplasia due to 21-hydroxylase deficiency | AR | unkc & pat | high |
| aortic coarctation | m | native Hawaiian or other Pacific Islander | G6PD (GenBank: NM_000402.3) | c.961G>A (p.Val321Met), (LP) | hem | glucose-6-phosphate dehydrogenase deficiency | XLR | mat | moderate |
| tetralogy of Fallot, pulmonic stenosis, and cryptorchidism | m | white | GLMN (GenBank: NM_053274.2) | c.554_558delinsG (p.Lys185Serfs∗19), (LP) | het | glomuvenous malformations | AD | pat | high |
| respiratory distress (surfactant deficiency) and hypoglycemia | f | multi-racial | MSH2b (GenBank: NM_000251.2) | c.1637_1638insA (p.Asn547Glufs∗4), (P) | het | Lynch syndrome | AD | mat | high |
| neonatal pneumonia and meconium aspiration | m | white | SLC7A9 (GenBank: NM_014270.4) | c.614dupA (p.Asn206Glufs∗3), (P) | het | cystinuria | AD | mat | moderate |
Abbreviations are as follows: m = male; f = female; AD = autosomal-dominant; AR = autosomal-recessive; XLR = X-linked recessive; P = pathogenic; LP = likely pathogenic; het = heterozygous; hom = homozygous; hem = hemizygous; comp het = compound-heterozygous; mat = maternal; and pat = paternal, unk = unknown.
Estimated penetrance for the gene was defined on the basis of curated literature for reported individuals with pathogenic variants in the gene. It was classified as “high” if ≥80% of individuals were symptomatic, “moderate” if 20%–80% of individuals were symptomatic, and “low” if <20% of individuals were symptomatic, as described.6
Actionable adult-onset finding; please see text for explanation.
Although the p.Pro483Ser variant was confirmed to be paternally inherited, the p.Val282Leu variant could not be confirmed in either parent via standard Sanger sequencing. On the basis of multiple occurences in the literature of the two variants on separate chromosomes, these variants were reported to confer disease risk in the newborn in this study. The need to confirm their phase in the newborn by parental testing using targeted CYP21A2 long-range PCR assay was explained in the newborn’s nGS report.
Five genes identified as conferring a risk of childhood-onset disease were reported to have high (>80%) penetrance in the literature. Three of these were associated with autosomal-dominant (AD) conditions. KCNQ4 (MIM: 603537) is associated with non-syndromic hearing loss (MIM: 600101) that typically has a post-lingual presentation within the second decade of life.12 GLMN (MIM: 601749) is associated with glomuvenous malformations (MIM: 138000), which are vascular lesions with a cobblestone appearance and are painful on palpation.13 A NICU newborn with an anteriorly displaced and imperforate anus had a de novo, likely pathogenic variant in ANKRD11 (MIM: 611192), which has been previously associated with KBG syndrome (MIM: 148050), a disorder characterized by macrodontia, distinctive craniofacial features, skeletal anomalies, and developmental delay.14 Although one patient with KBG syndrome has been recently reported as having an anteriorly displaced anus,15 because anorectal malformations were not part of the known phenotypic spectrum of KBG syndrome at the time of our analysis, this variant was initially returned as an incidental finding unrelated to the newborn’s clinical indication; it was later considered to be diagnostically relevant. Two other newborns were found to have bi-allelic variants in genes associated with autosomal-recessive (AR) conditions: biotinidase deficiency (BTD [MIM: 253260]) and congenital adrenal hyperplasia due to 21-hydroxylase deficiency (CAH [MIM: 201910]).
Three of the above disease-risk findings were related to conditions that are tested for by standard newborn screening (NBS) and were identified in newborns who had passed their NBS. As a result of the postlingual onset of KCNQ4-related hearing loss, the effects of a likely pathogenic variant in this gene are not expected to be detected by audiological screening at birth. In another newborn enrolled from the well-baby nursery,16 nGS identified compound heterozygosity for two BTD variants; one was classified as pathogenic (GenBank: NM_000060.4; c.1612C>T [p.Arg538Cys]) and the other (c.44+1G>A (p.?)) as a VUS during the initial assessment on the basis of the existence of transcripts without the relevant exon 1 that might abrogate the effects of predicted splicing disruption. To clarify the clinical significance of the c.44+1G>A variant, we further investigated the baby’s NBS results and discovered borderline NBS results for BTD; a subsequent diagnostic measure of enzyme levels also confirmed partial BTD.16 As a result, we classified the c.44+1G>A variant as likely pathogenic, and the newborn was placed on biotin supplementation. Two variants in CYP21A2 were identified in a female baby with severe chronic lung disease. These two variants have previously been reported in trans with each other in several individuals with nonclassic CAH,17, 18, 19, 20, 21, 22 suggesting that compound heterozygosity for these variants is associated with the nonclassic form of the disease. Females with nonclassic CAH present postnatally with hyperandrogenism signs, such as hirsutism, menstrual irregularities, and infertility, all features that would not be expressed in infancy.23
Eleven variants were found in genes that were previously reported to have moderate (20%–80%) penetrance, and these were disclosed in our study because knowledge about disease risk could allow early interventions during childhood to reduce morbidity and mortality. These variants included six that were associated with dilated (MIM: 604145, 611407) or hypertrophic cardiomyopathies (MIM: 115197) in TTN (MIM: 188840) (four newborns), VCL (MIM: 193065), and MYBPC3 (MIM: 600958). There were also one variant each in ELN (MIM: 130160), CD46 (MIM: 120920), SLC7A9 (MIM: 604144), and G6PD (MIM: 305900), variants in which are associated with supravalvular aortic stenosis (SVAS [MIM: 185500]), atypical hemolytic-uremic syndrome (aHUS [MIM: 612922]), type I cystinuria (MIM: 220100), and G6PD deficiency (MIM: 300908), respectively. It should be noted that the rate of TTN variants was quite high, raising concern of false positives because of the challenges in interpreting predicted loss-of-function (LOF) variants in TTN.24 However, we followed the current best practice for ensuring the LOF variants were located in exons that are not alternatively spliced in cardiac tissue.25 Furthermore, two of the TTN variants have been previously reported in patients with dilated cardiomyopathy (see Table S1 for detailed variant interpretations).
For analysis of the risk of adult-onset disease, variants in five genes associated with conditions that are medically actionable during adulthood, have at least moderate penetrance, and are amenable to testing by WES were also assessed: BRCA1, BRCA2, MLH1, MSH2, and MSH6. Two infants had pathogenic BRCA2 variants associated with increased risk for breast, ovarian, prostate, and pancreatic cancers,26 and one infant had a MSH2 variant pathogenic for Lynch syndrome (MIM: 120435), characterized by elevated risk for colorectal, endometrial, gastric, ovarian, and other cancers.27
None of the infants who were found to be at risk for childhood-onset disease were known to be affected with these conditions at the times of enrollment or interpretation of the nGS results. 14 newborns were heterozygous for AD-condition-associated variants (in BRCA2, CD46, ELN, GLMN, KCNQ4, MSH2, MYBPC3, SLC7A9, TTN, and VCL) inherited from parents who reported themselves as healthy during pre-enrollment genetic counseling. Therefore, we classified variants in the absence of relevant clinical or family histories in the subjects, and we assessed the available evidence from the literature to determine whether the absence of a phenotype in the newborn period and/or absence of family history of disease would exclude a pathogenic role for these variants. Two genes, KCNQ4 and GLMN, are reported to have high penetrance; however, the age of onset and severity of KCNQ4-related hearing loss might be variable, and glomuvenous malformations might appear later in life and express only as single small lesions, which could be missed without focused clinical examination.12, 13 The other genes are known to have moderate or age-dependent penetrance and variable expressivity. Therefore, the identification of variants in these genes in reportedly healthy newborns and parents did not exclude a pathogenic role for these variants.
Carrier Status
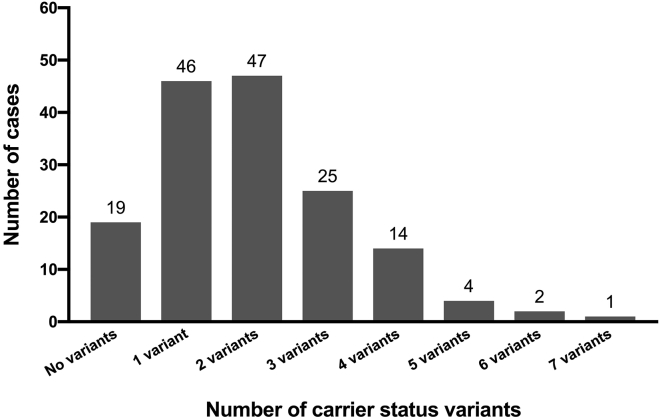
At least one variant conferring carrier status for a recessive childhood-onset disorder8 was identified in 140 of 159 (88%) newborns in the nGS group, and the median was two variants per newborn (Table S2). The number of carrier-status variants ranged from one variant each in 46 newborns to seven variants in a single newborn (Figure 1). Of the 310 variants reported for carrier status, 225 (73%) were identified only once in our cohort. The most common genes and variants returned for carrier status are listed in Table 2. Eleven genes and five specific variants were reported in more than three newborns each (Table 2). The most frequently identified variant was the c.1330G>C (p.Asp444His) variant in BTD. This pathogenic variant was detected in 15 newborns, including one homozygote. The p.Asp444His variant is predicted to cause a ∼25% reduction in biotinidase activity, such that heterozygotes have 75% of normal activity and homozygotes have 50% of normal activity; the latter is similar to that in heterozygotes for a severe variant. This variant has been reported to cause partial BTD when in trans with a severe BTD variant28, 29 and therefore was reported for carrier status in both heterozygotes and homozygotes. The genes that were most frequently reported for carrier status in our study are known to have high carrier frequency in the general population;30, 31, 32, 33 the exception was RBM8A, which is not commonly tested for in routine carrier screening. The RBM8A (MIM: 605313) c.−21G>A (p.?) variant has only been reported in trans with variants that are expected to cause complete loss of RBM8A function in individuals with thrombocytopenia and absent radius (TAR [MIM: 274000]) syndrome;34, 35 Such variants are extremely rare in the general population (gnomAD, see Web Resources). Therefore, although the c.-21G>A variant is common in the population (detected in ∼2.8% of European chromosomes in gnomAD), the reproductive risk is low for carriers of this variant.
Figure 1.
Number of Carrier-Status Variants Reported per Newborn
Number of carrier-status variants reported per newborn is displayed. The numbers above the bars represent the number of newborns who had the specified number of carrier-status variants reported.
Table 2.
Common Variants Reported for Carrier Status in the BabySeq Project
| Gene (Transcript) | Disease | Number of Newborn Carriers | Classification | Variant |
|---|---|---|---|---|
| Genes Reported for Carrier Status in More Than Three Newborns | ||||
| BTD | biotinidase deficiency | 15a | – | – |
| RBM8A | thrombocytopenia with absent radius (TAR) syndrome | 11 | – | – |
| GJB2 | GJB2-related nonsyndromic hearing loss | 10 | – | – |
| CFTR | cystic fibrosis | 6 | – | – |
| MUTYH | MUTYH-related attenuated familial adenomatous polyposis | 6 | – | – |
| ABCA4 | Stargardt disease | 5 | – | – |
| DHCR7 | Smith-Lemli-Opitz | 5 | – | – |
| TYR | oculocutaneous albinism type 1 | 5 | – | – |
| ACADM | medium-chain acyl-CoA dehydrogenase (MCAD) deficiency | 4 | – | – |
| NPC1 | Niemann-Pick disease type C | 4 | – | – |
| SI | congenital sucrase-isomaltase deficiency | 4 | – | – |
| Variants Reported for Carrier Status in More Than Two Newborns | ||||
| BTD (GenBank: NM_000060.2) | biotinidase deficiency | 15a | pathogenic | c.1330G>C (p.Asp444His) |
| RBM8A (GenBank: NM_005105.4) | thrombocytopenia with absent radius (TAR) syndrome | 8 | likely pathogenic | c.−21G>A (p.?) |
| CFTR (GenBank: NM_000492.3) | cystic fibrosis | 4 | pathogenic | c.1521_1523delCTT (p.Phe508del) |
| GJB2 (GenBank: NM_004004.5) | GJB2-related nonsyndromic hearing loss | 4 | pathogenic | c.35delG (p.Gly12Valfs*2) |
| MUTYH (GenBank: NM_001128425.1) | MUTYH-related attenuated familial adenomatous polyposis | 4 | pathogenic | c.1187G>A (p.Gly396Asp) |
| CNGB3 (GenBank: NM_019098.4) | achromatopsia | 3 | pathogenic | c.1148delC (p.Thr383Ilefs∗13) |
| DHCR7 (GenBank: NM_001360.2) | Smith-Lemli-Opitz | 3 | likely pathogenic | c.724C>T (p.Arg242Cys) |
| GJB2 (GenBank: GNM_004004.5) | GJB2-related nonsyndromic hearing loss | 3 | pathogenic | c.101T>C (p.Met34Thr) |
| RBM8A (GenBank: NM_005105.4) | thrombocytopenia with absent radius (TAR) syndrome | 3 | likely pathogenic | c.67+32G>C (p.?) |
Includes one subject homozygous for the p.Asp444His variant and one subject who had the c.511G>A (p.Ala171Thr) variant in cis with this variant.
Although it is unlikely that carrier-status variants would impact phenotypic expression, certain variants associated with AR disorders have been associated with symptoms in the heterozygous state in rare cases of so-called “manifesting heterozygotes.” Out of the 140 newborns who were identified as carriers, six (4.3%) were heterozygous for variants that have been previously associated with mild presentations in carriers (Table S2). These include two female infants with G6PD variants and one female infant with an F8 (MIM: 300841) variant associated with X-linked recessive hemophilia A (MIM: 306700); these variants might lead to mild phenotypes in females with skewed X inactivation, although carrier females for these disorders are typically not affected.36, 37, 38 Two newborns had pathogenic DUOX2 (MIM: 606759) variants causative for congenital hypothyroidism (MIM: 607200) in a bi-allelic state; these variants might lead to mild transient hypothyroidism in manifesting carriers, although this was reportedly not detected in NBS of the two carrier newborns identified in our study.39, 40 One newborn carried an MYBPC3 c.3628−41_3628−17del variant that has been associated with increased risk for milder and late-onset cardiomyopathy in heterozygotes,41 but it is also known to have severe effects in the homozygous state and therefore was classified as LP for early-onset AR cardiomyopathy. The late onset and low-penetrance risk in the heterozygous state was noted in the evidence summary. When variants of this type were reported in the carrier-status section of the report, information about the rare possibility of manifesting symptoms and the limited understanding of their penetrance and expressivity in carriers because of the absence of large numbers of phenotyped carriers and functional studies was included in the evidence description.
For genes that are associated with both AD and AR disorders, individuals with monoallelic pathogenic variants might be at risk for one disease while being a carrier for another disease. Three newborns had TTN variants that were classified as P/LP for AD cardiomyopathy,42 and these variants were also likely pathogenic for AR centronuclear myopathy.43 Therefore, they were described as conferring both risk for cardiomyopathy and carrier status for centronuclear myopathy, and they were reported in both sections of the NGSR. In contrast, the more mildly manifesting carrier variants were only reported in the carrier section.
Although the prior probability of identifying bi-allelic pathogenic variants that confer disease risk in a well newborn is extremely low, it should be kept in mind that a second, pathogenic variant in genes where a monoallelic variant is identified cannot be ruled out by GS, particularly when the associated phenotypes are expected to present later in life. First, only SNVs and small insertions and deletions are reliably detected, and GS might miss other types of pathogenic variation, such as copy-number events, larger indels, or repeat variation. GS also has limited utility for genes that have high homology with pseudogenes or other regions and therefore require other targeted assays for reliable testing. Second, many genes might have incomplete coverage in GS. Among the 310 variants reported for carrier status in our study, 168 (54%) resided in genes with 100% coverage of the target exonic and splice (+/−1,2) regions, whereas 46% had reduced coverage ranging from 59.6% to 99.9% (average 92.7%) (Table S2). Additionally, the clinical significance of many variants remains uncertain. 8 of 140 (6%) newborns with carrier-status variants also had a VUS in one of the reported carrier genes (PCNT [MIM: 605925], MMACHC [MIM: 609831], G6PD, MUTYH [MIM: 604933], SLC22A5 [MIM: 603377], TTN, DYNC2H1 [MIM: 603297], and USH2A [MIM: 608400)] data not shown). In a healthy adult carrier of a highly penetrant recessive disease variant, a second variant detected in trans with a pathogenic variant in the same gene is considered to be benign if the individual does not have any symptoms of the associated disease. However, because the features associated with many recessive disorders are not apparent at birth, interpreting the clinical significance of second variants identified in the carrier genes is more challenging in newborns. In our project, we are continuing to explore whether any of the carrier-status findings might have revealed disease risk due to missing a second pathogenic variant in the gene. However, the frequency of pathogenic variants in these genes, as it is for most monogenic recessive disease genes, is low; therefore, the probability that they have a second pathogenic variant remains very low.
Indication-Based Analyses
At the time of enrollment, an indication-based analysis (IBA) was requested for 29 newborns in the NICU cohort for the following presentations: congenital heart defects (CHDs) (11 newborns), multiple congenital anomalies (11 newborns), severe lung disease, encephalopathy, laryngomalacia, congenital anemia, hemivertebrae, esophageal atresia, and anorectal malformation (one newborn each, Table 3). For three newborns with clinical diagnoses of prematurity (two newborns) and neonatal pneumonia due to meconium aspiration (one newborn), the study physicians did not request an IBA.
Table 3.
Results of Indication-Based Analyses
| Sex | Ethnicity/Race | Indication | Day IBA Ordereda(DOL) | Number of Genes Analyzed | Result | Gene (Transcript) | Variant(s) (Classification) (Zygosity) | Disease (Inheritance) | Penetrance |
|---|---|---|---|---|---|---|---|---|---|
| Well-Baby Cohort | |||||||||
| f | white | bilateral hip dysplasia | 47 | 52 | neg | – | – | – | – |
| f | white | atrial septal defect (PDA) | 4 | 94 | neg | – | – | – | – |
| m | multi-racial | hyperbilirubinemia (DOL 4–6) | 90 | 103 | neg | – | – | – | – |
| f | white | ventricular septal defect | 7 | 97 | neg | – | – | – | – |
| f | multi-racial | cavernous malformation | 400 | 102 | neg | – | – | – | – |
| f | white | liver disease | 212 | 758 | neg | – | – | – | – |
| NICU Cohort | |||||||||
| f | white | hypoplastic left heart | – | 93 | VUS | NKX2-5 (GenBank: NM_004387) | c.111G>A p.Leu37Leu (VUS) (het) | congenital heart disease (AD) | unknown |
| m | white | multiple congenital anomalies with possible diagnosis of VACTERL w/ hydrocephalus | – | 148 | VUS | FANCE (GenBank: NM_021922) | c.1331T>C p.Leu444Pro (VUS) (het) | Fanconi anemia (AR) | high |
| m | native Hawaiian or other Pacific Islander | aortic coarctation | – | 93 | VUS | NOTCH1 (GenBank: NM_017617) | c.4880G>A p.Arg1627His (VUS) (het) | congenital heart disease (AD) | unknown |
| m | white | tetralogy of Fallot, pulmonic stenosis, and cryptorchidism | – | 356 | VUS | NOTCH1 (GenBank; NM_017617) | c.4168C>A p.Pro1390Thr (VUS) (het) | congenital heart disease (AD) | unknown |
| m | white | encephalopathy | – | 459 | incb | GLDC (GenBank: NM_000170) | c.128delA p.Asp43Alafs∗48 (P) (het) | glycine encephalopathy (AR) | high |
| f | white | multiple congenital anomalies including TOF, pulmonary stenosis, TET spells, duodenal atresia, anteriorly displaced anus, and failure to thrive | – | 142 | neg | – | – | – | – |
| f | white | Pierre Robin sequence (micrognathia, cleft palate, glossoptosis), hooded eyes, tubular nose | – | 266 | neg | – | – | – | – |
| f | white | hemivertebrae | – | 1 | neg | – | – | – | – |
| m | white | double outlet right ventricle, atrioventricular canal defect, recurrent respiratory infections, laryngomalacia, enterocolitis, hypocal–cemia, short stature | – | 0 | neg | – | – | – | – |
| m | white | hypoplastic left heart | – | 94 | neg | – | – | – | – |
| f | white | tetralogy of Fallot with absent pulmonary valve | – | 94 | neg | – | – | – | – |
| f | white | anteriorly displaced anus (anorectal malformations) | – | 45 | neg | – | – | – | – |
| m | white | hypoplastic left heart | – | 93 | neg | – | – | – | – |
| f | white | dextrotransposition of the great arteries | – | 93 | neg | – | – | – | – |
| f | white | tricuspid atresia and ventricular septal defect | – | 93 | neg | – | – | – | – |
| f | multi-racial | respiratory distress (surfactant deficiency) and hypoglycemia | – | 169 | neg | – | – | – | – |
| m | white | hypoplastic left heart | – | 93 | neg | – | – | – | – |
| m | white | transposition of great arteries | – | 106 | neg | – | – | – | – |
| f | white | interstitial lung disease and facial dysmorphia | – | 366 | neg | – | – | – | – |
| m | white | liver disease, thrombocytopenia/anemia, hyperbilirubinemia, and hypoglycemia | – | 224 | neg | – | – | – | – |
| f | unspecified | congenital severe chronic lung disease | – | 387 | neg | – | – | – | – |
| f | white | aortic coarctation and ventricular septal defect | – | 93 | neg | – | – | – | – |
| f | unspecified | flat facial profile, preauricular pits, macroglossia, and hemangioma | – | 109 | neg | – | – | – | – |
| m | white | encephalopathy and hemangioma | – | 247 | neg | – | – | – | – |
| m | multi-racial | single ventricle, double inlet left ventricle with normally related great arteries | – | 186 | neg | – | – | – | – |
| m | white | laryngomalacia | – | 79 | neg | – | – | – | – |
| f | white | hypoglycemia and large for gestational age | – | 110 | neg | – | – | – | – |
| f | white | congenital anemia | – | 400 | neg | – | – | – | – |
| f | white | esophageal atresia with tracheoesophageal fistula | – | 251 | neg | – | – | – | – |
Abbreviations are as follows: m = male; f = female; DOL = day of life; neg = negative; VUS = variant of uncertain significance; inc = inconclusive; P = pathogenic; het = heterozygous; inh = inheritance; AD = autosomal dominant; and AR = autosomal recessive. aDay IBA ordered in the well-baby cohort.bSingle pathogenic variant associated with AR disease.
For the IBAs, genes that have been associated with the newborns’ reported clinical features, (including those with limited evidence for disease association and/or low penetrance), were identified, and all variants in these genes were reviewed to assess their clinical significance, as well as relevance to the newborns’ indications. The number of genes specifically interrogated in the IBAs ranged from one (TBX6 [MIM: 602427], for an IBA of hemivertebrae) to 758 (for an IBA of liver disease); the median was 106 genes per analysis.
WES did not identify any variants that unequivocally explained the indications in newborns from the NICU cohort. Inconclusive results, including VUSs in genes that might be related to the indication or monoallelic variants in genes associated with AR conditions, were identified in 5 of 29 (17%) infants (Table 3). Three newborns with CHDs, one of whom also had cryptorchidism, were heterozygous for VUSs in genes associated with AD CHDs. These variants were inherited from parents who did not report having CHDs; however, because of the incomplete penetrance of the phenotypes associated with these genes, the clinical significance of the variants remained uncertain. One newborn with a possible diagnosis of VACTERL (vertebral defects, anal atresia, cardiac defects, tracheo-esophageal fistula, renal anomalies, and limb abnormalities) association and hydrocephalus was heterozygous for a VUS in FANCE (MIM: 613976), a gene associated with AR Fanconi anemia (MIM: 600901), which shares some features with VACTERL. Finally, a newborn with encephalopathy was heterozygous for a pathogenic variant in GLDC (MIM: 238300), a gene that can cause AR glycine encephalopathy (MIM: 605899). No second variant was identified in FANCE or GLDC in these newborns, reducing the likelihood that variants identified in these genes were relevant for their phenotypes, although copy-number variants could not be detected by our test. An infant who had an anteriorly displaced anus had a likely pathogenic de novo ANKRD11 variant identified in the NGSR analysis; this variant, was reported in the Monogenic Disease Risk section of the report rather than in the Indication-Based Analysis results, as described above. The ANKRD11 gene was not known to be associated with the reported phenotype of the infant at the time of analysis and therefore was not included in the 45 genes that were thought to be potentially related to anorectal malformations and were targeted in the IBA. However, this variant was later considered to be relevant on the basis of the recent association of the gene with this feature.
Additionally, 6 of the 127 newborns (5%) in the well-baby cohort had indications that prompted an IBA during the course of our study. For two of them, an IBA was requested at the time of enrollment on the basis of the discovery of a patent ductus arteriosus and a ventricular septal defect. For the other four infants, an IBA was requested after enrollment for the following indications: bilateral hip dysplasia, hyperbilirubinemia (diagnosed at day of life [DOL] 4), abnormal liver function, and seizure resulting from bleeding of a cavernous malformation; the IBAs were requested at DOL 47, 108 (following nGS results disclosure), 212, and 400, respectively. No variants with a potential relationship to their indication were identified in these infants.
PGx Variants
Return of PGx results was limited to genes with substantial evidence of association with atypical responses to drugs that might be used in the pediatric population (Table 4). Variants identified in three genes were determined to be in this category: DPYD (MIM: 612779), TPMT (MIM: 187680), and G6PD. PGx variants identified in these genes were returned in 8 of 159 (5%) newborns who received nGS. Four newborns had DPYD variants associated with increased risk for toxicity from the use of fluoropyrimidines and therefore with a decreased dose requirement for these medications. Three newborns had TPMT variants associated with higher risk of life-threatening myelosuppression when treated with standard doses of thiopurines and therefore had a decreased dose requirement for thiopurines. The G6PD variant was associated with G6PD deficiency and was identified in a hemizygous male infant. It was returned in the disease risk section of NGSR because the disease could be triggered by factors other than medications, as well. The PGx association for this variant was described in the variant summary.
Table 4.
Reported Pharmacogenomic Variants
| Gene (Transcript) | Variant | Drug | Dosing Information | Number of Newborns |
|---|---|---|---|---|
| DPYD (GenBank: NM_000110.3) | c.1905+1G>A (p.?) | fluoropyrimidines | decreased dose requirement | 2 |
| DPYD (GenBank: NM_000110.3) | c.2846A>T (p.Asp949Val) | fluoropyrimidines | decreased dose requirement | 2 |
| TPMT (GenBank: NM_000367.4) | c.460G>A (p.Ala154Thr) | thiopurines | decreased dose requirement | 3 |
| G6PDa (GenBank: NM_000402.3) | c.961G>A (p.Val321Met) | certain antimalarials such as primaquine; antibiotics such as quinolones and sulfonamides, and methylene blue. (See reference 68 and G6PD Deficiency Favism Association in Web Resources) | contraindicated | 1 |
Reported in Monogenic Disease Risk section of NGSR.
Parental Sample Testing to Help Interpret nGS Results
Interpreting nGS results has unique challenges because of the absence of a phenotype in newborns for non-congenital diseases. To help interpret and communicate nGS findings, we sometimes tested parental samples to establish phase, assess for de novo occurrence, and otherwise clarify the significance of variants and/or explain familial risk to the parents. During the course of the project, 37 variants identified in NGSR analysis of 28 newborns were tested in parental samples. 16 P/LP variants conferring disease risk (AD and X-linked) and four carrier-status variants for which adult carriers could present symptoms were tested for in parents so that the associated disease risk could be better interpreted and communicated to the families. For 17 other variants, parental testing results contributed to the decisions made for whether and how to report variants by helping us to determine the variants’ clinical significance, phase, and/or mode of inheritance. Seven P/LP variants in genes that have been associated with both AD and AR modes of inheritance (in BEST1 [MIM: 607854], COL6A2 [MIM: 120240], GLRA1 [MIM: 138491], MYH7 [MIM: 160760], RNASEH2B [MIM: 610326], TECTA [MIM: 602574], and VWF [MIM: 613160]) were tested so that their inheritance pattern could be determined. These variants either were novel truncating variants or had been reported in both heterozygous and homozygous or compound-heterozygous affected individuals in the literature, and their identification in healthy parents was considered to be evidence supporting a recessive mode of inheritance, favoring the decision to report these for carrier status. Seven P/LP variants in AR BTD and CYP21A2 were tested for phasing, and their allelic states were reported accordingly. Three VUSs identified in NGSR analyses were tested so that their clinical significance could be clarified, and their identification in a healthy parent was considered evidence in support of a benign role. Variants in EXT2 (MIM: 608210) and RB1 (MIM: 614041), associated with highly penetrant AD disorders, and in a female newborn, a paternally inherited BRWD3 (MIM: 300553) varaint (associated with an X-linked recessive disorder), were classified as VUSs on the basis of their identification in healthy parents and other lines of evidence, and they were excluded from the NGSRs. Three VUSs identified in IBAs (described above) were also tested in parents; however, because they were in genes associated with moderate penetrance and/or variable expressivity, their identification in reportedly healthy parents did not alter their classification. Overall, parental testing contributed to determining whether or how a variant was reported in 13 of 159 (8%) of the newborns who received nGS and helped with interpretation and communication of nGS results in a total of 28 of 159 (18%) of the newborns.
Discussion
Because of its potential to target a wide range of disorders for screening and diagnostic purposes with a single test, nGS can be a powerful tool for improving the future healthcare of infants. However, the application of newborn sequencing poses several challenges, including how to interpret variants associated with conditions that might not be apparent in the infant at the time of testing and the potential costs and psychosocial impacts.44 Our data from 159 newborns sequenced in the BabySeq Project help illustrate the range of situations that might arise from nGS. They also highlight factors that need to be considered for the interpretation and reporting of nGS results, including the age of onset, penetrance, and inheritance patterns of identified variants, and their relevance to the clinical and family histories of the newborns at the time of analysis.
Our study had several limitations. First, we had a small cohort size, which was particularly limited for the NICU group. Second, the fact that participants were randomized to either receive or not receive nGS in our study might have discouraged parents of ill newborns who could receive diagnostic nGS clinically or as part of another non-randomized study. This might have created a self-selection for parents whose newborn was less likely to receive GS in other settings on the basis of their phenotype and therefore might have created an enrichment of phenotypes that were less likely to benefit from nGS. Additionally, our proband-only sequencing approach using phenotype-driven gene filtering had limited ability to detect de novo variants in genes that were recently described or had limited association with the infant’s indication at the time of our analysis.
The prior probability of a genetic disorder is assumed to be low in healthy newborns. However, nGS identified risk for childhood-onset diseases in 9.4% and risk for actionable adult-onset diseases in 3.5% of the newborns sequenced in the BabySeq Project. Eleven newborns had variants that were expected to have moderate penetrance or variable expressivity on the basis of previous reports in the literature, but these variants were considered as medically actionable during childhood. These include seven newborns who were discovered to have risk for cardiomyopathies or SVAS, for which increased surveillance by regular echocardiograms and EKGs might allow timely interventions that would significantly reduce the risk for heart failure and sudden cardiac death.45, 46, 47 Knowledge about risk for these conditions could also allow informed clinical and lifestyle choices (such as participation in sports or the use of stimulant medications) to further reduce the risk for devastating events.48, 49 Other conditions identified in our cohort, such as aHUS, G6PD deficiency, and cystinuria could also benefit from avoidance of precipitating factors.50, 51, 52 Because many of the conditions for which we have detected risk might have incomplete penetrance, later onset, and/or uncertain immediate medical actionability, it is possible that identifying their risk later in life rather than during the newborn period might also be beneficial for health outcomes. This might also avoid the possibility of negative psychosocial implications or increased medical interventions and healthcare costs when detected within the first days of life. On the other hand, in the absence of a significant family history, a genomic screening approach might be the only setting where an individual’s risk gets identified before any symptoms arise. The results of our study and other studies on the use of GS in newborns and other populations will help develop best practices for the optimal timing and application of such a screen in an individual’s life.
None of the disease risk findings were predicted on the basis of known clinical and family histories of the newborns at the time of testing. Our results prompted follow-up studies to search for evidence of disease and/or family history that was not appreciated during enrollment. After the disclosure of the nGS results, the parents of three infants expressed that they had a family history of the disease for which their newborn was identified to be at risk (a grandparent of a newborn with TTN variant had dilated cardiomyopathy, a grandparent of a newborn with KCNQ4 variant had hearing loss, and a parent of a newborn with BRCA2 variant had family history of breast cancer). Clinical follow-up with the infants and their parents harboring the disease-risk variants is ongoing so that clinicians can assess whether there are any symptoms of disease. Because many of the genes we detected are known to have incomplete penetrance or might present later in life with variable expressivity, the absence of a phenotype or family history in the parents did not exclude a pathogenic role for the variants, although it was informative to predict the likelihood of disease in the newborns who had these variants.
Interestingly, P/LP variants in genes related to cardiomyopathies and SVAS were found in 7 of 159 (4%) newborns, a rate that is higher than the known prevalence of these conditions in the general populationand which emphasizes the incomplete and age-dependent penetrance of these conditions. The ELN and MYBPC3 variants were classified as pathogenic on the basis of a truncating effect or segregation in multiple families, respectively (Table S1). Truncating VCL variants, such as the one identified in our study, are currently considered likely pathogenic for DCM on the basis of their identification and segregation in affected families (LMM internal data, Table S1), although additional studies are needed to clarify this gene’s penetrance. Four newborns had truncating TTN variants that were classified as P/LP for DCM. Two of these variants have previously been reported in multiple DCM patients and/or have been found to segregate with disease in affected family members (Table S1), providing further support for their pathogenicity. TTN truncating variants are prevalent in the general population,24, 53 which makes it challenging to interpret their clinical significance. It has been demonstrated that truncating variants in control individuals were more likely to affect minor TTN isoforms and occur in alternatively spliced exons, whereas those in constitutively expressed exons are enriched in DCM patients as compared to controls.24, 53, 54, 55, 56 Two truncating TTN variants identified in our study (p.Pro4115Glnfs∗14 and p.Met11632Serfs∗8) have not been previously reported in individuals with DCM, and although they are located in the I-band, where alternative splicing occurs frequently, the exons they are located in have been demonstrated to be not alternatively spliced in cardiac tissue.24 These variants were classified as LP for DCM on the basis of the current best practice of classifying as LP the truncating TTN variants located in exons that are not alternatively spliced.25 The penetrance of TTN truncating variants has been demonstrated to be ∼60% in a study of family members of affected individuals,56 although it is possible that the penetrance might vary depending on the location of these variants. Analyses in larger cohorts are needed to clarify the penetrance of truncating TTN variants located in various regions of this gene.
Our results suggest that nGS might also expand the detectable phenotypic spectrum of disorders that are targeted by current NBS, although the identification of these conditions at birth might or might not provide additional benefit. In three newborns who passed NBS, nGS identified risk for NBS-targeted conditions (hearing loss, BTD and CAH). Postlingual hearing loss due to KCNQ4 variants is not expected to be detected by audiological screening at birth. However, recognizing early stages of hearing loss in children is challenging and can often delay diagnosis and interventions. Information about this risk could allow additional vigilance and screening to provide timely interventions and reduce its impact on the child’s development and social skills, particularly if the onset of hearing loss is during childhood. Detecting risk for later-onset hearing loss in presymptomatic individuals might have less significance for the individual’s health and quality of life. Partial BTD identified on the basis of the nGS results might be missed in NBS, as it was in our subject, although it might be clinically significant, particularly at times of stress. Although many individuals with partial BTD might not experience any symptoms throughout their lifetime and detecting partial BTD in the newborn period might not be critical, symptoms can effectively be prevented with a simple and inexpensive treatment of biotin supplementation, as was prescribed in this case.16 Finally, NBS rarely detects nonclassic CAH.57 Identifying individuals at risk for nonclassic CAH might be beneficial for facilitating early diagnosis and therapies, if needed, although many individuals with this condition might not need treatment. Therefore, our results serve as a reminder that negative NBS results do not rule out pathogenic variants in genes associated with NBS conditions, and they suggest that nGS might identify individuals with milder or later-onset phenotypes of NBS conditions, whose detection might not be as critical in the newborn period.
Currently, there is ongoing debate about whether adult-onset disease risk should be returned to children and whether nondisclosure of particularly actionable adult-onset disease risk might do more harm to the children and families.58, 59, 60 In our study, families were offered the option to receive information regarding risk for medically actionable adult-onset conditions in their infant. Three of 85 (3.5%) infants whose parents consented to receive this information harbored pathogenic variants associated with adult-onset conditions for which early knowledge leading to increased surveillance and preventative treatments might be lifesaving.26, 27 These variants were also identified in the mothers of the three children, and early interventions based on this knowledge might also have lifesaving consequences for the child’s parent, which undoubtedly could impact the child’s quality of life. The risks and benefits of returning adult-onset disease risk to children will continue to be discussed on the basis of the results of studies that address this question.5
Recent studies using GS in adult cohorts reported a rate of 3%–5.6% for secondary findings in the ACMG5911 genes or in other small groups of actionable genes determined by the authors.61, 62, 63, 64 Our reporting criteria were much broader and included a higher number of genes to be returned for disease risk findings (>900 genes met criteria for reporting in our initial curation efforts).8 In our study, four newborns had a disease risk variant in one of the ACMG59 genes: three of those were in adult-onset disease genes (BRCA2 and MSH2) and one (MYBPC3) was considered a childhood-onset disease gene. This corresponds to a combined rate of 4.1% ([3/85] + [1/159]), a rate that is similar to the rate of incidental findings in ACMG59 genes reported in adult cohorts.61, 62, 63
nGS also allows detection of carrier status for a wide range of disorders that are not included in currently available expanded carrier screening panels. We identified at least one carrier-status variant in 88% of the newborns and up to seven variants per subject. The majority (73%) of these variants were identified only once in our cohort, suggesting that returning only common pathogenic variants with high frequency in the general population would miss the majority of carriers for childhood-onset diseases. In addition, each of these novel variants required considerable manual curation to determine their clinical significance, implying that returning carrier status in nGS would significantly increase the amount of work done by clinical laboratories; this increase might impact the turnaround time and cost for reporting nGS results. Carrier-status information is mostly relevant for future reproductive planning for the infant and the parents because genetic testing in the newborn nearly always reveals variants that are also carried by one of the parents. An estimate of a couple’s reproductive risk can be provided on the basis of assumptions that (1) the parents are not related and (2) the probability for the other parent to be a carrier for the same gene is equal to the gene’s known or estimated carrier frequency in their ethnic subpopulation. However, to determine the actual reproductive risk, couples might want to pursue carrier testing for both the identified variants and subsequently the full gene in the non-carrier parent. Effectively determining carrier status in the parents would require targeted gene tests in the case of many genes that have been identified for carrier status in our cohort; such tests might have varying availability in clinical laboratories and therefore pose a challenge for future reproductive planning. Our study continues to review what portion of our participants are pursuing such targeted carrier testing on the basis of our nGS results and the outcomes of these tests to assess the utility and impact of returning carrier-status information in nGS.
nGS has recently been shown to have a high clinical yield in critically ill newborns who had been admitted to a NICU and were suspected of having a genetic disorder.3, 4 In contrast, we observed a lower rate of positive findings in our NICU cohort. Several reasons might account for this difference. First, the NICU patients in our study were not pre-selected for a suspected genetic disorder but were chosen with minimal exclusion criteria to represent a more general population. Additionally, our randomized study design might have led to some self-selection of families whose newborns had a lower likelihood of benefitting from nGS. Because our participants had a 50% probability of receiving nGS, it is possible that parents of newborns who could receive diagnostic GS as part of their standard care or in a non-randomized nGS study might have had less interest in enrolling in the BabySeq Project. In our study, 22 of 29 ill newborns (76%) had nonsyndromic congenital heart defects or multiple congenital anomalies. Monogenic diseases usually have a small contribution to these conditions, and they might also frequently be explained by chromosome abnormalities and structural alterations that are not reliably detected by WES.65, 66, 67 Although our study design might have led to self-selection of NICU newborns who were less likely to have a monogenic disease etiology, our results suggest that the diagnostic yield of nGS might depend on the phenotype of the subjects. Finally, we performed proband-only GS and used a phenotype-driven gene-filtering approach focusing on genes with known association with the infant’s features, a method that is limited to detecting de novo and other variants in genes that had recent or limited association with the disease of interest at the time of our analysis. The aim of our study was to explore the use of singleton nGS in newborn care, although trio sequencing is known to have a higher clinical yield for a wide range of indications.3, 68 Our identification of a likely pathogenic de novo variant in ANKRD11 in the NGSR analysis as opposed to the IBA highlights the limitations of performing phenotype-driven gene and/or variant assessments and of proband-only sequencing. This variant was detected as a novel LOF variant in a disease gene and was initially interpreted as an incidental finding; therefore, it was reported under the Monogenic Disease Risk section rather than IBA results and was later thought to be diagnostically relevant in light of recent studies. Although all variants in genes known to be relevant for the indication were analyzed in our IBAs regardless of their reporting status and predicted impact on the protein, because ANKRD11 was not known to be associated with anorectal malformations at the time of our analysis, it was not included in the phenotype-driven gene list for the IBA of this infant. In the absence of their known association with the infant’s phenotype, VUSs in this gene would not have been captured or returned in our study, and a clinically relevant variant could be missed.
In addition to discovering short-term disease risk and diagnosing existing but clinically unsuspected disease, nGS allows genomic information that can be specifically interrogated for new indications and inform personalized medicine applications to be accessible throughout an individual’s lifetime. During the course of our project, 5% of infants enrolled from the well-baby nursery developed an indication that prompted an IBA. Because our subjects are currently between 8 months and 3 years old, it is likely that additional participants will eventually develop indications that benefit from genomic IBAs. Although GS might not be the most appropriate test for all presentations, an IBA on already available GS data will be a rapid, first-tier approach when gene sequencing is indicated and can be supplemented or followed by additional tests. An IBA for a new presentation in an individual who has already been sequenced will allow for the review of genes that are recently associated with the disease and for the full assessment of all variants in relevant disease genes. Other uses of nGS data in a newborn’s future life might include analyses for a wide range of adult-onset disease risk, polygenic risk estimates for complex traits, and PGx for drugs used in the adult population.
Having access to the known clinical and family histories of our subjects, as well as to samples from their parents, was invaluable for the interpretation of nGS results in our study. Parental sample testing, when available, is frequently performed in diagnostic GS; however, its utility for interpreting nGS findings in a screening setting has not, to our knowledge, been previously addressed. Results of parental testing in light of provided family histories helped determine whether and how a variant was reported for 8% of our participants; all of these variants were part of the NGSR analyses directed to screening purposes. Although obtaining detailed clinical and family history information might be challenging in a population-wide application of nGS, the collection of parental samples and informing laboratories of existing or newly diagnosed conditions in the newborn and family members should be performed to help better interpret nGS results.
In summary, we present our nGS findings from 159 newborns sequenced in the BabySeq Project. Although detecting disease risk for many actionable early-onset conditions would be beneficial in improving health outcomes, potential healthcare costs and psychosocial impacts need to be considered in the development of best practices for nGS. Our study continues to explore the medical, behavioral, and economic impacts of our nGS reports on the basis of medical observations and post-disclosure surveys in parents and clinicians. As these newborn cohorts age, future analyses of economic and healthcare utilization patterns in our nGS and control cohorts will allow for the eventual assessment and quantification of both costs and benefits of GS in the newborn setting. The results from our study, as well as future efforts to prospectively analyze the long-term implications of nGS in larger cohorts, will help inform the effective and responsible application of nGS in wider medical practice.
Consortia
The members of the BabySeq Project Team are as follows: Pankaj B. Agrawal, Alan H. Beggs, Wendi N. Betting, Ozge Ceyhan-Birsoy, Kurt D. Christensen, Dmitry Dukhovny, Shawn Fayer, Leslie A. Frankel, Casie A. Genetti, Chet Graham, Robert C. Green, Amanda M. Guiterrez, Maegan Harden, Ingrid A. Holm, Joel B. Krier, Matthew S. Lebo, Harvey L. Levy, Xingquan Lu, Kalotina Machini, Amy L. McGuire, Jaclyn B. Murry, Medha Naik, Tiffany T. Nguyen, Richard B. Parad, Hayley A. Peoples, Stacey Pereira, Devan Petersen, Uma Ramamurthy, Vivek Ramanathan, Heidi L. Rehm, Amy Roberts, Jill O. Robinson, Serguei Roumiantsev, Talia S. Schwartz, Tina K. Truong, Grace E. VanNoy, Susan E. Waisbren, and Timothy W. Yu.
Declaration of interests
Dr. Green receives compensation for consultation from AIA, Helix, Ohana, Prudential, and Veritas, and is co-founder, advisor, and equity holder in Genome Medical, Inc. The remaining authors declare no competing interests.
Acknowledgments
The authors would like to thank the families for their participation in the BabySeq Project. Research reported in this publication was supported by the National Institutes of Health under award numbers U19HD077671, R01HD075802, and U41HG006834. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Published: January 3, 2019
Footnotes
Supplemental Data include two tables and can be found with this article online at https://doi.org/10.1016/j.ajhg.2018.11.016.
Contributor Information
Alan H. Beggs, Email: beggs@enders.tch.harvard.edu.
The BabySeq Project Team:
Pankaj B. Agrawal, Alan H. Beggs, Wendi N. Betting, Ozge Ceyhan-Birsoy, Kurt D. Christensen, Dmitry Dukhovny, Shawn Fayer, Leslie A. Frankel, Casie A. Genetti, Chet Graham, Robert C. Green, Amanda M. Guiterrez, Maegan Harden, Ingrid A. Holm, Joel B. Krier, Matthew S. Lebo, Harvey L. Levy, Xingquan Lu, Kalotina Machini, Amy L. McGuire, Jaclyn B. Murry, Medha Naik, Tiffany T. Nguyen, Richard B. Parad, Hayley A. Peoples, Stacey Pereira, Devan Petersen, Uma Ramamurthy, Vivek Ramanathan, Heidi L. Rehm, Amy Roberts, Jill O. Robinson, Serguei Roumiantsev, Talia S. Schwartz, Tina K. Truong, Grace E. VanNoy, Susan E. Waisbren, and Timothy W. Yu
Accession Numbers
The data/analyses reported in this paper have been deposited in the NBSTRN LPDR under accession identifier nbs000002.v1.p1.
Web Resources
OMIM, http://www.omim.org/
G6PD Deficiency Favism Association, https://www.g6pd.org
gnomAD, http://gnomad.broadinstitute.org
NBSTRN LPDR, https://nbstrn.org/research-tools/longitudinal-pediatric-data-resource
Supplemental Data
References
- 1.Kingsmore S.F. Newborn testing and screening by whole-genome sequencing. Genet. Med. 2016;18:214–216. doi: 10.1038/gim.2015.172. [DOI] [PubMed] [Google Scholar]
- 2.Bailey D.B., Jr., Gehtland L. Newborn screening: Evolving challenges in an era of rapid discovery. JAMA. 2015;313:1511–1512. doi: 10.1001/jama.2014.17488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng L., Pammi M., Saronwala A., Magoulas P., Ghazi A.R., Vetrini F., Zhang J., He W., Dharmadhikari A.V., Qu C. Use of exome sequencing for infants in intensive care units: Ascertainment of severe single-gene disorders and effect on medical management. JAMA Pediatr. 2017;171:e173438. doi: 10.1001/jamapediatrics.2017.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrikin J.E., Cakici J.A., Clark M.M., Willig L.K., Sweeney N.M., Farrow E.G., Saunders C.J., Thiffault I., Miller N.A., Zellmer L. The NSIGHT1-randomized controlled trial: Rapid whole-genome sequencing for accelerated etiologic diagnosis in critically ill infants. NPJ Genom Med. 2018;3:6. doi: 10.1038/s41525-018-0045-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg J.S., Agrawal P.B., Bailey D.B., Jr., Beggs A.H., Brenner S.E., Brower A.M., Cakici J.A., Ceyhan-Birsoy O., Chan K., Chen F. Newborn sequencing in genomic medicine and public health. Pediatrics. 2017;139:e20162252. doi: 10.1542/peds.2016-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holm I.A., Agrawal P.B., Ceyhan-Birsoy O., Christensen K.D., Fayer S., Frankel L.A., Genetti C.A., Krier J.B., LaMay R.C., Levy H.L., BabySeq Project Team The BabySeq project: Implementing genomic sequencing in newborns. BMC Pediatr. 2018;18:225. doi: 10.1186/s12887-018-1200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Genetti C.A., Schwartz T.S., Robinson J.O., VanNoy G.E., Petersen D., Pereira S., Fayer S., Peoples H.A., Agrawal P.B., Betting W.N., BabySeq Project Team Parental interest in genomic sequencing of newborns: Enrollment experience from the BabySeq Project. Genet. Med. 2018 doi: 10.1038/s41436-018-0105-6. Published online September 13, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceyhan-Birsoy O., Machini K., Lebo M.S., Yu T.W., Agrawal P.B., Parad R.B., Holm I.A., McGuire A., Green R.C., Beggs A.H., Rehm H.L. A curated gene list for reporting results of newborn genomic sequencing. Genet. Med. 2017;19:809–818. doi: 10.1038/gim.2016.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duzkale H., Shen J., McLaughlin H., Alfares A., Kelly M.A., Pugh T.J., Funke B.H., Rehm H.L., Lebo M.S. A systematic approach to assessing the clinical significance of genetic variants. Clin. Genet. 2013;84:453–463. doi: 10.1111/cge.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaughlin H.M., Ceyhan-Birsoy O., Christensen K.D., Kohane I.S., Krier J., Lane W.J., Lautenbach D., Lebo M.S., Machini K., MacRae C.A., MedSeq Project A systematic approach to the reporting of medically relevant findings from whole genome sequencing. BMC Med. Genet. 2014;15:134. doi: 10.1186/s12881-014-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Green R.C., Berg J.S., Grody W.W., Kalia S.S., Korf B.R., Martin C.L., McGuire A.L., Nussbaum R.L., O’Daniel J.M., Ormond K.E., American College of Medical Genetics and Genomics ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet. Med. 2013;15:565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naito T., Nishio S.Y., Iwasa Y., Yano T., Kumakawa K., Abe S., Ishikawa K., Kojima H., Namba A., Oshikawa C., Usami S. Comprehensive genetic screening of KCNQ4 in a large autosomal dominant nonsyndromic hearing loss cohort: Genotype-phenotype correlations and a founder mutation. PLoS ONE. 2013;8:e63231. doi: 10.1371/journal.pone.0063231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouillard P., Boon L.M., Revencu N., Berg J., Dompmartin A., Dubois J., Garzon M., Holden S., Kangesu L., Labrèze C., GVM Study Group Genotypes and phenotypes of 162 families with a glomulin mutation. Mol. Syndromol. 2013;4:157–164. doi: 10.1159/000348675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sirmaci A., Spiliopoulos M., Brancati F., Powell E., Duman D., Abrams A., Bademci G., Agolini E., Guo S., Konuk B. Mutations in ANKRD11 cause KBG syndrome, characterized by intellectual disability, skeletal malformations, and macrodontia. Am. J. Hum. Genet. 2011;89:289–294. doi: 10.1016/j.ajhg.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Low K., Ashraf T., Canham N., Clayton-Smith J., Deshpande C., Donaldson A., Fisher R., Flinter F., Foulds N., Fryer A., DDD Study Clinical and genetic aspects of KBG syndrome. Am. J. Med. Genet. A. 2016;170:2835–2846. doi: 10.1002/ajmg.a.37842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murry J.B., Machini K., Ceyhan-Birsoy O., Kritzer A., Krier J.B., Lebo M.S., Fayer S., Genetti C.A., VanNoy G.E., Yu T.W., BabySeq Project Team Reconciling newborn screening and a novel splice variant in BTD associated with partial biotinidase deficiency: A BabySeq Project case report. Cold Spring Harb. Mol. Case Stud. 2018;4:a002873. doi: 10.1101/mcs.a002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balsamo A., Cacciari E., Baldazzi L., Tartaglia L., Cassio A., Mantovani V., Piazzi S., Cicognani A., Pirazzoli P., Mainetti B., Zappulla F. CYP21 analysis and phenotype/genotype relationship in the screened population of the Italian Emilia-Romagna region. Clin. Endocrinol. (Oxf.) 2000;53:117–125. doi: 10.1046/j.1365-2265.2000.01048.x. [DOI] [PubMed] [Google Scholar]
- 18.Barbaro M., Lajic S., Baldazzi L., Balsamo A., Pirazzoli P., Cicognani A., Wedell A., Cacciari E. Functional analysis of two recurrent amino acid substitutions in the CYP21 gene from Italian patients with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 2004;89:2402–2407. doi: 10.1210/jc.2003-031630. [DOI] [PubMed] [Google Scholar]
- 19.Fernández C.S., Bruque C.D., Taboas M., Buzzalino N.D., Espeche L.D., Pasqualini T., Charreau E.H., Alba L.G., Ghiringhelli P.D., Dain L. Misregulation effect of a novel allelic variant in the Z promoter region found in cis with the CYP21A2 p.P482S mutation: Implications for 21-hydroxylase deficiency. Endocrine. 2015;50:72–78. doi: 10.1007/s12020-015-0680-0. [DOI] [PubMed] [Google Scholar]
- 20.Finkielstain G.P., Chen W., Mehta S.P., Fujimura F.K., Hanna R.M., Van Ryzin C., McDonnell N.B., Merke D.P. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2011;96:E161–E172. doi: 10.1210/jc.2010-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livadas S., Dracopoulou M., Dastamani A., Sertedaki A., Maniati-Christidi M., Magiakou A.M., Kanaka-Gantenbein C., Chrousos G.P., Dacou-Voutetakis C. The spectrum of clinical, hormonal and molecular findings in 280 individuals with nonclassical congenital adrenal hyperplasia caused by mutations of the CYP21A2 gene. Clin. Endocrinol. (Oxf.) 2015;82:543–549. doi: 10.1111/cen.12543. [DOI] [PubMed] [Google Scholar]
- 22.Skordis N., Kyriakou A., Tardy V., Ioannou Y.S., Varvaresou A., Dracopoulou-Vabouli M., Patsalis P.C., Shammas C., Neocleous V., Phylactou L.A. Molecular defects of the CYP21A2 gene in Greek-Cypriot patients with congenital adrenal hyperplasia. Horm. Res. Paediatr. 2011;75:180–186. doi: 10.1159/000320040. [DOI] [PubMed] [Google Scholar]
- 23.New M.I. Extensive clinical experience: Nonclassical 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 2006;91:4205–4214. doi: 10.1210/jc.2006-1645. [DOI] [PubMed] [Google Scholar]
- 24.Roberts A.M., Ware J.S., Herman D.S., Schafer S., Baksi J., Bick A.G., Buchan R.J., Walsh R., John S., Wilkinson S. Integrated allelic, transcriptional, and phenomic dissection of the cardiac effects of titin truncations in health and disease. Sci. Transl. Med. 2015;7:270ra6. doi: 10.1126/scitranslmed.3010134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pugh T.J., Kelly M.A., Gowrisankar S., Hynes E., Seidman M.A., Baxter S.M., Bowser M., Harrison B., Aaron D., Mahanta L.M. The landscape of genetic variation in dilated cardiomyopathy as surveyed by clinical DNA sequencing. Genet. Med. 2014;16:601–608. doi: 10.1038/gim.2013.204. [DOI] [PubMed] [Google Scholar]
- 26.Petrucelli N., Daly M.B., Pal T. BRCA1- and BRCA2-associated hereditary breast and ovarian cancer. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., editors. GeneReviews. University of Washington; 1993. [Google Scholar]
- 27.Kohlmann W., Gruber S.B. Lynch Syndrome. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., editors. GeneReviews. University of Washington; 1993. [Google Scholar]
- 28.Hymes J., Stanley C.M., Wolf B. Mutations in BTD causing biotinidase deficiency. Hum. Mutat. 2001;18:375–381. doi: 10.1002/humu.1208. [DOI] [PubMed] [Google Scholar]
- 29.Norrgard K.J., Pomponio R.J., Swango K.L., Hymes J., Reynolds T., Buck G.A., Wolf B. Double mutation (A171T and D444H) is a common cause of profound biotinidase deficiency in children ascertained by newborn screening the United States. Mutations in brief no. 128. Online. Hum. Mutat. 1998;11:410. doi: 10.1002/(SICI)1098-1004(1998)11:5<410::AID-HUMU10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 30.Lazarin G.A., Haque I.S., Nazareth S., Iori K., Patterson A.S., Jacobson J.L., Marshall J.R., Seltzer W.K., Patrizio P., Evans E.A., Srinivasan B.S. An empirical estimate of carrier frequencies for 400+ causal Mendelian variants: Results from an ethnically diverse clinical sample of 23,453 individuals. Genet. Med. 2013;15:178–186. doi: 10.1038/gim.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lubbe S.J., Di Bernardo M.C., Chandler I.P., Houlston R.S. Clinical implications of the colorectal cancer risk associated with MUTYH mutation. J. Clin. Oncol. 2009;27:3975–3980. doi: 10.1200/JCO.2008.21.6853. [DOI] [PubMed] [Google Scholar]
- 32.Maugeri A., Flothmann K., Hemmrich N., Ingvast S., Jorge P., Paloma E., Patel R., Rozet J.M., Tammur J., Testa F. The ABCA4 2588G>C Stargardt mutation: Single origin and increasing frequency from South-West to North-East Europe. Eur. J. Hum. Genet. 2002;10:197–203. doi: 10.1038/sj.ejhg.5200784. [DOI] [PubMed] [Google Scholar]
- 33.Treem W.R. Clinical aspects and treatment of congenital sucrase-isomaltase deficiency. J. Pediatr. Gastroenterol. Nutr. 2012;55(Suppl 2):S7–S13. doi: 10.1097/01.mpg.0000421401.57633.90. [DOI] [PubMed] [Google Scholar]
- 34.Albers C.A., Paul D.S., Schulze H., Freson K., Stephens J.C., Smethurst P.A., Jolley J.D., Cvejic A., Kostadima M., Bertone P. Compound inheritance of a low-frequency regulatory SNP and a rare null mutation in exon-junction complex subunit RBM8A causes TAR syndrome. Nat Genet. 2012;44:435–439. doi: 10.1038/ng.1083. S1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bottillo I., Castori M., De Bernardo C., Fabbri R., Grammatico B., Preziosi N., Scassellati G.S., Silvestri E., Spagnuolo A., Laino L., Grammatico P. Prenatal diagnosis and post-mortem examination in a fetus with thrombocytopenia-absent radius (TAR) syndrome due to compound heterozygosity for a 1q21.1 microdeletion and a RBM8A hypomorphic allele: a case report. BMC Res. Notes. 2013;6:376. doi: 10.1186/1756-0500-6-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chu C.S., Bancone G., Moore K.A., Win H.H., Thitipanawan N., Po C., Chowwiwat N., Raksapraidee R., Wilairisak P., Phyo A.P. Haemolysis in G6PD heterozygous females treated with primaquine for plasmodium vivax malaria: A nested cohort in a trial of radical curative regimens. PLoS Med. 2017;14:e1002224. doi: 10.1371/journal.pmed.1002224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reclos G.J., Hatzidakis C.J., Schulpis K.H. Glucose-6-phosphate dehydrogenase deficiency neonatal screening: preliminary evidence that a high percentage of partially deficient female neonates are missed during routine screening. J. Med. Screen. 2000;7:46–51. doi: 10.1136/jms.7.1.46. [DOI] [PubMed] [Google Scholar]
- 38.Konkle B.A., Huston H., Nakaya Fletcher S. Hemophilia A. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., editors. GeneReviews. University of Washington; 1993. [Google Scholar]
- 39.De Marco G., Agretti P., Montanelli L., Di Cosmo C., Bagattini B., De Servi M., Ferrarini E., Dimida A., Freitas Ferreira A.C., Molinaro A. Identification and functional analysis of novel dual oxidase 2 (DUOX2) mutations in children with congenital or subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 2011;96:E1335–E1339. doi: 10.1210/jc.2010-2467. [DOI] [PubMed] [Google Scholar]
- 40.Moreno J.C., Bikker H., Kempers M.J., van Trotsenburg A.S., Baas F., de Vijlder J.J., Vulsma T., Ris-Stalpers C. Inactivating mutations in the gene for thyroid oxidase 2 (THOX2) and congenital hypothyroidism. N. Engl. J. Med. 2002;347:95–102. doi: 10.1056/NEJMoa012752. [DOI] [PubMed] [Google Scholar]
- 41.Dhandapany P.S., Sadayappan S., Xue Y., Powell G.T., Rani D.S., Nallari P., Rai T.S., Khullar M., Soares P., Bahl A. A common MYBPC3 (cardiac myosin binding protein C) variant associated with cardiomyopathies in South Asia. Nat. Genet. 2009;41:187–191. doi: 10.1038/ng.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerull B., Gramlich M., Atherton J., McNabb M., Trombitás K., Sasse-Klaassen S., Seidman J.G., Seidman C., Granzier H., Labeit S. Mutations of TTN, encoding the giant muscle filament titin, cause familial dilated cardiomyopathy. Nat. Genet. 2002;30:201–204. doi: 10.1038/ng815. [DOI] [PubMed] [Google Scholar]
- 43.Ceyhan-Birsoy O., Agrawal P.B., Hidalgo C., Schmitz-Abe K., DeChene E.T., Swanson L.C., Soemedi R., Vasli N., Iannaccone S.T., Shieh P.B. Recessive truncating titin gene, TTN, mutations presenting as centronuclear myopathy. Neurology. 2013;81:1205–1214. doi: 10.1212/WNL.0b013e3182a6ca62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnston J., Lantos J.D., Goldenberg A., Chen F., Parens E., Koenig B.A., members of the NSIGHT Ethics and Policy Advisory Board Sequencing newborns: A call for nuanced use of genomic technologies. Hastings Cent. Rep. 2018;48(Suppl 2):S2–S6. doi: 10.1002/hast.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bonow R.O., Carabello B.A., Chatterjee K., de Leon A.C., Jr., Faxon D.P., Freed M.D., Gaasch W.H., Lytle B.W., Nishimura R.A., O’Gara P.T., American College of Cardiology/American Heart Association Task Force on Practice Guidelines 2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2008;52:e1–e142. doi: 10.1016/j.jacc.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Burkett E.L., Hershberger R.E. Clinical and genetic issues in familial dilated cardiomyopathy. J. Am. Coll. Cardiol. 2005;45:969–981. doi: 10.1016/j.jacc.2004.11.066. [DOI] [PubMed] [Google Scholar]
- 47.Gersh B.J., Maron B.J., Bonow R.O., Dearani J.A., Fifer M.A., Link M.S., Naidu S.S., Nishimura R.A., Ommen S.R., Rakowski H., American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. American Association for Thoracic Surgery. American Society of Echocardiography. American Society of Nuclear Cardiology. Heart Failure Society of America. Heart Rhythm Society. Society for Cardiovascular Angiography and Interventions. Society of Thoracic Surgeons 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2761–2796. doi: 10.1161/CIR.0b013e318223e230. [DOI] [PubMed] [Google Scholar]
- 48.Gersh B.J., Maron B.J., Bonow R.O., Dearani J.A., Fifer M.A., Link M.S., Naidu S.S., Nishimura R.A., Ommen S.R., Rakowski H., American College of Cardiology Foundation/American Heart Association Task Force on Practice. American Association for Thoracic Surgery. American Society of Echocardiography. American Society of Nuclear Cardiology. Heart Failure Society of America. Heart Rhythm Society. Society for Cardiovascular Angiography and Interventions. Society of Thoracic Surgeons 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Thorac. Cardiovasc. Surg. 2011;142:e153–e203. doi: 10.1016/j.jtcvs.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 49.Vetter V.L., Elia J., Erickson C., Berger S., Blum N., Uzark K., Webb C.L., American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee. American Heart Association Council on Cardiovascular Nursing Cardiovascular monitoring of children and adolescents with heart disease receiving medications for attention deficit/hyperactivity disorder [corrected]: A scientific statement from the American Heart Association Council on Cardiovascular Disease in the Young Congenital Cardiac Defects Committee and the Council on Cardiovascular Nursing. Circulation. 2008;117:2407–2423. doi: 10.1161/CIRCULATIONAHA.107.189473. [DOI] [PubMed] [Google Scholar]
- 50.Beutler E. G6PD deficiency. Blood. 1994;84:3613–3636. [PubMed] [Google Scholar]
- 51.Goldstein B., Goldfarb D.S. Early recognition and management of rare kidney stone disorders. Urol. Nurs. 2017;37:81–89. 102. [PMC free article] [PubMed] [Google Scholar]
- 52.Kavanagh D., Goodship T.H., Richards A. Atypical haemolytic uraemic syndrome. Br. Med. Bull. 2006;77-78:5–22. doi: 10.1093/bmb/ldl004. [DOI] [PubMed] [Google Scholar]
- 53.Tabish A.M., Azzimato V., Alexiadis A., Buyandelger B., Knöll R. Genetic epidemiology of titin-truncating variants in the etiology of dilated cardiomyopathy. Biophys. Rev. 2017;9:207–223. doi: 10.1007/s12551-017-0265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akinrinade O., Alastalo T.P., Koskenvuo J.W. Relevance of truncating titin mutations in dilated cardiomyopathy. Clin. Genet. 2016;90:49–54. doi: 10.1111/cge.12741. [DOI] [PubMed] [Google Scholar]
- 55.Akinrinade O., Koskenvuo J.W., Alastalo T.P. Prevalence of titin truncating variants in general population. PLoS ONE. 2015;10:e0145284. doi: 10.1371/journal.pone.0145284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Franaszczyk M., Chmielewski P., Truszkowska G., Stawinski P., Michalak E., Rydzanicz M., Sobieszczanska-Malek M., Pollak A., Szczygieł J., Kosinska J. Titin truncating variants in dilated cardiomyopathy - prevalence and genotype-phenotype correlations. PLoS ONE. 2017;12:e0169007. doi: 10.1371/journal.pone.0169007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Votava F., Török D., Kovács J., Möslinger D., Baumgartner-Parzer S.M., Sólyom J., Pribilincová Z., Battelino T., Lebl J., Frisch H., Waldhauser F., Middle European Society for Paediatric Endocrinology -- Congenital Adrenal Hyperplasia (MESPE-CAH) Study Group Estimation of the false-negative rate in newborn screening for congenital adrenal hyperplasia. Eur. J. Endocrinol. 2005;152:869–874. doi: 10.1530/eje.1.01929. [DOI] [PubMed] [Google Scholar]
- 58.Botkin J.R., Belmont J.W., Berg J.S., Berkman B.E., Bombard Y., Holm I.A., Levy H.P., Ormond K.E., Saal H.M., Spinner N.B. Points to consider: Ethical, legal, and psychosocial implications of genetic testing in children and adolescents. Am. J. Hum. Genet. 2015;97:6–21. doi: 10.1016/j.ajhg.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross L.F., Saal H.M., David K.L., Anderson R.R., American Academy of Pediatrics. American College of Medical Genetics and Genomics Technical report: Ethical and policy issues in genetic testing and screening of children. Genet. Med. 2013;15:234–245. doi: 10.1038/gim.2012.176. [DOI] [PubMed] [Google Scholar]
- 60.Wilfond B.S., Fernandez C.V., Green R.C. Disclosing secondary findings from pediatric sequencing to families: Considering the “benefit to families”. J. Law Med. Ethics. 2015;43:552–558. doi: 10.1111/jlme.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dewey F.E., Murray M.F., Overton J.D., Habegger L., Leader J.B., Fetterolf S.N., O’Dushlaine C., Van Hout C.V., Staples J., Gonzaga-Jauregui C. Distribution and clinical impact of functional variants in 50,726 whole-exome from the DiscovEHR study. Science. 2016;354:aaf6814. doi: 10.1126/science.aaf6814. [DOI] [PubMed] [Google Scholar]
- 62.Gambin T., Jhangiani S.N., Below J.E., Campbell I.M., Wiszniewski W., Muzny D.M., Staples J., Morrison A.C., Bainbridge M.N., Penney S. Secondary findings and carrier test frequencies in a large multiethnic sample. Genome Med. 2015;7:54. doi: 10.1186/s13073-015-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang Y., Muzny D.M., Xia F., Niu Z., Person R., Ding Y., Ward P., Braxton A., Wang M., Buhay C. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA. 2014;312:1870–1879. doi: 10.1001/jama.2014.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dorschner M.O., Amendola L.M., Turner E.H., Robertson P.D., Shirts B.H., Gallego C.J., Bennett R.L., Jones K.L., Tokita M.J., Bennett J.T., National Heart, Lung, and Blood Institute Grand Opportunity Exome Sequencing Project Actionable, pathogenic incidental findings in 1,000 participants’ exomes. Am. J. Hum. Genet. 2013;93:631–640. doi: 10.1016/j.ajhg.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fahed A.C., Gelb B.D., Seidman J.G., Seidman C.E. Genetics of congenital heart disease: The glass half empty. Circ. Res. 2013;112:707–720. doi: 10.1161/CIRCRESAHA.112.300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feldkamp M.L., Carey J.C., Byrne J.L.B., Krikov S., Botto L.D. Etiology and clinical presentation of birth defects: Population based study. BMJ. 2017;357:j2249. doi: 10.1136/bmj.j2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gelb B.D., Chung W.K. Complex genetics and the etiology of human congenital heart disease. Cold Spring Harb. Perspect. Med. 2014;4:a013953. doi: 10.1101/cshperspect.a013953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Posey J.E., Rosenfeld J.A., James R.A., Bainbridge M., Niu Z., Wang X., Dhar S., Wiszniewski W., Akdemir Z.H., Gambin T. Molecular diagnostic experience of whole-exome sequencing in adult patients. Genet. Med. 2016;18:678–685. doi: 10.1038/gim.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.