Abstract
AIM
To determine the best option for bowel preparation [sodium picosulphate or polyethylene glycol (PEG)] for elective colonoscopy in adult outpatients.
METHODS
A systematic review of the literature following the PRISMA guidelines was performed using Medline, Scopus, EMBASE, Central, Cinahl and Lilacs. No restrictions were placed for country, year of publication or language. The last search in the literature was performed on November 20th, 2017. Only randomized clinical trials with full texts published were included. The subjects included were adult outpatients who underwent bowel cleansing for elective colonoscopy. The included studies compared sodium picosulphate with magnesium citrate (SPMC) and PEG for bowel preparation. Exclusion criteria were the inclusion of inpatients or groups with specific conditions, failure to mention patient status (outpatient or inpatient) or dietary restrictions, and permission to have unrestricted diet on the day prior to the exam. Primary outcomes were bowel cleaning success and/or tolerability of colon preparation. Secondary outcomes were adverse events, polyp and adenoma detection rates. Data on intention-to-treat were extracted by two independent authors and risk of bias assessed through the Jadad scale. Funnel plots, Egger’s test, Higgins’ test (I2) and sensitivity analyses were used to assess reporting bias and heterogeneity. The meta-analysis was performed by computing risk difference (RD) using Mantel-Haenszel (MH) method with fixed-effects (FE) and random-effects (RE) models. Review Manager 5 (RevMan 5) version 6.1 (The Cochrane Collaboration) was the software chosen to perform the meta-analysis.
RESULTS
662 records were identified but only 16 trials with 6200 subjects were included for the meta-analysis. High heterogeneity among studies was found and sensitivity analysis was needed and performed to interpret data. In the pooled analysis, SPMC was better for bowel cleaning [MH FE, RD 0.03, IC (0.01, 0.05), P = 0.003, I2 = 33%, NNT 34], for tolerability [MH RE, RD 0.08, IC (0.03, 0.13), P = 0.002, I2 = 88%, NNT 13] and for adverse events [MH RE, RD 0.13, IC (0.05, 0.22), P = 0.002, I2 = 88%, NNT 7]. There was no difference in regard to polyp and adenoma detection rates. Additional analyses were made by subgroups (type of regimen, volume of PEG solution and dietary recommendations). SPMC demonstrated better tolerability levels when compared to PEG in the following subgroups: “day-before preparation” [MH FE, RD 0.17, IC (0.13, 0.21), P < 0.0001, I2 = 0%, NNT 6], “preparation in accordance with time interval for colonoscopy” [MH RE, RD 0.08, IC (0.01, 0.15), P = 0.02, I2 = 54%, NNT 13], when compared to “high-volume PEG solutions” [MH RE, RD 0.08, IC (0.01, 0.14), I2 = 89%, P = 0.02, NNT 13] and in the subgroup “liquid diet on day before” [MH RE, RD 0.14, IC (0.06,0.22), P = 0.0006, I2 = 81%, NNT 8]. SPMC was also found to cause fewer adverse events than PEG in the “high-volume PEG solutions” [MH RE, RD -0.18, IC (-0.30, -0.07), P = 0.002, I2 = 79%, NNT 6] and PEG in the “low-residue diet” subgroup [MH RE, RD -0.17, IC (-0.27, 0.07), P = 0.0008, I2 = 86%, NNT 6].
CONCLUSION
SPMC seems to be better than PEG for bowel preparation, with a similar bowel cleaning success rate, better tolerability and lower prevalence of adverse events.
Keywords: Sodium picosulphate, Polyethylene glycol, Bowel cleaning success, Tolerability, Colonoscopy, Randomized clinical trials, Meta-analysis
Core tip: Previous meta-analyses did not consider patient status (if inpatient or outpatient) for inclusion in the studies and grouped different types of patients. They also failed to conduct analyses by subgroups (regimen schedule, volume of polyethylene glycol solution, dietary restriction) in order to elucidate confounding factors. This is the first systematic review and meta-analysis for this specific group of patients and the first to communicate effectiveness by NNT.
INTRODUCTION
Colonoscopy is the gold-standard method for polyp and adenoma detection and can reduce both incidence and mortality for colorectal cancer[1,2]. Different devices and tools were created to improve mucosal exposure and the detection of neoplastic lesions[3] and carbon dioxide insufflation used to increase tolerance to colonoscopy[4]. Even so, bowel cleaning is still the cornerstone for optimizing colonoscopy.
Cleaning efficacy is the most important characteristic of bowel cleansers as the quality of cleaning directly impacts on evaluation, difficulty, speed, and completeness of colonoscopy[5,6]. As inadequate bowel preparation results in missing pre-cancerous lesions and increases the costs related to repetition of colonoscopy, the choice of the product should aim to achieve high-quality bowel preparation and optimize the evaluation[7,8].
Polyethylene glycol (PEG)-based solutions are the most widely used and studied bowel cleansers. PEG is an isosmotic laxative which achieves high-quality bowel preparations through the ingestion of large volumes of the solution (approximately four liters). Their poor palatability and the volume to be ingested increase the incidence of adverse events and decrease full intake of the medication[9-11].
Among purgatives that have been recently developed to overcome these limitations is sodium picosulphate with magnesium citrate (PICO or SPMC), a low-volume dual laxative which may cause less gastrointestinal symptoms. It promotes colon cleansing by retaining fluids in the colon and by increasing the frequency and the force of peristalsis; however, due to electrolyte exchanges it can cause dehydration and biochemical impairments[12,13].
PEG solutions trials date from 1982 and have contributed to their consolidation as the most widely used solutions[14]. Although sodium picosulphate has been used for several years in the United Kingdom and Australia, large randomized clinical trials evaluating its efficacy are recent and usually compare it to PEG solution[13,15]. Other solutions that have already been compared to SPMC are oral sulfate solution and mannitol[16,17].
The highest level of evidence for medical practice is found in meta-analyses of randomized clinical trials. Among meta-analyses that compared PEG and sodium picosulphate for bowel preparation before colonoscopy[18-20], the largest one (Jin et al[20]) included 21 studies and showed no difference in bowel cleaning efficacy between them. Unfortunately, inclusion criteria for population did not specify patient status (their condition of inpatient or outpatient), which impaired the quality of the results obtained to be applied in medical practice as inpatient status is an independent risk factor for inadequate bowel preparation[11,21].
As most colonoscopies are performed in outpatients and there is no established evidence comparing sodium picosulphate and PEG cleaning efficacy and tolerability in this subset of patients, we therefore conducted this meta-analysis. Regimens adopted for bowel preparation were also considered for analysis since there are studies demonstrating differences in cleaning efficacy depending on the kind of the regimen adopted[22].
MATERIALS AND METHODS
Protocol and registration
Strategies for the search, selection and analysis were pre-specified as stated in Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and documented in a protocol registered in International Prospective Register of Systematic Reviews (PROSPERO) database (CRD 42016050059)[23].
Eligibility criteria
Eligibility criteria were based on PICOS (population, intervention, comparison, outcomes and study design) strategy. Only randomized clinical trials with full texts published were included irrespective of language or time of publication. The subjects included were adult outpatients who underwent bowel cleaning for elective colonoscopy. The included studies compared sodium picosulphate/magnesium citrate and PEG. Exclusion criteria were inpatient status, groups with specific comorbidities, combination of different products for the preparation of the solution, association with enema or enteroclysis and the absence of dietary restrictions on the day prior to the exam. Primary outcomes evaluated were efficacy and tolerability. Secondary outcomes were prevalence of adverse events, and polyp and adenoma detection rates.
Search strategy and study selection
Two independent authors identified records in the following electronic databases: Medline, Scopus, EMBASE, Central, Cinahl and Lilacs. No limits were applied for country, year of publication or language. The last search in the literature was performed on November 20th, 2017. Search keywords were “colonoscopy”, “colonosc*”, “sodium picosulphate”, “sodium picosulfate”, “polyethylene glycol”, “polyethylene glycols” and “random*”. Full search strategy for each database is shown in Supplementary material (Appendix 1). Medline was the main database for the development of the search strategy, as follows: “(colonoscopy OR colonoscopies OR bowel preparation OR bowel prep* OR bowel cleansing OR bowel clean* OR colon preparation OR colon prep* OR colon cleansing OR colon clean’ AND (sodium picosuffate OR sodium picosulphate OR picosulfate OR picosulphate) AND (polyethylene OR polyethylene glycol OR polyethylene glycols OR polyethylene glicol OR polyethylene glicols)”.
Two independent authors performed eligibility assessment and studies selection. Duplicated references were excluded and the remaining ones were screened by title and abstract. Those that met any of the exclusion criteria were disregarded. The full texts of the remaining records were assessed and the studies that met the eligibility criteria were included for the meta-analysis. The gray literature search was made in the references of the included studies and a third author solved disagreements between the other two.
Data collection process
Data available in texts, charts or tables were extracted by two independent authors using a previously devised form. Data presented in percentage were converted into frequency and rounded up if the number obtained was not an integer.
Data items: The following data were collected for each trial: (1) characteristics of participants; (2) type of intervention; (3) outcomes; and (4) type of outcome measurement (including definition, score adopted, bowel cleansing success, tolerability, adverse events prevalence, polyp and adenoma detection rate).
The following definitions standardizations were previously established for outcomes: (1) bowel cleaning success, defined as the number of patients with successful cleaning by either the study or by the assessment score as “excellent, adequate, good or clean”; (2) tolerability, defined as the number of patients who ingested the entire bowel cleaning preparation or the minimum established by the study as acceptable; (3) adverse events prevalence, defined as the number of patients affected by at least one adverse event; (4) polyp detection rate (PDR), defined as the number of patients with at least one polyp detected during colonoscopy; (5) adenoma detection rate (ADR), defined as the number of patients with at least one adenoma detected during colonoscopy.
Risk of bias in individual studies
As treatment effect size may differ due to selection, performance, detection and attrition bias, the methodological evaluation of the studies was performed. Two authors working independently determined the adequacy of randomization, adequacy of blinding, and the description of withdrawals and dropouts using the Jadad scale[24] for the evaluation of the randomized clinical trials.
Summary measures
Meta-analysis was preferably performed using intention-to-treat (ITT) data. Per-protocol (PP) data were only used when ITT data were not available. Outcomes evaluated were dichotomous (bowel cleansing success, tolerability, adverse events prevalence, polyp and adenoma detection rate). Risk difference (RD) with 95% confidence intervals (CI) was calculated for each outcome.
Synthesis of results
Meta-analyses were performed by computing RD for dichotomous outcomes using Mantel-Haenszel method (MH) with fixed-effects (FE) and random-effects (RE) models. Heterogeneity was assessed by Cochran’s Q test (P value), which examines the null hypothesis that all studies are evaluating the same effect, and by Higgins’ test (I2), which quantifies inconsistency across studies and describes the percentage of the variability in effect estimates that is due to heterogeneity[25]. FE model was used in the presence of null or low heterogeneity (I2 < 50%) assuming the true effect size did not differ across studies. However, an I2 value equal to or greater than 50% was considered substantial heterogeneity and RE model was preferred to FE as true effect size varied from one study to another and a more conservative approach for statistical significance was needed. The number needed to treat (NNT) for each outcome with statistical difference was also calculated. Review Manager 5 (RevMan 5) Version 6.1 (by the Cochrane Collaboration, 2015) was the software chosen to run the meta-analysis.
Risk of bias across studies
Reporting bias across studies was evaluated by a graphic diagnostic tool named funnel plot. For each trial, the treatment effect was plotted against the measure of study precision (represented by the inverse of its standard error) and the symmetry of scatter plot assessed by Egger’s test[26]. Asymmetrical funnel plot suggests the presence of reporting bias (absence of low-precision studies that have negative or non-significant results), methodological bias or true heterogeneity between smaller and larger studies.
Additional analysis
In the presence of an asymmetrical funnel plot or high heterogeneity, (I2 ≥ 50%) a sensitivity analysis was conducted to explore how the results of the meta-analysis change under different assumptions[27]. Heterogeneity and funnel plot before and after the removal of each study from the meta-analysis was assessed to identify the study accounting for the inconsistency among trials (usually due to a markedly different intervention effect or an undue influence on the summary results). If heterogeneity was reduced to below 50% after the removal of the outlier, the corrected intervention effect estimate was applied and the interpretation of results made with caution. If inconsistency did not decrease, it was considered true heterogeneity.
Subgroup analyses were performed for variables that could knowingly influence the effect sizes: (1) types of regimen [(A) full intake on the day prior the exam; (B) intake split into the day prior and the same day of the exam; and (C) intake only on the same day of the exam); (2) volume of PEG-based solution [(A) low-volume group - 2L or less; and (B) high-volume group - more than 2L]; (3) dietary restrictions on day before [(A) low fiber or low residue diet; and (B) liquid diet].
RESULTS
Search and study selection
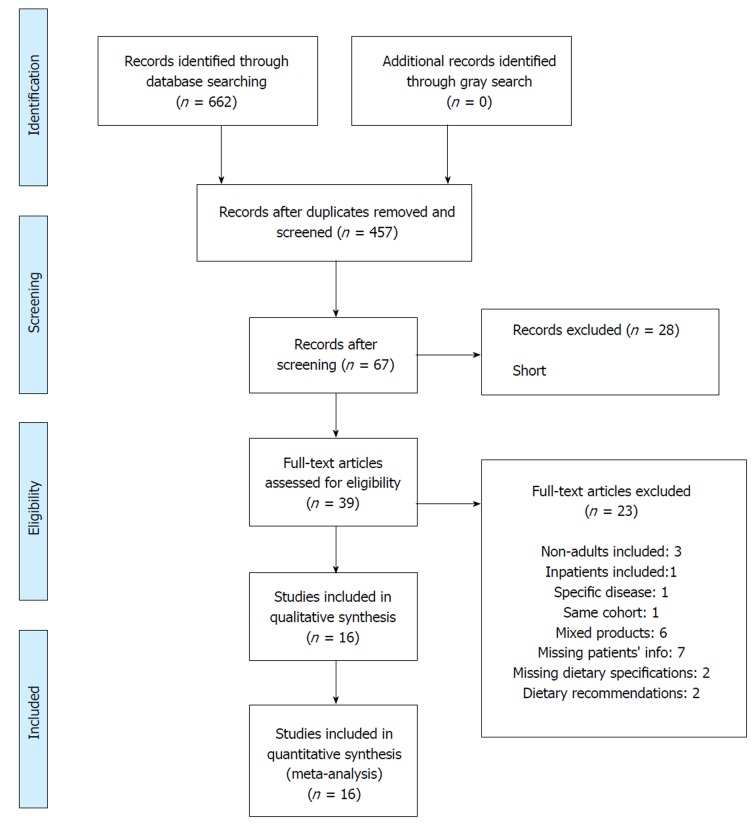
A total of 662 records were identified through a search in the databases (57 in MEDLINE, 128 in EMBASE, 384 in Scopus, 85 in CENTRAL, 8 in CINAHL and none in LILACS) (Figure 1). After adjusting for duplicates, 457 records remained and were evaluated by title and abstract. 390 records were excluded because they met one or more exclusion criteria. Of the 67 remaining, 28 were then excluded (2 were short communications and 26 were congress abstracts). The full texts of the remaining 39 records were examined and 23 were rejected. Reasons are presented in Supplementary material (Appendix 2). At the end, 16 studies were included in the meta-analysis[9,11,23-36].
Figure 1.
Flow diagram of included studies.
Studies characteristics
All 16 studies selected were RCTs, with full text available, published in English between 1996 and 2017. Included studies involved 6200 participants, from 18 to 86 years of age. Main patient exclusion criteria were age, renal insufficiency, congestive heart failure, recent myocardial infarction, constipation, gastrointestinal or colon disorders, and previous colorectal surgery (Table 1).
Table 1.
Studies characteristics
| STUDY | SITE | POPULATION | INTERVENTION (ITT/PP) | COMPARISON (ITT/PP) | OUTCOMES |
| Regev et al[34] | 1 | 29 to 86 y | Pico-Salax (1+1+1)/0 sachets | New-Meroken® 3/0 L | Bowel cleansing quality |
| Israel | + (200/h for 16h)/0 mL of water | Cecal intubation | |||
| ITT: 68 | + clear liquid diet | + clear liquid diet | General discomfort | ||
| PP: 68 | 39/39 | 29/29 | Medication Intake | ||
| Adverse events | |||||
| Lawrance et al[35] | 1 | 18 to 75 y | Pico® (1+1)/0 sachets for morning procedure or | ColonLytely® 4/0 L for morning procedure or | Bowel cleansing quality |
| Australia | |||||
| Pico 1/1 sachets for afternoon procedure | ColonLytely® 4/0 L for afternoon procedure | ||||
| + liquid | + clear liquid 2/0 | ||||
| ITT: 634 | + low residue diet | 284/279 | Medication Intake | ||
| PP: 625 | 171/169 | ||||
| CB fleet® (45+45)/0 mL | |||||
| + (750+750)/0 mL of water for morning procedure or | Mucosal inflammation | ||||
| CB fleet® 45/45 mL | |||||
| + 750/750 mL of water for afternoon procedure | |||||
| + low residue diet | |||||
| 179/177 | |||||
| Kao et al[36] | 1 | 18 to 75 y | SPMC (1+1+MC)/0 sachets for morning procedure or | PEG 4/0 L for morning procedure or | Bowel cleansing quality |
| Canada | |||||
| SPMC (1+1)/MC sachets for afternoon procedure | PEG 2/2 L for afternoon procedure | Bowel cleansing quality according to the procedure time | |||
| ITT: 834 | + liquid | + liquid | Tolerability | ||
| PP: 790 | + clear liquid diet | + clear liquid diet | |||
| 194/194 | 218/210 | Adherence | |||
| NaP 45/45/0 mL for morning procedure or | PEG 2/0 L + bisacodyl 20/0 mg for morning procedure or | Sleeping hours | |||
| NaP 0/45/45 mL for afternoon procedure | PEG 0/2 L + bisacodyl 20/0 mg for afternoon procedure | Willingness to reuse | |||
| + liquid | + liquid | Safety | |||
| + clear liquid diet | + clear liquid diet | ||||
| 164/164 | 214/210 | Ischemic colitis | |||
| Katz et al[15] | 12 | 18 to 80 y | Prepopik® (1+1)/0 sachets | Half-Lytely e bisacodyl Tablets® 2/0 L | Bowel cleansing quality |
| USA | |||||
| + (1200+720)/0 mL of liquid | + 10/0 mg bisacodyl | Acceptability | |||
| Tolerability | |||||
| ITT: 603 | + clear liquid diet | + clear liquid diet | Medication Intake | ||
| PP: 598 | Ease to use medication | ||||
| 300/296 | 303/302 | General experience | |||
| Taste | |||||
| Willingness to reuse | |||||
| Adverse events | |||||
| Manes et al[37] | 3 | 18 to 85 y | CitraFleet® (1+1)/0 sachets | Moviprep® 2/0 L | Bowel cleansing quality |
| Italy | |||||
| + 3/0 L of liquid for morning procedure or | + 1/0 L of liquid for morning procedure or | Bowel cleansing quality of right colon | |||
| ITT: 293 | CitraFleet® 1/1 sachets | Moviprep® 1/1 L | Polyps detected | ||
| PP: 285 | + 1.5/1.5 L of liquid for afternoon procedure | + 500/500 mL of liquid for afternoon procedure | Acceptance | ||
| + low-fiber diet | + low-fiber diet | Tolerability | |||
| 145/140 | 148/145 | Adherence | |||
| Adverse events | |||||
| Rex et al[15] | 10 | 18 to 80 y | Prepopik® 1/1 sachets | Half-Lytely e bisacodyl Tablets® 2/0 L | Bowel cleansing quality |
| USA | |||||
| + 1200/720 mL of liquid | + bisacodyl 10/0 mg | Acceptability | |||
| + clear liquid diet | Tolerability | ||||
| ITT: 608 | + clear liquid diet | Ease to use medication | |||
| PP: 601 | Medication Intake | ||||
| Taste | |||||
| NA/305 | NA/298 | Willingness to reuse medication | |||
| Adverse events | |||||
| Colonoscopy before | |||||
| Jeon et al[38] | 1 | 20 to 80 y | Picolight powder® (1+1)/1 sachet | Coolprep powder® 1/1 L | Bowel cleansing quality |
| South Korea | |||||
| + (1+1)/1 L of water | + 500/500 mL of water | Cecal intubation | |||
| + low-fiber diet | + low-fiber diet | Withdrawal time | |||
| ITT: 430 | ADR | ||||
| PP: 388 | PDR | ||||
| Tolerability | |||||
| 215/195 | 215/193 | Satisfaction | |||
| Adverse events | |||||
| Kang et al[39] | 1 | 18 to ou mais | Picolight® 0/(1+1) | Colyte® 2/2 L | Bowel cleansing quality |
| South Korea | |||||
| + 0/≥ 1 L of water | + low-fiber diet | Tolerability | |||
| ITT: 197 | + low-fiber diet | 99/99 | Adverse events | ||
| PP: 197 | 98/98 | Sleep time quantity | |||
| PDR | |||||
| ADR | |||||
| Kim et al[40] | 1 | 18 to 75 y | SPMC 1/1 sachets | PEG 0/4 L | Bowel cleansing quality |
| South Korea | |||||
| + 1/1 L of liquid | Adherence | ||||
| + low-fiber diet | + low-fiber diet | Medication Intake | |||
| ITT: 200 | 50/50 | 50/50 | |||
| PP: 200 | SPMC (1+1)/1 sachets | PEG 2/2 L | Tolerability | ||
| + (1+1)/1 L of liquid | Taste | ||||
| + low-fiber diet | + low-fiber diet | Biochemical changes | |||
| 50/50 | 50/50 | Adverse events | |||
| Kim et al[41] | 1 | 18 to 80 y | Picolight (1+1)/0 sachets | Standard PEG 4/0 L | Acceptability |
| South Korea | + 2L of water | Adherence to instructions | |||
| ITT: 194 | + bisacodyl 10/0 mg | + bisacodyl 10/0 mg | Bowel cleansing quality | ||
| PP: 184 | + low-fiber diet (ZeroCol) | + semi-fluid diet | Adverse events | ||
| 97/94 | 97/90 | Willingness to reuse medication | |||
| Leitao et al[28] | 3 | 18 to 80 y | CitraFleet® 1/1 sachets | Enddealk® 2/1L | Bowel cleansing quality |
| Norway | |||||
| + 2/2 L of water | + 0/1 L of liquid | Tolerability | |||
| ITT: 368 | + no-grains diet | + diet without crops | Adherence | ||
| PP: 368 | PDR | ||||
| 179/179 | 189/189 | Cecal intubation time | |||
| Cecal intubation | |||||
| Kim et al[29] | 13 | 20 to 75 y | sachets | Standard PEG 2/2 L | Bowel cleansing quality |
| South Korea | |||||
| + 2/2 L of water | no bisacodyl | Satisfaction | |||
| + bisacodyl 10/0 mg | + low-fiber diet | Tolerability | |||
| + low-fiber diet | Medication Intake | ||||
| ITT: 387 | 193/181 | 194 / 184 | Ease to use | ||
| PP: 365 | Taste | ||||
| Willingness to reuse | |||||
| Adverse events | |||||
| Munsterman et al[30] | 1 | 18 to 80 y | Picoprep® 1/1 sachets for morning procedure or | Kleanprep® 3/1 L for morning procedure or | Bowel cleansing quality |
| Netherlands | |||||
| ITT: 173 | Picoprep® 1/1 sachets for afternoon procedure | Kleanprep® 2/2 L for afternoon procedure | |||
| PP: 172 | + 2/2 L of water | + additional liquid | Tolerability | ||
| + low-fiber diet | + low-fiber diet | ||||
| 88/87 | 85/85 | ||||
| Pohl et al[31] | 17 | 40 to 80 y | CitraFleet® (1+1)/0 sachets | Moviprep® 1/1 L | Patients with at least one polyp or flat lesion |
| Germany | |||||
| ITT: 399 | + 250mL/h of water after sachet | + 500/500 mL of liquid | Patients with at least one adenoma | ||
| PP: 398 | Cancer detection rate | ||||
| + fibers restriction diet | + fibers restriction diet | Flat lesion detection rate | |||
| NA/197 | NA/201 | Advanced lesions detection rate | |||
| Yoo et al[32] | 1 | 18 to 80 y | Picolight® 1/1 sachets | Coolprep® 1/1 L | Bowel cleansing quality |
| South Korea | |||||
| + 1/1 L of water | + 500/500 mL of water | Bubble score | |||
| ITT: 200 | + low-fiber diet | + low-fiber diet | Tolerability | ||
| PP: 200 | |||||
| 100/100 | 100/100 | Satisfaction | |||
| Kojecky et al[33] | 3 | 18 to 99 y | Picoprep® 2/0 sachets | Fortrans™ 4/0 L | Length of preparation |
| Czech Republic | + 2L of water | 94/102 | |||
| ITT: 612 | 92/102 | Fortrans™ 3/1 L | Time to colonoscopy | ||
| 87/102 | |||||
| PP: 584 | OR | Moviprep™ 2/0 L | |||
| + 1/0 L of fluids | |||||
| Picoprep® 1/1 sachets | 96/102 | Bowel cleansing quality | |||
| + 1/1 L of water | |||||
| 86/102 | Moviprep™ 1/1 | ||||
| + 0.5/0.5 L of fluids | |||||
| 93/102 | Tolerability score | ||||
| + low residue diet | + low residue diet |
* In intervention and control columns, slash separates different days and plus sign separates different doses on the same day; * ITT: number of randomized patients (intention to treat); * PP: number of treated patients (per protocol); * NR: not reported in full-text; * PDR: polyp detection rate; * ADR: adenoma detection rate.
Thirteen of 16 studies were multicenter. Six studies were conducted in South Korea, two in the United States and eight in different countries. Brand names of sodium picosulphate based products were CitraFleet®, Pico®, Pico-Salax®, Picolax®, Picoprep®, Picolight powder® and Prepopik®, while polyethylene glycol-based products were Colyte®, ColonLytely®, Coolprep powder®, Endofalk®, Half-Lytely®, Kleanprep®, Moviprep®, New Meroken® and FortransTM.
Seven studies compared split dose regimens[28-30,32,33,38,40] and 4 studies compared day-before dose[13,33,34,41]. None of the included studies compared same-day dose. Four studies compared different regimens of bowel cleaning between the two products[15,31,39,40] and three others[35-37] according to the interval time to colonoscopy.
Two different adjuvants were used in 5 studies. Four studies[13,15,36,41] used Bisacodyl with PEG and 2 studies used it with SPMC[29,41]. Magnesium citrate was also used separated from sodium picosulphate in one study[36].
Dietary restrictions on the day prior to the procedure were considered in all studies. In four studies, patients were given liquid diet[13,15,34,36] and in twelve studies a low-fiber or low-residue diet[23-26,28,30,32-36] was allowed.
Outcomes
Sixteen studies evaluated bowel cleaning success. Efficacy was measured by five different bowel preparation scales: a 4-point scale[42], Boston bowel preparation scale[29,30,32,37,38], Aronchick scale[13,15,32,33,40,41], Harefield scale[32] and Ottawa bowel preparation scale[15,35,36,39,40]. Tolerability was evaluated in 12 studies[9,11,23,24,28-30,32-36], adverse events prevalence in 13 studies[13,15,28,29,31,34,37-39,41], PDR in seven[28,31,37-41] and ADR in five studies[31,32,38,39,41].
Risk of bias within studies
The maximum Jadad score obtained was three, since patient blinding was not possible due to the different characteristics of cleaning protocols (Table 2). Eleven studies scored three points[9,11,26-28,30-34,36], four studies scored two points[28-30,34] and one study scored just one point[40]. All of them were randomized, but Kim et al[40] did not describe the randomization method and Regev et al[34] randomized patients inappropriately. Kim et al[40], Leitao et al[28], Kim et al[29] and Munsterman et al[30] also failed to describe losses.
Table 2.
Assessment of risk of bias by JADAD scale
| # | STUDY | Randomized? (1 pt) | Randomization method | Adequate randomization? (1 pt) | Double blind? (1 pt) | Masking method | Adequate masking? (1 pt) | Loss description? (1 pt) | Jadad (0-5 pts) | GENERAL QUALITY |
| 1 | Regev et al[34] | Yes | Randomization per patients' ID (odd or even numbers) | No | No | Endoscopist blind for the preparation regimen | No | Yes | 2 | Intermidiate |
| 2 | Lawrance et al[35] | Yes | Randomized using Generator Pro 1.69 (Segobit software) in ratio 2:1:1 (PEG:NaP:Pico) | Yes | No | Endoscopist blind for the preparation regimen | No | Yes | 3 | High |
| 3 | Kao et al[36] | Yes | Randomization in blocks of 8 and stratified per AM/PM using a computer-generated table | Yes | No | Endoscopist blind for the preparation regimen | No | Yes | 3 | High |
| 4 | Katz et al[15] | Yes | Randomization numbers allocated sequentially by voice system | Yes | No | Endoscopist blind for the preparation regimen | No | Yes | 3 | High |
| 5 | Manes et al[37] | Yes | Randomization by computer-generated sequence | Yes | No | Endoscopist blind for the preparation regimen | No | Yes | 3 | High |
| 6 | Rex et al[15] | Yes | Randomization numbers allocated sequentially by voice system | Yes | No | Endoscopist blind for the preparation regimen | No | Yes | 3 | High |
| 7 | Jeon et al[38] | Yes | Randomization by computer-generated table | Yes | No | Endoscopist blind for the preparation regimen | No | Yes | 3 | High |
| 8 | Kang et al[39] | Yes | Randomization in blocks using website randomization.com | Yes | No | Endoscopist blind for the preparation regimen | No | Yes | 3 | High |
| 9 | Kim et al[40] | Yes | Not described | No | No | Endoscopist blind for the preparation regimen | No | No | 1 | Low |
| 10 | Kim et al[41] | Yes | Randomization by computer-generated sequence | Yes | No | Endoscopist blind for the preparation regimen | No | Yes | 3 | High |
| 11 | Leitao et al[28] | Yes | Randomization 1:1 with blocks of 10 by endoscopy-unit secretary | Yes | No | Endoscopist blind for the preparation regimen | No | No | 2 | Intermidiate |
| 12 | Kim et al[29] | Yes | Randomization by computer-generated table | Yes | No | Not described | No | No | 2 | Intermidiate |
| 13 | Munsterman et al[30] | Yes | Randomization by computer-generated 1:1 stratified by age (18-64) or (65-80) | Yes | No | Endoscopist blind for the preparation regimen | No | No | 2 | Intermidiate |
| 14 | Pohl et al[31] | Yes | Randomization 1:1 in blocks of 4 by statistician list-generated | Yes | No | Endoscopist blind for the preparation regimen | No | Yes | 3 | High |
| 15 | Yoo et al[32] | Yes | Randomization 1:1 in blocks of 4 by a a computer-generated list | Yes | No | Endoscopist blind for the preparation regimen | No | Yes | 3 | High |
| 16 | Kojecky et al[33] | Yes | Randomization 1:1 using a software generated random table | Yes | No | Endoscopist blind for the preparation regiment | No | Yes | 3 | High |
Results of individual studies
Raw data of included studies are presented in Supplementary material (appendix 3). One study (Kim et al[40]) presented four different treatment arms, two of which were SPMC arms (with 2 or 3 sachets in split dose regimen) and two others of PEG arms (4 L of solution in a split or in a same-day dose regimen). For the analysis, the study was dismembered into two based on treatment regimens (Kim et al[40], SPMC split dose arms vs PEG split dose; and Kim et al[40], SPMC split dose vs PEG same-day dose).
Another study (Kojecky et al[33]) presented three different treatment arms (PEG, PEG plus ascorbic acid and SPMC) with two subgroups each (day-before dose and split dose). PEG and PEG-A treatment arms were grouped and the study dismembered into two according to the regimen (Kojecky et al[33], day-before dose; and Kojecky et al[33], split dose).
Bowel cleansing success: Twelve studies (corresponding to fourteen comparisons) demonstrated that sodium picosulphate and PEG had the same efficacy in bowel cleaning[13,29,33-40], two studies demonstrated that sodium picosulphate was better[15,41] and one study demonstrated that PEG was better[31].
Tolerability: Three studies (four comparisons) demonstrated that both obtained the same tolerability level[37,39,40], seven demonstrated that SPMC was better tolerated[13,15,28,34,35,40,41] and one that PEG was better than SPMC[39].
Adverse events prevalence: Eight studies reported adverse events prevalence as a dichotomous outcome. Five of them showed that both products regimens presented the same adverse events prevalence[13,15,28,34,38] and three of them that SPMC regimens achieved fewer adverse events[29,31,41].
PDR: Five studies (corresponding to six comparisons) demonstrated that PDR was the same with both products regimens[31,37,38,40,41]. In one study there was statistical difference in favor of SPMC[28].
ADR: Only five studies assessed ADR and none of them showed difference between SPMC and PEG[31,32,38,39,41].
Syntheses of results
The overall meta-analysis for each outcome was performed with heterogeneity assessment and cumulative treatment effect.
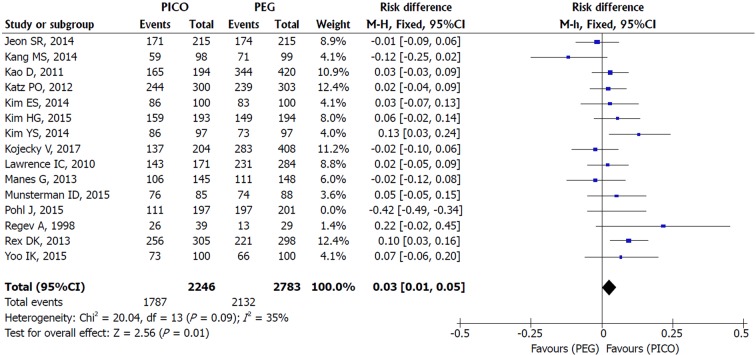
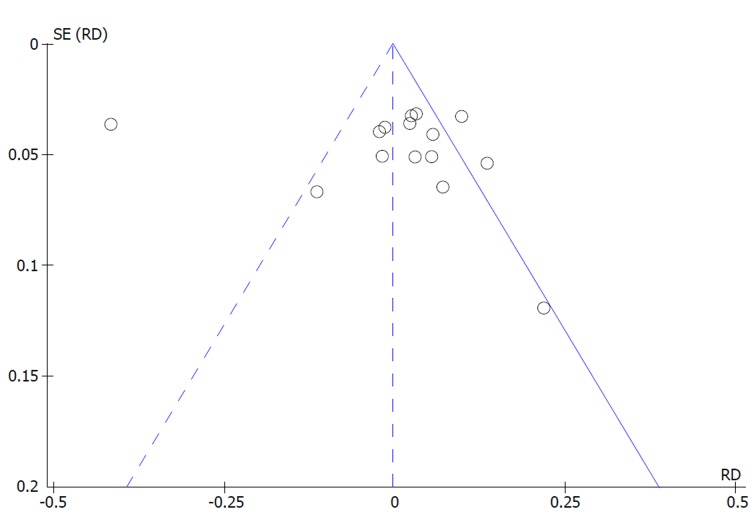
Bowel cleaning success: An asymmetrical funnel plot and high heterogeneity (I2 = 91%, P < 0.00001) were observed among the 15 studies included. An outlier study responsible for reporting bias was identified through sensitivity analysis (Pohl et al[31]). After its exclusion (I2 = 35%, P = 0.09) and through the use of FE model, there was statistical difference in favor of SPMC. More cases of success were obtained with SPMC compared to PEG [MH FE, RD 0.03, IC (0.01, 0.05), P = 0.003, I2 = 33%] with a NNT of 34 (34 people need to be treated with SPMC to obtain 1 additional benefit over PEG) (Figures 2 and 3).
Figure 2.
Metanalysis forest plot of bowel cleaning success.
Figure 3.
Metanalysis funnel plot of bowel cleaning success.
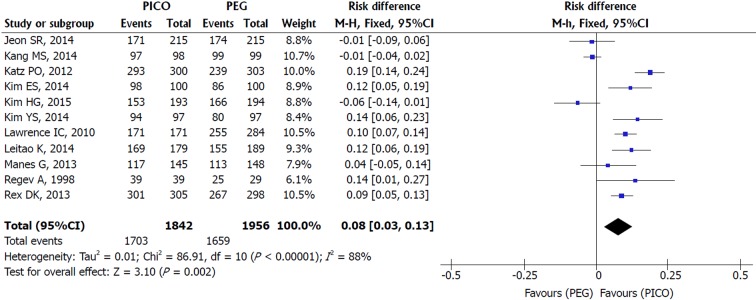
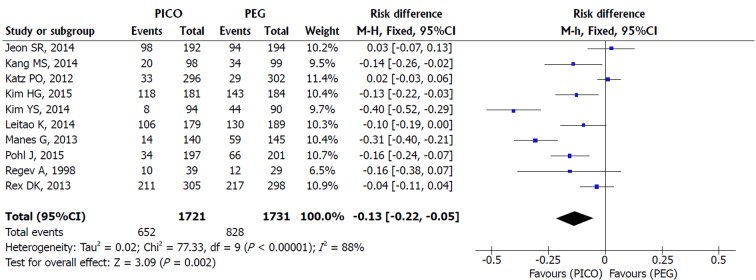
Patient tolerability: Sensitivity analysis of the eleven included studies revealed true heterogeneity (I2 = 88%, P < 0.00001) and RE model was adopted. SPMC was better tolerated than PEG [MH RE, RD 0.08, IC (0.03, 0.13), P = 0.002, I2 = 88%], with a NNT of 13 (Figure 4). As Manes et al[37] and Jeon et al[38] criteria for completion of intake were different from other studies (failure was defined as lower than 70% and 50% of ingestion of the solutions, respectively), additional analysis was performed without them. The result still favored SPMC [MH RE, RD 0.09, IC (0.03, 0.15), P = 0.002, I2 = 91%] and lower NNT (NNT of 11).
Figure 4.
Metanalysis forest plot of tolerability.
Adverse events prevalence: A RE model analysis was conducted due to the high heterogeneity among the ten included studies (I2 = 88%, P < 0.00001). Fewer adverse events occurred using SPMC [MH RE, RD 0.13, IC (0.05, 0.22), P = 0.002, I2 = 88%], and the NNT obtained was 7 (7 people need to be treated with SPMC to avoid 1 adverse event over PEG) (Figure 5).
Figure 5.
Metanalysis forest plot of adverse events.
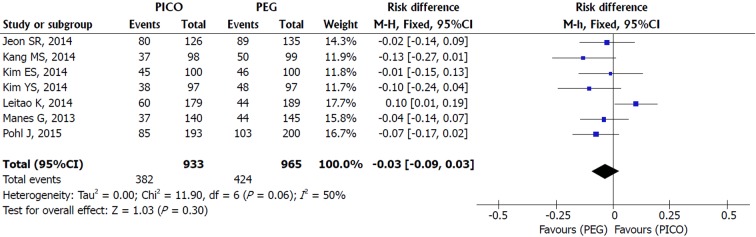
PDR: An asymmetric funnel and inconsistency in the upper limit (I2 = 50%, P = 0.06) were observed among the seven included studies. Sensitivity analysis identified the study responsible for the heterogeneity (Leitao et al[28]). The study was not excluded due to the small number of studies included (fewer than 10) and a RE model analysis was conducted. There was no difference between SPMC and PEG for polyp detection [MH RE, RD -0.03, IC (-0.09, 0.02), P = 0.30, I2 = 50%] (Figure 6).
Figure 6.
Metanalysis forest plot of polyp detection rate.
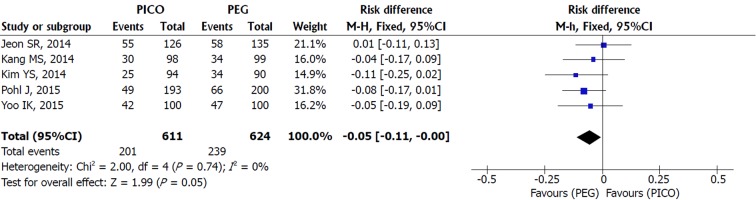
ADR: Heterogeneity was null among the five studies included and a FE model analysis showed no statistical difference between SPMC and PEG, but a trend in favor of PEG was present [MH FE, RD -0.05, IC (-0.11, -0.00), P = 0.05, I2 = 0%] (Figure 7).
Figure 7.
Metanalysis forest plot of adenoma detection rate.
Subgroups analyses
Additional analyses were performed by subgroups based on type of regimen, volume of PEG solution and dietary recommendations for the day prior to colonoscopy.
Per type of regimen: Studies were divided into four subgroups according to regimens compared: day-before dose[13,33,34,41]; split dose[28-30,32,33,38,40]; according to interval time for colonoscopy[35-37]; and comparison of different regimens[15,31,39].
Bowel cleansing success: SPMC was better than PEG for bowel cleaning in day-before dose comparison [MH RE, RD 0.06, IC (0.01, 0.11), P = 0.02, I2 = 38%], with an NNT of 17. No difference was observed in the split dose regimen [MH FE, RD 0.01, IC (-0.03, 0.05), P = 0.56, I2 = 29%], in the according-to-interval-time regimens [MH FE, RD 0.02, IC (-0.03, 0.06), P = 0.45, I2 = 0%] and in the different regimens subgroup [MH RE, RD -0.14, IC (-0.50, 0.21), P = 0.42, I2 = 98%].
Additional sensitivity analysis by subgroups showed that inconsistency among all studies included in the overall meta-analysis decreased from 91% to 19% after the removal of different regimens subgroup, in which the previous outlier study for the outcome was identified (Pohl et al[31]). Without this subgroup, the statistical difference disappeared and there was only a trend in favor of SPMC [MH FE, RD 0.03, IC (0.00, 0.05), P = 0.03, I2 = 19%] (Appendix 4 - Figure 1).
Patient tolerability: No difference was observed in tolerability in the split dose regimen (MH RE, RD 0.04, IC [-0.05, 0.14], P = 0.38, I2 = 86%) and in the different regimens subgroup [MH RE, RD 0.04, IC (-0.09, 0.17), P = 0.54, I2 = 97%]. In the day-before dose regimen [MH FE, RD 0.17, IC (0.13, 0.21), P < 0.0001, I2 = 0%] and in the according-to-interval-time subgroups [MH RE, RD 0.08, IC (0.01, 0.15), P = 0.02, I2 = 54%], SPMC was better tolerated than PEG, with an NNT of 6 and 13, respectively. Sensitivity analysis by subgroups did not change the overall meta-analysis results either (Appendix 4 - Figure 2).
Adverse events: Three subgroups were available (day-before dose, split dose and different regimens). No difference was found in day-before dose [MH RE, RD -0.18, IC (-0.50, 0.14), P = 0.26, I2 = 96%] and in split dose subgroups [MH RE, RD -0.07, IC (-0.16, 0.02), P = 0.15, I2 = 62%], but there were fewer adverse events with SPMC in the different regimens subgroup [MH RE, RD -0.10, IC (-0.19, -0.02), P = 0.01, I2 = 60%], with a NNT of 10(Appendix 4 - Figure 3).
PDR: The analysis showed no difference in PDR in the split dose subgroup [MH FE, RD 0.04, IC (-0.03, 0.10), P = 0.28, I2 = 43%] and superiority of PEG over SPMC in the different regimens subgroup [MH FE, RD -0.09, IC (-0.17, -0.01), P = 0.02, I2 = 0%], with a NNT of 12 (Appendix 4 - Figure 4).
ADR: Only two subgroups (split dose and different regimens) with 2 studies each were available. There was no statistical difference in ADR between them [split dose: MH FE, RD -0.02, IC (-0.11, 0.07), P = 0.70, I2 = 0%; different regimens: MH FE, RD -0.06, IC (-0.14, 0.01), P = 0.09, I2 = 0%] (Appendix 4 - Figure 5).
Per volume of PEG solution: Eight studies were included in low-volume subgroup[13,15,31-33,36-38] and nine in high-volume subgroup[29,30,33-36,39-41].
Bowel cleansing success: Low-volume PEG subgroup presented high heterogeneity (I2 = 96%, P < 0.00001) and sensitivity analysis identified one study (Pohl et al[31]) as the responsible for funnel asymmetry and high heterogeneity. Considering the small number of studies, it was maintained for the analysis and RE model was adopted. No difference was observed in bowel cleaning between SPMC and low-volume PEG [MH RE, RD -0.03, IC (-0.16, 0.09), P = 0.61, I2 = 95%]. High-volume subgroup analysis also showed no difference between them [MH FE, RD 0.03, IC (-0.01, 0.06), P = 0.09, I2 = 42%] (Appendix 4 - Figure 6).
Patient tolerability: SPMC was better tolerated than high-volume PEG solution [MH RE, RD 0.08, IC (0.01, 0.14), I2 = 89%, P = 0.02], with a NNT of 13, and a trend in favor of SPMC was observed in the low-volume PEG subgroup [MH RE, RD 0.08, IC (0.00, 0.16), I2 = 87%, P = 0.05]. (Appendix 4 - Figure 7).
Adverse events: After the performance of a sensitivity analysis, a study responsible for the heterogeneity in the high-volume subgroup was identified (Kim et al[41]), but was not excluded due to the small number of studies (fewer than 10 studies). RE model analysis showed SPMC caused fewer adverse events than PEG in the high-volume subgroup [MH RE, RD -0.18, IC (-0.30, -0.07), P = 0.002, I2 = 79%], with a NNT of 6. There was no difference in adverse events prevalence in the low-volume subgroup [MH RE, RD 0.09, IC (-0.02, 0.20), P = 0.12, I2 = 91%] (Appendix 4 - Figure 8).
PDR: Sensitivity analysis was carried out for high-volume subgroup and the study responsible for the inconsistency was identified (Leitao et al[28]). It was not removed due to the small number of included studies. There was no difference in PDR in the low-volume subgroup [MH FE -0.05, IC (-0.11, 0.01), P = 0.11, I2 = 0] or in the high-volume subgroup [MH RE, RD -0.03, IC (-0.14, 0.09), P = 0.65, I2 = 71%] (Appendix 4 - Figure 9).
ADR: No difference was observed between SPMC and PEG in both subgroups [low-volume: MH FE, RD -0.04, IC (-0.11, 0.02), P = 0.17, I2 = 0%; high-volume: MH FE, RD -0.07, IC (-0.17, 0.02), P = 0.12, I2 = 0%] (Appendix 4 - Figure 10).
Per dietary recommendations: Four studies were included in liquid diet subgroup[13,15,34,36] and twelve studies[23-28,30,32-36] in the low residue diet subgroup.
Bowel cleansing success: The analysis showed that SPMC was better than PEG for bowel cleaning in the liquid diet subgroup [MH FE, RD 0.06, IC (0.02, 0.09), P = 0.002, I2 = 40%], with a NNT of 17. In the low residue diet subgroup, high heterogeneity and an asymmetrical funnel plot were initially observed (I2 = 93%, P < 0.00001). After sensitivity analysis, one study (Pohl et al[31]) was identified as the responsible for reporting bias. After its exclusion, heterogeneity decreased to an acceptable level (I2 = 31%) and analysis using FE model showed that SPMC and PEG were similar in the low residue subgroup [MH FE, RD 0.01, IC (-0.02, 0.04), I2 = 30%, P = 0.38] (Appendix 4 – Figure 11).
Patient tolerability: SPMC was better tolerated than PEG in the liquid diet group [MH RE, RD 0.14, IC (0.06, 0.22), I2 = 81%, P = 0.0006], with an NNT of 8, and a trend in favor of SPMC was identified in the low residue subgroup [MH RE, RD 0.06, IC (0.00, 0.11), I2 = 86%, P = 0.05] (Appendix 4 - Figure 12).
Adverse events: There was low heterogeneity (I2 = 43%, P = 0.17) among the three studies included in the liquid diet subgroup and high heterogeneity (I2 = 86%, P < 0.00001) among the seven studies included in the low residue subgroup. FE and RE models were used for liquid diet and low residue subgroups, respectively. There was no difference between SPMC and PEG in the liquid diet subgroup [MH FE, RD -0.02, IC (-0.08, 0.05), P = 0.59, I2 = 43%], but the low residue subgroup SPMC presented fewer adverse events than PEG [MH RE, RD -0.17, IC (-0.27, -0.07), P = 0.0008, I2 = 86%], with a NNT of 6 (Appendix 4 - Figure 13).
Polyp and adenoma detection rates: PDR and ADR subgroups were the same for SPMC vs PEG comparison because all the included trials in this comparison recommended only low residue diet on the day before.
DISCUSSION
Summary of evidence
Results from the meta-analysis of the 16 included studies (with 6200 subjects from ten different countries) indicate that for adult outpatients before elective colonoscopy, SPMC is at least similar to PEG in bowel cleaning efficacy, better in tolerability and in adverse events prevalence and similar in polyp and adenoma detection rate.
As high inconsistency and true heterogeneity were present among the included studies despite the strict inclusion criteria adopted, caution for interpretation of data is recommended. Populations of different countries with different dietary patterns, different options of dosage and schedule for bowel preparation and different scales and different instruments to measure outcomes may have contributed to increase heterogeneity. As bowel cleaning protocols vary between different institutions worldwide, variations across trials are inherent and expected.
As this meta-analysis provided an overall impression by grouping different bowel cleaning protocols and did not consider confounding factors, such as type of regimen, volume of solution ingested and dietary restrictions, additional analyses by subgroups were conducted to elucidate these aspects and to help decision-making in daily clinical practice.
Sensitivity analyses provided additional information on the influence of the studies in the meta-analysis, helping with the confounding factors. Pohl et al[31] was identified as the main outlier study for bowel cleaning due to its methodological bias of treatment: the comparison of different regimens of bowel preparation.
As previously known in a meta-analysis by Bucci et al[43], the interval time between the last drink of bowel preparation and the beginning of colonoscopy (also known as “runway time”) is a key factor for cleaning quality. When Pohl et al[31] compared different regimens (a split regimen of PEG and a day-before regimen of SPMC), the difference between treatment effects was increased and favored that one with the shorter “runway time” (PEG).
The sensitivity analysis by subgroups of regimen confirmed the impact of including trials comparing different regimens. Through the exclusion of this subgroup (Rex et al[15], Kang et al[39], Kim et al[40] and Pohl et al[31]), a more reliable analysis with less heterogeneity was obtained and the difference in bowel cleaning and the trend in favor of PEG for adenoma detection disappeared. Hence, the more rational approach was to assume SPMC and PEG were similar for both outcomes.
Statistical difference in favor of SPMC was also identified in the sub-analysis in the following situations: (1) bowel preparation was made on the day before (better bowel cleaning success and better tolerability); (2) bowel preparation was made based on the interval time to colonoscopy (also better tolerability); (3) when compared to high-volume solution of PEG (better tolerability and fewer adverse events); (4) liquid diet was the option on the day before (with better bowel cleaning success and better tolerability); and (5) low residue diet was the option on the day before (fewer adverse events).
Although there was statistical difference in these outcomes, it is also important to observe the number needed to treat to evaluate treatment effectiveness properly and to help deciding about changes in daily clinical practice. If the NNT is high, there is low chance of benefits for the patient with the alternative treatment, which might not justify its adoption.
The high NNTs of SPMC for bowel cleaning (NNT of 34) and for tolerability (NNT of 13) result in a small chance of benefit for the patient (2.9% and 7.6%, respectively). However, the small NNT for adverse events (NNT of 7) reveals a significant reduction of 14.2% when SPMC is used, this being its main advantage and the reason for its adoption over PEG.
Benefits of using SPMC are also obtained in day-before preparations (16.6% more chance of tolerability), against high-volume solutions of PEG (reduction of 16.6% in chance of adverse events) and with prior-day dietary restrictions (a 12.5% greater chance of tolerability with the use of liquid diet and a 16.6% reduction in the chance of adverse events with low residue diet).
Despite the potential benefits of SPMC demonstrated in this meta-analysis, care should be taken in regard to some of the product faults. Because of the potential electrolyte shifts, SPMC is not recommended in patients with renal insufficiency, end-stage liver disease, heart failure and electrolyte abnormalities[44,45]. PEG is the product of choice for those patients as it is an inert molecule and isosmotic solution, which also induces less mucosal damage (inflammation or ulceration) by ten times when compared to SPMC[35].
The main disadvantage of PEG consists in the amount of solution to be ingested as observed in the meta-analysis by Xie et al[46]. Further sub-analyses by volume of PEG solution presented in this meta-analysis reinforce this drawback. High-volume PEG presented less tolerability and more adverse events than SPMC whereas no difference was found between low-volume PEG and SPMC. As tolerability and adverse events are correlated factors that can affect bowel cleaning, SPMC appears as an interesting alternative.
An extensive search strategy, well-defined eligibility criteria, careful inclusion of the studies and analyses based on “intention-to-treat” data are the strength of this study. Results obtained by additional analyses focusing on subgroups based on regimen schedule, volume of PEG solution and dietary restriction bring new information and complement two recent meta-analyses.
Jin et al[20] and van Lieshout et al[47] showed that SPMC was equally effective or slightly superior to PEG in terms of bowel cleaning efficacy and that it was better tolerated than PEG. However, they did not consider patient status (if inpatient or outpatient) for studies selection and grouped different types of patients. This is the first meta-analysis for this specific group of patients and the first communicating effectiveness of bowel preparation using NNT.
Limitations
Nine full-text trials identified in the search were not included in this meta-analysis due to the lack of essential information concerning eligibility criteria[48-56]. Their absence may have contributed to borderline results in some sub-analyses with few included studies, but it assured the assertiveness of the results for this specific population.
Quality of bowel cleaning measured by different cleanliness scores and patients’ preferences and impressions of the products are other important outcomes that were not evaluated. Due to the different instruments to collect data used by trials, matching these data is prejudiced.
The type and severity of adverse events were also not explored. Owing to the methodological feature of RCTs and the characteristics of those products, the events are generally mild to moderate gastrointestinal symptoms (nausea, vomiting, abdominal pain, bloating and dizziness). Serious adverse events after bowel preparation are rare[57].
In addition, results obtained by this meta-analysis should be only inferred to healthy patients or those with mild disease as the included trials excluded other types of patients. This is especially important for the use of SPMC, as it is known for the occurrence of electrolyte disturbances which could have a repercussion in moderately or severely diseased patients.
Finally, although all the included studies were randomized clinical trials, five of them presented problems regarding randomization and masking, the description of losses and failure in reporting the outcomes, which compromised the quality of the evidence. Therefore, the quality of the evidence obtained was moderate for bowel cleaning efficacy, tolerability and adverse events prevalence, and low for polyp and adenoma detection rates[58]. Future studies might influence some outcomes and sub-analyses, especially those with borderline differences, with high NNTs or few studies included.
Conclusion
According to data published until now, SPMC seems to be a better product than PEG for bowel preparation in healthy or mildly diseased adult outpatients before colonoscopy as its bowel cleaning efficacy is at least equal to that of PEG, its tolerability is better and adverse events prevalence is lower. The latter corresponds to the main advantage of using SPMC instead of PEG. Both SPMC and PEG can be used for split preparations as there are no difference in bowel cleaning success, tolerability and adverse events prevalence, but SPMC should be the choice for day-before preparations because of its better tolerability.
ARTICLE HIGHLIGHTS
Research background
Colonoscopy reduces the incidence and mortality for colorectal cancer. Bowel preparation is the cornerstone for colonoscopy as the quality of bowel cleaning directly affects the effectiveness for detecting neoplastic lesions. Different options of purgatives exist as a result of the search for the ideal product and none of them have all the ideal features. PEG solutions are the most widely used and studied bowel cleanser, while SPMC is a recently developed one to overcome PEG’s poor palatability and large volume of solution to be ingested. Meta-analyses of RCTs are the best evidence for medical practice, but none of them compared SPMC and PEG for outpatients before colonoscopy, leaving a gap in the literature.
Research motivation
Most of elective colonoscopies are performed in outpatients and inpatient status is an independent risk factor for inadequate bowel preparation. As previous meta-analyses comparing SPMC and PEG before elective colonoscopy did not consider patient status for inclusion criteria, there is no established evidence for this subset of patients.
Research objectives
To determine the best option for bowel preparation in adult outpatients before elective colonoscopy by comparing cleaning efficacy, tolerability, AE prevalence, PDR and ADR between SPMC and PEG. This is the first meta-analysis to include only outpatients and to communicate effectiveness using NNT.
Research methods
Systematic review and meta-analysis followed PRISMA Statement. Eligibility criteria were based on PICOS strategy. Search was performed in MEDLINE, Scopus, EMBASE, CENTRAL/Cochrane, CINAHL and LILACS. Jadad scale was the tool adopted to evaluate the methodological quality of included RCTs and heterogeneity among studies was assessed by Higgins’ test (I2). Meta-analysis was preferably performed using intention-to-treat data by computing risk difference (RD) for dichotomous outcomes using Mantel-Haenszel (MH) method and NNT calculated for each outcome with statistical difference.
Research results
Sixteen RCTs with 6200 subjects were included for the meta-analysis and high heterogeneity was found among them. Sensitivity analysis and sub analysis by type of regime, volume of PEG solution and dietary recommendations were performed to interpret data. In the overall analysis, SPMC was better for bowel cleaning [RD 0.03, IC (0.01, 0.05), NNT 34], for tolerability [RD 0.08, IC (0.03, 0.13), NNT 13] and for adverse events [RD 0.13, IC (0.05, 0.22), NNT 7]. The small NNT for adverse events (NNT of 7) reveals a reduction of 14.2% when SPMC is used. Better tolerability for SPMC was also found in “Day-before preparations” [RD 0.17, IC (0.13, 0.21), NNT 6], “According to interval time” [RD 0.08, IC (0.01, 0.15), NNT 13], “Against high-volume of PEG” [RD 0.08, IC (0.01, 0.14), NNT 13] and “Liquid diet subgroup” [RD 0.14, IC (0.06,0.22), NNT 8].
Research conclusions
Data from published RCTs suggests SPMC is a better bowel cleanser than PEG before elective colonoscopy for healthy and mildly diseased adult outpatients because of its better tolerability, lower AE prevalence and cleaning efficacy at least equal to that of PEG. For split preparations, SPMC and PEG can be equally use, but for day-before preparations SPMC should be the standard choice.
Research perspectives
Future RCTs might influence the outcomes of this meta-analysis with few studies included and/or with borderline differences obtained (e.g., PDR, ADR, per type of regimen and per dietary recommendations) since Meta-analyzes are limited by the number of studies available and by the quality of the studies included. More homogeneous and definitive results should be obtained through a large intercontinental multi-center RCT, with the same bowel preparation protocol and tools for evaluating results. Although expensive and hard-working, it would be the best study format to compare purgatives and determine the best conditions for each of the available purgatives.
Footnotes
Conflict-of-interest statement: The authors deny any conflict of interest.
PRISMA Checklist: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Manuscript source: Unsolicited manuscript
Peer-review started: August 9, 2018
First decision: October 4, 2018
Article in press: December 5, 2018
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hosoe N, Fiori E S- Editor: Dou Y L- Editor: A E- Editor: Tan WW
Contributor Information
Rodrigo Silva de Paula Rocha, Gastrointestinal Endoscopy Unit, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo 05403-010, Brazil.
Igor Braga Ribeiro, Gastrointestinal Endoscopy Unit, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo 05403-010, Brazil. igorbraga1@gmail.com.
Diogo Turiani Hourneaux de Moura, Gastrointestinal Endoscopy Unit, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo 05403-010, Brazil; Division of Gastroenterology, Hepatology and Endoscopy, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA 02115, United States.
Wanderley Marques Bernardo, Gastrointestinal Endoscopy Unit, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo 05403-010, Brazil.
Maurício Kazuyoshi Minata, Gastrointestinal Endoscopy Unit, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo 05403-010, Brazil.
Flávio Hiroshi Ananias Morita, Gastrointestinal Endoscopy Unit, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo 05403-010, Brazil.
Júlio Cesar Martins Aquino, Gastrointestinal Endoscopy Unit, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo 05403-010, Brazil.
Elisa Ryoka Baba, Gastrointestinal Endoscopy Unit, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo 05403-010, Brazil.
Nelson Tomio Miyajima, Gastrointestinal Endoscopy Unit, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo 05403-010, Brazil.
Eduardo Guimarães Hourneaux de Moura, Gastrointestinal Endoscopy Unit, Hospital das Clínicas da Faculdade de Medicina da Universidade de São Paulo, São Paulo 05403-010, Brazil.
References
- 1.Navarro M, Nicolas A, Ferrandez A, Lanas A. Colorectal cancer population screening programs worldwide in 2016: An update. World J Gastroenterol. 2017;23:3632–3642. doi: 10.3748/wjg.v23.i20.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ribeiro IB, Bernardo WM, Martins BDC, de Moura DTH, Baba ER, Josino IR, Miyahima NT, Coronel Cordero MA, Visconti TAC, Ide E, et al. Colonic stent versus emergency surgery as treatment of malignant colonic obstruction in the palliative setting: a systematic review and meta-analysis. Endosc Int Open. 2018;6:E558–E567. doi: 10.1055/a-0591-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rex DK. Polyp detection at colonoscopy: Endoscopist and technical factors. Best Pract Res Clin Gastroenterol. 2017;31:425–433. doi: 10.1016/j.bpg.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Coronel M, Korkischko N, Marques Bernardo W, Lordello Passos M, Cavalheiro Bonifacio P, Valente de Matos M, de Moura DTH, Ide E. Comparison between Carbon Dioxide and Air Insufflation in Colonoscopy: A Systematic Review and Meta-Analysis Based On Randomized Control Trials. J Gastroenterol Pancreatol Liver Disord. 2017:1–11. [Google Scholar]
- 5.Rex DK, Imperiale TF, Latinovich DR, Bratcher LL. Impact of bowel preparation on efficiency and cost of colonoscopy. Am J Gastroenterol. 2002;97:1696–1700. doi: 10.1111/j.1572-0241.2002.05827.x. [DOI] [PubMed] [Google Scholar]
- 6.Froehlich F, Wietlisbach V, Gonvers J-J, Burnand B, Vader J-P. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 7.Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest Endosc. 2011;73:1207–1214. doi: 10.1016/j.gie.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76–79. doi: 10.1067/mge.2003.294. [DOI] [PubMed] [Google Scholar]
- 9.Parra-Blanco A, Ruiz A, Alvarez-Lobos M, Amorós A, Gana JC, Ibáñez P, Ono A, Fujii T. Achieving the best bowel preparation for colonoscopy. World J Gastroenterol. 2014;20:17709–17726. doi: 10.3748/wjg.v20.i47.17709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson DA, Barkun AN, Cohen LB, Dominitz JA, Kaltenbach T, Martel M, Robertson DJ, Boland CR, Giardello FM, Lieberman DA, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US multi-society task force on colorectal cancer. Gastroenterology. 2014;147:903–924. doi: 10.1053/j.gastro.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Hassan C, Bretthauer M, Kaminski MF, Polkowski M, Rembacken B, Saunders B, Benamouzig R, Holme O, Green S, Kuiper T, et al. Bowel preparation for colonoscopy: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2013;45:142–150. doi: 10.1055/s-0032-1326186. [DOI] [PubMed] [Google Scholar]
- 12.Hoy SM, Scott LJ, Wagstaff AJ. Sodium picosulfate/magnesium citrate: a review of its use as a colorectal cleanser. Drugs. 2009;69:123–136. doi: 10.2165/00003495-200969010-00009. [DOI] [PubMed] [Google Scholar]
- 13.Katz PO, Rex DK, Epstein M, Grandhi NK, Vanner S, Hookey LC, Alderfer V, Joseph RE. A dual-action, low-volume bowel cleanser administered the day before colonoscopy: results from the SEE CLEAR II study. Am J Gastroenterol. 2013;108:401–409. doi: 10.1038/ajg.2012.441. [DOI] [PubMed] [Google Scholar]
- 14.Thomas G, Brozinsky S, Isenberg JI. Patient acceptance and effectiveness of a balanced lavage solution (Golytely) versus the standard preparation for colonoscopy. Gastroenterology. 1982;82:435–437. [PubMed] [Google Scholar]
- 15.Rex DK, Katz PO, Bertiger G, Vanner S, Hookey LC, Alderfer V, Joseph RE. Split-dose administration of a dual-action, low-volume bowel cleanser for colonoscopy: the SEE CLEAR I study. Gastrointest Endosc. 2013;78:132–141. doi: 10.1016/j.gie.2013.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Rex DK, DiPalma JA, McGowan J, Cleveland Mv. A comparison of oral sulfate solution with sodium picosulfate: magnesium citrate in split doses as bowel preparation for colonoscopy. Gastrointest Endosc. 2014;80:1113–1123. doi: 10.1016/j.gie.2014.05.329. [DOI] [PubMed] [Google Scholar]
- 17.de Moura DT, Guedes H, Tortoretto V, Arataque TP, de Moura EG, Román JP, Rodela GL, Artifon EL. [Comparison of colon-cleansing methods in preparation for colonoscopy-comparative of solutions of mannitol and sodium picosulfate] Rev Gastroenterol Peru. 2016;36:293–297. [PubMed] [Google Scholar]
- 18.Tan JJ, Tjandra JJ. Which is the optimal bowel preparation for colonoscopy - a meta-analysis. Colorectal Dis. 2006;8:247–258. doi: 10.1111/j.1463-1318.2006.00970.x. [DOI] [PubMed] [Google Scholar]
- 19.Belsey J, Crosta C, Epstein O, Fischbach W, Layer P, Parente F, Halphen M. Meta-analysis: the relative efficacy of oral bowel preparations for colonoscopy 1985-2010. Aliment Pharmacol Ther. 2012;35:222–237. doi: 10.1111/j.1365-2036.2011.04927.x. [DOI] [PubMed] [Google Scholar]
- 20.Jin Z, Lu Y, Zhou Y, Gong B. Systematic review and meta-analysis: sodium picosulfate/magnesium citrate vs. polyethylene glycol for colonoscopy preparation. Eur J Clin Pharmacol. 2016;72:523–532. doi: 10.1007/s00228-016-2013-5. [DOI] [PubMed] [Google Scholar]
- 21.Dik VK, Moons LM, Hüyük M, van der Schaar P, de Vos Tot Nederveen Cappel WH, Ter Borg PC, Meijssen MA, Ouwendijk RJ, Le Fèvre DM, Stouten M, et al. Predicting inadequate bowel preparation for colonoscopy in participants receiving split-dose bowel preparation: development and validation of a prediction score. Gastrointest Endosc. 2015;81:665–672. doi: 10.1016/j.gie.2014.09.066. [DOI] [PubMed] [Google Scholar]
- 22.Martel M, Barkun AN, Menard C, Restellini S, Kherad O, Vanasse A. Split-Dose Preparations Are Superior to Day-Before Bowel Cleansing Regimens: A Meta-analysis. Gastroenterology. 2015;149:79–88. doi: 10.1053/j.gastro.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Copas J, Shi JQ. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics. 2000;1:247–262. doi: 10.1093/biostatistics/1.3.247. [DOI] [PubMed] [Google Scholar]
- 28.Leitao K, Grimstad T, Bretthauer M, Holme Ø, Paulsen V, Karlsen L, Isaksen K, Cvancarova M, Aabakken L. Polyethylene glycol vs sodium picosulfate/magnesium citrate for colonoscopy preparation. Endosc Int Open. 2014;2:E230–E234. doi: 10.1055/s-0034-1377520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HG, Huh KC, Koo HS, Kim SE, Kim JO, Kim TI, Kim HS, Myung SJ, Park DI, Shin JE, et al. Sodium Picosulfate with Magnesium Citrate (SPMC) Plus Laxative Is a Good Alternative to Conventional Large Volume Polyethylene Glycol in Bowel Preparation: A Multicenter Randomized Single-Blinded Trial. Gut Liver. 2015;9:494–501. doi: 10.5009/gnl14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munsterman ID, Cleeren E, van der Ploeg T, Brohet R, van der Hulst R. ‘Pico-Bello-Klean study’: effectiveness and patient tolerability of bowel preparation agents sodium picosulphate-magnesium citrate and polyethylene glycol before colonoscopy. A single-blinded randomized trial. Eur J Gastroenterol Hepatol. 2015;27:29–38. doi: 10.1097/MEG.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 31.Pohl J, Halphen M, Kloess HR, Fischbach W. Impact of the quality of bowel cleansing on the efficacy of colonic cancer screening: a prospective, randomized, blinded study. PLoS One. 2015;10:e0126067. doi: 10.1371/journal.pone.0126067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoo IK, Lee JS, Chun HJ, Jeen YT, Keum B, Kim ES, Choi HS, Lee JM, Kim SH, Nam SJ, et al. A randomized, prospective trial on efficacy and tolerability of low-volume bowel preparation methods for colonoscopy. Dig Liver Dis. 2015;47:131–137. doi: 10.1016/j.dld.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 33.Kojecky V, Matous J, Keil R, Dastych M, Zadorova Z, Varga M, Kroupa R, Dolina J, Misurec M, Hep A, et al. The optimal bowel preparation intervals before colonoscopy: A randomized study comparing polyethylene glycol and low-volume solutions. Dig Liver Dis. 2018;50:271–276. doi: 10.1016/j.dld.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Regev A, Fraser G, Delpre G, Leiser A, Neeman A, Maoz E, Anikin V, Niv Y. Comparison of two bowel preparations for colonoscopy: sodium picosulphate with magnesium citrate versus sulphate-free polyethylene glycol lavage solution. Am J Gastroenterol. 1998;93:1478–1482. doi: 10.1111/j.1572-0241.1998.00467.x. [DOI] [PubMed] [Google Scholar]
- 35.Lawrance IC, Willert RP, Murray K. Bowel cleansing for colonoscopy: prospective randomized assessment of efficacy and of induced mucosal abnormality with three preparation agents. Endoscopy. 2011;43:412–418. doi: 10.1055/s-0030-1256193. [DOI] [PubMed] [Google Scholar]
- 36.Kao D, Lalor E, Sandha G, Fedorak RN, van der Knoop B, Doornweerd S, van Kooten H, Schreuders E, Midodzi W, Veldhuyzen van Zanten S. A randomized controlled trial of four precolonoscopy bowel cleansing regimens. Can J Gastroenterol. 2011;25:657–662. doi: 10.1155/2011/486084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manes G, Amato A, Arena M, Pallotta S, Radaelli F, Masci E. Efficacy and acceptability of sodium picosulphate/magnesium citrate vs low-volume polyethylene glycol plus ascorbic acid for colon cleansing: a randomized controlled trial. Colorectal Dis. 2013;15:1145–1153. doi: 10.1111/codi.12246. [DOI] [PubMed] [Google Scholar]
- 38.Jeon SR, Kim HG, Lee JS, Kim JO, Lee TH, Cho JH, Kim YH, Cho JY, Lee JS. Randomized controlled trial of low-volume bowel preparation agents for colonic bowel preparation: 2-L polyethylene glycol with ascorbic acid versus sodium picosulfate with magnesium citrate. Int J Colorectal Dis. 2015;30:251–258. doi: 10.1007/s00384-014-2066-9. [DOI] [PubMed] [Google Scholar]
- 39.Kang MS, Kim TO, Seo EH, Jung DK, Kim MS, Heo NY, Park JH, Park SH, Moon YS. Comparison of the Efficacy and Tolerability between Same-day Picosulfate and Split-dose Polyethylene Glycol Bowel Preparation for Afternoon Colonoscopy: A Prospective, Randomized, Investigator-blinded Trial. Intest Res. 2014;12:53–59. doi: 10.5217/ir.2014.12.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim ES, Lee WJ, Jeen YT, Choi HS, Keum B, Seo YS, Chun HJ, Lee HS, Um SH, Kim CD, et al. A randomized, endoscopist-blinded, prospective trial to compare the preference and efficacy of four bowel-cleansing regimens for colonoscopy. Scand J Gastroenterol. 2014;49:871–877. doi: 10.3109/00365521.2014.910543. [DOI] [PubMed] [Google Scholar]
- 41.Kim YS, Hong CW, Kim BC, Han KS, Park JW, Seong Choi H, Joo J, Sohn DK. Randomized clinical trial comparing reduced-volume oral picosulfate and a prepackaged low-residue diet with 4-liter PEG solution for bowel preparation. Dis Colon Rectum. 2014;57:522–528. doi: 10.1097/DCR.0000000000000066. [DOI] [PubMed] [Google Scholar]
- 42.Regev A, Fraser G, Delpre G, Laiser A, Neeman A, Maoz E, Anikin V, Niv Y. Efficacy and tolerability of sodium picosulphate with magnesium citrate versus polyethylene glycol electrolyte lavage solution for colouoscopy preparation. Gastrointest Endosc. 1996;43:320. [Google Scholar]
- 43.Bucci C, Rotondano G, Hassan C, Rea M, Bianco MA, Cipolletta L, Ciacci C, Marmo R. Optimal bowel cleansing for colonoscopy: split the dose! A series of meta-analyses of controlled studies. Gastrointest Endosc. 2014;80:566–576.e2. doi: 10.1016/j.gie.2014.05.320. [DOI] [PubMed] [Google Scholar]
- 44.Lim YJ, Hong SJ. What is the best strategy for successful bowel preparation under special conditions? World J Gastroenterol. 2014;20:2741–2745. doi: 10.3748/wjg.v20.i11.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bechtold ML, Mir F, Puli SR, Nguyen DL. Optimizing bowel preparation for colonoscopy: a guide to enhance quality of visualization. Ann Gastroenterol. 2016;29:137–146. doi: 10.20524/aog.2016.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xie Q, Chen L, Zhao F, Zhou X, Huang P, Zhang L, Zhou D, Wei J, Wang W, Zheng S. A meta-analysis of randomized controlled trials of low-volume polyethylene glycol plus ascorbic acid versus standard-volume polyethylene glycol solution as bowel preparations for colonoscopy. PLoS One. 2014;9:e99092. doi: 10.1371/journal.pone.0099092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lieshout I V, Munsterman ID, Eskes AM, Maaskant JM, van der Hulst R. Systematic review and meta-analysis: Sodium picosulphate with magnesium citrate as bowel preparation for colonoscopy. United Eur Gastroenterol J. 2017;5:917–943. doi: 10.1177/2050640616684696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Worthington J, Thyssen M, Chapman G, Chapman R, Geraint M. A randomised controlled trial of a new 2 litre polyethylene glycol solution versus sodium picosulphate + magnesium citrate solution for bowel cleansing prior to colonoscopy. Curr Med Res Opin. 2008;24:481–488. doi: 10.1185/030079908x260844. [DOI] [PubMed] [Google Scholar]
- 49.Kojecký V, Mišurec M, Varga M. Comparison of the tolerance and quality of bowel preparation before colonoscopy using picosulphate / magnesium citrate or polyethylene glycol in different dosing regimens [Porovnání tolerance a kvality přípravy střeva před kolonoskopií pomocí pikosulfát/citrátu hořečnatého nebo polyetylenglykolu v různém dávkování]. Gastroent Hepatol 2012; 66: 470-474 Available from: https://www.medvik.cz/bmc/link.do?id=bmc13003003 [Google Scholar]
- 50.Voiosu T, Ratiu I, Voiosu A, Iordache T, Schipor A, Baicus C, Sporea I, Voiosu R. Time for individualized colonoscopy bowel-prep regimens? A randomized controlled trial comparing sodium picosulphate and magnesium citrate versus 4-liter split-dose polyethylene glycol. J Gastrointestin Liver Dis. 2013;22:129–134. [PubMed] [Google Scholar]
- 51.Kojecky V, Dolina J, Kianicka B, Misurec M, Varga M, Latta J, Vaculin V. A single or split dose picosulphate/magnesium citrate before colonoscopy: comparison regarding tolerance and efficacy with polyethylene glycol. A randomized trial. J Gastrointestin Liver Dis. 2014;23:141–146. doi: 10.15403/jgld.2014.1121.232.vk1. [DOI] [PubMed] [Google Scholar]
- 52.Gweon TG, Kim SW, Noh YS, Hwang S, Kim NY, Lee Y, Lee SW, Lee SW, Lee JY, Lim CH, et al. Prospective, randomized comparison of same-day dose of 2 different bowel cleanser for afternoon colonoscopy: picosulfate, magnesium oxide, and citric acid versus polyethylene glycol. Medicine (Baltimore) 2015;94:e628. doi: 10.1097/MD.0000000000000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muñoz-Navas M, Calleja JL, Payeras G, Hervás AJ, Abreu LE, Orive V, Menchén PL, Bordas JM, Armengol JR, Carretero C, et al. A randomized trial to compare the efficacy and tolerability of sodium picosulfate-magnesium citrate solution vs. 4 L polyethylene glycol solution as a bowel preparation for colonoscopy. Int J Colorectal Dis. 2015;30:1407–1416. doi: 10.1007/s00384-015-2307-6. [DOI] [PubMed] [Google Scholar]
- 54.Heetun Z, Crowley R, Zeb F, Kearns D, Brennan MH, O’Connor C, Courtney G, Aftab AR. Comparison of polyethylene glycol vs sodium picosulphate vs sodium biphosphonate by efficacy in bowel cleansing and patients’ tolerability: a randomised trial. Ir J Med Sci. 2016;185:629–633. doi: 10.1007/s11845-015-1320-7. [DOI] [PubMed] [Google Scholar]
- 55.Choi HS, Chung JW, Lee JW, Lim MY, Park DK, Kim YJ, Kwon KA, Kim JH. Polyethylene glycol plus ascorbic acid is as effective as sodium picosulfate with magnesium citrate for bowel preparation: A randomized trial. J Dig Dis. 2016;17:268–273. doi: 10.1111/1751-2980.12337. [DOI] [PubMed] [Google Scholar]
- 56.Ruiz Zavala AM, García Guerrero VA, Zárate Guzmán Á M, Corral Medina A, Valdés Lías R. Tolerability and efficacy of sodium picosulphate and magnesium citrate compared with polyethyleneglycol in bowel cleaning. Endoscopia. 2016;28:148–153. [Google Scholar]
- 57.Anastassopoulos K, Farraye FA, Knight T, Colman S, Cleveland MV, Pelham RW. A Comparative Study of Treatment-Emergent Adverse Events Following Use of Common Bowel Preparations Among a Colonoscopy Screening Population: Results from a Post-Marketing Observational Study. Dig Dis Sci. 2016;61:2993–3006. doi: 10.1007/s10620-016-4214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490–1494. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]