Abstract
AIM
To construct a non-invasive prediction algorithm for predicting non-alcoholic steatohepatitis (NASH), we investigated Japanese morbidly obese patients using artificial intelligence with rule extraction technology.
METHODS
Consecutive patients who required bariatric surgery underwent a liver biopsy during the operation. Standard clinical, anthropometric, biochemical measurements were used as parameters to predict NASH and were analyzed using rule extraction technology. One hundred and two patients, including 79 NASH and 23 non-NASH patients were analyzed in order to create the prediction model, another cohort with 77 patients including 65 NASH and 12 non-NASH patients were analyzed to validate the algorithm.
RESULTS
Alanine aminotransferase, C-reactive protein, homeostasis model assessment insulin resistance, albumin were extracted as predictors of NASH using a recursive-rule extraction algorithm. When we adopted the extracted rules for the validation cohort using a highly accurate rule extraction algorithm, the predictive accuracy was 79.2%. The positive predictive value, negative predictive value, sensitivity and specificity were 88.9%, 35.7%, 86.2% and 41.7%, respectively.
CONCLUSION
We successfully generated a useful model for predicting NASH in Japanese morbidly obese patients based on their biochemical profile using a rule extraction algorithm.
Keywords: Non-alcoholic steatohepatitis, Artificial intelligence, Rule extraction, Morbid obesity, Liver biopsy, Non-invasive prediction
Core tip: The prevalence of non-alcoholic steatohepatitis (NASH) in Japanese morbidly obese patients is extremely high, and early intervention should be undertaken. However, it is difficult to perform a percutaneous liver biopsy or elastography in routine medical care, especially in morbidly obese patients. We therefore attempted to construct a non-invasive prediction algorithm in order to predict NASH in Japanese morbidly obese patients using artificial intelligence with rule extraction technology. Although further studies with larger numbers of patients are needed to confirm the results, this algorithm may be useful for non-invasively predicting NASH in morbidly obese Japanese patients in the clinical setting.
INTRODUCTIO
Non-alcoholic fatty liver disease (NAFLD) includes non-alcoholic steatohepatitis (NASH) and non-alcoholic fatty liver (NAFL); a pathological diagnosis is the gold standard for the diagnosis of NASH[1,2]. The prevalence of NAFLD is reported to be 29.7% and is increasing in Japan[3]. The number of patients with morbid obesity is also increasing worldwide, and this increase is a major social problem[4]. Although the prevalence of morbid obesity is low in Japan (0.25%-0.3%) compared with to the United States[5,6], it is also increasing in Japan.
Morbid obesity is frequently complicated by metabolic diseases such as type 2 diabetes mellitus, hypertension, hyperlipidemia, and ischemic heart disease. The prevalence of NASH in patients with morbid obesity is also high. We previously reported that the prevalence of NASH in Japanese morbidly obese patients was extremely high (77.5%)[7]. Among non-morbidly-obese patients, the survival rate of NASH patients is significantly lower than in the general population[8]. As a result, an early diagnosis and early intervention are important for NASH patients[7].
A percutaneous liver biopsy is usually performed to diagnose liver diseases, including NASH[9]. Since a percutaneous liver biopsy requires the insertion of a long needle through the subcutaneous tissues to reach the liver, this method is difficult to perform in obese patients with a thick layer of subcutaneous fat. In addition, a percutaneous liver biopsy carries a risk of complications and sampling error in patients with a thick layer of subcutaneous fat. Elastography is non-invasive and sometimes used to estimate the liver stiffness or the extent of fibrosis in patients with liver disease, including NASH[9]. Since elastography is also performed percutaneously, a thick layer of subcutaneous fat is again an obstacle for the accurate measurement and sometimes leads to a misdiagnosis. Magnetic resonance (MR) elastography is useful for predicting fibrosis and NASH[10]. However, it is also difficult to perform in morbidly obese patients due to limitations in the size or the ability of MR equipment to accommodate the patient. Given the above limitations, a safer, non-invasive, convenient diagnostic method for predicting NASH in morbidly obese patients is needed.
Predictive calculation formulas, such as the NAFIC score, fibrosis-4 index, and NAFLD fibrosis score are usually used to diagnose fibrosis and NASH[11-16]. These formulas are usually constructed using the data from patients whose body weight is within the general range and are not specialized for patients with morbid obesity. The HAIR [hypertension, alanine aminotransferase (ALT), insulin resistance] score[16] and BARD score[17] [includes body mass index (BMI), aspartate aminotransferase (AST)/ALT ratio and presence of diabetes mellitus] were constructed using data of morbidly obese patients from Australia[16] and the United States[17], respectively. However, there are racial differences between Asian people (including Japanese) and Caucasians[7,18,19]. When the BMI is similar, the degree of liver dysfunction and prevalence of NASH is worse in Asian patients than in Caucasians[7,18,19].
Recently, there has been remarkable progress in artificial intelligence, and we are now able to generate highly accurate and interpretable predictive models[20,21]. Rule extraction is a technique that attempts to find a compromise between requirements by building a simple rule set that mimics how well-performing complex predictive models (black-box, i.e., not understandable) make their decisions for physicians and clinicians. We proposed continuous recursive-rule extraction (Re-RX) with J48graft as a promising algorithm for rule extraction[22]; this was based on the Re-RX algorithm[23] to simultaneously enhance the accuracy and interpretability of the extracted classification rules.
In the present study, we extracted new classification rules to predict NASH based on the biochemical profile of Japanese morbidly obese patients using Continuous Re-RX with J48graft. This predictive algorithm, which was named the Japanese algorithm of Morbid Obesity for NASH Prediction (JOMO algorithm), may be useful for predicting NASH in Japanese or Asian morbidly obese patients in the clinical setting.
MATERIALS AND METHODS
Study design
To create the prediction algorithm, we used the data of the cohort of Japanese morbidly obese patients who participated in our previous study[7]. The patient characteristics are shown in Supplemental Table 1 and Table 2 (modified from reference 7). One hundred and two patients, including 79 NASH and 23 non-NASH patients were analyzed using rule extraction technology. The main purpose of this study was to generate a highly accurate and interpretable predictive model for NASH in Japanese morbidly obese patients using an accuracy-priority rule extraction algorithm.
To validate the algorithm, another cohort of 77 patients was collected from October 2012 to September 2014. This cohort included 65 NASH and 12 non-NASH patients. Consecutive obese patients undergoing bariatric surgery for the management of morbid obesity at a single center (Weight Loss and Metabolic Surgery Center, Yotsuya Medical Cube, Tokyo, Japan) underwent a liver biopsy during the operation[7]. The precise methods for liver biopsies and sampling have been described in previous study[7]. NASH was histologically defined as steatosis with at least two of the following[24]: (1) Lobular necro-inflammatory foci; (2) Ballooning degeneration of hepatocytes with or without Mallory bodies; and (3) Perisinusoidal fibrosis. The indications for bariatric surgery followed the criteria established in 2005 by the Asia–Pacific Bariatric Surgery Group consensus meeting[18] as follows: Asian patients with a BMI of > 37 kg/m2 or > 32 kg/m2 (with diabetes or 2 other obesity-related comorbidities) and failure to show any improvement with nonsurgical treatment.
The standard clinical, anthropometric (weight, height, waist and hip circumferences) and biochemical measurements, including AST, ALT, γ-glutamyl transpeptidase (γ-GTP), cholinesterase (ChE), uric acid (UA), albumin (Alb), C-reactive protein (CRP), Fe (iron), fasting blood insulin, fasting plasma glucose (FPG), hemoglobin (Hb) A1c, triglyceride, and low density lipoprotein-cholesterol (LDL-cho) levels, were obtained before bariatric surgery. All blood samples were collected after overnight fasting. The homeostasis model assessment insulin resistance (HOMA-IR) index was used as an index of insulin resistance and was calculated by the following formula: glucose (mg/dL) × insulin (µU/mL) /405. Computed tomography was performed, and the areas of subcutaneous and visceral fat were calculated. The diagnosis of type 2 diabetes mellitus was based on the Japanese Diabetes Society criteria[25]. Hypertension was diagnosed if the patient had a history of hypertension and was on antihypertensive medication or if the patient had a resting recumbent blood pressure of ≥ 140/90 mmHg on two repeated occasions.
Written informed consent was obtained from every patient, and the study was approved by the institutional review boards (IRB) of Yotsuya Medical Cube (YMC-2009-3). The study was performed in accordance with the principles of the Declaration of Helsinki. For this study using artificial intelligence using rule extraction technology, new IRB statements (Gunma University-2017-004) and opt-out were obtained.
Continuous Re-RX with J48graft
We recently proposed a new accuracy-priority rule extraction algorithm using Re-RX with J48graft[26] and continuous attributes (Continuous Re-RX with J48graft[23,27]). In order to extract more accurate and concise classification rules, we proposed replacing the conventional Re-RX algorithm, which uses C4.5 as a decision tree[28], with Re-RX with J48graft[23,29]. The conventional pruning used in J48 both complements and contrasts with that used in J48graft. The performance of the Re-RX algorithm is thought to be greatly affected by the decision tree.
In contrast, Continuous Re-RX[29] is a rule extraction algorithm that aims to achieve very high accuracy rather than concision. We found that the combination of Continuous Re-RX with J48graft and Continuous Re-RX simultaneously enhanced the accuracy and interpretability[27] and was well suited to generating a considerably better predictive model in this cohort. The age, Sex, BMI, waist-hip ratio, visceral fat area, AST, ALT, γ-GTP, ChE, UA, Alb, CRP, Fe, platelets, LDL-cho, TG, FPG, HbA1c, HOMA-IR, type 2 diabetes mellitus, and hypertension were used as parameters.
Statistical analysis
All data are shown as the mean ± SD. Differences between the groups were analyzed by Fisher’s exact probability test and Mann-Whitney U tests when a significant difference was obtained by the Kruskal-Wallis test. A value of P < 0.05 was considered to be significant.
RESULTS
Patient characteristics
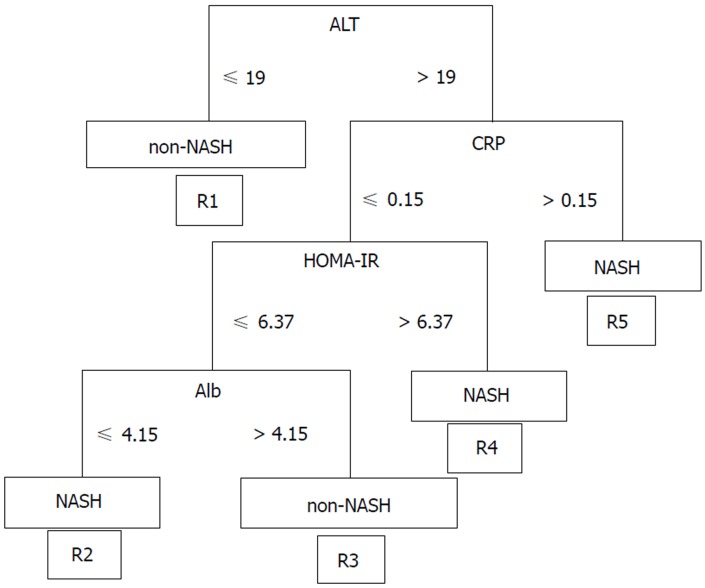
We classified the data of the pathological findings and the results of a blood test obtained from a routine medical examination in the NASH and Non-NASH groups and analyzed the data using Continuous Re-RX with J48graft. The algorithm demonstrated the following rules for classification: R1: ALT ≤ 19 then non-NASH; R2: ALT > 19; CRP ≤ 0.15, HOMA-IR ≤ 6.37, Alb ≤ 4.15 then NASH; R3: ALT > 19, CRP ≤ 0.15, HOMA-IR ≤ 6.37, Alb > 4.15 then Non-NASH; R4: ALT > 19, CRP ≤ 0.15, HOMA-IR > 6.37 then NASH; R5: ALT > 19, CRP > 0.15 then NASH (Figure 1). The patients classified in R1 and R3 were predicted to be non-NASH, while NASH was predicted in the patients who were classified into R2, R4 and R5. This algorithm was based on 10 runs of 5 cross validation (CV)[30].
Figure 1.
Prediction formula created using a highly accurate rule extraction algorithm. NASH: Non-alcoholic steatohepatitis; ALT: Alanine aminotransferase; CRP: C-reactive protein; Alb: Albumin; HOMA-IR: homeostasis model assessment insulin resistance.
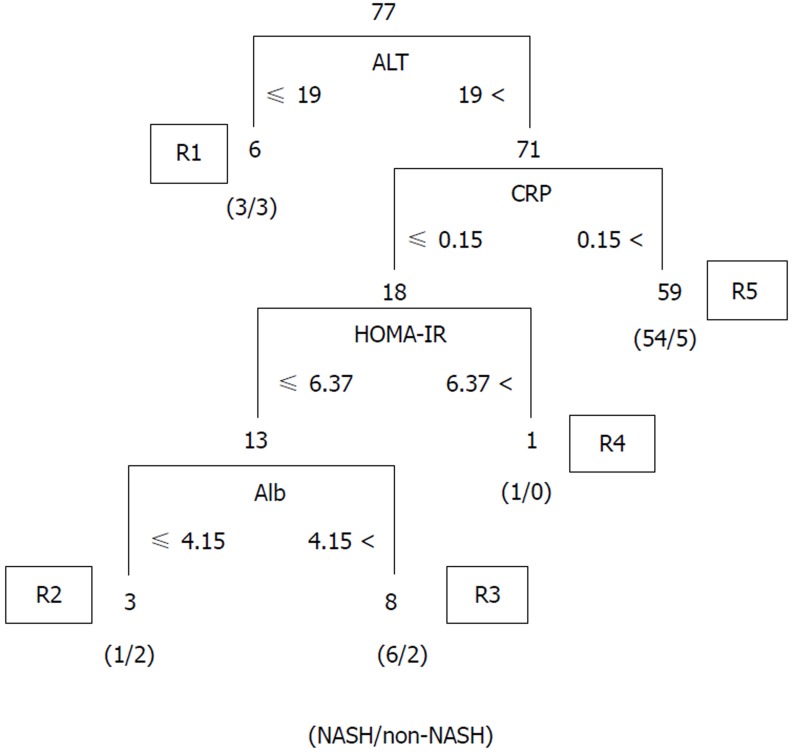
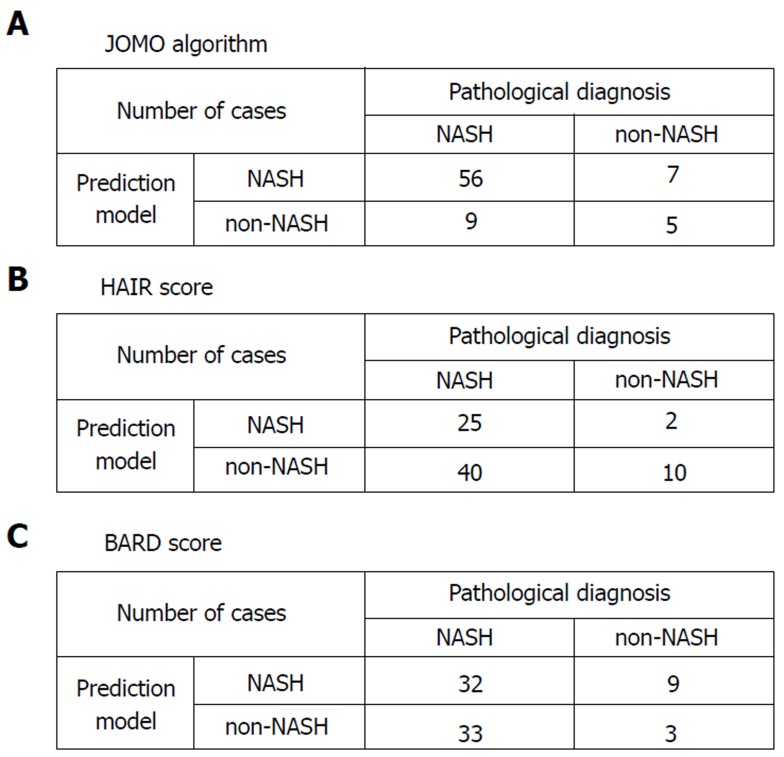
We validated the algorithm in the other cohort of 77 patients. To validate the performance of the extracted rules in classifying NASH patients, we confirmed the rate of agreement of the results. The 77 cases were classified using the prediction formula as follows: R1, n = 6; R2, n = 3; R3, n = 8; R4, n = 1; and R5, n = 59 (Figure 2). Fourteen cases were classified as non-NASH (R1 + R3) by the algorithm, and 5 cases were classified as non-NASH according to the results of the histopathological examinations. Sixty-three cases were classified into the NASH group (R2 + R4 +R5), while NASH was histopathologically diagnosed in 56 cases (Figure 2). Figure 3A shows the predictive value using our algorithm. The positive predictive value, negative predictive value, sensitivity and specificity were 88.9%, 35.7%, 86.2% and 41.7%, respectively (Table 1). The predictive accuracy rate was 79.2%.
Figure 2.
Predictive accuracy of the new developed JOMO algorithm in Japanese morbidly obese patients. NASH: Non-alcoholic steatohepatitis; ALT: Alanine aminotransferase; CRP: C-reactive protein; Alb: Albumin; HOMA-IR: Homeostasis model assessment insulin resistance.
Figure 3.
A cross table comparing the results of the prediction model and the pathological diagnosis. A: JOMO algorithm; B: HAIR score; C: BARD score. NASH: Non-alcoholic steatohepatitis.
Table 1.
The positive predictive value, negative predictive value, sensitivity, specificity and predictive accuracy rate of each category
| Positive predictive value | Negative predictive value | Sensitivity | Specificity | Predictive accuracy rate | |
| HAIR score | 92.6% | 20.0% | 38.5% | 83.3% | 45.5% |
| BARD score | 78.0% | 8.3% | 49.2% | 25.0% | 45.5% |
| JOMO algorithm | 88.9% | 35.7% | 86.2% | 41.7% | 79.2% |
Figure 3B shows the predictive value of the conventional predictive method using the HAIR score[16]. The HAIR score was constructed based on data from Australian patients with morbid obesity. The positive predictive value, negative predictive value, sensitivity and specificity were 92.6%, 20.0%, 38.5%, and 83.3%, respectively (Table 1). The predictive accuracy rate was 45.5%.
Figure 3C shows the prediction values using BARD score[17]. The BARD score was constructed based on a large cohort of patients from the United States with a median BMI of 33 kg/m2. The positive predictive value, negative predictive value, sensitivity and specificity were 78.0%, 8.3%, 49.2%, and 25.0%, respectively. The predictive accuracy rate was 45.5% (Table 1).
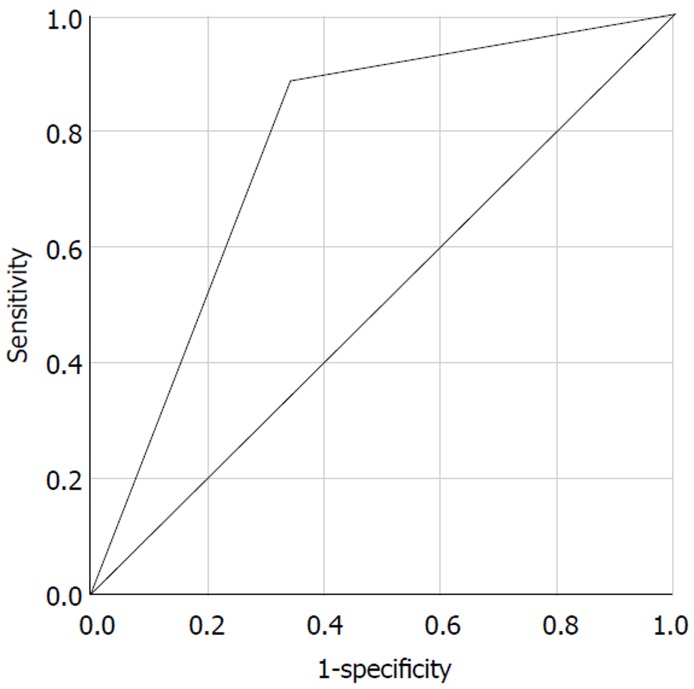
The Receiver Operating Characteristic (ROC) curve of our algorithm for all patients is shown in Figure 4. The area of ROC was 0.772.
Figure 4.
Receiver operating characteristic curve of the JOMO algorithm.
Our newly developed predictive algorithm showed high predictive accuracy in Japanese obese patients. We named our new predictive algorithm the JOMO algorithm.
DISCUSSION
It is difficult to perform a liver biopsy in morbidly obese patients during the course of a typical consultation. We therefore generated a new predictive model, the JOMO algorithm, to predict NASH in Japanese morbidly obese patients using Continuous Re-RX with J48graft. This algorithm showed substantially better results than conventional predictive formulae. We used 2 cohorts of 102 and 77 Japanese morbidly obese patients to generate and to validate the algorithm, respectively. Although a larger number of cases would enable us to improve the accuracy of the classification and interpretability, it is difficult to collect liver biopsy data from Japanese morbidly obese patients. These cohorts of 102 and 77 patients may be the largest cohorts of Japanese morbidly obese patients to have undergone a liver biopsy. For this reason, we adopted an artificial intelligence approach using Continuous Re-RX with J48graft. Continuous Re-RX with J48graft provided a more accurate predictive accuracy without the need for a validation cohort. Since we generated a predictive model by artificial intelligence using rule extraction technology, we were able to extract more accurate and interpretable classification rules from a limited cohort.
In this study, we performed k-fold cross-validation when crafting this algorithm[30,31]. Regarding the experimental settings and data splitting technique, k-fold CV[30] was applied, i.e., the original database was partitioned into k-subsets/folds of equal sizes where each subset had to be trained and tested. Accordingly, the final output was predicted by taking the averages of all partitions/folds that had been tested. Therefore, we applied 10 runs of 5-fold CV which was averaged to obtain the robust classification accuracy. Although the application of rule extraction technology is new in the medical field, strategies using Continuous Re-RX with J48graft, i.e., rule-based classifiers, have already been established in the field of artificial intelligence[23,27]. Our algorithm using Continuous Re-RX with J48graft can therefore be adapted to different cohorts without a loss of generality.
In our previous study[7], a multivariate analysis for predicting NASH indicated that the waist-hip ratio, ALT level, fasting plasma glucose level, and HOMA-IR were independent factors associated with NASH. However, the JOMO algorithm using Continuous Re-RX with J48graft selected the ALT level, HOMA-IR, CRP level, and Alb level as predictive values. The ALT level and HOMA-IR were both selected in our previous multivariate analysis as well as in this JOMO algorithm. However, the waist-hip ratio and fasting plasma glucose level were not chosen by the JOMO algorithm. On using the predictive factors determined in the previous multivariate analysis, the predictive accuracy rates of the waist-hip ratio, fasting plasma glucose level, ALT level and HOMA-IR were found to be 54.1%, 35.1%, 77.9%, and 79.2%, respectively. The predictive values of the JOMO algorithm were thus considerably better than those of the multivariate analysis. Furthermore, since the attributes selected by artificial intelligence in this study are more concise, they are easily checked by blood sampling. This is an advantage with the JOMO algorithm and fulfills the purpose of this study.
CRP was selected as a predictive parameter in this JOMO algorithm. The predictive value of CRP in the diagnosis of NASH remains controversial at present. Anty et al[32] reported the CRP levels were not predictive of the diagnosis of NASH in severely obese patients because the liver as well as the adipose tissue can produce CRP. However, Yoneda et al[33] demonstrated that high-sensitivity CRP was useful for making a prediction in cases of NASH compared with in cases of simple nonprogressive steatosis. They also suggested that high-sensitivity CRP might be a clinical feature that indicates the severity of hepatic fibrosis in cases of NASH[33]. It is of note that there was no statistically significant difference in the CRP levels between the NASH and non-NASH as groups in our cohort (Supplemental Table 2), and thus the introduction of CRP into the predictive model demonstrated the power and usefulness of artificial intelligence.
The level of CRP can be affected by many factors. However, the cohort used in this study was relatively homogenous: All patients were candidates for bariatric surgery, and blood samples were collected from all patients who were roughly in the same condition before surgery. As such, our cohort may be protected against miscellaneous factors that influence the CRP level. The prevalence of NASH patients was high, and the number of non-NASH patients was low in the cohort used in this study. This may have influenced the accuracy of our predictive algorithm. Therefore, further evaluation of the role of CRP in this algorithm will be needed before it should be applied to other cohorts. It the future, we plan to improve this algorithm further using a larger number of NASH and non-NASH patients.
In this study, we generated a new predictive model that used Continuous Re-RX with J48graft to predict NASH in Japanese morbidly obese patients. This algorithm enables the prediction of NASH using parameters available from blood tests in routine medical examinations. Although further studies with larger numbers of patients are needed to confirm the results, this algorithm may be useful for non-invasively predicting NASH in morbidly obese Japanese patients in the clinical setting.
ARTICLE HIGHLIGHTS
Research background
Pathological diagnosis is the gold standard for the diagnosis of non-alcoholic steatohepatitis (NASH). However, it is difficult to perform a percutaneous liver biopsy in routine medical care, especially in morbidly obese patients. Predictive calculation formulas are usually used to diagnose fibrosis and NASH.
Research motivation
Recently, there has been remarkable progress in artificial intelligence. We therefore attempted to construct a non-invasive prediction algorithm in order to predict NASH using artificial intelligence with rule extraction technology.
Research objectives
Morbidly obese Japanese patients who required bariatric surgery underwent a liver biopsy during the operation. Standard clinical, anthropometric, biochemical measurements were used as parameters for making prediction model.
Research methods
One hundred and two patients, including 79 NASH and 23 non-NASH patients were analyzed in order to create the prediction model, another cohort with 77 patients including 65 NASH and 12 non-NASH patients were analyzed to validate the algorithm. We used Continuous recursive-rule extraction with J48graft for rule extraction to predict NASH.
Research results
Alanine aminotransferase, C-reactive protein, homeostasis model assessment insulin resistance, albumin were extracted as predictors of NASH. When we adopted the extracted rules for the validation cohort, the predictive accuracy was 79.2%. The positive predictive value, negative predictive value, sensitivity and specificity were 88.9%, 35.7%, 86.2% and 41.7%, respectively.
Research conclusions
We successfully generated a useful model for predicting NASH in Japanese morbidly obese patients based on their biochemical profile using a rule extraction algorithm.
Research perspectives
Although further studies with larger numbers of patients are needed to confirm the results, this algorithm may be useful for non-invasively predicting NASH in morbidly obese Japanese patients in the clinical setting.
Footnotes
Institutional review board statement: This study was approved by institutional review boards of Yotsuya Medical Cube and Gunma University.
Informed consent statement: For this study using artificial intelligence using rule extraction technology, opt-out was obtained.
Conflict-of-interest statement: There are no conflicts of interest.
Data sharing statement: No additional data is available
Peer-review started: July 2, 2018
First decision: August 1, 2018
Article in press: October 17, 2018
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Ozenirler S, Namisaki T, Takahashi T, Soresi M, de Oliveira C, De Silva AP S- Editor: Dou Y L- Editor: A E- Editor: Tan WW
Contributor Information
Daisuke Uehara, Department of Medicine and Molecular Science, Gunma University Graduate School of Medicine, Maebashi, Gunma 371-8511, Japan; Department of Gastroenterology and Hepatology, Gunma University Graduate School of Medicine, Maebashi, Gunma 371-8511, Japan.
Yoichi Hayashi, Department of Computer Science, Meiji University, Tama-ku, Kawasaki, Kanagawa 214-8571, Japan.
Yosuke Seki, Weight Loss and Metabolic Surgery Center, Yotsuya Medical Cube, Tokyo 102-0084, Japan.
Satoru Kakizaki, Department of Gastroenterology and Hepatology, Gunma University Graduate School of Medicine, Maebashi, Gunma 371-8511, Japan. kakizaki@gunma-u.ac.jp.
Norio Horiguchi, Department of Gastroenterology and Hepatology, Gunma University Graduate School of Medicine, Maebashi, Gunma 371-8511, Japan.
Hiroki Tojima, Department of Gastroenterology and Hepatology, Gunma University Graduate School of Medicine, Maebashi, Gunma 371-8511, Japan.
Yuichi Yamazaki, Department of Gastroenterology and Hepatology, Gunma University Graduate School of Medicine, Maebashi, Gunma 371-8511, Japan.
Ken Sato, Department of Gastroenterology and Hepatology, Gunma University Graduate School of Medicine, Maebashi, Gunma 371-8511, Japan.
Kazuki Yasuda, Department of Metabolic Disorder, Diabetes Research Center, Research Institute, National Center for Global Health and Medicine, Tokyo 162-8655, Japan..
Masanobu Yamada, Department of Medicine and Molecular Science, Gunma University Graduate School of Medicine, Maebashi, Gunma 371-8511, Japan; Department of Internal Medicine, Division of Endocrinology and Metabolism, Gunma University Graduate School of Medicine, Maebashi, Gunma 371-8511, Japan.
Toshio Uraoka, Department of Gastroenterology and Hepatology, Gunma University Graduate School of Medicine, Maebashi, Gunma 371-8511, Japan.
Kazunori Kasama, Weight Loss and Metabolic Surgery Center, Yotsuya Medical Cube, Tokyo 102-0084, Japan.
References
- 1.Watanabe S, Hashimoto E, Ikejima K, Uto H, Ono M, Sumida Y, Seike M, Takei Y, Takehara T, Tokushige K, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. J Gastroenterol. 2015;50:364–377. doi: 10.1007/s00535-015-1050-7. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe S, Hashimoto E, Ikejima K, Uto H, Ono M, Sumida Y, Seike M, Takei Y, Takehara T, Tokushige K, et al. Evidence-based clinical practice guidelines for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. Hepatol Res. 2015;45:363–377. doi: 10.1111/hepr.12511. [DOI] [PubMed] [Google Scholar]
- 3.Eguchi Y, Hyogo H, Ono M, Mizuta T, Ono N, Fujimoto K, Chayama K, Saibara T; JSG-NAFLD. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 2012;47:586–595. doi: 10.1007/s00535-012-0533-z. [DOI] [PubMed] [Google Scholar]
- 4.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999-2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 5.Yoshiike N, Matsumura Y, Zaman MM, Yamaguchi M. Descriptive epidemiology of body mass index in Japanese adults in a representative sample from the National Nutrition Survey 1990-1994. Int J Obes Relat Metab Disord. 1998;22:684–687. doi: 10.1038/sj.ijo.0800651. [DOI] [PubMed] [Google Scholar]
- 6.Ohshiro Y, Ueda K, Nishi M, Ishigame M, Wakasaki H, Kawashima H, Furuta H, Sasaki H, Sanke T, Takasu N, et al. A polymorphic marker in the leptin gene associated with Japanese morbid obesity. J Mol Med (Berl) 2000;78:516–520. doi: 10.1007/s001090000143. [DOI] [PubMed] [Google Scholar]
- 7.Seki Y, Kakizaki S, Horiguchi N, Hashizume H, Tojima H, Yamazaki Y, Sato K, Kusano M, Yamada M, Kasama K. Prevalence of nonalcoholic steatohepatitis in Japanese patients with morbid obesity undergoing bariatric surgery. J Gastroenterol. 2016;51:281–289. doi: 10.1007/s00535-015-1114-8. [DOI] [PubMed] [Google Scholar]
- 8.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 9.Yoshioka K, Hashimoto S, Kawabe N. Measurement of liver stiffness as a non-invasive method for diagnosis of non-alcoholic fatty liver disease. Hepatol Res. 2015;45:142–151. doi: 10.1111/hepr.12388. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749–756. doi: 10.1148/radiol.11101942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, Eguchi Y, Suzuki Y, Imai S, Kanemasa K, Fujita K, Chayama K, Yasui K, Saibara T, Kawada N, Fujimoto K, Kohgo Y, Okanoue T; Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD) A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol. 2011;46:257–268. doi: 10.1007/s00535-010-0305-6. [DOI] [PubMed] [Google Scholar]
- 12.de Andrade AR, Cotrim HP, Alves E, Soares D, Rocha R, Almeida A, Almeida CG, de Freitas LA. Nonalcoholic fatty liver disease in severely obese individuals: the influence of bariatric surgery. Ann Hepatol. 2008;7:364–368. [PubMed] [Google Scholar]
- 13.Arora A, Sharma P. Non-invasive Diagnosis of Fibrosis in Non-alcoholic Fatty Liver Disease. J Clin Exp Hepatol. 2012;2:145–155. doi: 10.1016/S0973-6883(12)60103-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ; Nash Clinical Research Network. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5:211–218. doi: 10.1136/flgastro-2013-100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 17.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 18.Lee WJ, Wang W. Bariatric surgery: Asia-Pacific perspective. Obes Surg. 2005;15:751–757. doi: 10.1381/0960892054222614. [DOI] [PubMed] [Google Scholar]
- 19.Liang RJ, Wang HH, Lee WJ, Liew PL, Lin JT, Wu MS. Diagnostic value of ultrasonographic examination for nonalcoholic steatohepatitis in morbidly obese patients undergoing laparoscopic bariatric surgery. Obes Surg. 2007;17:45–56. doi: 10.1007/s11695-007-9005-6. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi Y, Fukunaga K. Accuracy of Rule Extraction Using a Recursive-Rule Extraction Algorithm with Continuous Attributes Combined with a Sampling Selection Technique for the Diagnosis of Liver Disease. Inf Med Unlocked. 2016;5:26–38. [Google Scholar]
- 21.Hayashi Y, Yukita S. Rule extraction using Recursive-Rule extraction algorithm with J48graft combined with sampling selection techniques for the diagnosis of type 2 diabetes mellitus in the Pima Indian dataset. Inf Med Unlocked. 2016;2:92–104. [Google Scholar]
- 22.Hayashi Y. Synergy effects between the grafting and the subdivision in the Re-RX with J48graft for the diagnosis of thyroid disease. Knowl-Based Syst. 2017;131:170–182. [Google Scholar]
- 23.Setiono R, Baesens B, Mues C. Recursive neural network rule extraction for data with mixed attributes. IEEE Trans Neural Netw. 2008;19:299–307. doi: 10.1109/TNN.2007.908641. [DOI] [PubMed] [Google Scholar]
- 24.Lee RG. Nonalcoholic steatohepatitis: tightening the morphological screws on a hepatic rambler. Hepatology. 1995;21:1742–1743. doi: 10.1002/hep.1840210636. [DOI] [PubMed] [Google Scholar]
- 25.The Japan Diabetes Society. 2013. Evidence-based Practice Guideline for the Treatment for Diabetes in Japan 2013. Nankodo, Tokyo, Japan. Available from: http://www.jds.or.jp/modules/en/index.php?content_id=33. [Google Scholar]
- 26.Hayashi T, Nakano S. Use of a Recursive-Rule eXtraction algorithm with J48graft to achieve highly accurate and concise rule extraction a large breast cancer dataset. Inf Med Unlocked. 2015;1:9–16. [Google Scholar]
- 27.Hayashi Y, Oishi T. High accuracy-priority rule extraction for reconciling accuracy and interpretability in credit scoring. New Gener Comput. 2018 [Google Scholar]
- 28.Quinlan JR. C4.5: Programs for Machine Learning. San Francisco: Morgan Kaufmann Pub; 1993. [Google Scholar]
- 29.Hayashi Y, Nakano S, Fujiwara S. Use of the recursive-rule extraction algorithm with Continuous attributes to improve diagnostic accuracy in thyroid disease. Inf Med Unlocked. 2015;1:1–8. [Google Scholar]
- 30.Salzberg SL. On comparing classifiers: Pitfalls to avoid and a recommended approach. Data Min Knowl Discov. 1997;1:317–328. [Google Scholar]
- 31.Ministry of economy, trade and industry/Japan agency for medical research and development. 2015. RD guideline for QA/QC of computer-aided detection and diagnosis software development; p. [in Japanese]. [Google Scholar]
- 32.Anty R, Bekri S, Luciani N, Saint-Paul MC, Dahman M, Iannelli A, Amor IB, Staccini-Myx A, Huet PM, Gugenheim J, et al. The inflammatory C-reactive protein is increased in both liver and adipose tissue in severely obese patients independently from metabolic syndrome, Type 2 diabetes, and NASH. Am J Gastroenterol. 2006;101:1824–1833. doi: 10.1111/j.1572-0241.2006.00724.x. [DOI] [PubMed] [Google Scholar]
- 33.Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y, et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol. 2007;42:573–582. doi: 10.1007/s00535-007-2060-x. [DOI] [PubMed] [Google Scholar]