Abstract
Metabolism drives function, on both an organismal and a cellular level. In T cell biology, metabolic remodeling is intrinsically linked to cellular development, activation, function, differentiation, and survival. After naive T cells are activated, increased demands for metabolic currency in the form of ATP, as well as biomass for cell growth, proliferation, and the production of effector molecules, are met by rewiring cellular metabolism. Consequently, pharmacological strategies are being developed to perturb or enhance selective metabolic processes that are skewed in immune-related pathologies. Here we review the most recent advances describing the metabolic changes that occur during the T cell lifecycle. We discuss how T cell metabolism can have profound effects on health and disease and where it might be a promising target to treat a variety of pathologies.
Keywords: immunometabolism, T cell function, plasticity, immunotherapy
INTRODUCTION
There is a growing appreciation that aberrant immune cell function underlies numerous pathologies that, even 10 years ago, were not linked to an immune component, as exemplified by Alzheimer disease (1, 2) and obesity (3, 4). Throughout the life of an immune cell, energy and substrate requirements change dramatically. Metabolic pathways must be engaged, or suppressed, to facilitate development and activation. As we uncover the links between metabolism and function, and the extent to which aberrant metabolic programming underlies dysfunction, there is hope that understanding immune cell metabolic engagement will yield targets for new therapies that will allow the augmentation or abrogation of immune responses in many disease settings.
Glycolysis and oxidative phosphorylation (OXPHOS) are the two main cooperative processes that supply ATP within cells. Glycolysis, the pathway in which glucose is broken down into pyruvate, is regulated by transcriptional, posttranslational, and metabolic cues (Figure 1). The production of lactate from pyruvate in the presence of oxygen by cells with sufficient mitochondrial capacity to utilize OXPHOS is known as the Warburg effect, or aerobic glycolysis, and is characteristic of many rapidly dividing cell types (5, 6). Glycolysis generates ATP but also provides metabolic intermediates that enter many other metabolic pathways (6).
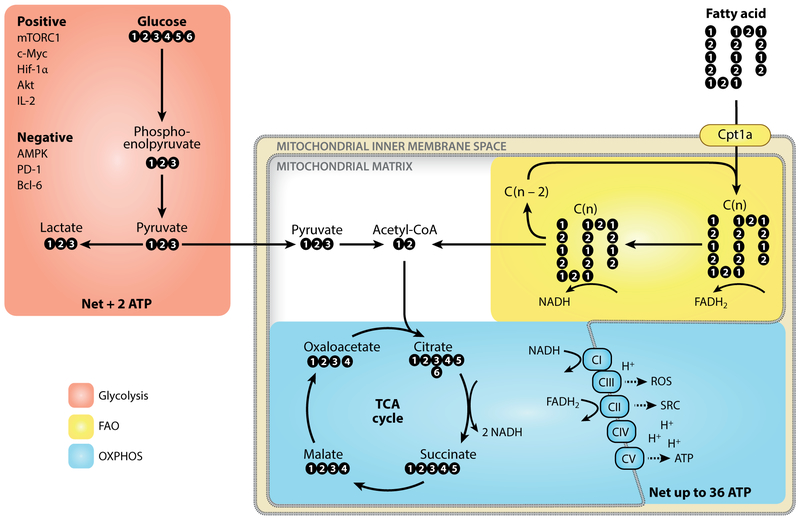
Figure 1.
Core metabolic pathways for T cell survival, activation, differentiation, and function. Glucose is metabolized to pyruvate through the glycolysis pathway, a process controlled by many positive and negative factors. Pyruvate is converted to lactate and exported from the cell or transported into the mitochondria to feed into the TCA cycle. The TCA cycle reduces NAD+ to NADH, which is in turn oxidized by complexes of the electron transport chain. The transferred electrons are used to generate a proton gradient across the mitochondrial inner membrane. Complex V uses the proton-motive force of this gradient to generate ATP in a process called OXPHOS. Glycolysis nets 2 ATP, whereas OXPHOS can yield up to 36 ATP per molecule of glucose. Other substrates, such as fatty acids, can also feed into the TCA cycle. Cpt1a regulates the entrance of long-chain fatty acids into the mitochondria, where they are cleaved to generate acetyl-CoA. Abbreviations: AMPK, adenosine monophosphate–activated protein kinase; Bcl-6, B cell CLL/lymphoma 6; Hif-1α, hypoxia-inducible factor 1 alpha; mTORC1, mechanistic target of rapamycin complex 1; PD-1, programmed cell death protein 1; ROS, reactive oxygen species; TCA, tricarboxylic acid; OXPHOS, oxidative phosphorylation; SRC, spare respiratory capacity.
In mitochondria, the tricarboxylic acid (TCA) cycle incorporates substrates from several cytosolic and mitochondrial metabolic pathways to generate reducing equivalents, which donate electrons to the electron transport chain (ETC) and lead to ATP generation through OXPHOS (reviewed in 7) (Figure 1). Substrates that are oxidized in the TCA cycle include pyruvate from glycolysis and metabolites derived from the oxidation of amino and fatty acids. Fatty acid oxidation (FAO) involves the translocation of free fatty acids into the mitochondrial matrix, where they are cleaved, producing acetyl-CoA that enters the TCA cycle (reviewed in 8) (Figure 1). FAO also generates NADH that can be reoxidized in the mitochondria by complex I of the ETC, which together with oxidation of FADH2 leads to the generation of a proton gradient across the inner mitochondrial membrane that is essential for OXPHOS. During this process, reactive oxygen species (ROS) are produced as a result of inefficient electron transfer in the ETC. ROS can have various effects within the cell, as described throughout this review (also reviewed in 9) (Figure 1). A specific metabolic pathway may be engaged for reasons other than ATP production, including biosynthesis, ROS titration, redox balance, generation of signaling molecules, and regulation of gene expression. The complexities of this system and how it relates to T cell metabolism and function are discussed in this review.
NAIVE T CELLS
Peripheral naive T (Tn) cells circulate throughout the body, surveying for antigens. These cells import small amounts of glucose to fuel the TCA cycle and OXPHOS to generate ATP (10). In addition to signals from cytokines such as IL-7, survival and homeostatic proliferation are maintained by weak T cell receptor (TCR) interactions with self-peptides presented on MHC molecules (11) (Figure 2). Sensitivity to these tonic signals is regulated by several mechanisms to ensure that the Tn cell metabolic state is maintained until a sufficiently strong TCR signal fully activates the cell.
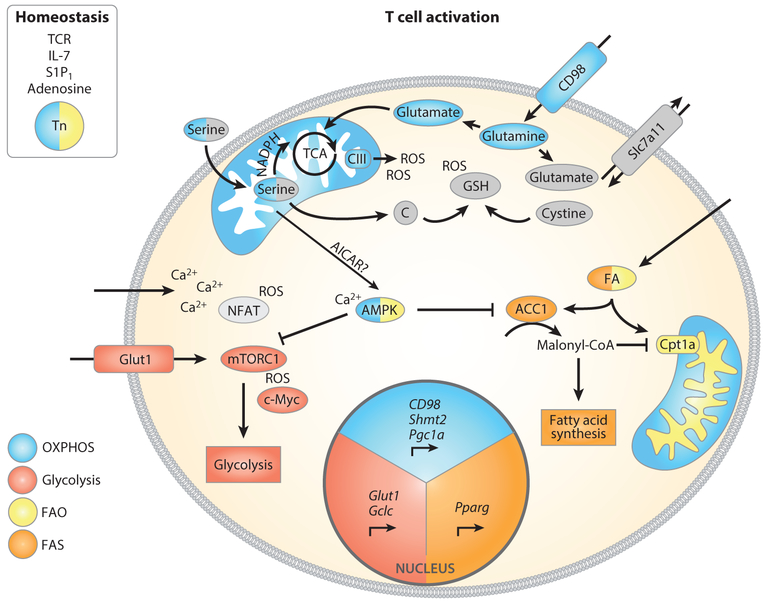
Figure 2.
A variety of factors control Tn cell survival and metabolic pathway engagement after T cell activation. After TCR stimulation, mTORC1 and c-Myc promote glucose and amino acid uptake by enhancing expression of Glut1 and CD98. Shortly after T cell activation, mitochondrial biogenesis is engaged by Pgc1α. Cells also augment acquisition of serine, which is metabolized by mitochondrial Shmt2, and upregulated Gclc expression and cystine uptake support glutathione synthesis. Glutathione limits the accumulation of ROS from complex III, thereby facilitating T cell activation. Calcium import leads to activation of NFAT and AMPK, the latter of which might limit mTOR activation to preserve Tm cell differentiation potential. During the first division, asymmetric inheritance of the glycolytic modulators mTOR and c-Myc leads to differential T cell fate for the two daughter cells. Activated AMPK limits ACC1 activity, and increased expression of Pparg leads to upregulated fatty acid import, thereby modulating fatty acid metabolism after activation. Abbreviations: ACC1, acetyl-CoA carboxylase 1; AICAR, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranosyl 5’-monophosphate; AMPK, adenosine monophosphate–activated protein kinase; FA, fatty acid; FAO, fatty acid oxidation; FAS, fatty acid synthesis; Gclc, glutamate-cysteine ligase catalytic subunit; mTORC1, mechanistic target of rapamycin complex 1; NFAT, nuclear factor of activated T cells; OXPHOS, oxidative phosphorylation; Pgc1a, peroxisome proliferator–activated receptor gamma coactivator 1 alpha; Pparg, peroxisome proliferator–activated receptor gamma; ROS, reactive oxygen species; S1P1, sphingosine 1-phosphate; Shmt2, serine hypoxymethyltransferase 2; TCA, tricarboxylic acid (cycle); TCRT cell receptor; Tm, memory T cell; Tn, naive T cell.
TCR sensitivity is partially mediated through control of the mechanistic target of rapamycin (mTOR), which exists in two distinct multiple-protein complexes [mechanistic target of rapamycin complex 1 (mTORC1) and mTORC2] that are key regulators of cellular metabolism (reviewed in 12). Loss of the tumor suppressor tuberous sclerosis complex 1 (TSC1) leads to activation of mTORC1, which enhances glycolytic metabolism, cell growth, and cell cycle entry, ultimately disrupting Tn cell homeostasis (13). This loss of quiescence, underscored by incorrect metabolic engagement, predisposes T cells lacking TSC1 to apoptosis and prevents the generation of an effective immune response. Intriguingly, another signaling axis controlled by the metabolite adenosine plays a role in maintaining the Tn cell pool. Extracellular adenosine via the adenosine 2A receptor (A2AR) decreases TCR sensitivity to tonic signals, and by doing so it sustains IL-7R expression and Tn cell survival (14) (Figure 2). Although adenosine provides an environmental cue to maintain immune homeostasis, the mechanisms regulating its production in this context remain unclear.
In the weeks immediately following their migration from the thymus, new Tn cells are less metabolically responsive when compared to more mature circulating Tn cells, as shown by less-marked increases in glycolysis after activation (15). This metabolic phenotype results from lower mTORC1 signaling and expression of the transcription factor c-Myc; these are two main positive regulators of glycolytic metabolism in T cells (16, 17) (Figure 2). This decreased mTOR and c-Myc activity can be alleviated by exposure to IL-2 from activated CD4+ effector T (Te) cells (18). This dependence on IL-2-producing CD4+ Te cells for optimal activation of recent thymic emigrants demonstrates how cytokines direct metabolism that regulates function. In addition to cytokines, signaling via the sphingosine 1-phosphate 1 receptor (S1P1R) also promotes Tn cell survival (Figure 2). S1P1 released by lymphatic endothelial cells provides a gradient that T cells require for lymph node egress. Perturbations of S1P1R signaling lead to decreased mitochondrial mass, metabolic dysfunction, and increased Tn cell apoptosis (19). Therefore, while metabolic processes supply circulating Tn cells with sufficient energy to move through tissues and prevent cell death, the active restraint of metabolic remodeling also maintains T cells in quiescence.
METABOLIC CHANGES DURING EARLY T CELL ACTIVATION
When Tn cells encounter their cognate antigen on an antigen-presenting cell (APC), the affinity of the TCR to the presented antigen causes TCR clustering and the formation of an immune synapse (20). In a proinflammatory environment, TCR signals along with costimulation lead to a growth phase in which T cells augment global metabolism (21, 22), a process dependent on phosphoinositide-3 kinase (PI3K)/AKT/mTOR signals (10, 23). In addition, TCR signals induce c-Myc, which enacts transcription of metabolic genes critical for T cell activation (16). Thus, TCR-mediated signals induce both glycolytic and mitochondrial metabolism, the latter of which leads to increased calcium-dependent ROS that are essential for T cell activation (24).
ROS and Glutathione
ROS generated in the mitochondria, or by NADPH oxidase expressed by CD4+ T cells, is postulated as signal 3 during T cell activation (25, 26). Mitochondrial ROS is critical for activation of nuclear factor of activated T cells (NFAT) and subsequent IL-2 production (24). A recent study highlighted the importance of ROS titration by the antioxidant glutathione, as failure to buffer accumulating ROS led to a block in T cell activation, in part by decreasing mTOR and c-Myc activity (27) (Figure 2). Glutathione production is in part mediated by glutamate-cysteine ligase catalytic subunit (Gclc), the rate-limiting enzyme for glutathione synthesis (27), and by the cystine/glutamate antiporter xCT (Slc7a11), which exports glutamate and leads to uptake of cystine (Figure 2), the oxidized dimer of cysteine that is used in glutathione synthesis (28). By using exogenous ROS scavengers, it was shown that ablation of cellular ROS leads to sustained adenosine monophosphate–activated protein kinase (AMPK) signals, thereby dampening mTOR activation (28) (Figure 2). Previous studies found that treating mice with antioxidants blocked T cell proliferation upon infection, further supporting the need for ROS in T cell activation (29). These observations show that titrating ROS levels during T cell activation, a process influenced by glutathione, is key for proper mTOR- and c-Myc-driven metabolic programming (30). The integration of these transcriptional, translational, and metabolic programs leads to the cell growth phase before the first cell division, which is in part mediated by activation of mTOR (reviewed in 22, 31).
Asymmetric Cell Division
Asymmetric cell division after T cell activation controls downstream fate (32). Two recent papers illustrated that this asymmetry is associated with distribution of metabolic mediators in CD8+ T cells, such that one daughter cell receives more activated mTORC1 and ultimately becomes a terminally differentiated, highly glycolytic Te cell, while the other daughter cell is primed to become a long-lived memory T (Tm) cell (33, 34). Inheritance of high mTORC1 activity is associated with increased expression of the large neutral amino acid transporter CD98 (33, 34), expression of which is critical for clonal expansion and Te cell differentiation (35) (Figure 2). CD98 is a target of c-Myc (16), which is also asymmetrically inherited and found in increased amounts in T cells that become highly glycolytic (33). These results show how metabolism integrates with transcriptional and translational programs to maintain the differential cell fates controlled by asymmetric cell division.
Mitochondrial Metabolism
Mitochondrial ATP production, or at least ATP synthase activity, during the first 24–48 h after T cell activation is critical for full Te cell activation and proliferative capacity, although continued proliferation of fully differentiated Te cells no longer depends on this mitochondrial function in vitro (36). A recent study integrating the proteome and phosphoproteome during T cell activation(37) revealed that many mitochondrial metabolic processes were altered, indicating that engaging mitochondrial metabolism is important for exiting quiescence. This finding is supported by data showing that T cell–specific knockdown of the complex IV subunit COX10 limits T cell activation(38), while loss of the complex III subunit Rieske iron sulfur protein (RISP) prevents expansion of antigen-specific T cells in vivo (26). T cells deficient for RISP lack the mitochondrial ROS-dependent signaling needed for T cell activation. In contrast to the importance of functional ETC components, expression of the rate-limiting enzyme for glycolysis, hexokinase 2 (HK2), is dispensable for early TCR-mediated activation (37).
Interestingly, there is a poor correlation between RNA expression changes after T cell activation and the levels of encoded proteins, suggesting that regulation of much of the proteome in these cells occurs posttranscriptionally (37, 39). Translation, and in particular posttranslational modification, of metabolic regulators is faster than their regulation through de novo transcription, adding to the speed of metabolic rewiring after T cell activation. In support of this notion, mitochondrial metabolism is regulated by posttranslational mechanisms. OXPHOS is altered by the phosphorylation of the fusion and fission machinery (40) and the proteolytic cleavage of mediators of mitochondrial cristae structure (41). In addition, there are conserved RNA-enzyme-metabolite (REM) networks, in which metabolic enzymes bind mRNA and thereby regulate expression of proteins (42). The RNA-binding ability of an enzyme is influenced by many factors, including the availability of its metabolites and posttranslational modifications. REM networks are an additional way in which metabolism regulates cell function in T cells (36) and are currently being studied by several groups.
Metabolites as Signaling Intermediates
Enhanced mitochondrial metabolism increases TCA metabolites and ATP, which can be used for posttranslational modification of proteins. In addition to the well-established phosphorylation (ATP) and acetylation of proteins (acetate), other TCA intermediates such as malate and succinate can be used to modify proteins through malonylation (43) and succinylation (44), respectively. The NAD+-dependent lysine deacylase SIRT5, which removes both succinyl and malonyl groups, targets GAPDH and alters its enzymatic activity, directly tying metabolites generated in the TCA cycle to a key glycolytic mediator (45). Also, the glycolytic metabolite 1,3-bisphosphoglycerate (1,3-BPG) reacts with select lysine residues to form 3-phosphoglyceryl-lysine (pgK), affecting protein structure and function (46). pgK modifications inhibit glycolysis enzymes, and when cells are exposed to high glucose levels these modifications accumulate, redirecting glycolytic intermediates to other biosynthetic pathways (46). Similarly, modification of metabolite transporters and cytokine receptors by sugar moieties generated in central carbon metabolism (N-linked glycosylation or O-GlcNAcylation) can modify subcellular localization and activity, thereby affecting T cell function (47–51). Furthermore, amino acids such as hypusine and citrulline are generated through polyamine and arginine metabolism, respectively, and also contribute to posttranslational modifications of proteins that influence cell function (52, 53). How metabolites acting as signaling intermediates contribute to the indispensable role of mitochondrial metabolism during early T cell activation, and for the later engagement of glycolytic metabolism and effector functions, remains to be resolved.
Serine and One-Carbon Metabolism
A recent study in CD4+ T cells showed that TCR signaling leads to mitochondrial replication, as well as instant engagement of mitochondrial one-carbon metabolism associated with induction of the folate cycle (54). Mitochondrial biogenesis as early as 4 h after activation coincides with increased TCA cycle intermediates. These events precede cell division and the engagement of aerobic glycolysis, as increased lactate production is apparent only >24 h after TCR stimulation. One-carbon units derived from serine are metabolized in the mitochondria, suggesting that serine import is critical for T cell activation (Figure 2), even when the glycolytic intermediate 3-phosphoglycerate is available for de novo serine synthesis. Mitochondrial serine metabolism governs T cell survival after activation, as SHMT2 knockdown leads to accumulation of DNA damage (54) and reduced viability (55). Enhanced mitochondrial serine metabolism contributes to the generation of redox equivalents and to one-carbon metabolism to generate glutathione (54, 55). The later engagement of glycolysis implies that serine import (55) allows the rapid mitochondrial proliferation during this early phase of T cell activation (54), while producing sufficient redox equivalents and one-carbon units to synthesize glutathione, thereby preventing the accumulation of ROS (Figure 2).
AMPK
T cells increase de novo purine synthesis shortly after activation, a process that leads to an ~10-fold increase in the intermediary metabolite for nucleotide synthesis, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranosyl 5-monophosphate (AICAR) (54, 55) (Figure 2). Increased AICAR during activation might play a role in activating AMPK, another crucial sensor of the cellular metabolic state. Whether the transient increase in AICAR during T cell activation directly activates AMPK is unclear, but the importance of AMPK during T cell activation has been well documented.
AMPK is activated by TCR signaling via two distinct signaling pathways. The first is LKB1 dependent: AMPK senses the cellular AMP-to-ATP ratio and acts as a break on metabolism to ensure there is sufficient ATP to progress through full activation (56), or to cope with metabolic stress (57). A second signal that activates AMPK is calcium import–mediated activation of calcineurin, and the downstream activation of calcium/calmodulin-activated kinase kinase (CAMKK) (58). In early T cell activation, the increase in intracellular calcium can activate AMPK, limiting mTOR signaling and precluding the activation of downstream mediators and premature engagement of the glycolytic anabolic metabolism associated with the proliferative phase of clonal expansion (59). The dependency on glycolysis of T cells lacking AMPK, and a failure to reengage the mitochondria upon glucose depletion, supports this idea (60). AMPK activation facilitates mitophagy (61), which limits ROS and ensures mitochondrial health during catabolism (59), and sustains expression of peroxisome proliferator–activated receptor (PPAR) gamma coactivator 1 alpha (PGC1α), a transcription factor important for mitochondrial biogenesis and health in CD8+ T cells (62). In mouse embryonic fibroblasts it was recently shown that mitochondrial ROS can also activate AMPK in a noncanonical way, sustaining PGC1α and thereby preserving mitochondrial function(63). Therefore, ROS generated from complex III during T cell activation (24) could be a third signal by which AMPK might be activated downstream of TCR signaling.
AMPK also controls fatty acid metabolism as its activation leads to the phosphorylation-mediated inhibition of acetyl-CoA carboxylase (ACC), an enzyme that catalyzes the conversion of acetyl-CoA to malonyl-CoA, and inhibits FAO (8) (Figure 2). Two isoforms exist, and deletion of ACC2 has no effect on Tn cell homeostasis, while ACC1 deletion decreases survival of circulating T cells (64, 65). ACC1 controls the cytosolic production of malonyl-CoA, which is utilized in fatty acid synthesis (FAS) (66) (Figure 2). An increase in FAS is also evident early after T cell activation (54). Thus, during T cell activation many upstream regulators modulate AMPK activity, and signals that emanate from this kinase influence many downstream processes, placing AMPK centrally in metabolic remodeling of T cells.
EFFECTOR T CELLS
Specific examples of the latest findings describing metabolism important for CD8+ Te cells and general Te cell function will be reviewed, followed by a discussion on metabolism in CD4+ Te cell differentiation.
Activated Te cells use glycolysis for proliferation and to maintain effector functions (Figure 3a). A recent study found that 10% of the cellular carbon in activated Te cells is traced from glucose, with another 10% from glutamine (67). These findings show that Te cells cultured in vitro use aerobic glycolysis and glutaminolysis mainly for ATP generation and redox balance to support rapid proliferation, rather than biomass accumulation. This metabolite allocation allows lipids and other amino acids to be redirected to generate biomass, such as nucleotides for DNA and lipids for membranes, to support cell division and Te cell functions (68, 69). Since glucose, glutamine, and other amino acids are supplied in excess to in vitro cultured T cells, it will be important to determine whether in vivo T cells also use glucose and glutamine predominantly for processes beyond biosynthesis and similarly allocate other metabolites to the generation of biomass.
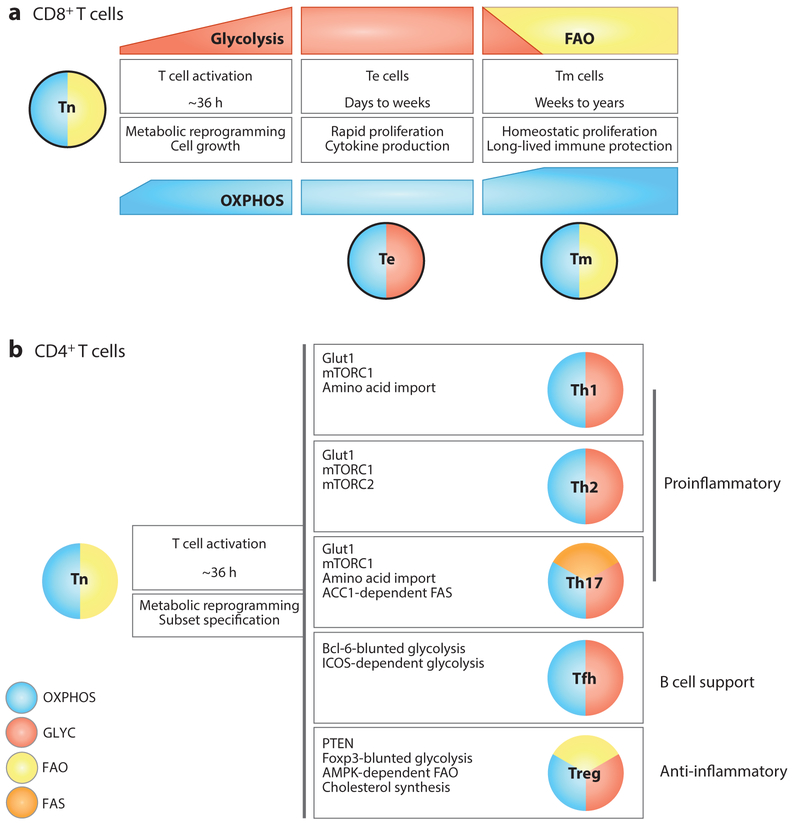
Figure 3.
Metabolic phenotypes of CD8+ and CD4+ T cell subsets. This schematic conveys the major pathways discussed in this review and is not exhaustive or exclusive of other pathways utilized by these T cell subtypes. (a) CD8+ Tn cells (outlined in black) rely on OXPHOS and use glucose to generate energy to maintain homeostatic proliferation and survival. Upon activation, CD8+ T cells increase OXPHOS and steadily increase their glycolytic rate, even before the first cell division. Fully differentiated CD8+ Te cells utilize glycolysis and OXPHOS. Tm cells decrease their glycolytic rate, coinciding with the engagement of FAO to fuel OXPHOS. Tm cells are endowed with more SRC than Tn cells, which underlies their rapid recall capacity upon challenge. (b) Like CD8+ Tn cells, CD4+ Tn cells (no outline) rely on OXPHOS and use glucose to generate energy to maintain homeostatic proliferation and survival. Upon activation, CD4+ T cells differentiate into diverse subsets with distinct roles and metabolic requirements. While all subsets engage glycolysis, Th2 and Th17 cells display high levels of glucose uptake. The master transcription factors of the Treg (Foxp3) and Tfh (Bcl-6) cell lineages repress glycolytic gene transcription, although depending on context Tregs also engage glycolysis. All subsets sustain OXPHOS, albeit at varying levels. Tregs engage FAO for suppressive function, whereas Th17 cells rely on de novo FAS. The balance of FAO and FAS contributes to the divergence between Th17 and Tregs. Abbreviations: ACC1, acetyl-CoA carboxylase 1; AMPK, adenosine monophosphate–activated protein kinase; Bcl-6, B-cell CLL/lymphoma 6; FAO, fatty acid oxidation; FAS, fatty acid synthesis; Foxp3, forkhead box P3; ICOS, inducible T cell costimulator; OXPHOS, oxidative phosphorylation; SRC, spare respiratory capacity; Te, effector T cell; Tfh, T follicular helper cell; Th, T helper cell; Tm, memory T cell; Tn, naive T cell; Treg, regulatory T cell.
In reductionist terms, all metabolism is regulated by available substrate; however, the complexity of the system means that actual substrate availability can be controlled at many levels. Substrate concentrations within distinct subcellular pools, metabolic enzyme expression, subcellular location of a given enzyme, enzyme activity, and nutrient transporter expression all contribute to how a cell utilizes substrate. For T cells, substrate utilization is influenced by growth factor cytokines, strength of TCR activation, inhibitory/costimulatory signals, as well as regulatory mechanisms imposed by other immune cells. In vitro, these various signals are easily controlled, as are nutrient and oxygen levels. However, what cells experience in culture is different from the in vivo niches where they reside. The reduced metabolic activity observed in T cells isolated ex vivo might reflect more realistic levels of metabolite usage in vivo (70–72). Recent studies have shown how a variety of signals can influence substrate utilization and Te cell functions (reviewed in 73).
Glucose and CD8+ Effector T Cell Function
A critical step in the activation and differentiation of T cells is the increase in the glucose transporter Glut1 expression and trafficking to the cell surface (74). Glut1 induction is required to mediate the sharp increase in glucose intake needed to facilitate cell growth, effector function, and proliferation of Te cells (75). The correlation between glycolytic metabolism and Te cell function was reinforced by a recent study showing that inhibition of mTORC1 activity blunts glycolysis and reduces effector functions, whereas deletion of mTORC2 enhances glycolysis and increases effector functions (17). Delving into the mechanism as to why Te cell function requires glycolysis, one study revealed that in the absence of glucose, GAPDH moonlights from its role as a glycolytic enzyme and instead binds to, and prevents the efficient translation of, IFN-γ mRNA (36). A more recent study focused on the role of glycolysis influencing epigenetics in Te cell function. Acetyl-CoA generated from pyruvate is oxidized in the mitochondria for energy but also contributes to a range of other cellular processes, including acetylation of proteins and histones (76). Functional lactate dehydrogenase (LDH) and aerobic glycolysis in Te cells is required for acetylation of the Ifng promoter and enhancer regions (77). Lack of LDH expression leads to increased OXPHOS and an ensuing decrease in the acetyl-CoA pool and IFN-γ expression.
This importance of glycolysis for optimal Te cell function was recently demonstrated in vivo using a sarcoma tumor model, where immune evasion of tumors directly correlates with their ability to compete for glucose with tumor-infiltrating lymphocytes (TILs) (78). Glucose competition in the tumor microenvironment (TME) leads to a reduction of the glycolytic metabolite phosphoenolpyruvate (PEP), which regulates the antitumor response by regulating intracellular calcium flux in TILs (79). High PEP levels in Te cells repress the sarco/endoplasmic reticulum calcium ATPase (SERCA), which allows cytosolic calcium-mediated sustained activation of NFAT (Figure 2). When extracellular glucose concentrations drop, so does PEP, leading to SERCA-mediated depletion of calcium and blunted NFAT-mediated effector functions. In the absence of glycolysis, the overexpression of the cytosolic pyruvate carboxykinase (PCK1) can rescue Te cell function by replenishing PEP levels from the TCA intermediate oxaloacetate, suggesting that increasing cytosolic PEP in cells used for adoptive transfer could be of therapeutic benefit.
Tumor-imposed glucose restriction on Te cells also affects cancer immunity by altering microRNA expression. When T cells are exposed to a low glucose environment, such as that encountered in ovarian tumors, microRNAs 101 and 26a are induced, which limit the expression of the methyltransferase EZH2 (80). EZH2 activates Notch by suppressing the expression of Notch repressors via histone methylation. Te cells require EZH2 expression for cytokine production and long-term survival of tumor-bearing mice and patients. In keeping with the idea that competing successfully for glucose is an important feature of protective T cells, it was shown that the inability of T cells to mediate clearance of B cell leukemia is due to chronic stimulation leading to Glut1 downregulation, thereby limiting the glycolytic rate. Indeed, enforced overexpression of Glut1 or increased Akt/mTORC1 signals rescued Te cell function in this model (81).
Persistent antigen exposure causes the functional impairment of Te cells, a phenotype associated with diminished glucose metabolism (82, 83). Illustrating how glycolysis is tightly linked to T cell function, mice with sustained activation of the glycolysis activator hypoxia-inducible factor 1 alpha (Hif-1α) in T cells exhibit better tumor control in a cancer model compared to control mice, but they also succumb to ongoing inflammation during chronic infection that ultimately leads to host death (84). Interestingly, T cells with heightened Hif-1α activation retain effector functions but do not terminally differentiate, as they persist and develop into long-term effector memory T (Tem) cells (85). Consistent with these findings, overexpression of Glut1 also generates a population of Tem-like cells, which exhibit increased cytokine production (74).
The importance of glycolysis induction in Te cell function is further supported by the requirement of this pathway for the rapid recall response of Tm cells as they differentiate into secondary Te cells. Reactivation of CD8+ Tm cells requires augmentation of glucose metabolism for their proliferation and ensuing Te cell response (86). An immediate-early glycolytic switch, marked by enhanced GAPDH activity at early time points, is required for the rapid production of IFN-γ by reactivated Tem cells, a process linked to mTOR signals and epigenetic modifications of the Ifng locus (87). Interestingly, during recall responses, Tm cells utilize extracellular-derived acetate to acetylate GAPDH, which leads to increased glycolytic flux and IFN-γ translation (88).
Costimulatory and inhibitory receptors expressed on T cells also influence T cell activation and metabolism. For example, CD28 costimulation during TCR stimulation enhances glycolytic metabolism and prevents anergy (89). Several recent studies have investigated how other accessory molecules regulate T cell metabolism. During T cell activation, ligation of the inhibitory receptor programmed cell death protein 1 (PD-1) sustains FAO and prevents glycolysis induction, decreasing Te cell function (82). However, when Tn cells are activated in the presence of ligand for 4–1BB, a costimulatory receptor, FAO and glycolytic metabolism increase, enhancing survival and proliferation of Te cells (90, 91). Currently there is considerable interest in resolving the impact of these accessory molecules on T cell metabolism given that activating or inhibiting the pathways they regulate with antibodies or ligands has proven efficacious in modulating immune responses in vivo.
Mitochondrial Changes in CD8+ Effector T Cells
Whereas glycolysis is important for full activation of Te cells, and lack of glycolytic flux can perturb function, mitochondrial morphology changes dynamically during the life of T cells, and these changes are associated with the engagement of distinct metabolic pathways. After activation, CD8+ Te cells fragment their mitochondria in a process known as fission (71). Mitochondrial fission is induced by activation of the GTPase dynamin-related protein 1 (Drp1), which recruits a complex of proteins that lead to fragmentation of the mitochondrial network (reviewed in 92). When mitochondrial fission is countered by pharmacological induction of mitochondrial fusion, Te cells reduce glycolysis and thereby activation, and adopt a Tm-like phenotype (71). These results indicate that blocking mitochondrial fission counters Te cell terminal differentiation, thereby abrogating inflammatory functions.
In addition to changes in mitochondrial dynamics, mitochondrial biogenesis is also induced after T cell activation and leads to increased mitochondrial mass, which is mediated by PGC1α. In activated CD8+ Te cells in the TME, chronic Akt–mediated FOXO inactivation decreases PGC1α expression, and this correlates with reduced Te cell responses (62). Overexpression of PGC1α enhances mitochondrial biogenesis and restores Te cell function, enhancing antitumor immunity (62). During chronic viral infection, PGC1α overexpression promotes viral clearance by overcoming PD-1-mediated suppression of mitochondrial metabolism, facilitating the glycolytic reengagement needed for Te cell function (93).
AMPK in the CD8+ Effector T Cell Response
AMPK has a central role in regulating T cell metabolism. In limiting glucose, AMPK signals drive expression of glutamate transporters and glutaminolysis genes to sustain Te cell metabolism (60). Mice with T cells deficient for AMPK exhibit decreased Te cell responses during primary infection and reduced inflammation during T cell–mediated colitis (60). A recent study uncovered a direct link between glucose restriction and AMPK activation. In the absence of the glucose-derived substrate fructose-1.6-bisphosphate, the glycolytic enzyme aldolase mediates the formation of a lysosomal complex that brings together LKB1 and AMPK and leads to AMPK activation (94). PI3K signals have also been shown to tether aldolase to the cytoskeleton and boost glycolytic efficiency, while the absence of PI3K signals leads to release of free aldolase into the cytoplasm (95). Whether aldolase acts as a PI3K-sensitive glycolytic sensor that is important for metabolic rewiring in glucose-deprived T cells has yet to be determined.
mTOR in CD4+ Effector T Cells
Engagement of distinct metabolic pathways is important for the differentiation of CD4+ Te cells into a spectrum of subsets that are marked by distinct functional outputs (Figure 3b). mTOR signaling, with its regulation of metabolic reprogramming, has a profound effect on CD4+ T cell fate. For example, mTOR signals promote Th1 and Th17 cell differentiation (96), while mTOR inhibition blocks effector-like T helper (Th) cells and skews differentiation toward forkhead box P3 (FoxP3)-expressing regulatory T cells (Tregs) (97–101). Th2 cells differentiate without Ras homolog enriched in brain (Rheb)-dependent activation of mTORC1, but they do require the mTORC1 subunit regulatory-associated protein of mTOR (RPTOR) and activation of the kinase serum/glucocorticoid regulated kinase 1 (SGK1) downstream of mTORC2 (96, 102, 103). In addition, Ras homolog gene family, member A (RhoA) signaling downstream of mTORC2 activation (104) is important for differentiation, but not maintenance, of Th2 cells (105). Linked with their reliance on mTOR signaling and glycolytic metabolism, the inflammatory CD4+ Te cell subsets require rapid expression of Glut1 (75) (Figure 2). In contrast, while also highly glycolytic, CD8+ Te cells have a reduced reliance on Glut1, perhaps due to the expression of other glucose transporters, such as Glut3 (39, 75, 106).
Recently it was shown that mTORC1 phosphorylates T box expressed in T cells (T-bet), the master transcription factor for Th1 cells, and inhibition of mTOR signaling reduces T-bet-dependent IFN-γ production (107). In a hypoxic environment, Hif-1α-deficient CD4+ Te cells produce more IFN-γ than wild-type Th1 cells (108), and Hif-1α stabilization in low oxygen inhibits Th1 function (109). These results indicate that while mTORC1 signaling is important for Th1 differentiation, the downstream target of mTOR, Hif-1α, may play a role in limiting effector function in these cells. Whether this limit of proinflammatory function in a hypoxic environment is related to the engagement of metabolic pathways that are not compatible with production of Th1 effector cytokine remains to be determined.
Glycolysis, Glutaminolysis, and the Hexosamine Pathway in CD4+ Effector T Cells
TCR signaling upregulates the import of both glucose and glutamine, which enforces Te cell differentiation and limits the induction of Tregs that have suppressive capabilities. In fact, glutamine depletion, or the inhibition of glutaminolysis, permits Treg differentiation, even in Th1-polarizing conditions (110), an effect rescued by addition of a cell permeable α-ketoglutarate analogue (dimethyl-2-oxoglutarate) (111). In addition, the differentiation of Th17 cells is associated with an increase in glutamine utilization through highly expressed glutamate oxaloacetate transaminase 1 (GOT1) and a profound accumulation of the metabolite 2-hydroxyglutarate (2-HG), a product of the error-prone dehydrogenation of α-ketoglutarate. 2-HG inhibits the α-ketoglutarate-dependent demethylation of the FoxP3 locus, thereby actively suppressing Treg development (112). In addition to contributing to ATP production, glutamine and glucose are required for O-GlcNAcylation, which is needed for T cell development and activation (47). Glycolysis and glutaminolysis modulate CD4+ T cell differentiation by limiting substrates (i.e., fructose-6-phosphate and glutamine, respectively) for the hexosamine pathway, which is required for protein glycosylation through the generation of UDP-N-acetylglucosamine (GlcNAc). Therefore, hexosamine biosynthesis is in substrate competition with glycolysis and glutaminolysis (113). Illustrating a role for N-glycosylation in modulating T cell differentiation, polarizing CD4+ Te cells in Th17 conditions decreases N-glycan branching, whereas enhancing this process, by over-expressing mannoside acetyl glucosaminyl transferase 5 (MGAT5), or adding GlcNAc, increases Treg development while inhibiting Th17-associated gene expression (48). N-glycan branching is needed for CD25 cell surface expression, and IL-2 signaling through this receptor is required for Treg development. In support of these findings, the amino-sugar glucosamine, which inhibits N-linked glycosylation of CD25, decreases Th1, Th2, and Treg, but not Th17, cell differentiation (49). In addition, the cell surface translocation of Glut1 and the glutamine transporters sodium-coupled neutral amino acid transporter (SNAT)1; SNAT2; and alanine-, serine-, and cysteine-preferring transporter 2 (ASCT2), which are expressed by activated Te cells, requires N-linked glycosylation, supporting the importance of N-linked glycosylation in modulating Th cell differentiation (50, 51, 114). A balance between glycolysis, glutaminolysis, and hexosamine metabolism is needed, whereby sufficient flux through the hexosamine pathway must be preserved to sustain receptor glycosylation to maintain substrate influx for all three metabolic systems. Perturbations of this balance can lead to skewed T cell differentiation and function.
Competition for metabolites like glucose in other settings can drive T cell differentiation. T follicular helper (Tfh) cells, unlike other CD4+ Te cell subsets, persist in the lymph node after activation, where they are essential for supporting B cell survival and antibody production (115). Due to their location within the follicle of the reactive lymph node, Tfh cells are likely to be exposed to metabolite competition with highly proliferative germinal center B cells. The master transcription factor for Tfh differentiation, B cell CLL/lymphoma 6 (Bcl-6), works in competition with IL-2 signaling to directly repress gene expression of many components of the glycolytic pathway (116, 117), perhaps limiting sensitivity to glucose competition. However, signaling through inducible T cell costimulator (ICOS), a receptor highly expressed by Tfh cells, promotes glycolysis in an mTOR-dependent fashion (118), suggesting that Tfh cells will engage glycolysis when directed upon ICOS stimulation. Tfh cells could function with a reduced reliance on glycolysis by enacting mitochondrial metabolism. T cell–specific deletion of the mitochondrial ETC component cytochrome c oxidase inhibits the development of germinal centers, indicating a functional deficiency in the Tfh cell compartment (38). Development of new Tfh cell reporter and selective knockout mice has increased our ability to probe the metabolic requirements of these specialized cells. These tools will help elucidate what metabolic pathways are engaged to support Tfh cell function and survival in the unique environment of the lymph node.
Fatty Acid Metabolism in CD4+ Effector T Cells
T cells deficient in ACC1, which drives FAS, fail to differentiate into Th17 cells and default into the Treg lineage (119). Th17 cells are important in intestinal homeostasis and host immunity, but pathogenic Th17 cells, which make IFN-γ in addition to IL-17, are the cause of a number of inflammatory diseases (120). One recent study found that mice fed a high-fat diet increased expression of ACC1 in CD4+ T cells, and this ACC activity augmented promoter site binding of RAR-related orphan receptor gamma (RORγt), the master transcription factor of Th17 fate (121). Precisely how ACC-mediated FAS directs this binding is not clear, but these results highlight how this intracellular metabolic pathway influences the activation of a transcription factor that directs T cell fate. Interestingly, obese individuals also maintain increased expression of ACC in CD4+ T cells, perhaps contributing to Th17-related pathologies in these people (121). Consistent with the role of FAS in Th17 cells, T cell–specific deletion of ACC1 protects mice from EAE, coincidentally reducing the number of IFN-γ-producing Th17 cells and increasing the number of Foxp3+ Treg cells in the spinal cord (119). Furthermore, in an adoptive transfer model of graft-versus-host disease, proinflammatory cytokine production was diminished and Treg numbers were increased in mice that received ACC1−/− Te cells compared to those that were given wild-type Te cells (122).
Th17 cells rely on de novo FAS, rather than acquisition of extracellular fatty acids to meet lipid requirements (119). It is not clear why Th17 cells do not acquire free fatty acids and only rely on the ATP-costly process of de novo FAS, particularly with the substantial lipid requirements of proliferating cells. CD8+ Te cells also require de novo FAS for optimal expansion, but they use exogenous fatty acids for cell growth and accumulation as well (64). During T cell activation, mTORC1 signals lead to nuclear translocation of the sterol regulatory element–binding proteins (SREBPs) that promote FAS and induce PPARγ, which controls expression of genes that regulate fatty acid uptake (123, 124). Early in the proliferative program, fatty acid uptake steadily increases in activated CD4+ T and CD8+ T cells (125), but blocking FAS with 5-(tetradecyloxy)-2-furoic acid (TOFA) during this time blunts proliferation and reduces glucose metabolism (124). Thus, FAS and fatty acid uptake are critically important for rapid T cell proliferation that occurs early after activation. A recent study assessed substrate uptake in T cells isolated from the brains of mice with orthotopic glioma and found that they exhibited decreased glucose uptake but increased fatty acid uptake compared to T cells in the lymphoid tissue (125). Whether or not available extracellular fatty acids can compensate for de novo FAS, or vice versa, remains to be determined, but these context-dependent differences likely influence the balance between these pathways.
Another intracellular metabolic pathway that influences Th17 cell development is cholesterol synthesis, as intermediates of this pathway bind to RORγt and promote IL-17 expression and Th17 function (126, 127). Indeed, inhibitors of cholesterol biosynthesis block Th17 cell development. Further outlining the important function of lipids in this cell type, a recent study demonstrated that the balance of intracellular saturated and polyunsaturated fatty acids, controlled by CD5L expression, is key for Th17 function and determines whether these cells become pathogenic or nonpathogenic (128). The downregulation of CD5L by IL-23 signals promotes pathogenic Th17 cells by changing the fatty acid competition and increasing cholesterol pathway intermediates that are RORγt ligands (128).
Leptin Signaling in CD4+ Effector T Cells
The adipocyte-produced hormone leptin is systemically increased in obesity and correlates with the proinflammatory phenotype of this disease (129). Demonstrating the cross talk between whole-body metabolism and the immune system, leptin is needed for mature T cell activation by promoting mTOR and the engagement of glucose metabolism through induction of Glut1 (130). This adipokine signals through the IL-6-type cytokine family receptor Lepr, which is expressed at low levels on Tn cells and increases upon TCR stimulation (131). Th17 cells also require leptin, as deletion of Lepr on T cells diminishes STAT3 signaling and Th17 cell function (132). Supporting these data, treatment with leptin rescues reduced splenic Th17 cell numbers evident in fasting mice, and T cell–specific knockout of Lepr inhibits glycolysis and Hif-1α expression in Th17 cells (133). Again highlighting the balance between Tregs and Th17 cells, human CD4+ Te cells produce leptin that blocks Treg proliferation in response to TCR stimulation (134). Targeting leptin signaling may be a way in which Treg and Th17 cell responses could be altered therapeutically in obesity and other inflammatory conditions.
REGULATORY T CELLS
Unlike proinflammatory Th cell subsets, Tregs, which express the transcription factor Foxp3, suppress immune responses by competing for IL-2 via high CD25 expression and produce anti-inflammatory mediators such as IL-10 (135). These cells are important in the resolution of immune responses, and also for maintaining homeostasis in a number of organs, including adipose tissue. Just as their functions are unique compared to CD4+ Te cell subsets, Tregs also have distinct metabolic requirements (Figure 3b).
Transforming growth factor beta (TGF-β) signals drive Treg differentiation (136) and activate AMPK (137), which promotes FAO and skews inflammatory Th17 cells toward a Treg phenotype (98, 138). The complexity of this regulatory network was highlighted in a recent study where Treg-specific LKB1 deletion led to functional and survival defects in Tregs, independent of its downstream target AMPK (139). However, Treg differentiation is dampened by the FAO inhibitor etomoxir, indicating a dependence on this pathway (98), whereas pharmacological or genetic inhibition of FAS has no effect on Treg development (119). Although these results are clear, the overall picture of how metabolism links to function in Tregs remains a subject of ongoing investigation.
Suppression of PI3K activity after T cell stimulation by phosphatase and tensin homolog (PTEN) diminishes activation of phosphoinositol (3,4,5)-trisphosphate (PIP3)-dependent kinases, such as Akt, and drives Treg differentiation. Mice lacking PTEN in T cells develop an autoimmune lymphoproliferative disease marked by unrestrained Th1, Tfh, and B cell responses (140, 141) (Figure 4). PTEN-deficient Tregs display elevated glycolytic activity, which correlates with the loss of Foxp3 stability and functional capacity. Supporting the observations that metabolic programing influences Treg development is a study showing that blocking Akt activity, either through PD-1-mediated activation of PTEN (inhibiting AktT308 phosphorylation) or via dendritic cell–mediated tryptophan catabolism inhibiting mTORC2-dependent phosphorylation of AktS473, enhances Treg differentiation (142). Molecules like PTEN and Akt ensure proper metabolic reprogramming in T cells, and the appropriate balance of these signals promotes the stable development of functional Tregs.
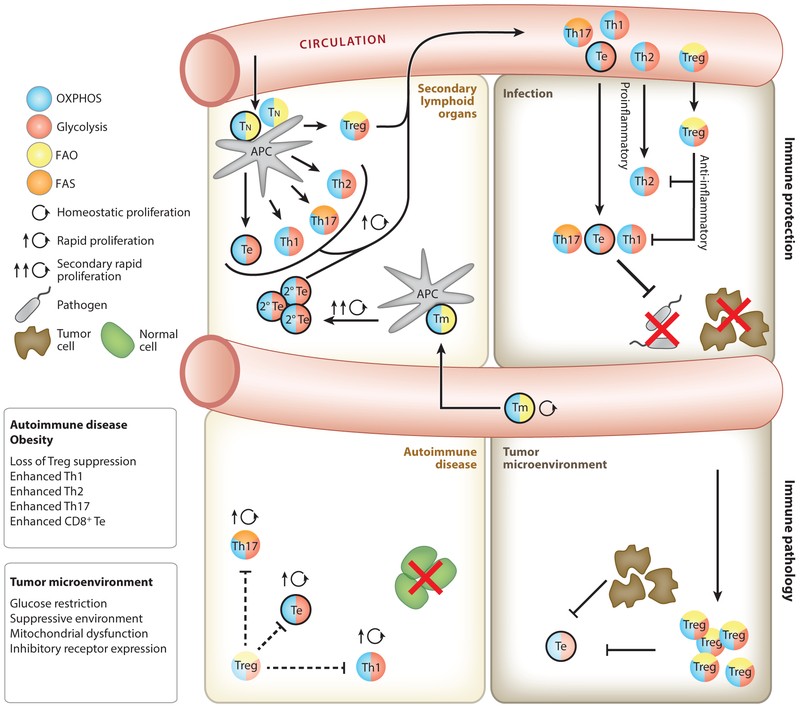
Figure 4.
T cell metabolism during an immune response. Tn cells are activated by mature APCs in secondary lymphoid organs (top left) and then proliferate and differentiate into Te cells that travel to peripheral tissues and mediate immune responses against pathogens or tumors. During an immune response Tregs regulate inflammation to limit tissue damage (top right). Upon resolution of an infection or tumor, long-lived CD8+ Tm cells (outlined in black) form and remain metabolically quiescent until they reencounter antigen and become reactivated, at which time they rapidly reengage glycolysis and promote OXPHOS (top left). Limited reliance on glycolysis allows Tregs to persist in the glucose-depleted tumor microenvironment, whereby they inhibit antitumor Te cell function. Te cell functions that require glycolytic engagement, such as the robust translation of IFN-γ, are also inhibited in the tumor microenvironment (bottom right). Cell-intrinsic disruptions to metabolic pathways in T cells, and systemic metabolic disease such as obesity, promote inappropriate T cell activation and differentiation (bottom left). Loss of suppressive function by Tregs leads to greater inflammatory function in CD4+ and CD8+ Te cells (outlined in black). In obesity, leptin signals during activation drive glycolysis and Th1/Th17 cells rather than Tregs. Abbreviations: APC, antigen-presenting cell; FAO, fatty acid oxidation; FAS, fatty acid synthesis; OXPHOS, oxidative phosphorylation; Te, effector T cell; Th, T helper cell; Tm, memory T cell; Tn, naive T cell; Treg, regulatory T cell.
While the absence of mTOR does not cause decreased Foxp3 expression, the loss of this kinase pathway does diminish cholesterol biosynthesis, which is required for suppressive Treg function in vivo (143). The T cell–specific deletion of ATP-binding cassette subfamily G member 1 (ABCG1), a transporter that mediates cholesterol efflux from cells, leads to increased Treg numbers and suppressive activity, which correlates with greater intracellular cholesterol (143). How these results marry with observations showing that statins, which block cholesterol synthesis, increase Tregs in vivo and in vitro is not yet clear (reviewed in 144).mTOR is also important for glycolytic metabolism in Tregs, and human-induced Tregs rely on glycolysis after weak TCR stimulation (145). However, the role of glycolysis in these cells is not straightforward, as deletion of the autophagy mediator autophagy related 7 (Atg7) enhances mTORC1-mediated glycolysis and instigates the loss of Foxp3 expression (146). In this study, mTORC1 inhibition by rapamycin reduced c-Myc expression, dampening glycolysis and promoting Foxp3 expression and associated suppressive function. Foxp3 directly interferes with c-Myc expression and thereby actively inhibits glycolytic engagement (147). However, in human Tregs, glycolysis is tightly linked to suppressive activity, whereby utilization of this pathway ensures the maintenance of the enzyme enolase-1, which mediates expression of a human-specific Foxp3 variant that is indispensable for their suppressive function (145). In addition to glycolysis, human Tregs also require FAO for proliferation and suppressive ability, in contrast to Te cells, in which FAO is dispensable for proliferation and effector function (148).
The differing evidence describing the reliance of Tregs on either glycolysis or FAO suggests a complex relationship between metabolism and function in these cells. One study suggests that glycolysis induces Treg proliferation, but this is inversely proportional to its suppressive capacity. Forced expression of Foxp3 suppresses glycolysis-related genes while inducing expression of genes associated with lipid and oxidative metabolism, the latter of which is required for their maximal suppressive ability (149). The utilization of OXPHOS is presumed to be dependent on FAO in these cells, although the FAO inhibitor etomoxir only partially blunts suppressive capacity. It remains to be determined whether proliferation and suppression in Tregs are invariably mutually exclusive or exceptions to this dichotomy exist.
The fact that Tregs populate a variety of peripheral tissues may suggest they need a certain degree of metabolic flexibility, depending on their environment. In support of this notion, a recent study showed that glycogen remobilization in a glucokinase-dependent manner fuels glycolysis to support Treg migration to inflammatory sites (150). Some studies have indicated that Tregs adopt phenotypes that reflect a relationship between their environment and metabolic pathway engagement. For example, Tregs that reside in visceral adipose tissue (VAT) acquire a unique VAT Treg phenotype characterized by PPARγ expression (151, 152). In lean individuals, these VAT Tregs maintain suppressive activity; however, in obesity, suppressive VAT Tregs are lost, which leads to the infiltration of inflammatory Te cells in the VAT. It is thought that the close contact between adipocytes and immune cells during dysregulated systemic metabolism alters immune function (4).
Tregs also inhabit tumors, and the accumulation of suppressive Tregs in the TME is associated with poor prognosis, as Tregs blunt Te cell responses (153) (Figure 4). In addition, the TME is often substrate limiting, further leading to Te cell dysfunction (reviewed in 73). However, tumor-associated Tregs use a unique mechanism to cope with the high-lactate, low-oxygen TME. A recent report demonstrated that in low-glucose/high-lactate conditions, Tregs import extracellular lactate, which is converted to pyruvate via LDH to fuel the TCA cycle (147). This enzymatic reaction requires NAD+, which is generated during OXPHOS, a metabolism driven by the action of Foxp3 in Tregs. High ROS in the TME can lead to apoptosis of Tregs, accompanied by release of ATP from dying cells. High expression of cell surface ectonucleotidases in Tregs leads to conversion of ATP to adenosine, causing immunosuppression and tumor progression (154). In naive T cells adenosine was shown to dampen TCR signals (14), which in this context could inhibit TCR-mediated tumor responses in situ (154). Due to their roles in cancer, infection, and tissue homeostasis, identifying how to modulate Treg numbers and function is a critical research goal. Teasing apart the distinct metabolic pathways that contribute to their generation and suppressive function will hopefully elucidate new targets.
MEMORY T CELLS
In contrast to CD8+ Te cells, CD8+ Tm cells reduce mTOR activity and aerobic glycolysis and instead rely on FAO and mitochondrial metabolism to support their persistence and function (155, 156) (Figure 3a). Restraining glycolysis after activation generates Tm cells with a more quiescent metabolic phenotype (157), which along with lower mitochondrial membrane potential and greater mitochondrial fitness are desirable metabolic features for long-lived Tm cells with protective capabilities (158, 159). One study showed how this effect could be achieved by treating T cells with an Akt inhibitor. Adoptive transfer of these T cells in a cancer model promoted TILs with a Tm-like phenotype, including increased mitochondrial function and antitumor protection (160). Interference of kinase activities, including Akt activity, by ablating the upstream kinase mTORC2 leads to greater Tm cell fitness and function that correlate with elevated mitochondrial metabolic activity (17). Modulating mitochondrial function by dampening pathways such as those driven by Akt may increase the efficacy of therapies based upon adoptive transfer of tumoricidal T cells.
Tm cells promote mitochondrial biogenesis and Cpt1a-dependent FAO, parameters associated with spare respiratory capacity (SRC) (161), which is the reserve energy–generating capacity available in the mitochondria beyond the basal state. In myocytes, complex II of the ETC mediates SRC, a process dependent on both glucose and FAO (162). Consistent with a link between FAO and SRC, promoting FAO in T cells enhances Tm cell generation after infection (155, 161). In concert with the maintenance of significant SRC and increased mitochondrial mass, Tm cells engage OXPHOS to generate ATP for their differentiation and survival. AMPK signals augment OXPHOS during Tm differentiation (60, 155, 163), perhaps by engaging FAO, and also promote mitochondrial clearance important for maintaining mitochondrial health in these cells (59). The significant SRC in CD8+ Tm cells is important not only for their development and persistence but also for their rapid recall ability. Upon restimulation, Tm cells rely on their mitochondria to provide the ATP needed for the rapid induction of glycolysis as they differentiate into secondary Te cells (86). These results show that mitochondrial capacity is directly linked to the glycolytic function in these cells. It was recently shown that CD4+ Tm cells have increased glycolytic reserve evident in response to hypoxia as compared with Tn cells (164). It is possible that greater mitochondrial metabolism in CD4+ Tm cells is also linked to their larger glycolytic capacity, as is the case for CD8+ Tm cells.
Mitochondrial Dynamics in Memory T Cells
Optic atrophy 1 (Opa1) mediates fusion of the inner mitochondrial membrane and is indispensable for the proper maintenance of mitochondrial cristae and the assembly and stability of the ETC complexes that increase respiratory efficiency (165). Upon transition to the Tm cell phenotype, CD8+ T cells require the expression of Opa1 for fusion of their mitochondria into networks in which cristae are tightly associated, thereby promoting efficient ETC activity (71). Loss of Opa1 leads to loose cristae and defective CD8+ Tm cell generation, whereas CD8+ Te cells treated with drugs that promote fusion adopt a Tm-like phenotype. In the absence of Opa1, Te cells remain able to engage FAO in vitro, suggesting that although both FAO and mitochondrial fusion regulate Tm cell functionality, they may not necessarily be directly connected and instead reflect two independent pathways that are important for Tm cell recall (71, 161). Morphological changes in mitochondrial cristae are mediated very early during T cell activation (166), and metabolic rewiring occurs before the first cell division; however, more research is needed to determine how these early events potentially influence later functional output of Te and Tm cells.
Colocalization of the ETC complexes augments OXPHOS by forming supercomplexes (165). Shedding further light on how this might affect T cell responses is a recent study showing that deletion of MCJ, a protein that interferes with ETC complex organization, increases basal mitochondrial respiration in Te cells and promotes cell survival during the contraction phase after infection (167). While MCJ deficiency has no effect on the differentiation of Tm cells, it does increase cytokine production and protective responses upon challenge infection. MCJ-deficient CD8+ T cells have decreased complex II activity, which leads to a buildup of succinate. The cause of the succinate accumulation is proposed to be the exclusion of complex II from the supercomplex, uncoupling complex II from the ETC and allowing direct flow of electrons from complex I to complex III (167). Regardless of the precise mechanisms, overall these data demonstrate the importance of efficient electron transfer between mitochondrial complexes in Tm cells.
Fatty Acid Metabolism in Memory T Cells
As mentioned, ACC1-mediated generation of malonyl-CoA is the first step in the generation of de novo fatty acids. FAS is required for Te cell activation, as it supplies intermediates for membrane synthesis as cell growth pathways are engaged (168). CD8+ Te cells also import fatty acids for biosynthesis during rapid replication, but CD8+ Tm cells rely on glucose to synthesize their fatty acids, which are subsequently broken down by cell-intrinsic lipolysis to provide substrates for FAO (72). Why Tm cells engage this futile cycle of building and then burning fatty acids is unclear; however, it may be engaged to continuously move substrates through these pathways, in order to maintain enzyme expression. If this were the case, it would keep the cells primed and ready for rapid recall in the event of pathogen reencounter, the hallmark of functional Tm cells. Supporting the importance of FAS and FAO in these cells, Tm cells upregulate expression of aquaporin 9 (AQP9) to enhance glycerol import, which is indispensable for triglyceride (TAG) synthesis and storage (169). The storage of TAG endows long-lived Tm cells with the capacity to deal with metabolic stress, as they could break down TAG, presumably through lipolysis, and use the liberated fatty acids to generate ATP via FAO (161, 169). It was recently shown that tissue-resident Tm cells in the skin and lung are also FAO dependent, but in contrast to what might be the case for central Tm cells in the lymph nodes, skin-resident Tm cells rely on the import of extracellular fatty acids for this process (170). Why different populations of Tm cells use different pathways to fuel FAO is not yet clear; however, it is possible that substrates available in differing tissue environments affect metabolic pathway engagement.
POTENTIAL FOR CLINICAL APPLICATIONS
In the last decade effort has gone into developing clinical therapies based on the ability of tumor antigen–specific CD8+ Te cells to kill tumors. Given that TILs can be rendered functionally insufficient in the TME by multiple factors, understanding the mechanistic basis of this hyporesponsiveness is critical for developing effective approaches (reviewed in 73, 171, 172) (Figure 4). T cell hyporesponsiveness can be overcome by checkpoint blockade antibodies, such as anti-PD-1 and anti-CTLA-4, which have been applied with varying success in certain malignancies (83). Checkpoint blockade therapy can reinvigorate Te cells present in the TME and is used in combination with many other treatment modalities, such as chemotherapy, radiotherapy, and targeted cancer therapies (173). While positive advances have been made, there have been reports of adverse effects leading to autoimmune pathologies (174).
In a different strategy, patient-derived T cells that express chimeric antigen receptors (CARs) engineered to target tumor antigens have proven useful in the treatment of B cell lineage leukemia (175). In addition, many phase 1 clinical trials are testing the efficacy of CAR therapy in solid malignancies by targeting key oncogenic drivers, such as human epidermal growth factor receptor 2 (HER2) and epithelial growth factor receptor (EGFR), which are amplified in many cancers (176). Recent studies have shown that the costimulatory domains in second- and third-generation CAR alter the metabolic features of the T cells that express them, and this can have beneficial long-term consequences for cell persistence and functional capacity in vivo (177). A better understanding of metabolic requirements of T cells, and how the multitude of costimulatory molecules and surface receptors expressed by these cells modulate their metabolism, may lead to new powerful approaches to prevent exhaustion and ensure maximal activation in vivo. For example, the costimulatory 4–1BB domain included in third-generation CAR constructs leads to reduced PD-1 expression when compared to second-generation CAR T cells (178), suggesting that 4–1BB signals enhance T cell function through dampened expression of receptors associated with T cell exhaustion.
Studies have hinted that T cell persistence and Tm cell development in vivo are linked to restrained activation, characterized by a lower glycolytic phenotype (157). Retraining activation is achieved by blocking AKT (179), suggesting that blunting signals that enhance glucose uptake or glycolytic flux promotes T cell persistence after adoptive cell transfer (157). Also, cells with decreased oxidative stress after activation mark Tm precursors, and these cells display superior antitumor activity (159). The treatment of T cells with mitochondrial fusion–promoting drugs also heightened antitumor activity after reinfusion into tumor-bearing mice (71). Furthermore, CD28 signals modulate glucose and fatty acid metabolism early after activation, coinciding with preservation of mitochondrial cristae flexibility and metabolic plasticity, which is crucial for the development of functional Tm cells capable of secondary immune responses (89, 166, 180). Taken together, these studies suggest that mitochondrial metabolism contributes to the functional capacity of long-lived Tm cells and this parameter should be considered as new therapies are developed.
The complex interplay between the specific TME and T cell metabolism was highlighted in one study assessing tumors in the lung, a common metastatic site for many cancers that contributes significantly to mortality (reviewed in 181). Beyond tumor cell–intrinsic features that allowed extravasation in the lung capillaries and survival of tumor cells at this site (181), T cell metabolism is altered in this highly oxygenated tissue, resulting in an immune-tolerant niche. High oxygen tension leads to prolyl-hydroxylase (PHD)-mediated inactivation of Hif-1α, inducing a preferential local differentiation of suppressive Tregs (182). Pharmacological interference with PHD activity in an adoptive T cell therapy protocol leads to better tumor control in the lung. This therapeutic strategy might be beneficial for the generation of CAR T cells when targeting tumors in highly oxygenated tissues.
The development of specific drugs that target metabolic regulators of T cell differentiation and function could lead to the generation of valuable therapeutics. Pharmacological modulation of single metabolic pathways or metabolites could allow enhanced T cell responses against chronic viral infections and cancer. Conversely, debilitating autoimmune diseases could be limited by reducing autoreactive T cells by interfering with proinflammatory metabolism.
As we have outlined in this review, the number of studies into T cell metabolism in relation to function is rapidly growing for a reason. We are coming into an exciting era in which we might be able to use the inherent flexibility of T cells and their metabolism to develop therapies that could improve the outcome of a wide variety of human pathologies.
ACKNOWLEDGMENTS
We would like to thank Ed Pearce and the members of the Pearce laboratories for helpful discussions, and would like to extend our apologies to our colleagues who have published relevant work in immunometabolism that we could not include due to space limitation.
Footnotes
DISCLOSURE STATEMENT
E.L.P. is on the scientific advisory board of Immunomet and has a patent titled “Methods of Enhancing T-Cell Longevity and Uses Thereof “ (US 20170101624 A1).
LITERATURE CITED
- 1.Weiner HL, Frenkel D. 2006. Immunology and immunotherapy of Alzheimer’s disease. Nat. Rev. Immunol 6:404–16 [DOI] [PubMed] [Google Scholar]
- 2.Heppner FL, Ransohoff RM, Becher B. 2015. Immune attack: the role of inflammation in Alzheimer disease. Nat. Rev. Neurosci 16:358–72 [DOI] [PubMed] [Google Scholar]
- 3.Saltiel AR, Olefsky JM. 2017. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig 127:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mathis D 2013. Immunological goings-on in visceral adipose tissue. Cell Metab. 17:851–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warburg O 1925. The metabolism of carcinoma cells. J. Cancer Res 9:148–63 [Google Scholar]
- 6.Vander Heiden MG, Cantley LC, Thompson CB. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills EL, Kelly B, O’Neill LAJ. 2017. Mitochondria are the powerhouses of immunity. Nat. Immunol 18:488–98 [DOI] [PubMed] [Google Scholar]
- 8.Houten SM, Violante S, Ventura FV, Wanders RJA. 2016. The biochemistry and physiology of mitochondrial fatty acid β-oxidation and its genetic disorders. Annu. Rev. Physiol 78:23–44 [DOI] [PubMed] [Google Scholar]
- 9.Schieber M, Chandel NS. 2014. ROS function in redox signaling and oxidative stress. Curr. Biol 24:R453–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacIver NJ, Michalek RD, Rathmell JC. 2013. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol 31:259–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brownlie RJ, Zamoyska R. 2013. T cell receptor signalling networks: branched, diversified and bounded. Nat. Rev. Immunol 13:257–69 [DOI] [PubMed] [Google Scholar]
- 12.Saxton RA, Sabatini DM. 2017. mTOR signaling in growth, metabolism, and disease. Cell 168:960–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang K, Neale G, Green DR, He W, Chi H. 2011. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat. Immunol 12:888–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cekic C, Sag D, Day YJ, Linden J. 2013. Extracellular adenosine regulates naive T cell development and peripheral maintenance. J. Exp. Med 210:2693–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunningham CA, Bergsbaken T, Fink PJ. 2017. Cutting edge: Defective aerobic glycolysis defines the distinct effector function in antigen-activated CD8+ recent thymic emigrants. J. Immunol 198:4575–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, et al. 2011. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 35:871–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pollizzi KN, Patel CH, Sun IH, Oh MH, Waickman AT, et al. 2015. mTORC1 and mTORC2 selectively regulate CD8+ T cell differentiation. J. Clin. Investig 125:2090–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyman O, Sprent J. 2012. The role of interleukin-2 during homeostasis and activation of the immune system. Nat. Rev. Immunol 12:180–90 [DOI] [PubMed] [Google Scholar]
- 19.Mendoza A, Fang V, Chen C, Serasinghe M, Verma A, et al. 2017. Lymphatic endothelial S1P promotes mitochondrial function and survival in naive T cells. Nature 546:158–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malissen B, Bongrand P. 2015. Early T cell activation: integrating biochemical, structural, and biophysical cues. Annu. Rev. Immunol 33:539–61 [DOI] [PubMed] [Google Scholar]
- 21.Esensten JH, Helou YA, Chopra G, Weiss A, Bluestone JA. 2016. CD28 costimulation: from mechanism to therapy. Immunity 44:973–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel CH, Powell JD. 2017. Targeting T cell metabolism to regulate T cell activation, differentiation and function in disease. Curr. Opin. Immunol 46:82–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. 2012. Regulation of immune responses by mTOR. Annu. Rev. Immunol 30:39–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, et al. 2013. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 38:225–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Previte DM, O’Connor EC, Novak EA, Martins CP, Mollen KP, Piganelli JD. 2017. Reactive oxygen species are required for driving efficient and sustained aerobic glycolysis during CD4+ T cell activation. PLOS ONE 12:e0175549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jackson SH, Devadas S, Kwon J, Pinto LA, Williams MS. 2004. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat. Immunol 5:818–27 [DOI] [PubMed] [Google Scholar]
- 27.Mak TW, Grusdat M, Duncan GS, Dostert C, Nonnenmacher Y, et al. 2017. Glutathione primes T cell metabolism for inflammation. Immunity 46:1089–90 [DOI] [PubMed] [Google Scholar]
- 28.Siska PJ, Kim B, Ji X, Hoeksema MD, Massion PP, et al. 2016. Fluorescence-based measurement of cystine uptake through xCT shows requirement for ROS detoxification in activated lymphocytes.J. Immunol. Methods 438:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laniewski NG, Grayson JM. 2004. Antioxidant treatment reduces expansion and contraction of antigen-specific CD8+ T cells during primary but not secondary viral infection. J. Virol 78:11246–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandel NS. 2015. Evolution of mitochondria as signaling organelles. Cell Metab. 22:204–6 [DOI] [PubMed] [Google Scholar]
- 31.Jones RG, Pearce EJ. 2017. MenTORing immunity: mTOR signaling in the development and function of tissue-resident immune cells. Immunity 46:730–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, et al. 2007. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science 315:1687–91 [DOI] [PubMed] [Google Scholar]
- 33.Verbist KC, Guy CS, Milasta S, Liedmann S, Kaminski MM, et al. 2016. Metabolic maintenance of cell asymmetry following division in activated T lymphocytes. Nature 532:389–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollizzi KN, Sun IH, Patel CH, Lo YC, Oh MH, et al. 2016. Asymmetric inheritance of mTORC1 kinase activity during division dictates CD8+ T cell differentiation. Nat. Immunol 17:704–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinclair LV, Rolf J, Emslie E, Shi YB, Taylor PM, Cantrell DA. 2013. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol 14:500–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang C-H, Curtis JD, Maggi LB Jr., Faubert B, Villarino AV, et al. 2013. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153:1239–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan H, Yang K, Li Y, Shaw TI, Wang Y, et al. 2017. Integrative proteomics and phosphoproteomics profiling reveals dynamic signaling networks and bioenergetics pathways underlying T cell activation. Immunity 46:488–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarasenko TN, Pacheco SE, Koenig MK, Gomez-Rodriguez J, Kapnick SM, et al. 2017. Cytochrome c oxidase activity is a metabolic checkpoint that regulates cell fate decisions during T cell activation and differentiation. Cell Metab. 25:1254–68.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hukelmann JL, Anderson KE, Sinclair LV, Grzes KM, Murillo AB, et al. 2016. The cytotoxic T cell proteome and its shaping by the kinase mTOR. Nat. Immunol 17:104–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomes LC, Di Benedetto G, Scorrano L. 2011. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat. Cell Biol 13:589–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, et al. 2006. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell 126:163–75 [DOI] [PubMed] [Google Scholar]
- 42.Hentze MW, Preiss T. 2010. The REM phase of gene regulation. Trends Biochem. Sci 35:423–26 [DOI] [PubMed] [Google Scholar]
- 43.Peng C, Lu Z, Xie Z, Cheng Z, Chen Y, et al. 2011. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol. Cell Proteom 10:M111.012658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. 2011. Identification of lysine succinylation as a new post-translational modification. Nat. Chem. Biol 7:58–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishida Y, Rardin MJ, Carrico C, He W, Sahu AK, et al. 2015. SIRT5 regulates both cytosolic and mitochondrial protein malonylation with glycolysis as a major target. Mol. Cell 59:321–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moellering RE, Cravatt BF. 2013. Functional lysine modification by an intrinsically reactive primary glycolytic metabolite. Science 341:549–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swamy M, Pathak S, Grzes KM, Damerow S, Sinclair LV, et al. 2016. Glucose and glutamine fuel protein O-GlcNAcylation to control T cell self-renewal and malignancy. Nat. Immunol 17:712–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Araujo L, Khim P, Mkhikian H, Mortales C-L, Demetriou M. 2017. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. eLife 6:e21330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chien M-W, Lin M-H, Huang S-H, Fu S-H, Hsu C-Y, et al. 2015. Glucosamine modulates T cell differentiation through down-regulating N-linked glycosylation of CD25. J. Biol. Chem 290:29329–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asano T, Takata K, Katagiri H, Ishihara H, Inukai K, et al. 1993. The role of N-glycosylation in the targeting and stability of GLUT1 glucose transporter. FEBS Lett. 324:258–61 [DOI] [PubMed] [Google Scholar]
- 51.Console L, Scalise M, Tarmakova Z, Coe IR, Indiveri C. 2015. N-linked glycosylation of human SLC1A5 (ASCT2) transporter is critical for trafficking to membrane. Biochim. Biophys. Acta 1853:1636–45 [DOI] [PubMed] [Google Scholar]
- 52.Puleston DJ, Villa M, Pearce EL. 2017. Ancillary activity: beyond core metabolism in immune cells. Cell Metab. 26:131–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mowen KA, David M. 2014. Unconventional post-translational modifications in immunological signaling. Nat. Immunol 15:512–20 [DOI] [PubMed] [Google Scholar]
- 54.Ron-Harel N, Santos D, Ghergurovich JM, Sage PT, Reddy A, et al. 2016. Mitochondrial biogenesis and proteome remodeling promote one-carbon metabolism for T cell activation. Cell Metab. 24:104–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma EH, Bantug G, Griss T, Condotta S, Johnson RM, et al. 2017. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 25:345–57. Correction. 2017. Cell Metab. 25:482 [DOI] [PubMed] [Google Scholar]
- 56.MacIver NJ, Blagih J, Saucillo DC, Tonelli L, Griss T, et al. 2011. The liver kinase B1 is a central regulator of T cell development, activation, and metabolism. J. Immunol 187:4187–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayer A, Denanglaire S, Viollet B, Leo O, Andris F. 2008. AMP-activated protein kinase regulates lymphocyte responses to metabolic stress but is largely dispensable for immune cell development and function. Eur. J. Immunol 38:948–56 [DOI] [PubMed] [Google Scholar]
- 58.Tamás P, Hawley SA, Clarke RG, Mustard KJ, Green K, et al. 2006. Regulation of the energy sensor AMP-activated protein kinase by antigen receptor and Ca2+ in T lymphocytes. J. Exp. Med 203:1665–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adams WC, Chen Y-H, Kratchmarov R, Yen B, Nish SA, et al. 2016. Anabolism-associated mitochondrial stasis driving lymphocyte differentiation over self-renewal. Cell Rep. 17:3142–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Blagih J, Coulombe F, Vincent EE, Dupuy F, Galicia-Vazquez G, et al. 2015. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 42:41–54 [DOI] [PubMed] [Google Scholar]
- 61.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, et al. 2011. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331:456–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scharping NE, Menk AV, Moreci RS, Whetstone RD, Dadey RE, et al. 2016. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity 45:374–88. Correction. 2016. Immunity 45:701–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rabinovitch RC, Samborska B, Faubert B, Ma EH, Gravel SP, et al. 2017. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 21:1–9 [DOI] [PubMed] [Google Scholar]
- 64.Lee J, Walsh MC, Hoehn KL, James DE, Wherry EJ, Choi Y. 2014. Regulator of fatty acid metabolism, acetyl coenzyme A carboxylase 1, controls T cell immunity. J. Immunol 192:3190–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee JE, Walsh MC, Hoehn KL, James DE, Wherry EJ, Choi Y. 2015. Acetyl CoA carboxylase 2 is dispensable for CD8+ T cell responses. PLOS ONE 10:e0137776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saha AK, Ruderman NB. 2003. Malonyl-CoA and AMP-activated protein kinase: an expanding partnership. Mol. Cell Biochem 253:65–70 [DOI] [PubMed] [Google Scholar]
- 67.Hosios AM, Hecht VC, Danai LV, Johnson MO, Rathmell JC, et al. 2016. Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev. Cell 36:540–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liberti MV, Locasale JW. 2016. The Warburg Effect: How does it benefit cancer cells? Trends Biochem. Sci 41:211–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fox CJ, Hammerman PS, Thompson CB. 2005. Fuel feeds function: energy metabolism and the T-cell response. Nat. Rev. Immunol 5:844–52 [DOI] [PubMed] [Google Scholar]
- 70.Franchi L, Monteleone I, Hao L-Y, Spahr MA, Zhao W, et al. 2017. Inhibiting oxidative phosphorylation in vivo restrains Th17 effector responses and ameliorates murine colitis. J. Immunol 198:2735–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang C-H, et al. 2016. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell 166:63–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Sullivan D, van der Windt GJW, Huang SC-C, Curtis JD, Chang C-H, et al. 2014. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41:75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buck MD, Sowell RT, Kaech SM, Pearce EL. 2017. Metabolic instruction of immunity. Cell 169:570–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobs SR, Herman CE, MacIver NJ, Wofford JA, Wieman HL, et al. 2008. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol 180:4476–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, et al. 2014. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell Metab. 20:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shi L, Tu BP. 2015. Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr. Opin. Cell Biol 33:125–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peng M, Yin N, Chhangawala S, Xu K, Leslie CS, Li MO. 2016. Aerobic glycolysis promotes T helper 1 cell differentiation through an epigenetic mechanism. Science 354:481–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang C-H, Qiu J, O’Sullivan D, Buck MD, Noguchi T, et al. 2015. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162:1229–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, et al. 2015. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162:1217–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhao E, Maj T, Kryczek I, Li W, Wu K, et al. 2016. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nature Immunol. 17:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Siska PJ, van der Windt GJW, Kishton RJ, Cohen S, Eisner W, et al. 2016. Suppression of Glut1 and glucose metabolism by decreased Akt/mTORC1 signaling drives T cell impairment in B cell leukemia.J. Immunol 197:2532–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, et al. 2015. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat. Commun 6:6692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baumeister SH, Freeman GJ, Dranoff G, Sharpe AH. 2016. Coinhibitory pathways in immunotherapy for cancer. Annu. Rev. Immunol 34:539–73 [DOI] [PubMed] [Google Scholar]
- 84.Doedens AL, Phan AT, Stradner MH, Fujimoto JK, Nguyen JV, et al. 2013. Hypoxia-inducible factors enhance the effector responses of CD8+ T cells to persistent antigen. Nat. Immunol 14:1173–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Phan AT, Doedens AL, Palazon A, Tyrakis PA, Cheung KP, et al. 2016. Constitutive glycolytic metabolism supports CD8+ T cell effector memory differentiation during viral infection. Immunity 45:1024–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van der Windt GJ, O’Sullivan D, Everts B, Huang SC, Buck MD, et al. 2013. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. PNAS 110:14336–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gubser PM, Bantug GR, Razik L, Fischer M, Dimeloe S, et al. 2013. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat. Immunol 14:1064–72 [DOI] [PubMed] [Google Scholar]
- 88.Balmer ML, Hess C. 2016. Feeling worn out? PGC1α to the rescue for dysfunctional mitochondria in T cell exhaustion. Immunity 45:233–35 [DOI] [PubMed] [Google Scholar]
- 89.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, et al. 2002. The CD28 signaling pathway regulates glucose metabolism. Immunity 16:769–77 [DOI] [PubMed] [Google Scholar]
- 90.Choi BK, Lee DY, Lee DG, Kim YH, Kim S-H, et al. 2017. 4–1BB signaling activates glucose and fatty acid metabolism to enhance CD8+ T cell proliferation. Cell Mol. Immunol 14:748–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H, et al. 2001. 4–1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy.J. Immunol 167:1313–24 [DOI] [PubMed] [Google Scholar]
- 92.Balmer ML, Ma EH, Bantug GR, Grählert J, Pfister S, et al. 2016. Memory CD8+ T cells require increased concentrations of acetate induced by stress for optimal function. Immunity 44:1312–24 [DOI] [PubMed] [Google Scholar]
- 93.Bengsch B, Johnson AL, Kurachi M, Odorizzi PM, Pauken KE, et al. 2016. Bioenergetic insufficiencies due to metabolic alterations regulated by the inhibitory receptor PD-1 are an early driver of CD8+ T cell exhaustion. Immunity 45:358–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang CS, Hawley SA, Zong Y, Li M, Wang Z, et al. 2017. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature 548:112–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hu H, Juvekar A, Lyssiotis CA, Lien EC, Albeck JG, et al. 2016. Phosphoinositide 3-kinase regulates glycolysis through mobilization of aldolase from the actin cytoskeleton. Cell 164:433–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, et al. 2011. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol 12:295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, et al. 2009. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity 30:832–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, et al. 2011. Cutting edge: Distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol 186:3299–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Battaglia M, Stabilini A, Roncarolo M-G. 2005. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105:4743–48 [DOI] [PubMed] [Google Scholar]
- 100.Valmori D, Tosello V, Souleimanian NE, Godefroy E, Scotto L, et al. 2006. Rapamycin-mediated enrichment of T cells with regulatory activity in stimulated CD4+ T cell cultures is not due to the selective expansion of naturally occurring regulatory T cells but to the induction of regulatory functions in conventional CD4+ T cells. J. Immunol 177:944–49 [DOI] [PubMed] [Google Scholar]
- 101.Kang J, Huddleston SJ, Fraser JM, Khoruts A. 2008. De novo induction of antigen-specific CD4+CD25+Foxp3+ regulatory T cells in vivo following systemic antigen administration accompanied by blockade of mTOR. J. Leukoc. Biol 83:1230–39 [DOI] [PubMed] [Google Scholar]
- 102.Yang K, Shrestha S, Zeng H, Karmaus PW, Neale G, et al. 2013. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity 39:1043–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heikamp EB, Patel CH, Collins S, Waickman A, Oh M-H, et al. 2014. The AGC kinase SGK1 regulates TH1 and TH2 differentiation downstream of the mTORC2 complex. Nat. Immunol 15:457–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, et al. 2004. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol 6:1122–28 [DOI] [PubMed] [Google Scholar]
- 105.Yang J-Q, Kalim KW, Li Y, Zhang S, Hinge A, et al. 2016. RhoA orchestrates glycolysis for TH2 cell differentiation and allergic airway inflammation. J. Allergy Clin. Immunol 137:231–45.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhao FQ, Keating AF. 2007. Functional properties and genomics of glucose transporters. Curr. Genom 8:113–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chornoguz O, Hagan RS, Haile A, Arwood ML, Gamper CJ, et al. 2017. mTORC1 promotes T-bet phosphorylation to regulate Th1 differentiation. J. Immunol 198:3939–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kurobe H, Urata M, Ueno M, Ueki M, Ono S, et al. 2010. Role of hypoxia-inducible factor 1αin T cells as a negative regulator in development of vascular remodeling. Arterioscler. Thromb. Vasc. Biol 30:210–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shehade H, Acolty V, Moser M, Oldenhove G. 2015. Cutting edge: Hypoxia-inducible factor 1 negatively regulates Th1 function. J. Immunol 195:1372–76 [DOI] [PubMed] [Google Scholar]
- 110.Metzler B, Gfeller P, Guinet E. 2016. Restricting glutamine or glutamine-dependent purine and pyrimidine syntheses promotes human T cells with high FOXP3 expression and regulatory properties.J. Immunol 196:3618–30 [DOI] [PubMed] [Google Scholar]
- 111.Klysz D, Tai X, Robert PA, Craveiro M, Cretenet G, et al. 2015. Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci. Signal 8:ra97. [DOI] [PubMed] [Google Scholar]
- 112.Xu T, Stewart KM, Wang X, Liu K, Xie M, et al. 2017. Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature 548:228–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abdel Rahman AM, Ryczko M, Pawling J, Dennis JW. 2013. Probing the hexosamine biosynthetic pathway in human tumor cells by multitargeted tandem mass spectrometry. ACS Chem. Biol 8:2053–62 [DOI] [PubMed] [Google Scholar]
- 114.Carr EL, Kelman A, Wu GS, Gopaul R, Senkevitch E, et al. 2010. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol 185:1037–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Crotty S 2014. T follicular helper cell differentiation, function, and roles in disease. Immunity 41:529–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oestreich KJ, Read KA, Gilbertson SE, Hough KP, McDonald PW, et al. 2014. Bcl-6 directly represses the gene program of the glycolysis pathway. Nat. Immunol 15:957–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ray JP, Staron MM, Shyer JA, Ho P-C, Marshall HD, et al. 2015. The interleukin-2-mTORc1 kinase axis defines the signaling, differentiation, and metabolism of T helper 1 and follicular B helper T cells. Immunity 43:690–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zeng H, Cohen S, Guy C, Shrestha S, Neale G, et al. 2016. mTORC1 and mTORC2 kinase signaling and glucose metabolism drive follicular helper T cell differentiation. Immunity 45:540–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Berod L, Friedrich C, Nandan A, Freitag J, Hagemann S, et al. 2014. De novo fatty acid synthesis controls the fate between regulatory T and T helper 17 cells. Nat. Med 20:1327–33. Erratum. 2015. Nat. Med. 21:414 [DOI] [PubMed] [Google Scholar]
- 120.Gaffen SL, Jain R, Garg AV, Cua DJ. 2014. The IL-23-IL-17 immune axis: from mechanisms to therapeutic testing. Nat. Rev. Immunol 14:585–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Endo Y, Asou HK, Matsugae N, Hirahara K, Shinoda K, et al. 2015. Obesity drives Th17 cell differentiation by inducing the lipid metabolic kinase, ACC1. Cell Rep. 12:1042–55 [DOI] [PubMed] [Google Scholar]
- 122.Raha S, Raud B, Oberdörfer L, Castro CN, Schreder A, et al. 2016. Disruption of de novo fatty acid synthesis via acetyl-CoA carboxylase 1 inhibition prevents acute graft-versus-host disease. Eur. J. Immunol 46:2233–38 [DOI] [PubMed] [Google Scholar]
- 123.Ye J, DeBose-Boyd RA. 2011. Regulation of cholesterol and fatty acid synthesis. Cold Spring Harb. Perspect. Biol 3:a004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Angela M, Endo Y, Asou HK, Yamamoto T, Tumes DJ, et al. 2016. Fatty acid metabolic reprogramming via mTOR-mediated inductions of PPARγ directs early activation of T cells. Nat. Commun 7:13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Muroski ME, Miska J, Chang AL, Zhang P, Rashidi A, et al. 2017. Fatty acid uptake in T cell subsets using a quantum dot fatty acid conjugate. Sci. Rep 7:5790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hu X, Wang Y, Hao L-Y, Liu X, Lesch CA, et al. 2015. Sterol metabolism controls TH17 differentiation by generating endogenous RORγagonists. Nat. Chem. Biol 11:141–47. Corrigendum. 2015. Nat. Chem. Biol. 11:741 [DOI] [PubMed] [Google Scholar]
- 127.Santori FR, Huang P, van de Pavert SA, Douglass EF, Leaver DJ, et al. 2015. Identification of natural RORγ ligands that regulate the development of lymphoid cells. Cell Metab. 21:286–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang C, Yosef N, Gaublomme J, Wu C, Lee Y, et al. 2015. CD5L/AIM regulates lipid biosynthesis and restrains Th17 cell pathogenicity. Cell 163:1413–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Makki K, Froguel P, Wolowczuk I. 2013. Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm. 2013:139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Saucillo DC, Gerriets VA, Sheng J, Rathmell JC, MacIver NJ. 2014. Leptin metabolically licenses T cells for activation to link nutrition and immunity. J. Immunol 192:136–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Papathanassoglou E, El-Haschimi K, Li XC, Matarese G, Strom T, Mantzoros C. 2006. Leptin receptor expression and signaling in lymphocytes: kinetics during lymphocyte activation, role in lymphocyte survival, and response to high fat diet in mice. J. Immunol 176:7745–52 [DOI] [PubMed] [Google Scholar]
- 132.Reis BS, Lee K, Fanok MH, Mascaraque C, Amoury M, et al. 2015. Leptin receptor signaling in T cells is required for Th17 differentiation. J. Immunol 194:5253–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gerriets VA, Danzaki K, Kishton RJ, Eisner W, Nichols AG, et al. 2016. Leptin directly promotes T-cell glycolytic metabolism to drive effector T-cell differentiation in a mouse model of autoimmunity. Eur. J. Immunol 46:1970–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.De Rosa V, Procaccini C, Calì G, Pirozzi G, Fontana S, et al. 2007. A key role of leptin in the control of regulatory T cell proliferation. Immunity 26:241–55 [DOI] [PubMed] [Google Scholar]
- 135.Newton R, Priyadharshini B, Turka LA. 2016. Immunometabolism of regulatory T cells. Nat. Immunol 17:618–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Travis MA, Sheppard D. 2014. TGF-βactivation and function in immunity. Annu. Rev. Immunol 32:51–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Xie M, Zhang D, Dyck JR, Li Y, Zhang H, et al. 2006. A pivotal role for endogenous TGF-β-activated kinase-1 in the LKB1/AMP-activated protein kinase energy-sensor pathway. PNAS 103:17378–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Gualdoni GA, Mayer KA, Goschl L, Boucheron N, Ellmeier W, Zlabinger GJ. 2016. The AMP analog AICAR modulates the Treg/Th17 axis through enhancement of fatty acid oxidation. FASEB J. 30:3800–9 [DOI] [PubMed] [Google Scholar]
- 139.He N, Fan W, Henriquez B, Yu RT, Atkins AR, et al. 2017. Metabolic control of regulatory T cell (Treg) survival and function by Lkb1. PNAS 114:12542–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, et al. 2015. Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat. Immunol 16:188–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shrestha S, Yang K, Guy C, Vogel P, Neale G, Chi H. 2015. Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell responses. Nat. Immunol 16:178–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sharma MD, Shinde R, McGaha TL, Huang L, Holmgaard RB, et al. 2015. The PTEN pathway in Tregs is a critical driver of the suppressive tumor microenvironment. Sci. Adv 1:e1500845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. 2013. mTORC1 couples immune signals and metabolic programming to establish Treg-cell function. Nature 499:485–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Forero-Peña DA, Gutierrez FRS. 2013. Statins as modulators of regulatory T-cell biology. Mediators Inflamm. 2013:167086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.De Rosa V, Galgani M, Porcellini A, Colamatteo A, Santopaolo M, et al. 2015. Glycolysis controls the induction of human regulatory T cells by modulating the expression of FOXP3 exon 2 splicing variants. Nat. Immunol 16:1174–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wei J, Long L, Yang K, Guy C, Shrestha S, et al. 2016. Autophagy enforces functional integrity of regulatory T cells by coupling environmental cues and metabolic homeostasis. Nat. Immunol 17:277–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, et al. 2017. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 25:1282–93.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Procaccini C, Carbone F, Di Silvestre D, Brambilla F, De Rosa V, et al. 2016. The proteomic landscape of human ex vivo regulatory and conventional T cells reveals specific metabolic requirements. Immunity 44:406–21. Correction. 2016. Immunity 44:712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gerriets VA, Kishton RJ, Johnson MO, Cohen S, Siska PJ, et al. 2016. Foxp3 and Toll-like receptor signaling balance Treg cell anabolic metabolism for suppression. Nature Immunol. 17:1459–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kishore M, Cheung KCP, Fu H, Bonacina F, Wang G, et al. 2017. Regulatory T cell migration is dependent on glucokinase-mediated glycolysis. Immunity 47:875–89.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, et al. 2015. Antigen- and cytokine-driven accumulation of regulatory T cells in visceral adipose tissue of lean mice. Cell Metab. 21:543–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, et al. 2012. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 486:549–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Munn DH, Bronte V. 2016. Immune suppressive mechanisms in the tumor microenvironment. Curr. Opin. Immunol 39:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maj T, Wang W, Crespo J, Zhang H, Wang W, et al. 2017. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat. Immunol 18:1332–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, et al. 2009. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460:103–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, et al. 2009. mTOR regulates memory CD8 T-cell differentiation. Nature 460:108–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, et al. 2013. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Investig 123:4479–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, et al. 2011. A human memory T cell subset with stem cell-like properties. Nat. Med 17:1290–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sukumar M, Liu J, Mehta GU, Patel SJ, Roychoudhuri R, et al. 2016. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell Metab. 23:63–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, et al. 2015. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 75:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.van der Windt GJW, Everts B, Chang C-H, Curtis JD, Freitas TC, et al. 2012. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36:68–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pfleger J, He M, Abdellatif M. 2015. Mitochondrial complex II is a source of the reserve respiratory capacity that is regulated by metabolic sensors and promotes cell survival. Cell Death Dis. 6:e1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rolf J, Zarrouk M, Finlay DK, Foretz M, Viollet B, Cantrell DA. 2013. AMPKα1: a glucose sensor that controls CD8 T-cell memory. Eur. J. Immunol 43:889–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dimeloe S, Mehling M, Frick C, Loeliger J, Bantug GR, et al. 2016. The immune-metabolic basis of effector memory CD4+ T cell function under hypoxic conditions. J. Immunol 196:106–14 [DOI] [PubMed] [Google Scholar]
- 165.Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, et al. 2013. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155:160–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Klein Geltink RI, O’Sullivan D, Corrado M, Bremser A, Buck MD, et al. 2017. Mitochondrial priming by CD28. Cell 171:385–97.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Champagne DP, Hatle KM, Fortner KA, D’Alessandro A, Thornton TM, et al. 2016. Fine-tuning of CD8+ T cell mitochondrial metabolism by the respiratory chain repressor MCJ dictates protection to influenza virus. Immunity 44:1299–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lochner M, Berod L, Sparwasser T. 2015. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 36:81–91 [DOI] [PubMed] [Google Scholar]
- 169.Cui G, Staron MM, Gray SM, Ho PC, Amezquita RA, et al. 2015. IL-7-induced glycerol transport and TAG synthesis promotes memory CD8+ T cell longevity. Cell 161:750–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pan Y, Tian T, Park CO, Lofftus SY, Mei S, et al. 2017. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 543:252–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.O’Sullivan D, Pearce EL. 2015. Targeting T cell metabolism for therapy. Trends Immunol. 36:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Minn AJ, Wherry EJ. 2016. Combination cancer therapies with immune checkpoint blockade: convergence on interferon signaling. Cell 165:272–75 [DOI] [PubMed] [Google Scholar]
- 173.Swart M, Verbrugge I, Beltman JB. 2016. Combination approaches with immune-checkpoint blockade in cancer therapy. Front. Oncol 6:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Byun DJ, Wolchok JD, Rosenberg LM, Girotra M. 2017. Cancer immunotherapy—immune checkpoint blockade and associated endocrinopathies. Nat. Rev. Endocrinol 13:195–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lim WA, June CH. 2017. The principles of engineering immune cells to treat cancer. Cell 168:724–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yu S, Li A, Liu Q, Li T, Yuan X, et al. 2017. Chimeric antigen receptor T cells: a novel therapy for solid tumors. J. Hematol. Oncol 10:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kawalekar OU, O’Connor RS, Fraietta JA, Guo L, McGettigan SE, et al. 2016. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 44:380–90 [DOI] [PubMed] [Google Scholar]
- 178.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, et al. 2016. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Investig 126:3130–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Crompton JG, Sukumar M, Restifo NP. 2016. Targeting Akt in cell transfer immunotherapy for cancer. Oncoimmunology 5:e1014776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zheng Y, Delgoffe GM, Meyer CF, Chan W, Powell JD. 2009. Anergic T cells are metabolically anergic.J. Immunol 183:6095–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nguyen DX, Bos PD, Massague J. 2009. Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9:274–84 [DOI] [PubMed] [Google Scholar]
- 182.Clever D, Roychoudhuri R, Constantinides MG, Askenase MH, Sukumar M, et al. 2016. Oxygen sensing by T cells establishes an immunologically tolerant metastatic niche. Cell 166:1117–31.e14 [DOI] [PMC free article] [PubMed] [Google Scholar]