Abstract
Purpose
To compare the treatment efficacy of percutaneous catheter drainage alone to catheter drainage combined with ozone in the management of pyogenic liver abscess (PLA).
Material and methods
This prospective study included 85 patients diagnosed with PLA. All patients were randomly divided into two groups: catheter drainage alone (Group I); catheter drainage combined with ozone (Group II). Drainage was considered successful when: 1) the abscess cavities were completely drained, and 2) clinical symptoms were resolved. Kruskall-Wallis nonparametric test was used to compare the success rates, length of stay (LOS), and need of further surgery. A value of p < 0.05 was considered significant for all statistical analyses.
Results
In all the patients’ percutaneous catheters were placed successfully under the guidance of computed tomography. All of the patients in Group I (43; 50.6%) were treated with percutaneous catheter drainage alone, while the patients in Group II (42; 49.4%) were treated with PCD combined with ozone. The success rates of Group I and II were 81% and 94%, respectively (p < 0.05). The duration of fever as well as LOS was longer for Group I when compared with Group II (p < 0.05).
Conclusions
Catheter drainage combined with ozone is an effective and safe treatment in PLA.
Keywords: pyogenic liver abscess, catheter drainage, interventional radiology, ozone,
Introduction
Pyogenic liver abscess (PLA) is a relatively uncommon lesion with a high mortality rate because of delayed detection and treatment [1,2]. Therapy includes antibiotics alone or in combination with percutaneous or drainage. A number of studies have shown that percutaneous abscess drainage is effective and safe, and it is minimally invasive, which means it does not require general anaesthesia [3]. But to date the choice of therapy for the first-line of treatment remains debatable [3-6].
Thomas et al. [7] reported that after the placement of a percutaneous drainage catheter (PCD) in a liver abscess, the risk of post-procedure sepsis is significant (26%). They speculated that bacteraemia, which is caused by the release of bacteria directly into the bloodstream during the procedure, leads to formation of sepsis. Furthermore, some of the patients have various infections, and use of antibiotics alone is often ineffective. Moreover, long-term use of antibiotics can cause bacterial resistance [6]. Ozone can inactivate bacteria, viruses, yeasts, protozoa, and fungi. It can also stimulate the immune system and oxygen metabolism. Therefore, ozone has been widely considered to be one of the best sterilisation, antifungal, and antiviral agents [8]. For some chronic wounds, such as nutrition ulcers, ischaemic ulcers, and diabetic wounds, ozone has also been empirically used as a clinical therapeutic agent [9-11].
In order to improve the therapeutic effect of PLA, this study compares catheter drainage alone and in combination with ozone therapy in the treatment of PLA.
Material and methods
This prospective study was approved by our Hospital Institutional Review Board (HIRB), and informed consent to undergo the procedure was obtained from all patients. Eighty-five patients who were diagnosed with PLA (45 males and 40 females, aged 35-72 years; median age 46.2 years) were enrolled prospectively between February 2014 and September 2017. Diagnosis of the pyogenic abscess was confirmed by aspiration or microbiologic findings in all patients.
Inclusion criteria: 1) liver abscess more than 4 cm in size, 2) liver abscess were at mature stage (a capsule was formed around the necrotic cavity).
Exclusion criteria: 1) multiple abscess, 2) abscess ruptured into thoracic and peritoneal cavity.
All PLAs were uniformly managed with antibiotics throughout the study period. Usually a combination of a third-generation cephalosporin along with metronidazole were used as an antibiotic of choice. Subsequently antibiotic therapy was tailored according to the culture sensitivity results of blood or pus.
All patients were randomly divided into two groups: catheter drainage alone (Group I); catheter drainage combined with ozone (Group II). Contrast-enhanced CT scans were performed in all patients to determine the size, location, and extent of the lesion, and choose the trans hepatic route to avoid injuring other organs, blood vessels, and biliary systems. All catheters were placed by CT-guidance, under local anaesthesia. 10F pigtail catheters were placed into the patients using the Seldinger technique. In order to avoid secondary liver infection, it was ensured that the side holes of the catheter were placed within the abscess cavity. Abdominal CT images were obtained immediately after abscess drainage through the drainage catheter to assess the catheter location within the abscess cavity, and to rule out any complications such as bleeding.
In Group II, in addition to the drainage catheter, oxygenozone gas mixture was injected through the catheter. The concentration of oxygen-ozone gas mixture ranged between 5.0 and 30.0 ml per day according to the size of the abscess cavity (ozone concentration 25 μg/ml, based on our previous experiments [8]). We used an ozone generator (Herrmann, Kleinwallstadt, Germany) to produce oxygen-ozone gas mixtures prior to injection. After oxygen-ozone gas mixture injection, the catheter was clamped for one hour and then left unclamped for 23 hours to allow free drainage.
The drainage was considered successful when clinical symptoms of patients were resolved and the abscess cavities were drained. However, if the abscess failed to resolve and the follow-up imaging (ultrasound or CT) showed a thicker abscess wall which could not be aspirated, or the patient suffered from ongoing sepsis after drainage, the patient was referred for further surgical treatment.
We evaluated the differences in patient characteristics, the technical success of catheter placement, as well as the length of hospital stay (LOS) between the two groups. Clinical history of the patients such as ongoing sepsis, duration of fever (morning oral temperature of > 37.5°C), and the need for further surgery treatment were also noted.
Statistical analysis
SPSS software (version 20.0; SPSS Corporation, New York, USA) were used for statistical analyses. The mean and standard deviation (SD) of each variable from the two groups was calculated. Kruskall-Wallis non-parametric tests was used to calculate statistical differences between the two groups. A p value of less than 0.05 was considered significant for each statistical analysis.
Results
All of the 85 patients’ catheters were successfully pla-ced under CT guidance (Figure 1). Group I contained 43 patients (50.6%) who were treated with catheters alone, while Group II contained 42 patients (49.4%) all of whom were treated with combined catheters and ozone therapy. Different variables of the two groups are shown in Table 1. There were no significant differences between the two groups in terms of age and sex (p = 0.437). Moreover, size and number of abscesses were also not significantly different between the two groups (p = 0.471; Table 1). Among the 85 patients 80.72% (68) were culture positive on bacteriologic study (Table 2). The remaining abscesses had negative pus/blood culture and were instead diagnosed based on increased white blood cell count in the aspirated fluid. Streptococcus species were the main bacteria isolated, followed by Enterococcus species, Escherichia coli, and Klebsiella species.
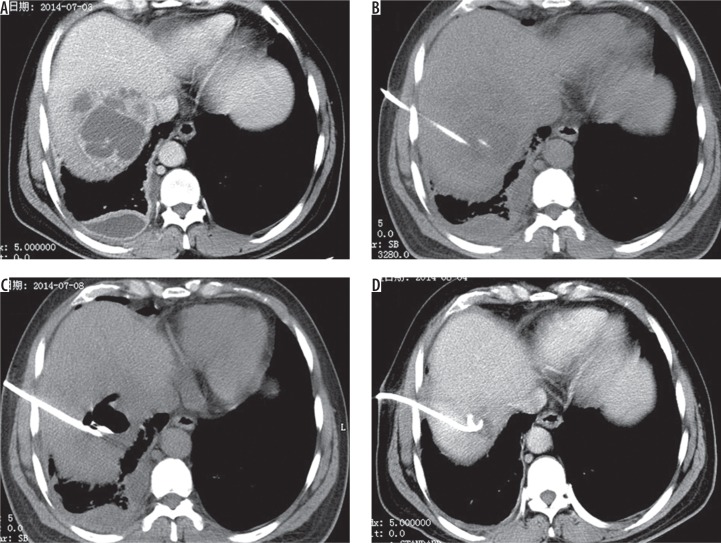
Figure 1.
A 38-year-old male presented with fever and abdominal pain for one week. A) Enhanced computed tomography (CT) shows lager liver abscess in right hepatic lobe. B) Catheter was placed under CT-guidance. C) Oxygen–ozone gas mixture was given through catheter. D) Abscesses were absorbed after three weeks
Table 1.
Comparison of preadmission variables among treatment groups
| Variable, mean (SD) | Group I | Group II |
|---|---|---|
| No. of patients | 43 | 42 |
| Average age (years) | 42.3 (13.2) | 39.6 (14.1) |
| Duration of symptoms (days) | 13.7 (9.2) | 14.8 (7.6) |
| Initial WBC (× 103) | 14.3 (9.7) | 15.8 (8.5) |
| Total lymphocytes (× 103) | 10.2 (6.3) | 11.4 (7.0) |
| Abscess size (cm) | 5.3 (1.9) | 6.2 (2.3) |
| Abscess number | 45 | 48 |
| Catheter size (Fr) | 10 | 10 |
Table 2.
Positive results of bacterial culture among treatment groups
| Variable, mean | Group I | Group II |
|---|---|---|
| Bacterial culture | 34 | 33 |
| Streptococcus species | 14 | 13 |
| Enterococcus species | 7 | 7 |
| Escherichia coli | 8 | 7 |
| Klebsiella species | 5 | 6 |
Initial white blood cell count (WBC), lymphocytes, and duration of symptoms, although not significant (p > 0.05), were different between the two groups. Success rates of Group I and II were 81% and 94%, respectively. The duration of fever as well as LOS was longer for Group I when compared with Group II (p < 0.05). Furthermore, the number of patients requiring further surgery was also significantly higher for Group I when compared with Group II (19% vs. 5%; p < 0.05) (Table 3). Three patients had minimal peri-hepatic bleeding post-intervention. No other complications were noted.
Table 3.
Comparison of hospitalization and outcome variables among treatment groups
| Variable, mean (SD) | Group I | Group II |
|---|---|---|
| Technical success of catheter placement | 100% | 100% |
| Success rate of management | 81% | 94% |
| Length of hospital stay (days) | 27.0 (8.6) | 18.7 (6.8) |
| Duration of fever (days) | 5.4 (2.8) | 4.1 (1.9) |
| Converted into further surgery | 8 (19%) | 2 (5%) |
| With ongoing sepsis | 7 (16%) | 2 (5%) |
Discussion
The aetiology and the management of PLA has changed considerably. At present, percutaneous catheter drainage is performed more often in the management of PLA [2]. Conventional treatment of PLA is antibiotic therapy and image-guided percutaneous drainage or aspiration [7]. However, percutaneous catheter drainage still has high reported failure rates [3]. Moreover, irrespective of types of intervention used, the probability of extravasation of bacteria into the bloodstream is high [12]. Some studies have shown the chance of post-procedure sepsis after liver abscess drainage [7,13,14].
In this study, we used catheter drainage combined with ozone to treat PLA, and achieved higher success rates (94%) than in the previous studies [7,15]. This higher success rate also led to significantly decreased LOS in the present study.
Clinical practice has proven that ozone is a powerful and reliable anti-microbial agent, which can inactivate bacteria, fungi, protozoa, and viruses [8,16,17]. For a century, ozone has been widely used and studied as an antibacterial agent. Its mechanism of action is through stimulation of the oxygen metabolism and the immune system of bacteria, viruses, fungi, yeast, and protozoa, to achieve inactivation [16]. Universally accepted theory is that the oxidant potential of ozone induces the destruction of cell walls and the cytoplasmic membranes of bacteria and fungi. In this process, ozone attacks glycolipids, glycoproteins, and other amino acids as well as inhibiting and blocking the cells’ enzymatic control system. This leads to increased membrane permeability, the key element of cell viability, leading to immediate functional cessation [9,17].
Usage of antibiotics sometimes fails to give satisfactory results because most PLA patients have mixed infections. In addition, long-term use of antibiotics can lead to bacterial resistance [2,6,18]. Identifying pathogenic organisms has great clinical importance. Experience, however, has shown that this was not always possible because many patients were treated before fluid was obtained for culture [2]. The use of ozone as a secondary antibacterial agent to treat PLA has achieved a better effect.
A study has found that the beneficial effects of ozone on wound healing might be due to increased oxygen tension by ozone exposure in the wound area [17]. Another study reported that ozone exposure could activate transcription factors that adjust inflammatory reactions and eventually the whole process of wound healing [16]. Therefore, ozone has also been used empirically as a clinical therapeutic agent for chronic wounds, such as ischaemic ulcers, trophic ulcers, and diabetic wounds [19]. Furthermore, ozone can be dispersed into the cavity of the abscess causing abscess wall dehydration [11]. Moreover, ozone can separate the adhesion thereby expanding the distribution of drug solution in the abscess cavity [8]. Therefore, the use of drainage combined with ozone has a synergistic effect on the management of liver abscess.
The drainage placement procedure in this study was guided by CT. CT imaging can be more accurate in displaying the lesion and thus is critical to the success rates of treatment. Abscess with small collections, with presence of air as well as those closely abutting the diaphragm, are poorly defined on US. CT imaging was found to be superior in depicting such abscesses [20].
Some complications from catheter drainage, such as haemorrhage due to intercostal vessel and liver parenchyma injury, catheter-related pain, and subcutaneous emphysema, have been reported [11]. In this study, we did not encounter any serious complications.
Conclusions
In short, catheter drainage combined with ozone is an effective and safe treatment procedure in PLA. This technique can reduce the rates of surgical intervention and LOS. However, further studies with larger sample sizes are warranted to validate these findings.
Acknowledgements
We thank English native speakers Susan for helping to polish the grammar and structure of the article.
Conflict of interest
The authors report no conflict of interest.
References
- 1.Dulku G, Mohan G, Samuelson S, et al. Percutaneous aspiration versus catheter drainage of liver abscess: A retrospective review. Australas Med J. 2015;8:7–18. doi: 10.4066/AMJ.2015.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jun CH, Yoon JH, Wi JW, et al. Risk factors and clinical outcomes for spontaneous rupture of pyogenic liver abscess. J Dig Dis. 2015;16:31–36. doi: 10.1111/1751-2980.12209. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed S, Chia CL, Junnarkar SP, et al. Percutaneous drainage for giant pyogenic liver abscess--is it safe and sufficient? Am J Surg. 2016;211:95–101. doi: 10.1016/j.amjsurg.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Lai KC, Cheng KS, Jeng LB, et al. Factors associated with treatment failure of percutaneous catheter drainage for pyogenic liver abscess in patients with hepatobiliary-pancreatic cancer. Am J Surg. 2013;205:52–57. doi: 10.1016/j.amjsurg.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Liao WI, Tsai SH, Yu CY, et al. Pyogenic liver abscess treated by percutaneous catheter drainage: MDCT measurement for treatment outcome. Eur J Radiol. 2012;81:609–615. doi: 10.1016/j.ejrad.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava A, Yachha SK, Arora V, et al. Identification of high-risk group and therapeutic options in children with liver abscess. Eur J Pediatr. 2012;171:33–41. doi: 10.1007/s00431-011-1481-y. [DOI] [PubMed] [Google Scholar]
- 7.Thomas J, Turner SR, Nelson RC, et al. Postprocedure sepsis in imaging-guided percutaneous hepatic abscess drainage: how often does it occur? AJR Am J Roentgenol. 2006;186:1419–1422. doi: 10.2214/AJR.04.1914. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Liu C, Li Y, et al. Computed tomography-guided catheter drainage with urokinase and ozone in management of empyema. World J Radiol. 2017;9:212–216. doi: 10.4329/wjr.v9.i4.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreula C. Ozone therapy. Neuroradiology. 2011;53:S207–S209. doi: 10.1007/s00234-011-0930-7. [DOI] [PubMed] [Google Scholar]
- 10.Li B, Xu XX, Du Y, et al. CT-guided chemonucleolysis combined with psoas compartment block in lumbar disc herniation: a randomized controlled study. Pain Med. 2014;15:1470–1476. doi: 10.1111/pme.12491. [DOI] [PubMed] [Google Scholar]
- 11.Re L, Rowen R, Travagli V. Ozone Therapy and Its Use in Medicine: Further Comments. Cardiology. 2016;136:269. doi: 10.1159/000452618. [DOI] [PubMed] [Google Scholar]
- 12.Sharma N, Kaur H, Kalra N, et al. Complications of Catheter Drainage for Amoebic Liver Abscess. J Clin Exp Hepatol. 2015;5:256–258. doi: 10.1016/j.jceh.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kusumoto K, Hamada A, Kusaka T, et al. A patient with sepsis and a gas-forming liver abscess caused by Clostridium perfringens treated with continuous perfusion drainage. Nihon Shokakibyo Gakkai Zasshi. 2014;111:1416–1423. [PubMed] [Google Scholar]
- 14.Liu CH, Gervais DA, Hahn PF, et al. Percutaneous hepatic abscess drainage: do multiple abscesses or multiloculated abscesses preclude drainage or affect outcome? J Vasc Interv Radiol. 2009;20:1059–1065. doi: 10.1016/j.jvir.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 15.Bari S, Sheikh KA, Malik AA, et al. Percutaneous aspiration versus open drainage of liver abscess in children. Pediatr Surg Int. 2007;23:69–74. doi: 10.1007/s00383-006-1812-7. [DOI] [PubMed] [Google Scholar]
- 16.Elvis AM, Ekta JS. Ozone therapy: A clinical review. J Nat Sci Biol Med. 2011;2:66–70. doi: 10.4103/0976-9668.82319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta G, Mansi B. Ozone therapy in periodontics. J Med Life. 2012;5:59–67. [PMC free article] [PubMed] [Google Scholar]
- 18.Lo JZ, Leow JJ, Ng PL, et al. Predictors of therapy failure in a series of 741 adult pyogenic liver abscesses. J Hepatobiliary Pancreat Sci. 2015;22:156–165. doi: 10.1002/jhbp.174. [DOI] [PubMed] [Google Scholar]
- 19.Yu G, Liu X, Chen Z, et al. Ozone therapy could attenuate tubulointerstitial injury in adenine-induced CKD rats by mediating Nrf2 and NF-kappaB. Iran J Basic Med Sci. 2016;19:1136–1143. [PMC free article] [PubMed] [Google Scholar]
- 20.Takeuchi Y, Okabe H, Myojo S, et al. CT-guided drainage of a mediastinal pancreatic pseudocyst with a transhepatic transdiaphragmatic approach. Hepatogastroenterology. 2002;49:271–272. [PubMed] [Google Scholar]