Summary:
Drugs of abuse, like alcohol, modulate gene expression in reward circuits and consequently alter behavior. However, the in vivo cellular mechanisms through which alcohol induces lasting transcriptional changes are unclear. We show that Drosophila Notch/Su(H) signaling, and the secreted fibrinogen-related protein Scabrous, in mushroom body (MB) memory circuitry, is important for the enduring preference of cues associated with alcohol’s rewarding properties. Alcohol exposure affects Notch responsivity in the adult MB and alters Su(H) targeting at the dopamine-2-like receptor (Dop2R). Alcohol-cue training also caused lasting changes to the MB nuclear transcriptome, including changes in the alternative splicing of Dop2R and newly implicated transcripts like Stat92E. Together, our data suggest that alcohol-induced activation of the highly conserved Notch pathway and accompanying transcriptional responses in memory circuitry contribute to addiction. Ultimately this provides mechanistic insight into the etiology and pathophysiology of Alcohol Use Disorder.
Keywords: Scabrous, Notch, Su(H), Dop2R, Dopamine, Alcohol, Memory, Addiction
Graphical Abstract
Introduction
Alcohol abuse is among the top five risk factors for disease, disability, and death (WHO 2014 Report). Chronic alcohol consumption can have lasting effects on the neurophysiology of motivation/reward brain circuitry, which contribute to addiction (Kalivas and Volkow, 2005). Remarkably, sensitization to drug-associated stimuli or cues often persists even after prolonged abstinence, contributing to drug-cravings and relapse (Robinson and Berridge, 2008). A mechanistic understanding of how these maladaptive memories form for cues associated with intoxication will provide therapeutic insight into alcohol abuse and other forms of addiction. However, we currently have very little understanding of the cellular pathways activated during this process, and consequent gene expression changes underlying these long-lasting memories.
The genetic pliability, molecular accessibility, and behavioral diversity of Drosophila melanogaster make it an ideal model organism with which to address this complex gap in knowledge (Devineni and Heberlein, 2013; Kaun et al., 2012; Robinson and Atkinson, 2013; Scaplen and Kaun, 2016). Drosophila develop long-lasting preference for odor cues associated with the pharmacological properties of alcohol, a behavior we term ‘alcohol associative preference’ (Figure 1A; Kaun et al., 2011). This alcohol associative preference requires a central associative brain structure called the mushroom bodies (MB) and dopaminergic neurotransmission. Dopaminergic innervation of memory-encoding circuits bears striking resemblance to the mammalian reward system (Figure 1B; Scaplen and Kaun, 2016). This genetically and anatomically defined circuit within the fly provides an ideal platform in which to investigate the cellular and transcriptional changes that underlie alcohol-associated memories.
Figure 1. Scabrous is required in adult MB neurons for alcohol associative preference.
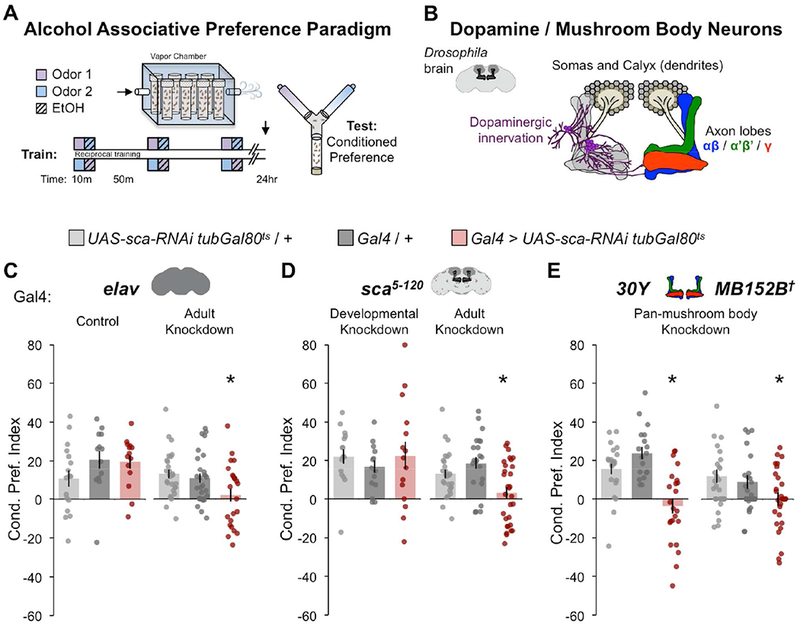
(A) Spaced olfactory memory paradigm for lasting cue-associated alcohol preference. A moderate dose of ethanol (90:60 EtOH:Air, 13.8 ± 3 mM internal concentration) was paired with either ethyl acetate or isoamyl alcohol. (B) The associative memory center mushroom body (MB) comprises intrinsic neurons (Kenyon cells) that have posteriorly positioned somas and dendritic arbors (calyx), and anteriorly positioned αβ, α’β’, γ axon bundles (blue, green, red). Representation of dopaminergic neurons innervating the MB axon bundles (purple). (C) Scabrous (Sca) knockdown in adult neurons (elav) impaired alcohol associative preference; Control (maintained at 18°C) n = 17, 13, 14; Adult knockdown (shifted after eclosion from 18→30°C) n = 27, 28, 23. (D) Sca knockdown in sca5-120-expressing cells during adulthood, but not development, impaired alcohol associative preference; Developmental Knockdown (shifted after eclosion from 30→18°C) n = 16, 13, 15; Adulthood Knockdown (shifted after eclosion from 18→30°C) n = 27, 22, 30. (E) Sca knockdown in all MB neurons (30Y or split gal4 MB152B) impaired alcohol associative preference; 30Y n = 22, 18, 23; MB152B n = 21, 20, 24. (C-E) Mean ± SEM with statistical significance evaluated by ANOVA, posthoc Bonferroni compared to experimental genotype, *p < 0.05, †Temperature insensitive split-GAL4. See also Figures S1, S2, S9.
Forward genetics approaches in Drosophila have been extremely valuable for investigating the molecular basis of behavior, including memory and addiction. We previously performed a screen of MB-expressed genes to identify molecular players underlying alcohol associative preference and uncovered a role for the secreted fibrinogen-related glycoprotein Scabrous (Sca) (Kaun et al., 2011). Throughout development, Sca is thought to anchor Notch at the membrane and influence Delta ligand-induced activity (Hu et al., 1995; Lee et al., 1996; Lee et al., 2000; Powell et al., 2001). It is currently unknown whether alcohol associative preference requires Notch, let alone how Sca might modulate Notch pathway activity. We hypothesized that lasting alcohol-induced cellular responses in reward memory circuits are the result of dysregulated Sca/Notch signaling.
The highly conserved Notch signal transduction pathway is involved in many crucial cellular processes, including stem cell fate determination and diversification during development (Hori et al., 2013). Canonical Notch signaling requires ligand-induced proteolytic cleavage of the Notch receptor, releasing the Notch intracellular domain, which then regulates target gene expression by interacting with the transcription factor Suppressor of Hairless (Su(H)), Recombination Binding Protein for immunoglobulin kappa J region (RBP-J/CBF1) in mammals. Notch/Su(H) signaling has been shown to regulate synaptic plasticity and long-term memory in adult mammalian and fly nervous systems (Alberi et al., 2011; Brai et al., 2015; Costa et al., 2003; Ge et al., 2004; Kidd et al., 2015; Matsuno et al., 2009; Presente et al., 2004; Song et al., 2009; Zhang et al., 2013b). However the limitations of conditional genetic tools have made it challenging to determine the spatio-temporal actions and dynamic signaling of the Notch pathway. Because of Notch’s underappreciated post-developmental functions, its in vivo contribution to transcriptional regulation of adult neuronal plasticity and behavior remains widely unexplored.
Intriguingly, there is accumulating evidence that Notch signaling is involved with changes in gene expression in the brain associated with Alcohol Use Disorder. For example, RNA-sequencing analyses in mammalian Alcohol Use Disorder models have identified dynamic changes in Notch-associated gene expression (Melendez et al., 2012; Smith et al., 2016; Tong et al., 2017). Furthermore, Notch activation can occur upon ethanol application in human endothelial cells and myoblasts (Arya et al., 2013; Morrow et al., 2008). We, therefore, reasoned that addiction-related behaviors could result from alcohol-induced changes in Notch signaling and gene expression within memory circuitry.
Here, we use a multifaceted approach to investigate the function of Sca/Notch/Su(H) signaling in alcohol associative preference. We show that alcohol acutely activates Notch signaling in vivo, and that the formation of alcohol associative preference coincides with lasting transcriptional effects within memory circuitry. We further find that the dopamine-2-like receptor (Dop2R) is targeted by Su(H) and differentially spliced following repeated alcohol experiences. Together, this evidence suggests that alcohol induces lasting Notch-dependent changes in gene expression underlying alcohol associative cue memories. This work provides crucial insight into the neuromolecular mechanisms governing persistent reward memories associated with alcohol addiction and relapse.
Results
Scabrous (Sca) is required in the adult MB for alcohol associative preference
During development, Sca specifies retinal precursor patterning (Gavish et al., 2016; Mlodzik et al., 1990) and refines epithethial cell adhesion during bristle patterning (Renaud and Simpson, 2001). As a short-range diffusible protein with the ability to sharpen proneural cluster boundaries (Powell et al., 2001), Sca may contribute to the structural plasticity of adult MB synapses required for alcohol associative preference.
To address the spatiotemporal requirement of Sca in alcohol associative preference, we thermo-genetically expressed sca-RNAi in adult neurons. Restricting pan-neuronal (elav-Gal4) knockdown of sca in adulthood significantly impaired alcohol associative preference, suggesting that adult neurons require Sca for alcohol associative preference (Figure 1C). Alcohol associative preference was also abolished when sca knockdown occurred in adult, but not developmental, sca-expressing cells (sca5-120-Gal4), which prominently include MB αβ, γ neurons (Figure 1D, Supplemental Figure 1). Further restriction of sca knockdown to pan-MB neurons (30Y-Gal4, and the more restrictive 152B split-Gal4) also significantly impaired alcohol associative preference (Figure 1E). Together, these experiments show that Sca produced by the adult MB is required for lasting preference of alcohol-associated cues.
The above, and subsequent, behavioral analyses were performed using reciprocally odor-trained flies receiving doses of alcohol sufficient to increase locomotion and behaviors associated with disinhibition (Kaun et al., 2011; Nunez et al., 2018; Park et al., 2017) (See Materials and Methods). All genotypes lacking alcohol associative preference were confirmed to have naïve odor responses (Supplemental Figure 2A). A negative RNAi control, pan-MB-specific MB010B split Gal4 driving GFP-RNAi, showed no changes in alcohol associative preference compared to genetic controls (Supplemental Figure 2B).
Sca promotes Notch activity in the adult brain
Sca functions as a secreted glycoprotein that likely binds to the extracellular domain of Notch in nearby cells to influence Delta/Notch signaling (Figure 2A; Baker et al., 1990; Powell et al., 2001). Specifically, Sca has been proposed to induce Delta/Notch activation, prevent Notch degradation, or reduce Notch availability in a ligand-dependent manner depending on the spatial and temporal context (Gavish et al., 2016; Giagtzoglou et al., 2013; Lee et al., 1996; Lee et al., 2000; Munoz-Soriano et al., 2016; Renaud and Simpson, 2001). Because the function of the Notch pathway is highly context specific (Bray, 2016), it is critical to understand the relationship between Sca and Delta/Notch in the adult brain.
Figure 2. Scabrous promotes Notch activation in adult heads.
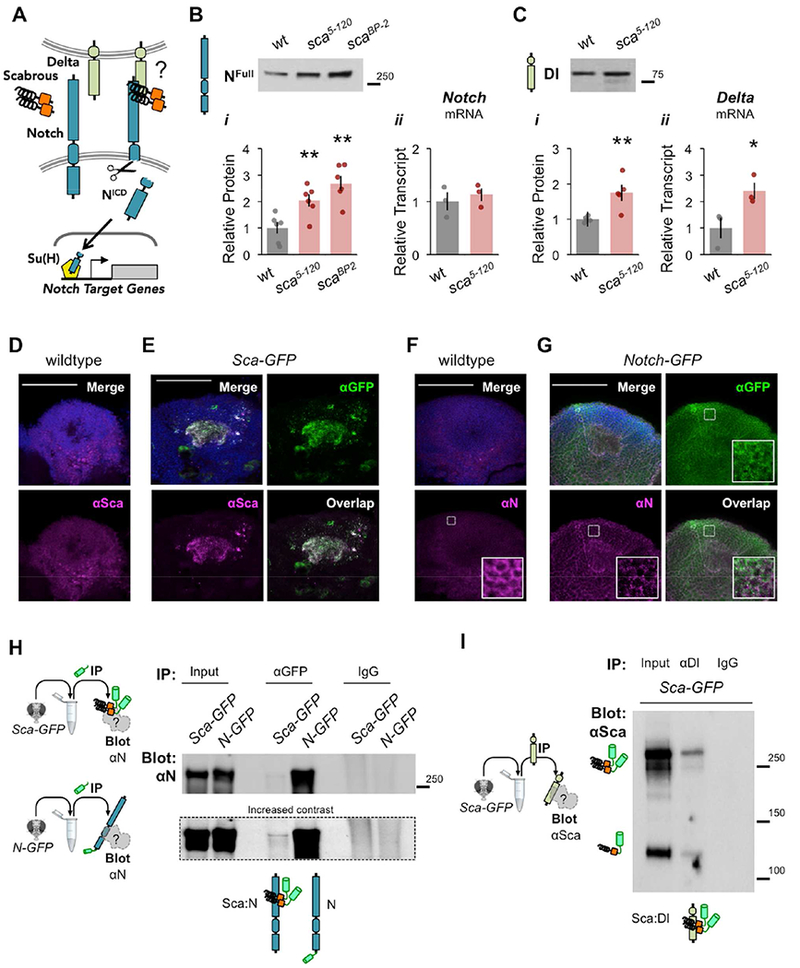
(A) Schematic of Scabrous/ Delta/Notch/Su(H) pathway. Extracellular Sca may influence DI ligand-induced activation of Notch, and Su(H) transcription factor- dependent changes in gene expression. (B) NFull protein (i) and Notch transcript (ii) levels in wildtype, hypomorphic sca5-120, and null scaBP-2 heads; n = 6, 6, 6; ΔΔCT n = 3, 3. (C) Delta protein (i) and Delta transcript (ii) levels in wildtype and sca5-120 heads; n = 5, 5; ΔΔCT n = 3, 3. (B-C) Data normalized to wildtype. Mean ± SEM with statistical significance evaluated by ANOVA, posthoc Bonferroni compared to wildtype, *p < 0.05, **p < 0.01. (D-E) αSca (magenta) in the MB soma regions of wildtype and Sca-GFP (sca5-120/+ ; UAS- sca-GFP/+) flies. (D) Sca signal in wildtype flies is low, diffuse, and (E) colocalizes with GFP in the MB calyx (dendrites) of Sca-GFP flies. (F-G) αNotch (magenta) in the MB soma regions of wildtype and Notch-GFP (N55e11 ; N-GFP) flies. (F) Notch in wildtype flies is low, diffuse, and localizes to cell membranes (inset: enhanced brightness/contrast). (E) αNotch colocalizes with GFP in N- GFP flies. (D-G) All images are 5-10 μm max projection stacks with Dapi (blue); scale bars 50 μm. (H-I) Scabrous binds Delta and Notch in adult heads. (H) Immunoprecipitation (IP) with αGFP and blotting (Blot) with αNotch in Sca-GFP (sca5-120/+ ; sca-GFP/+) and N-GFP (N55e11 ; N-GFP) heads. IP with αGFP, Blot with αNotch in Sca-GFP and N-GFP heads suggests an interaction between Sca:Notch, and includes a positive control for Notch pulldown and negative control with IgG. (I) IP with αDelta, non-reducing Blot with αSca in Sca-GFP heads supports an interaction between Sca:Delta. See also Figures S1, S3, S4, S5.
To determine whether Sca affects Notch activity in adults, we examined the protein levels of uncleaved Notch (NFull) receptor in the heads of wildtype and sca mutants. Relative to wildtype flies, hypomorphic transposon insertion sca5-120 and null deletion scaBP-2 mutants had significantly more NFull (Figure 2Bi, Data File S1). Since few homozygous scaBP-2 mutants survived to adulthood in our hands, subsequent experiments used sca5-120 flies. Increased NFull protein levels were not the result of enhanced Notch transcription in sca5-120 mutants (Figure 2Bii, Data File S1), suggesting that sca mutants have reduced Notch activity.
Notch activation inhibits downstream proneural gene expression, including its Delta ligand (i.e. via impaired lateral inhibition) (Heitzler and Simpson, 1991). We, therefore, expected that reduced Notch activity would promote Delta expression. Indeed, both Delta protein and transcript levels in adult heads were significantly increased in sca5-120 mutants as compared to wildtype flies (Figure 2C, Data File S1). These findings are consistent with sca mutants having reduced Notch activity, suggesting that Sca normally functions to promote Notch signaling in adult brains.
To understand the neuronal context of Sca, we examined protein localization in the adult brain. In developing tissue Sca is secreted and diffuses up to three cell diameters away from its origin, which makes it challenging to determine its source and site of action (Lee et al., 1996). Diffuse endogenous Sca protein (αSca) was observed in the MB soma region of wildtype flies, whereas abnormal expression was observed in sca5-120 mutants (Figure 2D, Supplemental Figure 3A). However, the precise cellular localization of Sca was difficult to ascertain, likely due to its short half-life and sensitivity to fixative (Lee et al., 1996). To address this limitation, we instead examined the localization of Sca GFP fusion proteins (Chou and Chien, 2002) in sca5-120-expressing cells of sca5-120/+ heterozygotes. Sca-GFP (C-terminal tag) localized to MB dendrites (calyx) and to the membranes of distinct somas (Figure 2E, Supplemental Figure 3B), whereas GFP-Sca (N-terminal tag) primarily localized to all MB somas (Supplemental Figure 3B). We postulate that N-terminal fusion disrupts Sca secretion by interfering with its predicted signal peptide (1-51aa) (Signal Peptide Database), and thus suggest that the C-terminal Sca fusion more likely reflects endogenous Sca localization. Immunostaining with αSca further confirmed Sca-GFP localization within the MB soma region (Figure 2E). Subsequent experiments were performed with C-terminal tagged Sca-GFP expressed by sca5-120.
We next sought to investigate the endogenous localization of Notch in the adult brain. Notch was widely expressed at low levels and localized to membranes in the MB soma region (Figure 2F, inset shows enhanced signal contrast). This expression pattern was confirmed by αNotch recognition of a previously constructed N-GFP fusion protein expressed in a Notch loss-of-function (N55e11) mutant background (Figure 2G; Couturier et al., 2014). Together, these findings suggest that Sca and Notch proteins are endogenously distributed throughout the MB soma region, and that Sca localizes to MB dendrites and a few distinct somas.
Sca dimers bind to Notch and Delta in the adult brain
Sca fibrinogen-like peptides are glycosylated and form disulfide-linked dimers in developing imaginal eye discs (Lee et al., 1996), but the form and function of Sca in the post-developmental brain is unknown. We identified the presence of endogenous Sca dimers in wildtype, sca5-120, and Sca-GFP (sca5-120 > sca-GFP) adult head lysates (Supplemental Figure 4A,B). Various Sca:Sca-GFP heterodimers and possible glycospecies were observed, and there was no evidence of artificial in vitro dimerization (Supplemental Figure 4C). These findings thus confirm the presence of functional Sca protein in adult head tissue.
Despite genetic and immunohistochemical evidence that Sca and Notch may form a ligand-receptor pair during development, biochemical efforts to confirm their interaction have been unsuccessful (Lee et al., 1996; Powell et al., 2001). To address possible protein-protein interaction in adult head tissue, we immunoprecipitated GFP-tagged Sca and Notch with αGFP from Sca-GFP (sca5-120 > sca-GFP) and N-GFP (N55e11 ; N-GFP) heads and blotted for αNotch or αDelta (Figure 2H, Supplemental Figure 4D-E). Notch co-precipitated modestly with Sca (Figure 2H) and Delta co-precipitated to a lesser extent with Sca and Notch (Supplemental Figure 4E). A reciprocal immunoprecipitation experiment, using αDelta for pulldown and αSca for blotting from Sca-GFP heads, further supported a Delta:Sca interaction (Figure 2I). These findings demonstrate associations between Notch:Delta, Notch:Sca, and Delta:Sca proteins in adult head tissue. Therefore, our working model is that Sca dimers bind both Notch and Delta to promote Notch activity, and that loss of Sca reduces Notch activation (Supplementary Figure 3C).
Notch is required in adult MBs for alcohol associative preference
In adult flies, Notch knockdown or overexpression results in the reduction or enhancement of long-term aversive shock memory, respectively (Ge et al., 2004; Presente et al., 2004; Zhang et al., 2013b). It is unclear, though, whether Notch is required for alcohol associative preference, and whether it acts only in specific adult circuits to influence memory. To determine the necessity of Notch in our behavioral paradigm we thermo-genetically expressed Notch-RNAi in adult neurons. Similar to our findings with sca, Notch knockdown in the adult MB (30Y-Gal4) impaired alcohol associative preference (Figure 3A, left). Conversely, Notch knockdown in the eyes (gmr-Gal4), an unrelated brain region, did not disrupt alcohol associative preference (Figure 3A, right). These findings highlight the necessity of Notch in adult MB neurons for the formation of alcohol associative preference.
Figure 3. Notch and Su(H) are required in MB neurons for alcohol associative preference.
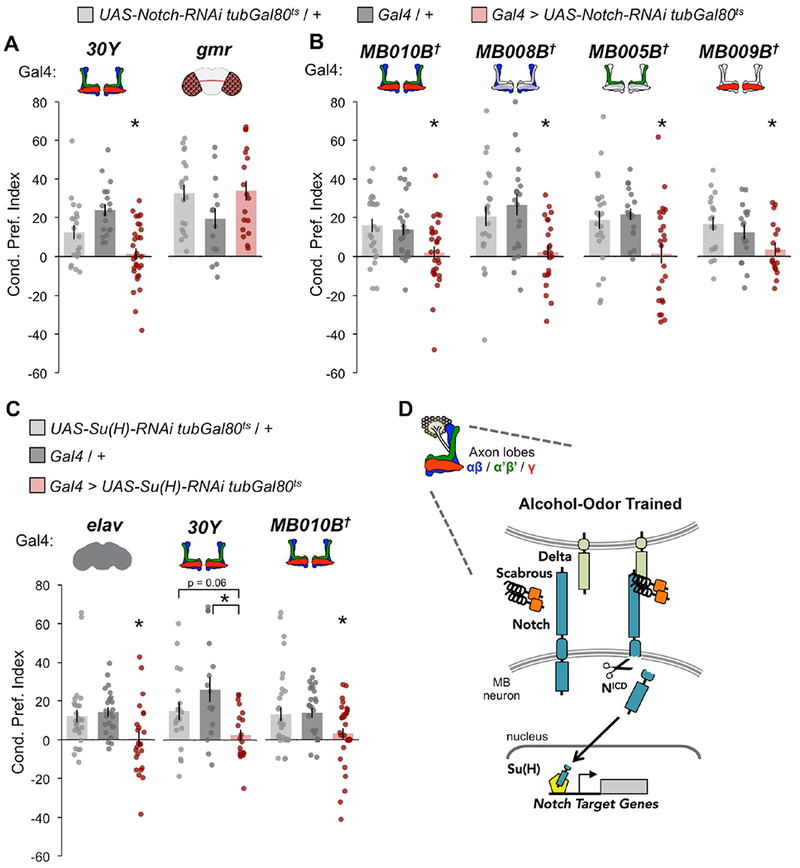
(A) Notch knockdown in all MB lobes (30Y), but not in the eye (gmr), impaired alcohol associative preference; 30Y n = 20, 18, 30; gmr n = 19, 15, 18. (B) Knockdown of Notch in all MB lobes (MB010B), αβ lobes (MB008B), α’β’ lobes (MB005B), or γ lobes (MB009B) impaired alcohol associative preference; MB010B n = 25, 26, 28; MB008B n = 24, 20, 22; MB005B n = 24, 18, 24; MB009B n = 25, 26, 28. (C) Su(H) knockdown in all neurons (elav) or MB lobes (30Y or MB010B) tended to impair alcohol associative preference; elav n = 27, 23, 24; 30Y n = 19, 16, 20; MB010B n = 33, 27, 33. All flies for behavioral experiments were shifted after eclosion from 18°C to 30°C. (A-C) Mean ± SEM with statistical significance evaluated by ANOVA, posthoc Bonferroni compared to experimental, *p < 0.05; †Temperature insensitive split-GAL4. (D) Working model where alcohol-odor training induces mis-activation of Notch/Su(H) in adult MBs. See also Figures S2, S9.
We have previously shown that neurotransmission from discrete mushroom body lobes is required for the acquisition (γ), consolidation (α’β’), and retrieval (αβ) of alcohol-associated memory (Kaun et al., 2011). To determine whether Notch was specifically required in these neuronal subsets, Notch-RNAi expression was restricted using refined split-Gal4 drivers (Figure 3B). Compared to genetic controls, alcohol associative preference was abolished when Notch-RNAi was expressed in all αβ, α’β’, γ (MB010B-Gal4), αβ (MB008B-Gal4), α’β’ (MB005B-Gal4), or γ (MB009B-Gal4) neurons. These findings suggest that Notch function is generalized to all, and not particular subtypes of, MB neurons for mediating lasting alcohol associative preference.
Su(H) is required in adult MBs for alcohol associative preference
Both canonical (Su(H)-dependent) and non-canonical (Su(H)-independent) Notch signaling have been implicated in Drosophila long-term memory (Song et al., 2009; Zhang et al., 2013b, 2015). Unlike Notch, either too little or too much Su(H) disrupts long-term odor-associated shock memory. Additionally, post-developmental knockout of the mammalian Su(H) homolog, Recombination Binding Protein for immunoglobulin kappa J region (RBP-J or CBF1), significantly influences synaptic plasticity and memory (Liu et al., 2015). To address the spatiotemporal requirement of Su(H) in alcohol associative preference, we thermo-genetically expressed Su(H)-RNAi in adult neurons. Adult pan-neuronal (elav-Gal4) or pan-MB (30Y-Gal4 or MB010B-Gal4) knockdown of Su(H) impaired alcohol associative preference (Figure 3C, See Supplemental Figure 2C for thermogenetic adult-specificity controls). Together, these findings illustrate that alcohol associative preference requires Sca / Delta / Notch / Su(H) signaling in adult MB neurons (Figure 3D).
Alcohol increases Notch activity in the adult brain
Alcohol has been shown to stimulate Notch signaling in human endothelial cells and myoblasts (Arya et al., 2013; Morrow et al., 2008), and can markedly alter neural firing and network activity in the mammalian prefrontal cortex and limbic brain regions (Ehlers et al., 2012; Pleil et al., 2015; Tu et al., 2007). Furthermore, Notch activation is required for activity- and experience-dependent plasticity in the mouse hippocampus, fly olfactory neurons and at the larval neuromuscular junction (Alberi et al., 2011; de Bivort et al., 2009; Kidd et al., 2015). We reasoned that alcohol exposure may disrupt neuronal Notch activity in vivo to produce maladaptive changes in gene transcription.
To test this hypothesis, we assessed Notch activity in the heads of flies exposed to either air or alcohol (Figure 4 top). Immediately following exposure, NFull levels were significantly reduced as compared to air-treated controls (Figure 4A, Data Files S3–5). This phenomenon was not merely the result of being exposed to an odorant (Supplemental Figure 5A). Interestingly, providing flies an hour rest after exposure allowed NFul1 to recover to control levels following one or two, but not three, rounds of exposure (Figure 4A). This suggests that alcohol transiently induces Notch cleavage, and that repeated exposures cause lasting changes in Notch activity.
Figure 4. Alcohol exposure induces Notch activity in vivo.
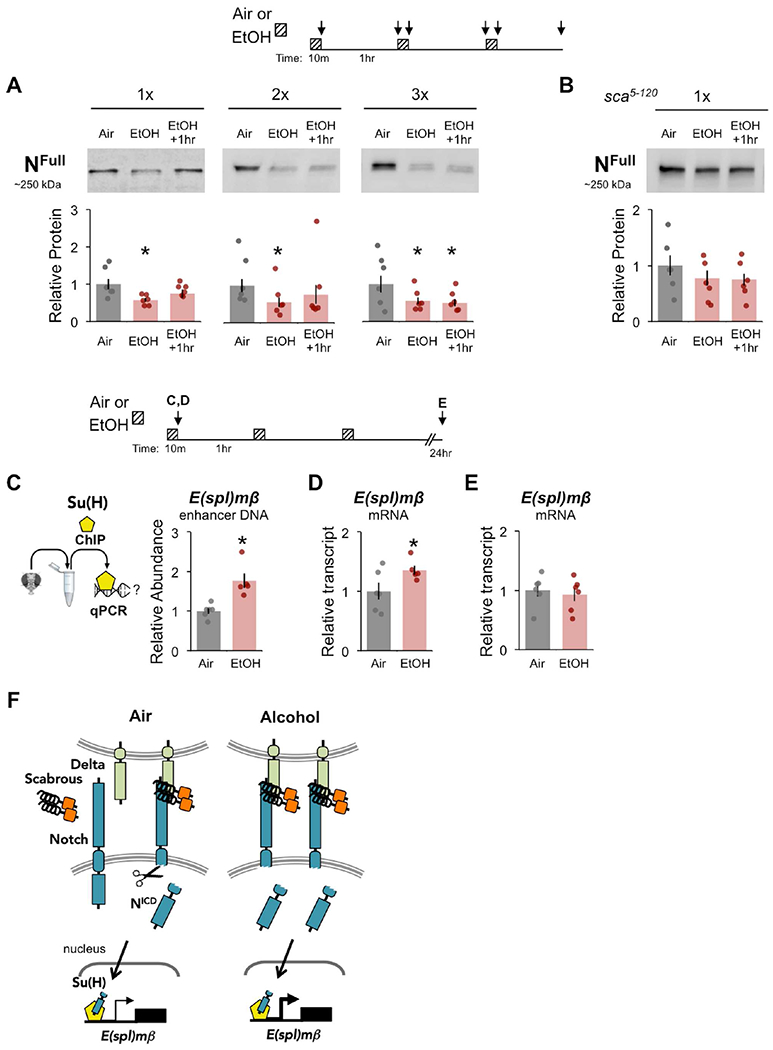
(A-B) Flies were exposed to air or alcohol (90:60 EtOH:Air), or rested 1 hr after exposure across multiple trials (1×, 2×, 3×), modeling the alcohol associative memory training paradigm; see diagram above. (A) NFull protein in wildtype heads was significantly reduced in response to alcohol; 1× n = 7, 7, 7; 2× n = 9, 9, 9; 3× n = 8, 8, 8. (B) NFull protein in sca5-120 mutant heads does not change in response to alcohol exposure; 1× n = 12, 12, 12. (A-B) Protein levels normalized to air. Mean ± SEM with statistical significance evaluated by ANOVA, posthoc Bonferroni compared to air, *p < 0.05. (C) Chromatin immunoprecipitation (ChIP) with αSu(H) followed by qPCR showed that alcohol significantly enriched Su(H) at an E(spl)mβ enhancer sequence, n = 6, 6; see diagram above. (D-E) E(spl)mβ mRNA expression increased in fly heads after acute 10 min alcohol exposure (D), but not 24 hr after repeated exposures (E); 10 min n = 6, 6; 24 hr n = 6, 6; data normalized to air. (A- E) Mean ± SEM with statistical significance evaluated by ANOVA, posthoc Bonferroni compared to air, or two-tailed T-test, *p < 0.05. (F) Working model where alcohol exposure activates canonical Notch/Su(H) signaling in adult head tissue. See also Figure S5.
Since sca5-120 mutants displayed reduced Notch activity (Figure 2B-C), we predicted that unlike in wildtype flies a single alcohol exposure would not activate Notch signaling. Indeed, NFull levels showed no significant reduction in sca5-120 mutants exposed to alcohol, suggesting that Sca is required for alcohol-induced Notch activation (Figure 4B). No significant changes were observed in Sca-GFP dimers in heads following acute alcohol exposure (Supplemental Figure 5B). Together, these findings support a role for Sca-dependent Notch activation in alcohol associative preference.
To detect the downstream consequence of alcohol-induced Notch activation, we attempted to examine the levels of Notch intracellular domain (NICD) in the heads of wildtype flies. Cleaved NICD was undetectable using the αNotch (mC17) antibody either due to low levels or poor antibody recognition - possibly due to post-translational modifications. NICD was weakly detected in N-GFP fly heads using αGFP (Supplemental Figure 5C), although significant changes following alcohol exposure were not observed (Supplemental Figure 5D).
To investigate whether alcohol-induced Notch activation caused downstream Su(H)-mediated transcriptional activity in vivo, we examined the recruitment of Su(H) to the known Notch target gene Enhancer of split-mβ (E(spl)mβ). Specifically, to determine whether Su(H) occupancy was affected by alcohol exposure at this locus we performed chromatin immunoprecipitation followed by qPCR (ChIP–qPCR) with heads of acutely exposed flies (Figure 4C, Supplemental Figure 5E). In response to alcohol exposure, Su(H) occupancy expectantly increased in abundance upstream of E(spl)mβ (Figure 4C, right). Furthermore, consistent with alcohol-induced Notch activation and Su(H) expression of target genes, acute alcohol exposure significantly increased E(spl)mβ mRNA transcript levels just after exposure (Figure 4D), but not 24 hours after repeated exposure (Figure 4E), in whole head tissue. Together, these findings illustrate that acute alcohol exposure induces Sca / Delta / Notch / Su(H) signaling causing E(spl)mβ expression detectable at the whole head tissue level (Figure 4F).
We next wondered whether Notch activity, specifically in MB neurons, was affected by alcohol-odor training. To address this, we used a genetically engineered ubiquitously expressed Notch-LexA/VP16 reporter that drives expression of lexAop-destabilized-GFP (N-LV > dGFP) (Figure 5A, left; Barolo et al., 2000; Lieber et al., 2011) and a MB neuron marker MB247-DsRed (Pech et al., 2013). Many dGFP+ cells, a proxy for Notch-competency, were observed throughout the anterior and posterior central adult brain (Figure 5A, right). We noted that 24 hours after odor only exposure (‘Odors’) there were on average ~10 Notch active dGFP+ MB neurons, which mildly increased with alcohol-odor training (‘Trained’, Figure 5B). This phenotype was also observed in the absence of the MB247-DsRed marker and using a computational method for cell counting (Supplemental Figure 6). These findings suggest that repeated alcohol-odor training has lasting effects on Notch-competency, which may contribute to lasting alcohol associative preference.
Figure 5. Alcohol-odor training causes lasting changes to Notch in MB neurons.
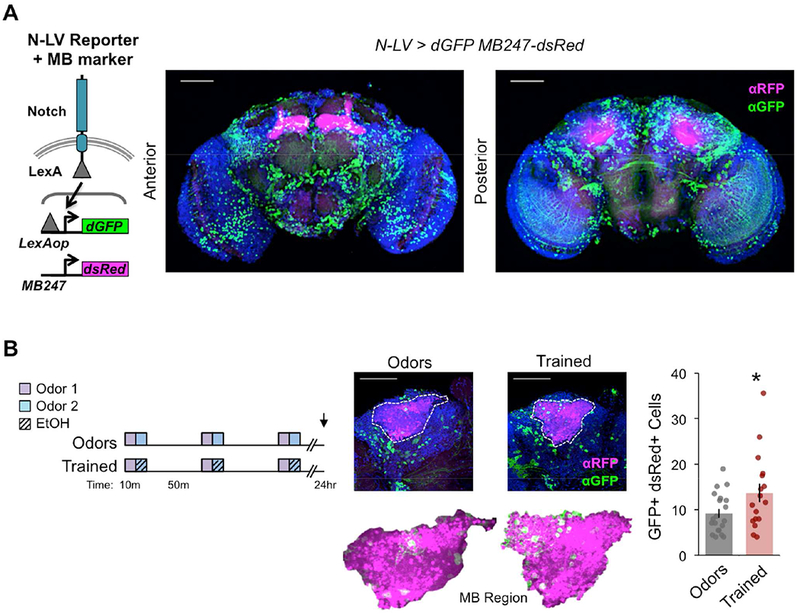
(A) Schematic of Notch-LexA:VP16 (N-LV) reporter driving destabilized GFP LexAop-dGFP and MB247-dsRed MB marker. GFP signal, a proxy of Notch activity, was extensive and broadly observed in the anterior and posterior central brain. (B) Trained N-LV>dGFP MB247-dsRed (all transgenes heterozygous) flies showed more GFP+ dsRed+ cells as compared to Odors only treatment 24 hours after training; n = 20, 17 flies (averaged across left and right hemispheres scored from confocal series). Mean ± SEM with statistical significance evaluated by two-tailed T- test, *p < 0.05. (A-B) Images are max projection stacks with Dapi (blue), dGFP (green), and dsRed (magenta); scale bar 50 μm. See also Figure S6.
Dop2R is required in adult MBs for alcohol associative preference
Our data thus far demonstrate that alcohol can activate the Notch pathway, a major transcriptional program. However, the lasting gene expression and behavioral consequences of this response are unknown. MB neurons are highly innervated by dopaminergic neurons (Figure 1B) (Aso et al., 2014; Waddell, 2010), and express the four known Drosophila dopamine receptors (D1 -like: Dop1R1, Dop1R2, DopEcR, and D2-like: Dop2R) (Crocker et al., 2016). D2-like receptors have been implicated in mammalian (El-Ghundi 2007) and Drosophila (Scholz-Kornehl and Schwarzel, 2016) learning and memory. Changes in human Dopamine D2-like receptor (DRD2) expression and function are famously associated with addiction, including AUD (Volkow et al., 1996). However, the mechanism by which alcohol regulates DRD2 expression or activity is unclear. Considering that Su(H) can act as both a transcriptional repressor or activator (Borggrefe and Oswald, 2009, 2016), and that alcohol could induce Notch activity in adult Drosophila heads (Figure 4A), we hypothesized that Dop2R may be a novel target of Su(H) downstream of adult Notch activity.
We identified predicted Su(H) binding sites upstream and within Dop2R using the computational transcription factor binding site tool, JASPAR (Mathelier et al., 2014) (For gene map see Supplemental Figure 5E). We then determined whether Su(H) occupancy at Dop2R was affected by alcohol exposure by performing ChIP–qPCR on heads acutely exposed to air or alcohol (Figure 6A). In response to alcohol exposure, Su(H) occupancy significantly decreased at an upstream predicted Su(H) binding site. There was no change at a predicted Su(H) site within the first intron of Dop2R, suggesting regional specificity of alcohol-induced loss of Su(H) abundance. Despite alcohol-induced changes in Notch activation and Su(H) mobilization, Dop2R mRNA transcript levels did not change immediately after acute exposure (Figure 6B), nor 24 hours after repeated alcohol exposure (Figure 6C) in head tissue. These findings show that acute alcohol exposure dynamically affects Su(H) occupancy at Dop2R in adult heads, but that no corresponding transcriptional effects are readily observed in total head Dop2R expression.
Figure 6. Alcohol exposure alters Su(H) binding at Dop2R and Dop2R is required for alcohol associative preference.
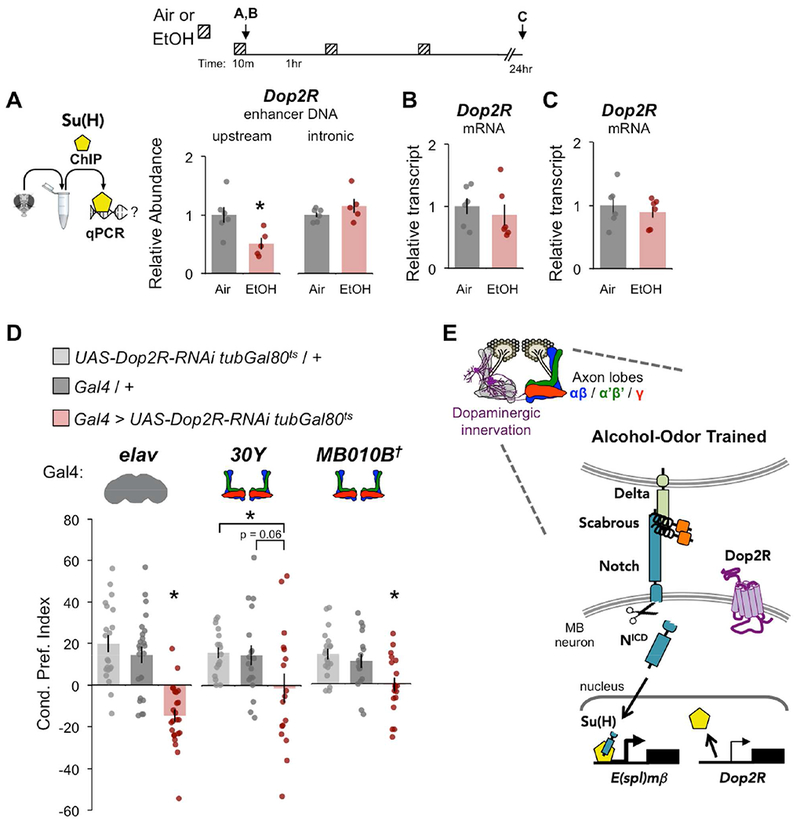
(A) Chromatin immunoprecipitation (ChIP) with αSu(H) followed by qPCR showed that alcohol experience reduced Su(H) occupancy at an upstream, but not intronic, Dop2R sequence, n = 6, 6. (B-C) Dop2R mRNA expression did not change in fly heads after acute 10 min alcohol exposure (C), nor 24 hr after repeated exposures (D); 10 min n = 6, 6; 24 hr n = 6, 6; see diagram above; data normalized to air. Mean ± SEM with statistical significance evaluated by ANOVA, posthoc Bonferroni compared to air, *p < 0.05. (D) Dop2R knockdown in all neurons (elav) or MB lobes (30Y or MB010B) impaired alcohol associative preference; elav n = 21, 24, 24; 30Y n = 17, 18, 17; MB010B n = 19, 17, 18; Mean ± SEM with statistical significance evaluated by ANOVA, posthoc Bonferroni compared to experimental, *p < 0.05, †Temperature insensitive split-GAL4. (E) Working model of alcohol-induced Notch activation in MB neurons causes changes in Su(H) occupancy at E(spl)mβ and Dop2R. See also Figures S2, S7, S9.
We hypothesized that use of whole head tissue obscures discrete MB-specific changes in Dop2R regulation that serve an important role in alcohol associative preference. To investigate this possibility, we thermo-genetically expressed Dop2R-RNAi in all adult neurons (elav-GAL4), or MB neurons (30Y-Gal4 or MB010B-Gal4), and found that knockdown of Dop2R impaired alcohol associative preference (Figure 6D, See Supplemental Figure 2C for thermogenetic adult-specificity controls). Together the data support a working model where alcohol-odor training induces Sca / Delta / Notch / Su(H) signaling in adult MB neurons, affects Dop2R regulation, and ultimately contributes to alcohol associative preference (Figure 6E).
Alcohol-odor training induces MB specific transcriptomic changes
Based on our previous findings, we hypothesized that alcohol-odor training induces changes in the MB-specific transcriptome, including changes in Dop2R regulation. To learn more about the lasting effects of alcohol-odor training, we set out to characterize experience-dependent transcriptomic changes in MB neurons.
To date, few studies have investigated memory-related transcriptome changes in MB neurons, and these efforts have primarily focused on gene, not transcript, level analyses (Crocker et al., 2016; Perrat et al., 2013). Unlike previous studies, we sought to determine changes in transcript expression 24 hours after alcohol-odor associative training, when flies show a preference for alcohol-associated cues. We chose to perform RNA-sequencing of MB nuclei using the INTACT method (Henry et al., 2012; Pankova and Borst, 2016). Before the isolation of MB nuclei, flies were exposed to ‘Odors’ or ‘Trained’ exposure paradigms (Figure 7A). MB INTACT (MB010B > unc84-GFP2x) flies were confirmed to have normal MB morphology (Supplemental Figure 7A) and alcohol associative preference (Supplemental Figure 7B). Read libraries were processed and analyzed with the ‘New Tuxedo’ bioinformatics pipeline (Pertea et al., 2016) (See Materials and Methods) and summarized in Supplemental Figure 7C.
Figure 7. Repeated alcohol exposures alter Dop2R in MB nuclear transcriptome.
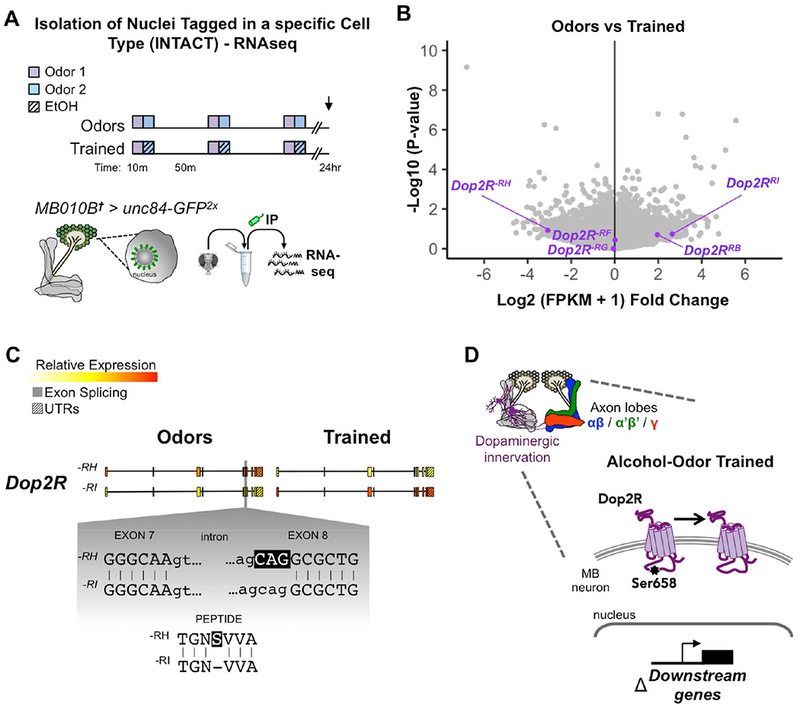
(A) Transcriptomic analyses were performed on MB nuclei isolated by the INTACT method 24 hours after flies were conditioned with Odors (odor only) or Trained (odors + ethanol) paradigms. Volcano plot displays fold change vs p-value statistics between Odors vs Trained libraries; Dopamine-2 receptor transcripts (purple). (B) Expression (FPKM) of Dop2R-RH transcript switched to Dop2R-RI in response to training. (C) Schematic of Dop2R gene (5’à3’) highlighting the single codon difference between –RH and –RI transcripts causing a Ser 658aa exclusion in the third cytoplasmic loop of Dop2R. (D) Working model of alcohol-induced change in Dop2R transcript usage in MB neurons. See also Figure S7, S8.
We observed high expression of common MB markers – Oamb, Fas2, VAChT, Pka-R2, prt, sNPF, and the INTACT transgene – in the MB nuclei (Supplemental Figure 8, blue). We also noted that sca is more highly expressed than Notch, Delta, and Su(H) transcripts (Supplemental Figure 8B,C, green), and that all four dopamine receptors are expressed in the MB, including various transcript isoforms of Dop2R (Supplemental Figure 8B,C, purple).
Alcohol-odor training alters isoform regulation of Dop2R in MB nuclei
Considering the strong association of D2-like receptors with addiction, and our finding that Su(H) targeting of Dop2R is affected by alcohol exposure, we first asked whether alcohol-odor training influenced the transcript regulation of Dop2R. Interestingly, the Dop2R−RH and Dop2R−RI transcripts showed large negative and positive fold change responses, respectively, suggesting a switch in isoform expression from Dop2R−RH to Dop2R−RI 24 hours after alcohol-odor training (Figure 7B). The only difference between the –H and –I isoforms is the inclusion of a Serine at 658aa in the Dop2R−PH protein (Figure 7C). This amino acid resides in the 3rd cytoplasmic loop of the fly and human D2-type GPCR (Figure 7D), and suggests that alcohol causes a lasting functional change to Dop2R in MB neurons. Importantly, performing a gene rather than transcript level analysis missed alternative isoform regulation of Dop2R (data not shown).
Identification of lasting MB transcriptomic changes following odor-alcohol training
To identify new transcripts that may play a role in alcohol associative behavior, we examined differential expression of transcripts affected by alcohol-odor training (‘Trained’) compared to odor only controls (‘Odors’) in MB nuclei. Using a false discovery rate of q-value < 0.05, the expression of eight transcripts were found to be significantly altered–SREBP−RC, shr−RP, CG9098−RA, CG3558−RB, CG9098−RB, Sec31−RA, Sdc−RK, Stat92E−RH (Figure 8A,B; Supplemental Figure 8B,C, red). Interestingly, there were notable changes in alternative isoform regulation in response to alcohol-odor training (Figure 8C). For example, both CG9098 and Stat92E showed conditional promoter usage, thus altering the expression of 5’ exonic sequences. In sum, these bioinformatic profiles provide evidence of lasting experience-dependent changes to the MB transcriptome.
Figure 8. Repeated alcohol exposures changes expression in MB nuclear transcriptome.
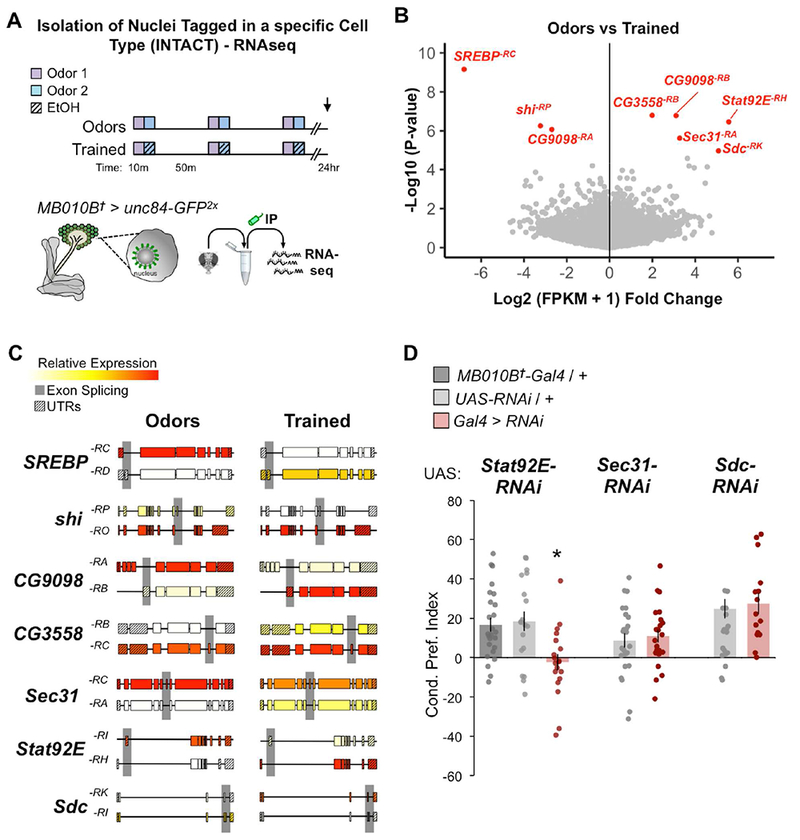
(A) Same transcriptomic approach as Figure 7A. (B) Same volcano plot of fold change comparing fold change and p-value statistics of Odors vs Trained libraries as Figure 7A; statistically significant q-value < 0.05 transcripts (red). (C) Schematic of significantly altered transcripts (5’→3’) highlighting notable exon splicing differences. D) Knockdown of Stat92E, but not Sec31, or Sdc with RNAi in MB lobes (MB010B) impaired alcohol associative preference; Stat92Ei n = 25, 19, 19; Sec31i n = 25, 23; Sdci n = 16, 15; Mean ± SEM with statistical significance evaluated by ANOVA, posthoc Bonferroni compared to experimental, *p < 0.05; †Temperature insensitive split-GAL4. See also Figure S2, S7, S8, S9.
Despite it being difficult to achieve isoform-specific manipulation, we behaviorally assessed flies expressing available RNAi lines targeting three upregulated transcripts - Stat92E, Sec3T, and Sdc in MB neurons (MB010B-Gal4). Knockdown of Stat92E, but not Sec31 or Sdc, impaired alcohol associative preference compared to genetic controls (Figure 8D) suggesting that Stat92E is required in the MB neurons for lasting alcohol associative preference. It remains unclear what role isoform-specific transcripts of Sec31 or Sdc may have in this behavior or if compensatory mechanisms exist in their absence.
Discussion
A mechanistic understanding of how preference for alcohol-associated cues arises and persists at the neuromolecular level is currently lacking. Our approach to address this process uniquely intersects the fields of alcohol addiction, learning and memory, and conserved Notch signaling. It also necessitates molecular, circuit, and behavioral level perspective. We found that long-term preference for alcohol-associated cues in Drosophila requires Scabrous / Delta / Notch / Su(H) signaling, and that repeated alcohol experiences alter transcription in memory-encoding mushroom body neurons. These findings offer a molecular framework for how dysregulation of a conserved transcriptional program contributes to maladaptive memories associated with addiction.
The undervalued role of Notch in adulthood
The Notch transduction pathway is widely recognized for its conserved developmental role in neuroectoderm differentiation via lateral inhibition, a process that essentially links the fate of cells to their neighbors (Artavanis-Tsakonas and Muskavitch, 2010). Often overlooked, however, is the profound impact of Notch on adult cellular physiology, particularly in the nervous system (Ables et al., 2011). Both canonical (Su(H)-dependent) and noncanonical (Su(H)-independent) Notch signaling are implicated in long-term associative memory (Alberi et al., 2011; Brai et al., 2015; Ge et al., 2004; Song et al., 2009; Wang et al., 2004; Zhang et al., 2015). Furthermore, Notch underlies experience-dependent changes in neuronal morphology and physiology, including long-term potentiation and depression, and expression of synaptic vesicle proteins (Alberi et al., 2011; Kidd et al., 2015; Lieber et al., 2011; Wang et al., 2004). Consistent with Notch’s critical role in adult neuronal physiology, we found that post-developmental Notch / Su(H) signaling is required in the Drosophila MB neurons for long-term associative memories of alcohol exposure. Acute alcohol exposure induced changes in Su(H) function, which may initiate or perpetuate maladaptive transcriptional events including the regulation of known Notch target genes (i.e. E(spl)mβ) and novel targets (i.e. Dop2R).
This work emphasizes that Notch-dependent signaling is an important cellular program that coordinates the neuronal plasticity required for drug-associated long-term memory. Notably, it does not address the contribution of non-canonical (Su(H)-independent) Notch signaling, or coordinated cross-talk with other downstream signaling cascades that converge to regulate transcription. For instance, the Notch intracellular domain might interact with the highly-studied CREB (Ca2+/cAMP-responsive element-binding protein) / CBP (CREB-binding protein) pathway to influence transcriptional output (Hallaq et al., 2015; Hirano et al., 2016; Zhang et al., 2013a; Zhang et al., 2015).
Changes in D2R following alcohol experience
A hallmark feature of addiction to drugs of abuse, including alcohol, is reduced striatal D2 dopamine receptor function (Heinz et al., 2004; Volkow et al., 1996). Our finding that Dop2R is crucial for alcohol associative preference (Figure 6) supports the fundamental importance of D2 regulation in response to alcohol. Interestingly, alcohol-odor training altered the MB expression of Dop2R isoforms (Figure 7), which is functionally reminiscent to mammalian alcohol-, and cocaine-associated splicing of 29 third cytoplasmic loop amino acids in short versus long DRD2 isoforms (Moyer et al., 2011; Sasabe and Ishiura, 2010). Interestingly, a Su(H) binding site is predicted near the intronic branch point between Dop2R’s exons 7 and 8, which suggests a possible mechanism by which Notch activity could influence splicing machinery and result in the expression of alternative transcript isoforms. Although the functional relevance of the Dop2R−PH and Dop2R−PI isoforms is unknown, the single amino acid (Ser658) difference is conserved in the human DRD2 (Ser262) and is located in the receptor’s third cytoplasmic loop, where downstream Gαi protein interactions occur.
A recent mouse study has intriguingly identified a link between Notch/RBP-J and striatal dopamine function (Toritsuka et al., 2017). Conditional knockout of RBP-J (Su(H) in flies) in neurons decreased striatal dopamine release, increased D1 agonist responsivity, and affected the acquisition and maintenance of conditioned avoidance behavior (Toritsuka et al., 2017). Since D1 and D2 receptor binding and expression were not altered in the absence of RBP-J, the cellular mechanisms of how Notch/RBP-J signaling affects dopaminergic activity still remain unclear. Our work provides possible Su(H)-mediated changes that may alter downstream D2 receptor signaling, which offer novel avenues for future research.
Differential transcriptional state following repeated alcohol exposure
Alcohol-induced transcriptional changes in brain tissue are often mild, widespread, and both brain region- and time-specific (Melendez et al., 2012; Morud et al., 2017; Smith et al., 2016). We identified lasting changes in eight alternatively spliced transcripts – CG3558−RB, CG9098−RA, CG9098−RB, Sdc−RK, Sec31−RA, shi−RP, SREBP−RC, Stat92E−RH. Many forms of genetic variation, including alternative splicing, have been observed in human alcoholic and animal model brain tissue (Farris and Mayfield, 2014). We particularly observed differential usage of transcript’s first exons. These findings are strictly contextualized to MB nuclei, and require subsequent experimentation to functionally interpret, but are exciting to consider nonetheless. For instance, alternative first exon usage of the Stat92E transcription factor is a negative feedback mechanism that controls its own transcription (Henriksen et al., 2002), suggesting that alcohol training causes lasting changes in Stat92E activity in the MB neurons. Interestingly, the innate immunity JAK/Stat (Janus kinase-Signal transducer and activator of transcription) pathway shows extensive crosstalk with Notch signaling (Kamakura et al., 2004). Other differentially expressed transcripts in response alcohol-odor training are known to be involved in axon targeting, neuron projection morphogenesis, endocytosis, fatty acid biosynthesis, and innate immunity. Further isoform-specific investigation is required to understand the functional role of these enticing targets in the formation and maintenance of alcohol cue-associated reactivity.
Summary and conclusions
Using a combination of techniques in Drosophila, Scabrous, a secreted extracellular matrix peptide, is shown to regulate adult alcohol-induced Notch/Su(H) activity. We propose that alcohol influences gene expression by dysregulating this highly conserved pathway. Furthermore, alcohol-odor training caused lasting changes to transcript splicing in memory circuitry, including in the D2 dopamine receptor. This work stresses the importance of Notch signaling and lasting genetic consequences in adult alcohol-associated neural plasticity. These mechanistic insights warrant further investigation to provide traction for therapeutic approaches in mammalian models of Alcohol Use Disorder.
STAR Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Karla R. Kaun (karla_kaun@brown.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Fly Husbandry
Flies were raised in cornmeal agar food (without propionic acid) at 24°C and 70% humidity under a 14:10 Light:Dark cycle. Male flies were used throughout the entirety of this work. Unless otherwise stated, 3-7 day-old male flies were isolated under light CO2, given 1 day to recover, and then used for behavioral or molecular assays. Information on RNAi validation can be found in Supplemental Figure 9 and Data Files S7. Fly lines used in this manuscript are listed in the KEY RESOURCES TABLE.
METHOD DETAILS
Alcohol associative preference paradigm
All flies were trained and tested as previously described (Kaun et al., 2011). A minor difference was the use of smaller training/testing vials with 20-30 flies/vial resulting in 90:60 (EtOH:Air) exposure causing a 13.8 ± 3 mM (0.01 g/dL) internal body ethanol concentration. As mentioned in text, all behavioral experiments were performed in reciprocal (averaged between alternative order of odors) alongside genetic controls, and although Gal80 does not effectively inhibit the split-Gal4 protein (Luan et al., 2006), temperature shifting paradigms were maintained for experiments using split-Gal4 genotypes to control for temperature effects on Gal4 and behavior. Each n is the average across reciprocals for ~50 flies, and data from entire experiments were discarded from analysis when genetic controls showed no alcohol associative preference memory.
Immunohistochemistry
To avoid rapid protein degradation, whole flies were immediately fixed in 4% PFA PBT (0.1%Triton) for 20 min on ice before brain dissection in 1× PBS, then brains were fixed in 2% PFA PBT overnight at 4°C. Brains were continuously rocked on a nutator throughout the remainder of the protocol. Brains were washed 4× 15 min with PBT, blocked at room temperature 1 hr in PBT-5% Goat serum. Primary antibodies (1:400) in PBT-Goat were incubated overnight at 4°C. Brains were then washed 4× 15 min with PBT and secondary antibodies (1:500) in PBT-Goat were incubated at room temperature 1 hr. Again, brains were washed 4× 15 min with PBT, then mounted in DAPI Fluoromount-G® (SouthernBiotech). See Key Resources for more information.
Confocal Microscopy
Images were obtained using a Zeiss LSM 800 Confocal Laser Scanning Microscope and Zeiss Blue software. Non-saturating laser power and gain were determined for each channel and held constant throughout an experiment. FIJI was used to define regions of interest (ROI manager), count 3D objects (“3D Objects Counter” plug-in), determine co-localization (“Colocalization” plug-in) to create Z-stack images, and apply contrast/brightness settings for visualization purposes.
Specifics: 2 μm Z-sections were used with a 20× objective, 1 μm Z-sections for a 40×oil objective and 0.5μm Z-sections for a 63×oil objective. Max stack images vary in depth shown – Z-series are available upon request. Any ROIs drawn and saved reflect the anatomical localization of Kenyon cells (KCs). In N-LV > dGFP experiments, manual scoring (blinded to genotype) or computational quantification was used – ROIs were background subtracted, signals dilated using Gray Morphology, 3D extended MinMax using MorphoLib, watershed, and objects counted with 3D Object Counter (default threshold, size criterion 100-5000) (Supplemental Figure 6). The two hemispheres of a fly were averaged for n = 1.
Western blots
For head collections, flies were frozen in liquid nitrogen, decapitated by brief vortexing, and heads were partitioned using chilled metal sieves (Humboldt Mfg. Co., Elgin, IL). Immediately after volatilized alcohol or air treatments, 20 frozen heads per sample were homogenized in 34 uL 2× Laemmli buffer (Bio-Rad) using a motorized tissue grinder and homogenate was separated on PROTEAN™ TGX Stain-Free™ native gels (Bio-Rad) or containing SDS for observing Sca dimers. Protein was transferred to a PVDF membrane, and then UV light was used to detect trihalo reactions with tryptophan in a Gel Doc EZ imager (Bio-rad), thus providing a stain-free measures of total protein. Membranes were blocked with 5% nonfat soy milk (WestSoy) in TBSTween-20 (1%), and probed with primary antibodies (1:100 αNotch, 1: 1000 αGFP) overnight at 4 degrees. Membranes were washed with TBSTween-20 before incubating with secondary antibody (1:10000) at room temperature, then washed with TBSTween-20 again. Bands were visualized with SuperSignal™ West Femto ECL (ThermoFisher) and either CL-XPosure™ Film (ThermoFisher) or a c600 imager (Azure Biosystems). For densitometric quantification, protein levels were normalized to total protein and compared to wildtype/control average band intensity (Data File S1–6). This quantification method provided a more reliable measure for normalization since alcohol exposure can change the expression of common housekeeping proteins. Each n in blots corresponds to biologically distinct samples of ~20 fly heads, often run on at least more than one gel and across different days.
Co-immunoprecipitation
~1,200 N-GFP (N55e11 ; N-GFP) or Sca-GFP (sca5-120 > UAS-ScaGFP) heads were homogenized in ice cold co-IP lysis buffer (50 mM HEPES (pH 7.2), 150 mM NaCL, 2 mM MgCl2, 2 mM CaCl2, 5 mM KCl, 0.1% Igepal, 20% glycerol) with protease inhibitors (Sigma I3911). Insoluble material was pelleted by centrifugation at 5,000 rpm for 5 min. Supernatants were incubated with either rabbit αGFP or mock IgG for 2 hr. Protein-bound antibody was collected by incubating with 10 uL of Dynabeads Protein A for 1 hr and beads were collected with a magnet. Protein-bound beads were gently washed once with co-IP lysis buffer with no Igepal or protease inhibitors, then eluted with 2× Laemmli sample buffer (Bio-Rad). Samples were reduced with 1% beta-mercaptoethanol and boiled for 5 min. Samples used for Scabrous blots were not reduced, and were incubated with 10 mM N-ethylmaleimide (NEM) to prevent artificial dimerization.
qRT-PCR
Following specified treatment conditions, total RNA was extracted from roughly 100 heads using TRIzol (Ambion, Life Technologies) and treated with DNase (Ambion DNA-Free Kit). Equal amounts of RNA were reverse-transcribed into cDNA (Applied Biosystems), then analyzed with Sybr Green Real-Time PCR (BioRad, ABI PRISM 7700 Sequence Detection System). Biological (≥3) and technical (≥2) replicates were performed and PCR conditions were as follows: 15 sec 95°C, 1 min 55°C, 40×, primer sequences in supplemental material. Across all samples and targets Ct threshold was set to 0.6 and amplification start/stop were manually adjusted. For comparative ΔCt method analysis all target genes were first normalized to CG13646 – a lowly expressed gene that does not change with alcohol exposure and is not targeted by Su(H)) – expression, then compared to control treatment or genotype to assess fold enrichment (ΔΔ CT method).
ChIP-qPCR
Roughly 1000 liquid nitrogen frozen fly heads were homogenized in 1× PBS + 1× protease inhibitors + 1.8% formaldehyde, allowed to cross-link for 20 min with gentle agitation at room temperature, then quenched by adding 0.60 g glycine. Samples were sequentially washed and spun down before being sonicated for 25 sec 5× each with a Fisher FB505 sonicator and FB4418 microtip with 1/8” diameter at 20% amplitude. Sheared chromatin samples were precleared with magnetic beads (half Dynabead A and half Dynabead G). 250 μl samples were then incubated with 20 μL αSu(H), or mock IgG, overnight at 4°C, then followed by an overnight at 4°C incubation with 50 μL of Dyn abead A and G magnetic beads. Beads were washed 4× with IP buffer (1× TE, 140mM NaCl, 1% Triton X-100, 0.1% SDS, 0.1% sodium deoxycholate, protease inhibitors) then twice with 1× TE buffer. DNA was then eluted off the beads through two sequential incubations at 65Ό in 125 μL of bicarbonate elution buffer for 15 min. Aliquots were combined and kept at 65°C overnight to undo crosslinking, followed by incubation at 42°C for 2-3 hrs by adding 1 μL Prote inase K to degrade remaining protein. A phenol/chloroform extraction followed by ethanol extraction was performed. DNA pellets were resuspended in 50 μL of autoclaved water for use in qPCR. SsoAdvanced Universal SYBRGreen Supermix (Bio-Rad) was used to perform qPCR at conditions stated above. ChIP samples using Su(H) pulldown for each paired condition were normalized to average mock IgG pulldown signal as a measure of background signal for each sequence target. Fold enrichment for each target was then normalized to CG13646 – a lowly expressed gene that does not change with alcohol exposure and is not targeted by Su(H)) – and compared to control treatment within each biological replicate (ΔΔ CT method).
INTACT
Isolation of Nuclei Tagged in specific Cell Types (INTACT) was adapted from the method described by (Pankova and Borst, 2016) and pioneered in flies using UAS-unc84-2xGFP by (Henry et al., 2012). The heads of flies sacrificed 24 hr after Odors or Trained paradigms (Figure 6A, 7A) were collected with biological replicates n = 4, 4, respectively, and with reciprocal odor groups (n = 2 each) combined for bioinformatic analysis. For each sample, two thousand snap frozen male fly heads were homogenized in a Kontes glass homogenizer (Sigma, D9938-1SET) with 10 mL of dounce buffer (10 mM β-glycerophosphate, 2 mM MgCl2, 0.5% Igepal buffer) and dounced gently for approximately 2 min with the loose A pestle. Homogenate was passed through a 190 μm nylon net filter (Small Parts, CMN-0185-C) and then the filter was rinsed with 2 mL of the same buffer before discarding. Homogenate was poured back into the rinsed homogenizer and dounced gently 6-7 times with the tight B pestle. Homogenate was passed through a 20 μm filter (Small Parts F020N-12-C), and volume was brought to 50 mL by adding sucrose buffer (10 mM ß-glycerophosphate, 2 mM MgCl2, 25 mM KCl, 250 mM sucrose) to the resulting filtrate. 300 μL of Dynabead A magnetic beads pre-incubated with 10 μL rabbit aGFP were incubated with the filtrate for 30 min at 4°C with gentle agitation. Beads were cap tured with a magnet for 15 min, then washed five times with 600 μL of sucrose buffer for 5 min at 4°C with gentle agitation. RNA was extracted from bead-bound nuclei using TRIzol (Ambion, Life Technologies) and DNAse treated (Ambion DNA-Free Kit).
RNA-seq
RNA library preparation and Illumina HiSeq sequencing was performed by GENEWIZ (South Plainfield, NJ). PolyA enrichment and single-end 1×50bp reads were acquired to encapsulate the processed mRNAs likely to be translated. Trimmomatic-0.36 (parameters: TruSeq3-SE.fa:2:30:10:8:true LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:36), HISAT2-2.0.5 (aligned to Ensembl BDGP6_transcriptome reference, modified to include unc-84-2xGFP to confirm INTACT pulldown), samtools-1.3.1 (samtools sort), stringtie-1.3.3 (aligned to BDGP6_transcriptome). RStudio (v1.0.136) was used to assemble a Ballgown (Pertea et al., 2016).
STATISTICAL ANALYSIS
General Statistics
The vast majority of data sets had normal distributions (Shapiro-Wilk test), thus were assessed with parametric statistics. For all experiments raw data, data mean, and standard error of the mean are shown. Behavioral experiences and blot quantifications were statistically evaluated by ANOVA, posthoc Bonferroni compared to the experimental genotype, *p < 0.05, **p < 0.01, ***p < 0.001.
Bioinformatic Statistics
Bioinformatic analyses were performed using a flexible linear model framework in the R package called Ballgown (Frazee et al., 2015). Default library-size adjustment was defined by the sum of a sample’s log expression below the 75th percentile and used for linear modeling; no cutoffs were made for lowly expressed transcripts.
DATA AVAILABILITY
Raw data
Raw data from Western blots and qPCR experiments are included in the Data Files S1–7. All other data is available upon request.
RNA-seq data
The nuclear mushroom body transcriptome .fastq and count data have been deposited in the Gene Expression Omnibus (GEO) under GSE108525.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Mouse anti-Notch | DSHB, University of IA | Cat# C17.9C6 |
| Rabbit anti-Elav | DSHB, University of IA | Cat# 7E8A10 |
| Mouse anti-Repo | DSHB, University of IA | Cat# 8D12 |
| Mouse anti-Delta | DSHB, University of IA | Cat# C594.9B |
| Rabbit anti-GFP | Thermo Fisher Scientific, CA | Cat# A-11122 |
| Rat anti-RFP | Chromotek | Cat# 5F8 |
| Mouse anti-Sca | DSHB, University of IA | Cat# mAb sca1 |
| Goat anti-SuH | Santa Cruz Biotech, CA | Cat# sc-15813 |
| HRP-Goat anti-Mouse | Thermo Fisher Scientific, CA | Cat# 626520 |
| HRP-Goat anti-Rabbit | Thermo Fisher Scientific, CA | Cat# 827036 |
| HRP-Rabbit anti-Mouse | Thermo Fisher Scientific, CA | Cat# A27025 |
| Goat AlexaFluor 405-conjugated anti-Mouse | Thermo Fisher Scientific, CA | Cat# A-31553 |
| Goat AlexaFluor 488-conjugated anti-Mouse | Thermo Fisher Scientific, CA | Cat# A-11029 |
| Goat AlexaFluor 488-conjugated anti-Rabbit | Thermo Fisher Scientific, CA | Cat# A-11008 |
| Goat AlexaFluor 568-conjugated anti-Rat | Thermo Fisher Scientific, CA | Cat# A-11077 |
| Goat AlexaFluor 647-conjugated anti-Mouse | Thermo Fisher Scientific, CA | Cat# A28181 |
| Critical Commercial Assays | ||
| High-Capacity cDNA Reverse Transcription Kit | Thermo Fisher Scientific, CA | Cat# 4368814 |
| SsoAdvanced Universal SYBR Green Supermix | BIO RAD | Cat# 1725274 |
| Deposited Data | ||
| MB010B > INTACT, raw .fastq and count data | (this paper) | GEO, GSE108525 |
| Experimental Models: Organisms/Strains | ||
| wt (Berlin) | Kaun et al. 2011 | N/A |
| sca5-120 | Kaun et al. 2011 | N/A |
| scaBP2 | Bloomington Stock Center | BL#7320 |
| elavc155-Gal4 | Bloomington Stock Center | BL#458 |
| 30Y-Gal4 | Bloomington Stock Center | BL# 30818 |
| GMR-Gal4 | Bloomington Stock Center | BL# 9146 |
| MB005B-Gal4 | Aso et al. 2014 | N/A |
| MB008B-Gal4 | Aso et al. 2014 | N/A |
| MB009B-Gal4 | Aso et al. 2014 | N/A |
| MB010B-Gal4 | Aso et al. 2014 | N/A |
| MB152B-Gal4 | Aso et al. 2014 | N/A |
| tubGal80ts | Bloomington Stock Center | BL#7019 |
| UAS-sca-RNAi | Vienna Drosophila Resource Center | VDRC#44527 |
| UAS-Notch-RNAi | Vienna Drosophila Resource Center | VDRC#1112 |
| UAS-Su(H)-RNAi | Vienna Drosophila Resource Center | VDRC#103597 |
| UAS-Dop2R-RNAi | Vienna Drosophila Resource Center | VDRC#11471 |
| N-GFP (N55e11 ; N-GFP) | Couturier et al., 2014 | N/A |
| UAS-sca-GFP | Chou and Chien, 2012 | N/A |
| UAS-GFP-sca | Chou and Chien, 2012 | N/A |
| tubNotch-LexAVP16 | Barolo et al., 2000 | N/A |
| lexAop-dGFP | Barolo et al., 2000 | N/A |
| MB247-DsRed | Pech et al., 2013 | N/A |
| UAS-unc84-5xGFP | Henry et al., 2012 | N/A |
| UAS-GFP-RNAi | Bloomington Stock Center | BL#41553 |
| UAS-Stat92E-RNAi | Vienna Drosophila Resource Center | VDRC#106980 |
| UAS-Sec31-RNAi | Vienna Drosophila Resource Center | VDRC#35867 |
| UAS-Sdc-RNAi | Vienna Drosophila Resource Center | VDRC#13322 |
| Oligonucleotides | ||
| Primers for RT-qPCR, see Table S1 | (this paper) | N/A |
| Primers for ChIP-qPCR, see Table S2 | (this paper) | N/A |
| mbetaF GACGCATTCGAAATGAGCTA | Skalska et al., 2015 | N/A |
| mbetaR GTCTTGGCCCGTTTATCAC | Skalska et al., 2015 | N/A |
| Software and Algorithms | ||
| Fiji-1.0 | Schindelin et al. 2012 | https://imagej.net/Fiji/Downloads |
| Trimmomatic-0.36 | Pertea et al. 2016 | http://www.usadellab.org/cms/?page=trimmomatic |
| HISAT2-2.0.5 | Pertea et al. 2016 | https://ccb.jhu.edu/software/hisat2/index.shtml |
| samtools-1.3.1 | Pertea et al. 2016 | https://www.htslib.org/doc/ |
| stringtie-1.3.3 | Pertea et al. 2016 | https://ccb.jhu.edu/software/stringtie/-install |
| RStudio-1.0.136 | Pertea et al. 2016 | https://download1.rstudio.org/RStudio-1.0.136.dmg |
| Ballgown-2.6.0 R package | Pertea et al. 2016 | https://www.bioconductor.org/packages/release/bioc/html/ballgown.html |
Supplementary Material
Data File S1. Data support ‘in text’ Figure 2B-C. Raw Western blots and corresponding Trihalo TGX Stain Free total protein gel images and raw densitometry.
Data File S2. Data support Supplemental Figure 5A. Raw Western blots and corresponding Trihalo TGX Stain Free total protein gel images and raw densitometry.
Data File S3. Data support ‘in text’ Figure 4A. Raw Western blots and corresponding Trihalo TGC Stain Free total protein gel images and raw densitometry.
Data File S4. Data support ‘in text’ Figure 4A. Raw Western blots and corresponding Trihalo TGC Stain Free total protein gel images and raw densitometry.
Data File S5. Data support ‘in text’ Figure 4A. Raw Western blots and corresponding Trihalo TGC Stain Free total protein gel images and raw densitometry.
Data File S6. Data support Supplemental Figure 5C-D. Raw Western blots and corresponding Trihalo TGC Stain Free total protein gel images and raw densitometry.
Data File S7. Data support Supplemental Figure 9A. Raw ΔΔCT values.
Acknowledgements
This work was supported by RI-INBRE (NIGMS P20GM103430 Project Leader K.R.K.), Smith Family Award for Excellence in Biomedical Research, NIAAA (R01AA024434 to K.R.K.), and BIBS Center for Nervous System Function COBRE (NIGMS P20GM103645, Project Leader K.R.K). We thank all of the Kaun lab members and the Brown community for fruitful discussions. We also appreciate the sharing of fly stocks from the Drosophila community, the Bloomington Stock Center, and the Vienna Drosophila RNAi Center. Additionally, we would like to thank Arjun Ray and Rachel Muster for transgenic stock generation and initial characterizations, Amanda Waterman and Katie McCullar for technical assistance, Nick Baker (Albert Einstein College of Medicine), Nic Giagtzoglou (Baylor University Medical Center) and Toby Lieber (NYU School of Medicine) for reagents and advice, Jason Wood (Brown University) for ChIP guidance, and Leila Reider (Brown University) for RNA-sequencing support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no competing financial interests.
References
- Ables JL, Breunig JJ, Eisch AJ, and Rakic P (2011). Not(ch) just development: Notch signalling in the adult brain. Nat Rev Neurosci 12, 269–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberi L, Liu S, Wang Y, Badie R, Smith-Hicks C, Wu J, Pierfelice TJ, Abazyan B, Mattson MP, Kuhl D, et al. (2011). Activity-induced Notch signaling in neurons requires Arc/Arg3.1 and is essential for synaptic plasticity in hippocampal networks. Neuron 69, 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, and Muskavitch MA (2010). Notch: the past, the present, and the future. Curr Top Dev Biol 92, 1–29. [DOI] [PubMed] [Google Scholar]
- Arya MA, Tai AK, Wooten EC, Parkin CD, Kudryavtseva E, and Huggins GS (2013). Notch pathway activation contributes to inhibition of C2C12 myoblast differentiation by ethanol. PLoS One 8, e71632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Hattori D, Yu Y, Johnston RM, Iyer NA, Ngo TT, Dionne H, Abbott LF, Axel R, Tanimoto H, et al. (2014). The neuronal architecture of the mushroom body provides a logic for associative learning. Elife 3, e04577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE, Mlodzik M, and Rubin GM (1990). Spacing differentiation in the developing Drosophila eye: a fibrinogen-related lateral inhibitor encoded by scabrous. Science 250, 1370–1377. [DOI] [PubMed] [Google Scholar]
- Barolo S, Walker RG, Polyanovsky AD, Freschi G, Keil T, and Posakony JW (2000). A notch-independent activity of suppressor of hairless is required for normal mechanoreceptor physiology. Cell 103, 957–969. [DOI] [PubMed] [Google Scholar]
- Brai E, Marathe S, Astori S, Fredj NB, Perry E, Lamy C, Scotti A, and Alberi L (2015). Notch1 Regulates Hippocampal Plasticity Through Interaction with the Reelin Pathway, Glutamatergic Transmission and CREB Signaling. Front Cell Neurosci 9, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray SJ (2016). Notch signalling in context. Nat Rev Mol Cell Biol. [DOI] [PubMed] [Google Scholar]
- Chou YH, and Chien CT (2002). Scabrous controls ommatidial rotation in the Drosophila compound eye. Dev Cell 3, 839–850. [DOI] [PubMed] [Google Scholar]
- Costa RM, Honjo T, and Silva AJ (2003). Learning and memory deficits in Notch mutant mice. Curr Biol 13, 1348–1354. [DOI] [PubMed] [Google Scholar]
- Couturier L, Trylinski M, Mazouni K, Darnet L, and Schweisguth F (2014). A fluorescent tagging approach in Drosophila reveals late endosomal trafficking of Notch and Sanpodo. J Cell Biol 207, 351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker A, Guan XJ, Murphy CT, and Murthy M (2016). Cell-Type-Specific Transcriptome Analysis in the Drosophila Mushroom Body Reveals Memory-Related Changes in Gene Expression. Cell reports 15, 1580–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bivort BL, Guo HF, and Zhong Y (2009). Notch signaling is required for activity-dependent synaptic plasticity at the Drosophila neuromuscular junction. J Neurogenet 23, 395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devineni AV, and Heberlein U (2013). The evolution of Drosophila melanogaster as a model for alcohol research. Annual review of neuroscience 36, 121–138. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wills DN, and Havstad J (2012). Ethanol reduces the phase locking of neural activity in human and rodent brain. Brain Res 1450, 67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SP, and Mayfield RD (2014). RNA-Seq reveals novel transcriptional reorganization in human alcoholic brain. Int Rev Neurobiol 116, 275–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazee AC, Pertea G, Jaffe AE, Langmead B, Salzberg SL, and Leek JT (2015). Ballgown bridges the gap between transcriptome assembly and expression analysis. Nat Biotechnol 33, 243–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavish A, Shwartz A, Weizman A, Schejter E, Shilo BZ, and Barkai N (2016). Periodic patterning of the Drosophila eye is stabilized by the diffusible activator Scabrous. Nature communications 7, 10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Hannan F, Xie Z, Feng C, Tully T, Zhou H, Xie Z, and Zhong Y (2004). Notch signaling in Drosophila long-term memory formation. Proc Natl Acad Sci U S A 101, 10172–10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagtzoglou N, Li T, Yamamoto S, and Bellen HJ (2013). Drosophila EHBP1 regulates Scabrous secretion during Notch-mediated lateral inhibition. J Cell Sci 126, 3686–3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallaq R, Volpicelli F, Cuchillo-Ibanez I, Hooper C, Mizuno K, Uwanogho D, Causevic M, Asuni A, To A, Soriano S, et al. (2015). The Notch intracellular domain represses CRE-dependent transcription. Cell Signal 27, 621–629. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, et al. (2004). Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. The American journal of psychiatry 161, 1783–1789. [DOI] [PubMed] [Google Scholar]
- Heitzler P, and Simpson P (1991). The choice of cell fate in the epidermis of Drosophila. Cell 64, 1083–1092. [DOI] [PubMed] [Google Scholar]
- Henriksen MA, Betz A, Fuccillo MV, and Darnell JE Jr. (2002). Negative regulation of STAT92E by an N-terminally truncated STAT protein derived from an alternative promoter site. Genes Dev 16, 2379–2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry GL, Davis FP, Picard S, and Eddy SR (2012). Cell type-specific genomics of Drosophila neurons. Nucleic Acids Res 40, 9691–9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y, Ihara K, Masuda T, Yamamoto T, Iwata I, Takahashi A, Awata H, Nakamura N, Takakura M, Suzuki Y, et al. (2016). Shifting transcriptional machinery is required for long-term memory maintenance and modification in Drosophila mushroom bodies. Nature communications 7, 13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K, Sen A, and Artavanis-Tsakonas S (2013). Notch signaling at a glance. J Cell Sci 126, 2135–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Lee EC, and Baker NE (1995). Molecular analysis of scabrous mutant alleles from Drosophila melanogaster indicates a secreted protein with two functional domains. Genetics 141, 607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, and Volkow ND (2005). The neural basis of addiction: a pathology of motivation and choice. The American journal of psychiatry 162, 1403–1413. [DOI] [PubMed] [Google Scholar]
- Kamakura S, Oishi K, Yoshimatsu T, Nakafuku M, Masuyama N, and Gotoh Y (2004). Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nat Cell Biol 6, 547–554. [DOI] [PubMed] [Google Scholar]
- Kaun KR, Azanchi R, Maung Z, Hirsh J, and Heberlein U (2011). A Drosophila model for alcohol reward. Nat Neurosci 14, 612–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaun KR, Devineni AV, and Heberlein U (2012). Drosophila melanogaster as a model to study drug addiction. Hum Genet 131, 959–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd S, Struhl G, and Lieber T (2015). Notch is required in 659 adult Drosophila sensory neurons for morphological and functional plasticity of the olfactory circuit. PLoS Genet 11, e1005244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EC, Hu X, Yu SY, and Baker NE (1996). The scabrous gene encodes a secreted glycoprotein dimer and regulates proneural development in Drosophila eyes. Mol Cell Biol 16, 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EC, Yu SY, and Baker NE (2000). The scabrous protein can act as an extracellular antagonist of notch signaling in the Drosophila wing. Curr Biol 10, 931–934. [DOI] [PubMed] [Google Scholar]
- Lieber T, Kidd S, and Struhl G (2011). DSL-Notch signaling in the Drosophila brain in response to olfactory stimulation. Neuron 69, 468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Wang Y, Worley PF, Mattson MP, and Gaiano N (2015). The canonical Notch pathway effector RBP-J regulates neuronal plasticity and expression of GABA transporters in hippocampal networks. Hippocampus 25, 670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan H, Peabody NC, Vinson CR, and White BH (2006). Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron 52, 425–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathelier A, Zhao X, Zhang AW, Parcy F, Worsley-Hunt R, Arenillas DJ, Buchman S, Chen CY, Chou A, Ienasescu H, et al. (2014). JASPAR 2014: an extensively expanded and updated open-access database of transcription factor binding profiles. Nucleic Acids Res 42, D142–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno M, Horiuchi J, Tully T, and Saitoe M (2009). The Drosophila cell adhesion molecule klingon is required for long-term memory formation and is regulated by Notch. Proc Natl Acad Sci U S A 106, 310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melendez RI, McGinty JF, Kalivas PW, and Becker HC (2012). Brain region-specific gene expression changes after chronic intermittent ethanol exposure and early withdrawal in C57BL/6J mice. Addiction biology 17, 351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M, Baker NE, and Rubin GM (1990). Isolation and expression of scabrous, a gene regulating neurogenesis in Drosophila. Genes Dev 4, 1848–1861. [DOI] [PubMed] [Google Scholar]
- Morrow D, Cullen JP, Cahill PA, and Redmond EM (2008). Ethanol stimulates endothelial cell angiogenic activity via a Notch- and angiopoietin-1-dependent pathway. Cardiovasc Res 79, 313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morud J, Ashouri A, Larsson E, Ericson M, and Soderpalm B (2017). Transcriptional profiling of the rat nucleus accumbens after modest or high alcohol exposure. PLoS One 12, e0181084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer RA, Wang D, Papp AC, Smith RM, Duque L, Mash DC, and Sadee W (2011). Intronic polymorphisms affecting alternative splicing of human dopamine D2 receptor are associated with cocaine abuse. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 36, 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Soriano V, Santos D, Durupt FC, Casani S, and Paricio N (2016). Scabrous overexpression in the eye affects R3/R4 cell fate specification and inhibits notch signaling. Dev Dyn 245, 166–174. [DOI] [PubMed] [Google Scholar]
- Nunez KM, Azanchi R, and Kaun KR (2018). Cue-Induced Ethanol Seeking in Drosophila melanogaster Is Dose-Dependent. Front Physiol 9, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankova K, and Borst A (2016). RNA-Seq Transcriptome Analysis of Direction-Selective T4/T5 Neurons in Drosophila. PLoS One 11, e0163986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A, Ghezzi A, Wijesekera TP, and Atkinson NS (2017). Genetics and genomics of alcohol responses in Drosophila. Neuropharmacology 122, 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pech U, Dipt S, Barth J, Singh P, Jauch M, Thum AS, Fiala A, and Riemensperger T (2013). Mushroom body miscellanea: transgenic Drosophila strains expressing anatomical and physiological sensor proteins in Kenyon cells. Front Neural Circuits 7, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrat PN, DasGupta S, Wang J, Theurkauf W, Weng Z, Rosbash M, and Waddell S (2013). Transposition-driven genomic heterogeneity in the Drosophila brain. Science 340, 91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea M, Kim D, Pertea GM, Leek JT, and Salzberg SL (2016). Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc 11, 1650–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil KE, Lowery-Gionta EG, Crowley NA, Li C, Marcinkiewcz CA, Rose JH, McCall NM, Maldonado-Devincci AM, Morrow AL, Jones SR, et al. (2015). Effects of chronic ethanol exposure on neuronal function in the prefrontal cortex and extended amygdala. Neuropharmacology 99, 735–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell PA, Wesley C, Spencer S, and Cagan RL (2001). Scabrous complexes with Notch to mediate boundary formation. Nature 409, 626–630. [DOI] [PubMed] [Google Scholar]
- Presente A, Boyles RS, Serway CN, de Belle JS, and Andres AJ (2004). Notch is required for long-term memory in Drosophila. Proc Natl Acad Sci U S A 101, 1764–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renaud O, and Simpson P (2001). scabrous modifies epithelial cell adhesion and extends the range of lateral signalling during development of the spaced bristle pattern in Drosophila. Dev Biol 240, 361–376. [DOI] [PubMed] [Google Scholar]
- Robinson BG, and Atkinson NS (2013). Is alcoholism learned? Insights from the fruit fly. Curr Opin Neurobiol 23, 529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, and Berridge KC (2008). Review. The incentive sensitization theory of addiction: some current issues. Philosophical transactions of the Royal Society of London Series B, Biological sciences 363, 3137–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasabe T, and Ishiura S (2010). Alcoholism and alternative splicing of candidate genes. Int J Environ Res Public Health 7, 1448–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaplen KM, and Kaun KR (2016). Reward from bugs to bipeds: a comparative approach to understanding how reward circuits function. J Neurogenet 30, 133–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz-Kornehl S, and Schwarzel M (2016). Circuit Analysis of a Drosophila Dopamine Type 2 Receptor That Supports Anesthesia-Resistant Memory. J Neurosci 36, 7936–7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Lopez MF, Archer KJ, Wolen AR, Becker HC, and Miles MF (2016). Time-Course Analysis of Brain Regional Expression Network Responses to Chronic Intermittent Ethanol and Withdrawal: Implications for Mechanisms Underlying Excessive Ethanol Consumption. PLoS One 11, e0146257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Q, Sun K, Shuai Y, Lin R, You W, Wang L, and Zhong Y (2009). Suppressor of Hairless is required for long-term memory formation in Drosophila. J Neurogenet 23, 405–411. [DOI] [PubMed] [Google Scholar]
- Tong M, Gonzalez-Navarrete H, Kirchberg T, Gotama B, Yalcin EB, Kay J, and de la Monte SM (2017). Ethanol-Induced White Matter Atrophy Is Associated with Impaired Expression of Aspartyl-Asparaginyl-beta-Hydroxylase (ASPH) and NotchSignaling in an Experimental Rat Model. J Drug Alcohol Res 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toritsuka M, Kimoto S, Muraki K, Kitagawa M, Kishimoto T, Sawa A, and Tanigaki K (2017). Regulation of striatal dopamine responsiveness by Notch/RBP-J signaling. Transl Psychiatry 7, e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Kroener S, Abernathy K, Lapish C, Seamans J, Chandler LJ, and Woodward JJ (2007). Ethanol inhibits persistent activity in prefrontal cortical neurons. J Neurosci 27, 4765–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, and Piscani K (1996). Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res 20, 1594–1598. [DOI] [PubMed] [Google Scholar]
- Waddell S (2010). Dopamine reveals neural circuit mechanisms of fly memory. Trends Neurosci 33, 457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chan SL, Miele L, Yao PJ, Mackes J, Ingram DK, Mattson MP, and Furukawa K (2004). Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci U S A 101, 9458–9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Little CJ, Tremmel DM, Yin JC, and Wesley CS (2013a). Notch-inducible hyperphosphorylated CREB and its ultradian oscillation in long-term memory formation. J Neurosci 33, 12825–12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yin JC, and Wesley CS (2013b). From Drosophila development to adult: clues to Notch function in long-term memory. Front Cell Neurosci 7, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yin JC, and Wesley CS (2015). Notch Intracellular Domain (NICD) Suppresses Long-Term Memory Formation in Adult Drosophila Flies. Cell Mol Neurobiol 35, 763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data File S1. Data support ‘in text’ Figure 2B-C. Raw Western blots and corresponding Trihalo TGX Stain Free total protein gel images and raw densitometry.
Data File S2. Data support Supplemental Figure 5A. Raw Western blots and corresponding Trihalo TGX Stain Free total protein gel images and raw densitometry.
Data File S3. Data support ‘in text’ Figure 4A. Raw Western blots and corresponding Trihalo TGC Stain Free total protein gel images and raw densitometry.
Data File S4. Data support ‘in text’ Figure 4A. Raw Western blots and corresponding Trihalo TGC Stain Free total protein gel images and raw densitometry.
Data File S5. Data support ‘in text’ Figure 4A. Raw Western blots and corresponding Trihalo TGC Stain Free total protein gel images and raw densitometry.
Data File S6. Data support Supplemental Figure 5C-D. Raw Western blots and corresponding Trihalo TGC Stain Free total protein gel images and raw densitometry.
Data File S7. Data support Supplemental Figure 9A. Raw ΔΔCT values.
Data Availability Statement
Raw data
Raw data from Western blots and qPCR experiments are included in the Data Files S1–7. All other data is available upon request.
RNA-seq data
The nuclear mushroom body transcriptome .fastq and count data have been deposited in the Gene Expression Omnibus (GEO) under GSE108525.


