SUMMARY
Spt6 is an essential histone chaperone that mediates nucleosome reassembly during gene transcription. Spt6 also associates with RNA polymerase II (RNAPII) via a tandem Src2 homology domain. However, the significance of Spt6-RNAPII interaction is not well understood. Here, we show that Spt6 recruitment to genes and its nucleosome reassembly functions are largely independent of association with RNAPII. Instead, the Spt6-RNAPII association mediates recruitment of the Ccr4-Not de-adenylation complex to transcribed genes for essential degradation of a range of mRNAs, including mRNAs required for cell cycle progression. These findings reveal an unexpected control mechanism for mRNA turnover during transcription facilitated by a histone chaperone.
INTRODUCTION
Spt6 is an essential replication-independent histone chaperone that was discovered by its ability to alter RNA Polymerase II (RNAPII) transcription start-site selection (Clark-Adams and Winston, 1987; Neigeborn et al., 1987; Swanson et al., 1990). Spt6, which binds H3/H4 dimers and/or tetramers (Bortvin and Winston, 1996; Kaplan et al., 2005; Kaplan et al., 2000), associates with elongating RNAPII and reassembles nucleosomes in the wake of transcription (Diebold et al., 2010; Hartzog et al., 1998; Sdano et al., 2017; Sun et al., 2010). In addition to Spt6, nucleosome reassembly also requires the H2A/H2B-binding FACT (facilitates chromatin transcription) complex, which like Spt6, functions co-transcriptionally (McCullough et al., 2015). As Spt6 maintains chromatin structure in the transcribed region of genes, a hallmark of Spt6 absence or inactivation is inappropriate (cryptic) transcription initiation from within gene bodies (Cheung et al., 2008; DeGennaro et al., 2013; Hainer et al., 2011; Ivanovska et al., 2011; Kaplan et al., 2005; Kaplan et al., 2003). Cryptic transcription, caused by the inactivation of Spt6, was first documented in Saccharomyces cerevisiae, but also occurs in the distantly related yeast Schizosaccharomyces pombe (Kaplan et al., 2003; Kato et al., 2013a; Kiely et al., 2011). Intriguingly, cryptic transcription in the absence of Spt6 may be bi-directional, producing antisense transcripts that interfere with sense transcription (DeGennaro et al., 2013; Kato et al., 2013a; Kato et al., 2013b). These studies have revealed many facets of Spt6 activity; however, we do not know the complete set of Spt6 functions that contributes to transcriptional regulation.
Spt6 has a key function in transcription elongation by interacting with the phosphorylated serine 2 and tyrosine 1 repeats within the C-terminal domain (CTD) of RNAPII, and with a phosphorylated linker region preceding the CTD (Ardehali et al., 2009; Kwak and Lis, 2013; Mayer et al., 2012; Sdano et al., 2017; Sun et al., 2010). In yeast, Spt6 interaction with phosphorylated tyrosine 1 of RNAPII CTD prevents pre-mature recruitment of termination factors to genes (Mayer et al., 2012). The highly acidic N-terminus of Spt6 interacts with histones and with Spn1/IWS1, another conserved and stable Spt6 binding partner (Mayer et al., 2010; McDonald et al., 2010; Zhang et al., 2008). Although RNAPII interaction provides an attractive explanation for recruitment functions of Spt6 during transcription, significance of this interaction for its functions are less well understood. Likewise, we do not know the precise mechanism by which the Spt6 N-terminus coordinates histone deposition with RNAPII elongation.
In this study, we used a mutant of Spt6 (spt6tSH2Δ) that cannot interact with RNAPII to demonstrate that Spt6-RNAPII interaction is not essential for recruitment of Spt6 to chromatin or for nucleosome deposition. Instead, we found that Spt6-RNAPII interaction is important for full recruitment of Spt6 and RNAPII across genes and for proper mRNA turnover; the latter activity results, in part, from Spt6’s recruitment of the Ccr4-Not complex to genes. Consistent with this finding, absence of Spt6-RNAPII interaction caused increased stability of many mRNAs that correlated with an increase of poly(A) tail lengths for a number of transcripts analyzed. Surprisingly, cell cycle-associated transcripts were among the most highly stabilized mRNAs in the absence of Spt6-RNAPII interaction, and, as cell cycle mRNAs must be degraded for cells to progress through the cell cycle, the spt6tSH2Δ mutant showed cell cycle progression defects. In sum, our findings reveal a critical mRNA stability control mechanism operating during transcription and an unexpected function for the Spt6 histone chaperone in this mechanism.
RESULTS
Uncoupling Spt6 from RNAPII Reveals Distinct Functions of the Spt6 Chaperone
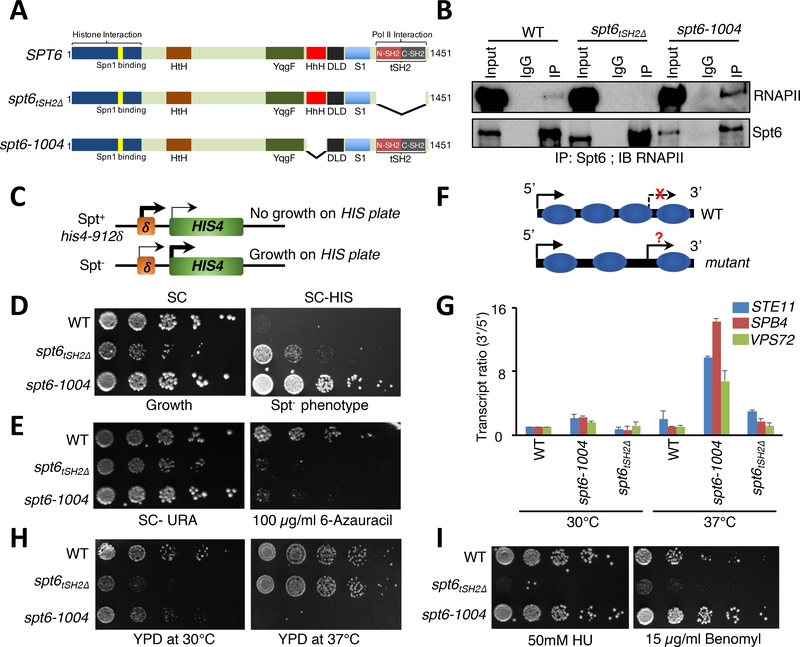
Spt6 is a transcription-coupled histone chaperone that contains multiple domains, most of which do not have established functions (Figure 1A). Deletion of the Spt6 Helix-hairpin-Helix domain in mutant spt6–1004 causes nucleosome reassembly defects, cryptic transcription, and decreased Spt6 protein (Cheung et al., 2008; Kaplan et al., 2005; Kaplan et al., 2003). However, spt6–1004 still interacts with RNAPII (Figure 1B). To determine which functions of Spt6 require its association with RNAPII, we generated a C-terminal truncation of Spt6 (spt6tSH2Δ) that selectively removes the tSH2 RNAPII interaction domain (Figure 1A and 1B) (Dengl et al., 2009; Diebold et al., 2010; Sun et al., 2010; Yoh et al., 2007). We first asked whether spt6tSH2Δ possesses a Suppressor of Ty (Spt) phenotype. An Spt– phenotype monitors the ability of RNAPII to utilize an alternate transcription start site to bypass the insertion of a transposable element in the promoters of certain auxotrophic genes such as HIS4 (Figure 1C), which is otherwise silent under WT conditions (Clark-Adams and Winston, 1987). As expected, spt6–1004 cells exhibited an Spt– phenotype (Figure 1D). Intriguingly, however, spt6tSH2Δ cells showed only a subtle Spt– phenotype (Figure 1D), indicating that the association of Spt6 with RNAPII is not the critical determinant in Spt6’s ability to disrupt Ty element-mediated silencing.
Figure 1. Uncoupling Spt6-RNAPII interaction reveals distinct Spt6 phenotypes.
(A) Domain organization and forms of Spt6 used in this study. HtH (Helix-turn-Helix domain); YqgF (RNase H-like domain); HhH (Helix-hairpin-Helix domain); S1 (S1 domain); DLD (Death-like-Domain); and tSH2 (Tandem Src2 homology domain). (B) Spt6 interacts with RNAPII via a tSH2 domain. Co-immunoprecipitation experiments were performed for Spt6 and RNAPII, and interaction was assessed by immunoblot analysis with various phospho-Ser2-CTD specific antibodies. (C) Schematic of the Spt- phenotype. Insertion of a Ty element at the HIS4 locus suppresses expression. Spt- mutants allow expression of HIS4 and growth on medium lacking histidine. (D) Spt- phenotypic assay. 5-fold serial dilutions of the indicated strains were spotted on synthetic complete (SC) and SC medium lacking histidine. (E) Spt6 is required for transcription elongation. Spotting assays showing the sensitivity of spt6 mutants on 6-azauracil plates. (F) Schematic illustrating normal and cryptic (intragenic) transcription. In wild-type (WT) cells, intragenic sites of transcription are normally suppressed but can be activated in mutants that perturb chromatin integrity. (G) qRT-PCR assay to validate the generation of intragenic sense transcripts using primers spanning the 5’ and the 3’ regions of STE11, SPB4, and VPS72 genes in WT, spt6–1004, and spt6tSH2Δ mutant cells. (H) and (I) Spotting assays showing functionally distinct phenotypes of the spt6–1004 and spt6tSH2Δ mutants upon exposure to heat and genotoxic agents, respectively. 5-fold serial dilutions were spotted on the indicated medium. Plates were incubated at 30°C or 37°C and photographs were taken after 4 days.
Because spt6tSH2Δ cells did not possess a strong Spt- phenotype, we asked whether this mutant might instead harbor a transcription elongation defect. To identify such a defect, we spotted wild-type, spt6–1004, and spt6tSH2Δ cells on plates containing 6-Azauracil (6-AU), a drug that depletes rNTPs and that inhibits growth when transcription elongation is otherwise undermined. Both Spt6 mutants were sensitive to 6-AU (Figure 1D) (Hyle et al., 2003), consistent with a function of Spt6 in transcription elongation.
A hallmark of Spt6 loss-of-function alleles is an inability to reassemble chromatin in the wake of RNAPII transcription, causing intragenic or cryptic transcription (DeGennaro et al., 2013; Kaplan et al., 2003; Kato et al., 2013a; Kato et al., 2013b). To test for cryptic transcription, we performed a quantitative real-time PCR (qRT-PCR) assay that monitored the transcript ratio between the 5’- and 3’-ends of genes (Figure 1F). Consistent with previous studies (Kaplan et al., 2003), spt6–1004 cells at 37°C showed a robust increase in cryptic transcription from several genes known to be susceptible to aberrant intragenic transcription (STE11, VPS72, and SPB4) (Figure 1G). Conversely, spt6tSH2Δ cells did not show significant evidence of cryptic transcripts at either 30°C or 37°C (Figure 1H). These data agree with findings of Dengl et al. (Dengl et al., 2009), and the data demonstrated that Spt6 interaction with RNAPII is not required to suppress cryptic transcription.
Finally, we examined additional phenotypes observed for Spt6 mutants to determine which phenotypes, if any, were dependent on Spt6’s association with RNAPII. As shown in Figure 1H and 1I, and in agreement with previous findings, the spt6–1004 mutant was temperature sensitive but not sensitive to several genotoxic agents, e.g., hydroxyurea (HU) and benomyl. Conversely, spt6tSH2Δ cells were heat-resistant and sensitive to the same genotoxic agents. Thus, uncoupling Spt6 from RNAPII creates phenotypes distinct from those associated with other Spt6 mutants that retain RNAPII binding.
The tSH2 domain of Spt6 Contributes to Spt6 and RNAPII Distribution Along Genes
We next assessed the effects of tSH2 domain deletion on Spt6 and RNAPII distribution across genes. Because the spt6tSH2Δ mutant showed weak Spt- and cryptic transcription phenotypes, we surmised that spt6tSH2Δ was still recruited to genes and performed at least some of the key transcription functions of Spt6. We used ChIP-qPCR to measure the levels and distribution of Spt6 and RNAPII at constitutively expressed genes (i.e., PMA1 and TDH3) in wild-type and spt6tSH2Δ cells. As expected for wild-type cells (see Supplementary Figure 1), Spt6 was recruited to both promoter regions and the open reading frames (ORFs) of actively transcribed genes. Consistent with other studies (Cui et al., 2016; Jeronimo et al., 2015; Kaplan et al., 2005; Kaplan et al., 2003; Kaplan et al., 2000; Mayer et al., 2010), the highest levels of Spt6 occurred in ORFs. In contrast, although spt6tSH2Δ was still recruited to the transcribed regions of genes, its levels were significantly lower than the levels in wild-type cells. Similarly, the levels of RNAPII were significantly lower in spt6tSH2Δ cells compared with wild-type cells at all the assayed genes (Supplementary Figure 1). These results are similar to a requirement of Spt5 in RNAPII recruitment and elongation functions (Shetty et al., 2017). We performed a similar analysis of the effects of the spt6–1004 allele on Spt6 and RNAPII distribution. Interestingly, on these same genes, the levels of Spt6 were decreased like the tSH2 domain deletion. In addition, the distributions of RNAPII were decreased on the PMA1 and TDH3 genes (Supplementary Figure 2). Thus, Spt6-RNAPII association is required for proper levels of the RNAPII complexes across transcribed regions, consistent with its role as a transcription elongation factor (Ardehali et al., 2009; Zobeck et al., 2010).
Spt6-RNAPII Interaction Regulates the Global Transcriptome
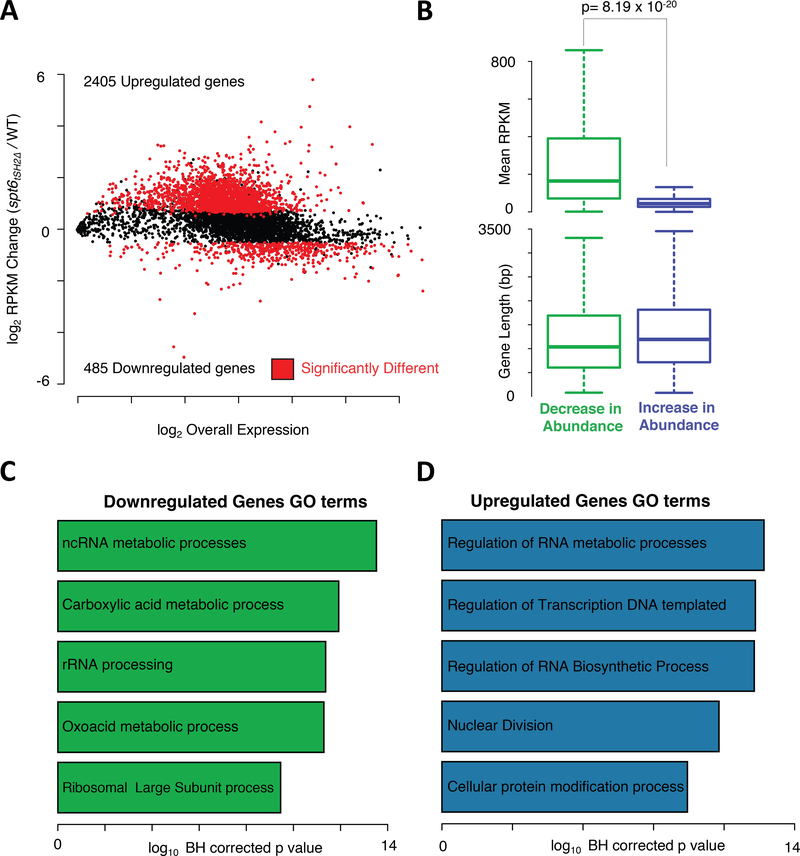
Because the absence of Spt6-RNAPII interaction led to decreases in Spt6 and RNAPII occupancy on genes, we next determined whether these decreases caused wide-spread changes in transcription. We performed steady state RNA-seq with wild-type and spt6tSH2Δ cells under asynchronously growing conditions. As shown in the Bland-Altman plot in Figure 2A and Supplementary Table 1, 2405 genes were upregulated and 485 genes were downregulated in the spt6tSH2Δ strain (p<0.05, and fold change > 1.4). We next asked whether there was a correlation between the genes that were up/downregulated and gene length or mRNA abundance. We did not detect an association between gene length and transcript abundance (p = 0.22; see Figure 2B). However, we observed a significant correlation (p= 8.19 × 10−20) between transcript abundance and changes in expression. Highly expressed genes were associated with decreases in mRNA levels, whereas weakly expressed genes displayed increased abundances. Intriguingly, transcripts downregulated in the spt6tSH2Δ mutant were enriched for genes in non-coding RNA metabolism pathways and rRNA processing (Figure 2C and Supplementary Table 1). Upregulated genes in the spt6tSH2Δ mutant were significantly enriched for processes including “regulation of RNA metabolism”, which includes genes involved in RNA synthesis and degradation, and “Nuclear Division”, which includes cell cycle-related genes. A further examination of the genes in the Nuclear Division class verified upregulation of many cell cycle regulators, including B-type Cyclins, DBF2, CDC28, SWE1, members of the anaphase promoting complex, and members of the condensin complex (Figure 2D). These results correlate with the observation that spt6tSH2Δ cells displayed a slow growth phenotype and an elongated bud morphology reminiscent of defects in Cdc28 activation during cell cycle progression (see Figure 3C and (Rudner et al., 2000).
Figure 2. Spt6-RNAPII Interaction Regulates Global Transcriptome.
(A) MA plot showing changes in the gene expression patterns in the spt6tSH2Δ mutant normalized to the WT transcriptome. (B) Box plots showing the correlation between the up/downregulated genes and expression levels and gene length. (C) and (D) Gene Ontology (GO) analysis of the downregulated and upregulated genes, respectively, in the spt6tSH2Δ mutant.
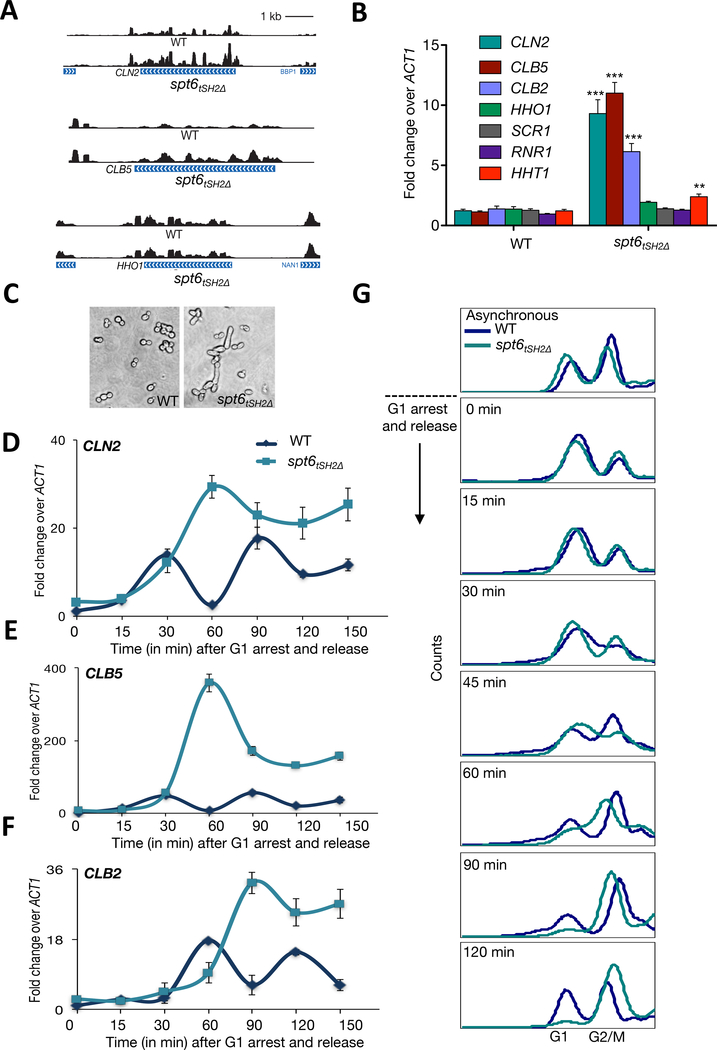
Figure 3. Spt6-RNAPII Interaction is required for cell cycle progression.
(A) Representative tracks of CLN2, CLB5, and HHO1 transcripts in the WT and spt6tSH2Δ mutant. (B) qRT-PCR analysis of the representative transcripts in the WT and spt6tSH2Δ mutant. Shown are the mean ± SD for three biological replicates. The p values were calculated using Student’s t test. ** represents a p value less than 0.01. (C) DIC image showing the morphology of the WT and spt6tSH2Δ mutant cells. (D-F) qRT-PCR quantitation of CLN2, CLB5, and CLB2 transcripts in WT and spt6tSH2Δ mutant cells arrested in G1 and released into fresh medium. Shown are the mean and SD for three biological replicates. (G) WT and spt6tSH2Δ mutant cells were arrested in G1 using α-factor and released into fresh medium; samples at different time points were analyzed by flow cytometry for DNA content.
Spt6-RNAPII Interaction is Required for Proper Cell Cycle Progression
Because of the large number of cell cycle-associated genes upregulated in the spt6tSH2Δ mutant, we tested for a link between Spt6 and cell cycle regulation. Figure 3A depicts the RNA-seq tracks for two representative cell cycle regulators (CLN2 and CLB5) that were upregulated in the spt6tSH2Δ mutant. CLN2 is critical for G1/S transition, whereas CLB5 is critical for S and G2/M phases (Bahler, 2005). An example of a gene unaffected by the spt6tSH2Δ mutant (linker histone HHO1) is also shown in Figure 3A. We next validated these findings by qRT-PCR, which confirmed the observation that cell cycle genes are selectively upregulated in the absence of Spt6-RNAPII interaction (Figure 3B).
Because RNA-seq analysis was performed with asynchronous cultures, we next examined the effect of Spt6-RNAPII uncoupling specifically on transcription of cell cycle genes across the cell cycle. We arrested wild-type and spt6tSH2Δ mutant cells in G1 with α-factor; following release into fresh medium, cells were collected at several time points across cell cycle. In wild-type cells, qRT-PCR analysis of CLN2, CLB5, and CLB2 (Figure 3D–3F) demonstrated a periodicity that reflected appropriate cell cycle regulation. Conversely, similar regulation was largely absent in the spt6tSH2Δ mutant. In the spt6tSH2Δ mutant, all three genes demonstrated a delayed induction and were minimally downregulated. Consistent with the changes in cyclin gene expression across the cell cycle, we found that spt6tSH2Δ cells had a 15-minute delayed entry into S phase (Figure 3G). Once in S phase, spt6tSH2Δ cells progressed into the G2/M phase. Significantly, however, spt6tSH2Δ cells did not exit G2/M at a wild-type rate (Figure 3G). Finally, and consistent with the defects in cell cycle progression and G2/M exit, spt6tSH2Δ cells exhibited a gross change in morphology (i.e., elongated buds) reminiscent of mutants that compromise Cdc28 activity and mitotic exit (Figure 3C) (Nasmyth, 1993).
To determine whether defects in cell cycle progression in the spt6tSH2Δ mutant were a direct effect of perturbed Spt6 function instead of a secondary effect of expressing the mutant, we used the anchor away (AA) technique to acutely deplete Spt6 from the nucleus (Haruki et al., 2008). By ChIP-qPCR, we observed that Spt6 levels on PMA1 and TDH3 chromatin were reduced to about 1.2% of the total levels after 90 minutes of Spt6 nuclear eviction (by rapamycin; Supplementary Figure 3A). We then arrested these cells with α-factor followed by the addition of rapamycin or vehicle control (DMSO) for an additional 90 minutes before cell cycle release (see schematic in Supplementary Figure 3B). After rapamycin treatment or vehicle control, cells were washed and subjected to flow cytometry. The DMSO-treated cells released from G1 arrest followed a normal cell cycle, entering G2/M phase by the expected 60 minutes (Supplementary Figure 3C). In contrast, Spt6 nuclear removal caused a delayed release from G1 arrest (Supplementary Figure 3C). Consistent with our previous qRT-PCR results showing an increased transcript abundance using the spt6tSH2Δ mutant, we found that cyclin gene expression levels were significantly increased at the 90-minute time points (Supplementary Figure 3D). These data demonstrate that Spt6 association with RNAPII is critical for cell cycle control and regulation of mRNA level.
Spt6-RNAPII interaction is not required for the nucleosome deposition function of Spt6
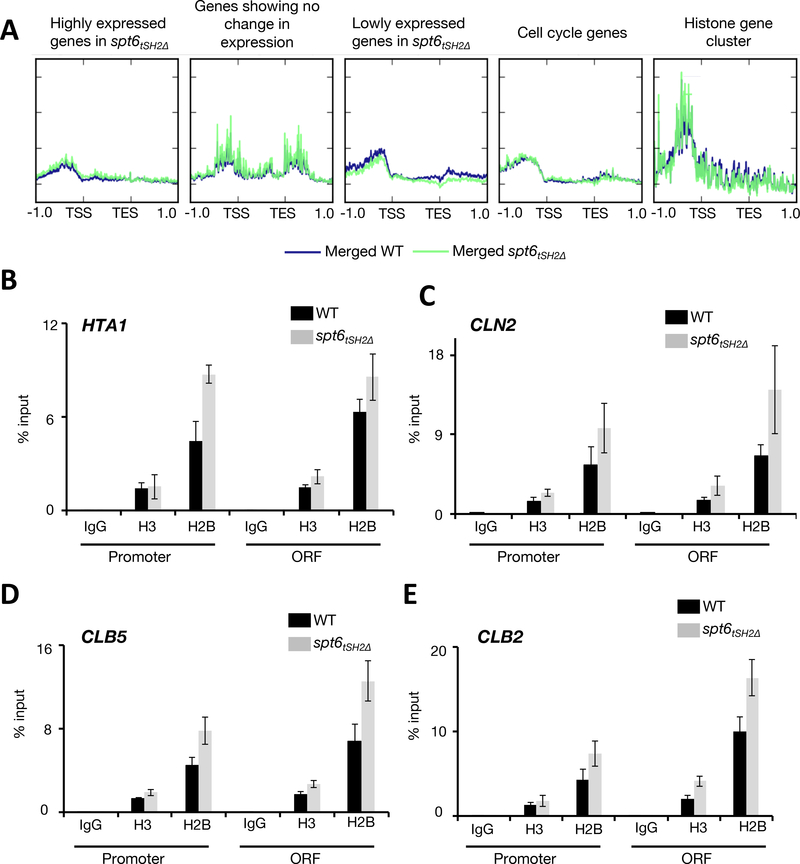
In light of Spt6’s function in nucleosome reassembly, particularly at gene promoters (Perales et al., 2013), we speculated that the upregulation of many genes, including cell cycle-associated genes, in the spt6tSH2Δ mutant may result from improper nucleosome deposition at promoters (an effect that may not be evident from our analyses of cryptic transcription). Control of nucleosome occupancy regulates cell cycle genes (Deniz et al., 2016; Flores et al., 2014; Hogan et al., 2006). To assess whether Spt6-RNAPII interaction might be critical for nucleosome deposition at cell cycle-associated genes, we performed Assay of Transposase-Accessible Chromatin (ATAC-seq) in asynchronously growing wild-type and spt6tSH2Δ cultures (Schep et al., 2015). Contrary to expectation, compared with wild-type cells, we did not observe changes in chromatin accessibility at the promoters of genes upregulated/downregulated in the spt6tSH2Δ mutant (Figure 4A). Specific examination of the ATAC-seq signals at all periodic genes (Spellman et al., 1998) also did not reveal changes in nucleosome occupancy. To confirm these ATAC-seq results, we performed ChIP-qPCR for histones at cell cycle-regulated genes that were upregulated in both asynchronous and synchronized cultures (i.e., CLN2, CLB5, and CLB2). In the spt6tSH2Δ strain, there were no significant alterations in the H3 and H2B levels in the promoters of these genes (Figure 4B-E). However, histone occupancy was slightly increased in the ORFs of all genes tested; this result was consistent with the finding that nucleosome occupancy is anti-correlated with RNAPII levels on genes (Gilchrist et al., 2010; Schwabish and Struhl, 2004), which we observed in the spt6tSH2Δ mutant (Supplementary Figure 1). Our results indicate that alterations in RNA abundance in the spt6tSH2Δ strain did not result from changes in nucleosome occupancy.
Figure 4. Spt6-RNAPII interaction is not required to maintain promoter fidelity.
(A) Metagene analysis of the ATAC-seq signals showing tracks for the changes in nucleosome occupancy in WT and the spt6tSH2Δ mutant under different conditions. The blue tracks represent the WT and the green tracks represent the spt6tSH2Δ mutant. Labels above each panel indicate the classification of genes based on their expression changes (B-E) ChIP-qPCR to detect the occupancy of histone H3 and H2B at the promoters and open reading frames (ORFs) of HTA1, CLN2, CLB5, and CLB2, respectively. Data are represented as mean ± SD of the percent input method of enrichments in three independent biological replicate experiments.
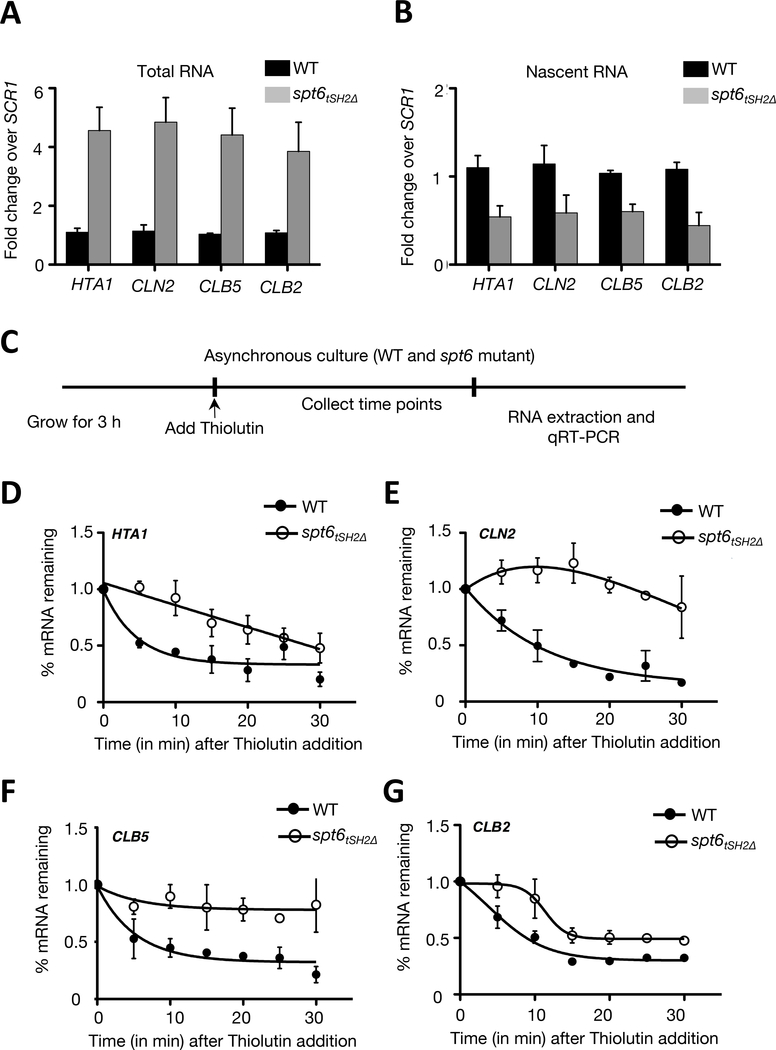
Spt6 is Required for Proper mRNA Turnover
mRNA abundance is a balance between rates of synthesis and degradation (Wilusz et al., 2001). Because the absence of Spt6-RNAPII interaction did not significantly affect nucleosome deposition at promoters, we analyzed nascent (chromatin-bound and un-spliced) and total RNA (processed and mature mRNA) fractions from wild-type and spt6tSH2Δ cells to determine whether there were changes in mRNA synthesis. We assessed the purity of the nascent RNA fraction by estimating the relative enrichment of intron containing RNAs in the nascent and total fractions (Supplementary Figure 4A) (Oesterreich et al., 2016). We subjected the nascent and total RNA fractions to qRT-PCR to measure the relative abundances of cell cycle-regulated transcripts, including HTA1, CLN2, CLB5, and CLB2. Surprisingly, increased cyclin and histone mRNAs in the spt6tSH2Δ strain was found only in the total fraction (Figure 5A); these same mRNAs were decreased in the nascent (i.e., newly synthesized) fraction (Figure 5B). These results implied that the absence of Spt6-RNAPII interaction caused a decrease in the rate of mRNA synthesis, but, once synthesized, the mRNAs became relatively more stable. The reduced polymerase detected at these genes was consistent with the finding of decreased mRNA synthesis (Supplementary Figure 1).
Figure 5. Spt6 is essential for regulation of mRNA turnover.
Spt6 is required for mRNA turnover. (A) qRT-PCR analysis of HTA1, CLN2, CLB5, and CLB2 from total RNA extracted from WT and spt6tSH2Δ mutant cells (B) The same transcripts analyzed from RNA extracted from the chromatin fraction (nascent chromatin) (C) Schematic representation of the experiment for the treatment of WT and spt6tSH2Δ mutant cells with Thiolutin to assess mRNA stability. (D-G) are the qRT-PCR results showing changes in HTA1, CLN2, CLB5, and CLB2 transcripts, respectively, at the indicated timepoints after Thiolutin addition. Data are the mean and SD for three biological replicates. The p values were calculated using Student’s t test.
Because mRNA decay is required for proper gene regulation and cell cycle progression (Das et al., 2017; Haimovich et al., 2013; Medina et al., 2014; Trcek et al., 2011), we next asked whether Spt6-RNAPII interaction regulates mRNA turnover. To measure turnover, we employed the rapid-acting RNA polymerase inhibitor Thiolutin, which enables monitoring of mRNA decay (Coller, 2008; Passos and Parker, 2008). Wild-type and spt6tSH2Δ cells were grown for 3h followed by addition of Thiolutin. Total cellular RNA was isolated at multiple time points and subjected to qRT-PCR for CLN2, CLB5, CLB2, and HTA1. Strikingly, in the spt6tSH2Δ strain, all of these genes exhibited increased transcript levels, and all of the transcripts had significantly increased half-lives (t1/2) (Figure 5D–5G). These results strongly suggested that the Spt6-RNAPII interaction has a critical function in regulation of mRNA turnover.
To independently verify that the effects on mRNA decay in the spt6tSH2Δ strain were attributable directly to Spt6 instead of to an indirect effect, we again employed the AA technique (see experimental scheme in Supplementary Figure 5A). We treated cells for 90 minutes with rapamycin or vehicle control followed by the addition of Thiolutin. Samples were collected at indicated time points, and CLN2, CLB5, and CLB2 transcripts were measured by qRT-PCR. The transcript levels of these mRNAs were significantly stabilized with acute nuclear eviction of Spt6 (Supplementary Figure 5B). These results strongly suggest that Spt6 directly regulates mRNA turnover.
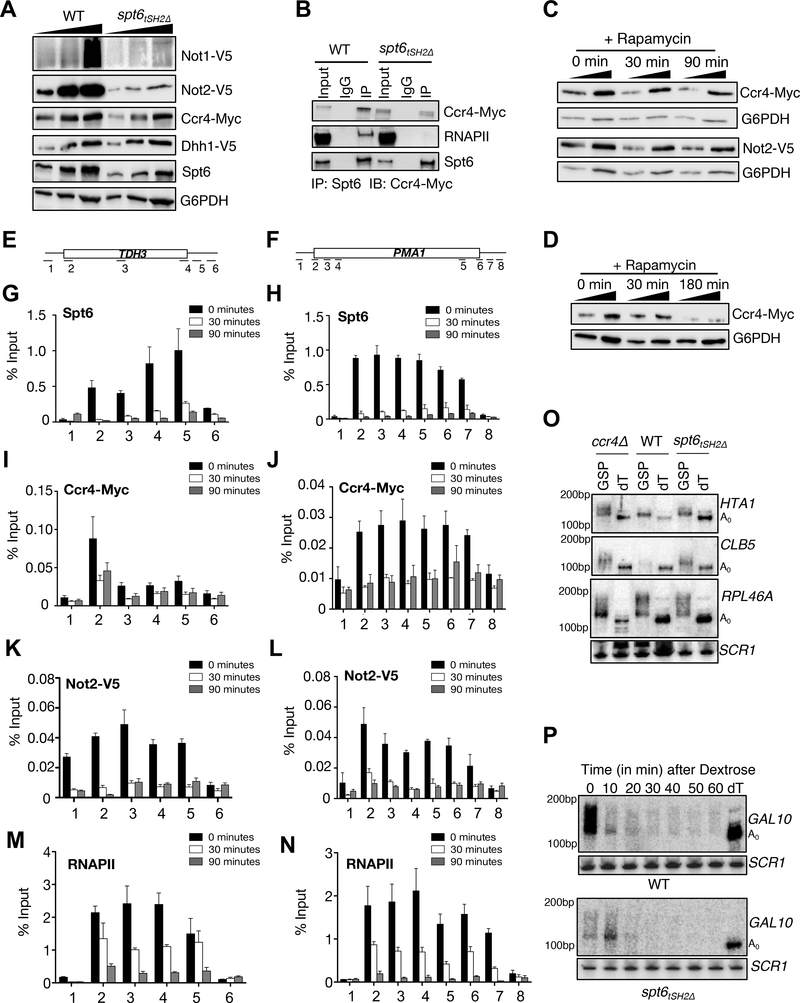
Spt6-RNAPII Interaction is Required for Recruitment of the Ccr4-Not Complex to Genes
Because Spt6-RNAPII interaction regulates mRNA turnover, we asked whether the absence of this interaction affects the factors that control mRNA decay. We focused on the Ccr4-Not complex because it functions in various aspects of mRNA metabolism and transcription elongation (Collart and Panasenko, 2012; Dutta et al., 2015; Kerr et al., 2011; Kruk et al., 2011; Miller and Reese, 2012; Reese, 2013; Villanyi and Collart, 2015). Strikingly, the levels of Ccr4, Not1, and Not2 proteins were significantly reduced, albeit to varying degrees, in the spt6tSH2Δ mutant (Figure 6A). Because the mRNA levels for all of the Ccr4-Not members were not affected (Supplementary Figure 6 and Supplementary Table 1), these data imply that Spt6-RNAPII interaction is required for the stability of the Ccr4-Not complex (Figure 6A).
Figure 6. Spt6 recruits the Ccr4-Not complex to chromatin.
Interaction of Spt6 with RNAPII is required for optimal levels of Ccr4-Not complex members. (A) Exponentially growing WT and spt6tSH2Δ mutant cells were subjected to immunoblot analysis (after TCA lysis) to detect changes in the levels of Ccr4-Not complex members (B) Co-immunoprecipitation showing an interaction between Spt6 and Ccr4 in WT and the spt6tSH2Δ mutant. (C) and (D) Immunoblots showing changes in the levels of Ccr4 and Not2 with Spt6 anchor-away at 30, 90 minutes and 3h, respectively. (E) and (F) Schematic representation of the primer locations across PMA1 and TDH3 genes. (G) and (H) ChIP-qPCR of Spt6 kinetics on PMA1 and TDH3 genes following rapamycin treatment. ChIP-qPCR showing changes in the occupancy of Ccr4 (I and J) and Not2 (K and L) and RNAPII (M and N) on PMA1 and TDH3 genes respectively, following Spt6 depletion. While Spt6 and Ccr4/Not2 are no longer detectable at the 30 minute time-point, we note that RNAPII is still detected, thereby suggesting a role for Spt6 in recruitment of Ccr4-Not (O) Spt6 is required for proper mRNA metabolism and poly(A) tail length. Total RNA was isolated using acid phenol method and 10μg was digested with RNAse H in the presence of gene specific primer (GSP) and/or oligo-dT12–16 (dT); A0 represents the completely deadenylated RNA species. Use of the GSP alone gives the length of the poly(A) tail whereas use of the GSP with dT allows for the determination of A0. Total RNA was separated in urea-PAGE, blotted on to membranes and probed with oligo probes. (P) High resolution PAGE Northern blot showing the rate of deadenylation of GAL10 mRNA.
Because the Ccr4-Not complex interacts with RNAPII (Dutta et al., 2015; Kruk et al., 2011), we next asked whether Ccr4-Not and Spt6 interact via their association with the RNAPII complex. Not1, 2 and 4 levels were extremely low in the spt6tSH2Δ strain, which excluded them from co-IP studies. Thus, we focused on Ccr4 (Figure 6A). Immunoprecipitation of Spt6 followed by immunoblot analysis for Ccr4 demonstrated an interaction between Ccr4 and Spt6 (Figure 6B). Significantly, deletion of the Spt6-tSH2 domain did not completely disrupt this interaction, albeit there were reduced the levels of co-immunoprecipitated Ccr4-Myc given the input levels were also lower. These results suggest that the association of Spt6 with Ccr4 is not mediated by co-association with RNAPII and point to a more direct interaction between Spt6 and the Ccr4-Not complex.
Because the input levels of Ccr4-Not complex members were affected in the spt6tSH2Δ strain, we again employed the AA technique to examine the stability and recruitment of Ccr4-Not members to genes upon acute nuclear depletion of Spt6. Treatment of cells with rapamycin for 30 or 90 minutes to deplete Spt6 from the nucleus did not affect the protein levels of Ccr4 and Not2 (Figure 6C). However, prolonged rapamycin treatment (>3h) resulted in a decrease of Ccr4 similar to that observed in the spt6tSH2Δ strain (Figure 6D). Because Ccr4-Not levels remained intact at early time points of Spt6 nuclear eviction, we performed ChIP-qPCR to measure the effect of Spt6 nuclear removal on Spt6, Ccr4, and Not2 occupancy at the PMA1 and TDH3 loci (Figure 6E and 6F). Within 30 minutes of rapamycin treatment, ~85% of Spt6 was depleted from PMA1 and TDH3 (Figure 6G and 6H). Strikingly, chromatin levels of Ccr4 (Figure 6I, 6J) and Not2 (Figure 6K, and 6L) were depleted with kinetics similar to that of Spt6. In contrast, RNAPII depletion from these same genes (Figure 6M, and 6N) occurred with slower kinetics and was detectable on these genes at the 30 min timepoint (a 11.3-fold reduction in the levels of Spt6 compared to 3-fold reduction in RNAPII at the 30 minute timepoint). These results provide further support for the idea that Spt6 directly recruits the Ccr4-Not complex to genes.
Spt6-RNAPII Interaction Regulates mRNA Deadenylation and Decay
In view of the unexpected connection between Spt6 and the Ccr4-Not complex, we asked whether disrupting Spt6-RNAPII interaction would result in RNA processing defects similar to defects observed in Ccr4 mutants (i.e., decreased deadenylation and slower mRNA decay). Thus, we performed high resolution Northern Blot analyses of the spt6tSH2Δ strain and a CCR4 deletion strain (ccr4Δ) to examine the poly(A) tail lengths of transcripts regulated by Spt6-RNAPII interaction (HTA1 and CLB5). For a control, we analyzed the mRNA levels of RPL46A, a transcript not found to be regulated by Spt6-RNAPII interaction. Poly(A) tail length was resolved by digesting total RNA with RNAse H in the presence of a gene specific 3’-end primer and oligo-dT to generate a completely deadenylated transcript (A0) or a gene specific primer to measure only the poly(A) tail length (Hu and Coller, 2013; Tucker et al., 2002; Tucker et al., 2001). We found that poly(A) tail lengths for HTA1 and CLB5 were increased in both the spt6tSH2Δ and ccr4Δ mutant strains compared with wild-type (Figure 6O). Conversely, no increases in poly(A) tail length in the RPL46A transcript were observed in any mutant strain (Figure 6M).
To ascertain whether the spt6tSH2Δ mutant also affected deadenylation kinetics, we employed the GAL gene model of transcriptional shut-off previously used to make kinetic assessments of mRNA turnover rates with mutants of the Ccr4-Not complex (Tucker et al., 2002; Tucker et al., 2001). As expected in wild-type cells, GAL10 mRNA was maximally induced within 15 minutes of galactose addition and was repressed (with its mRNA fully degraded) within 10 minutes following dextrose addition (Figure 6P). In stark contrast, induction of GAL10 was dramatically impaired in the spt6tSH2Δ strain, and, further, its rate of mRNA decay was also significantly delayed compared with wild-type (~10 minutes) (Figure 6N). These results reinforce the finding that Spt6 controls mRNA decay processes partly by deadenylation, in addition to the important function of Spt6-RNAPII interaction in transcriptional regulation.
DISCUSSION
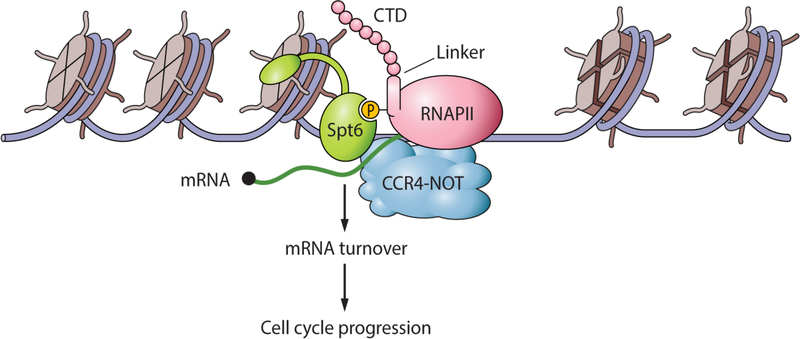
Although Spt6 has been well studied during transcription elongation, the full range of Spt6’s contributions to transcription have not been fully elucidated. In this study, we show that Spt6-RNAPII interaction is vital to the maintenance of mRNA turnover during transcription, a requirement that is likely explained by the fact that Spt6 recruits the Ccr4-Not mRNA deadenylation complex to genes (Figure 7). Until now, Spt6 was primarily thought to contribute to transcription by promoting RNAPII elongation and/or by reassembling nucleosomes in the wake of RNAPII transcription. Our data expand the repertoire of Spt6 transcriptional activities to include a function in mRNA homeostasis.
Figure 7. Model showing the connections between Spt6 and the regulation of mRNA turnover.
The model summarizes our finding that Spt6-RNAPII association is key to the recruitment and activity of the Ccr4-Not complex that regulates mRNA turnover. Uncoupling of Spt6 and RNAPII produces increased amounts of cell cycle-associated mRNAs (due to lack of turnover of a broad range of mRNAs) that cause cell cycle defects. Our model depicts Spt6 associating with a newly described phosphorylated linker region in RNAPII that has been found to bind Spt6; however, the Spt6 chaperone also interacts with phosphorylated residues in the RNAPII C-terminal domain (CTD) (not shown; see text for details). These results uncover a previously unknown function for the Spt6 histone chaperone in transcriptional regulation.
Although the function of the N-terminus of Spt6 in regulating histone/nucleosome reassembly has received much attention, the function of its C-terminus, which mediates RNAPII interaction via its tSH2 domain (Chiang et al., 1996; Close et al., 2011; Dengl et al., 2009; Diebold et al., 2010; Yoh et al., 2007), is less clear. We expected that Spt6-RNAPII interaction would be important for Spt6 recruitment to genes and nucleosome reassembly during transcription. However, our studies point to a completely unexpected Spt6 activity in that Spt6-RNAPII uncoupling caused defects in the global transcriptome without nucleosome reassembly change. Our studies explain how Spt6-RNAPII uncoupling causes significant upregulation of many weakly transcribed genes (i.e., increased mRNA stability); however, they do not explain why highly transcribed genes require Spt6-RNAPII interaction for optimal expression (Figure 2A). Perhaps highly transcribed genes are either less reliant on the Ccr4-Not pathway for their regulation or they are more dependent on the elongation-stimulating activity of Spt6.
Our finding that Spt6 associates with the Ccr4-Not complex provides a mechanistic basis for Spt6 control of mRNA turnover. The association between Spt6 and Ccr4-Not also likely explains how Spt6 recruits Ccr4-Not genes. However, more work is needed to determine whether Ccr4-Not interaction occurs directly with Spt6 or indirectly with other Spt6-associated factors (e.g., Spt4/Spt5). In addition, our Spt6/Ccr4-Not interaction findings contrast with those of Denis et al. who did not detect Spt6 in Ccr4 immunoprecipitates (Denis et al., 1994). The absence of Spt6-Ccr4 co-immunoprecipitation in the earlier study may have been due to the transient, and perhaps indirect, nature of the Spt6-Ccr4 interaction (Denis et al., 1994).
In addition to showing that Spt6 associates with Ccr4-Not, we found that Spt6-RNAPII uncoupling and long-term nuclear depletion of Spt6 impaired the stability of members of the Ccr4-Not complex. Although we do not know the mechanism of this accelerated protein turnover, these results suggest that recruitment of the Ccr4-Not complex to chromatin is critical for the stability and function of the complex. Intriguingly, Ccr4-Not is nuclear, where it functions in transcription elongation, and cytoplasmic, where it is involved in deadenylation (Collart, 2016; Siwaszek et al., 2014). Thus, our findings suggest that chromatin association is an important event in the life-cycle and long-term cytoplasmic function and stability of the Ccr4-Not complex. But how would this occur and why would this regulation be necessary? We speculate that loading of Ccr4-Not onto genes undergoing transcription may facilitate subsequent mRNA binding (via its numerous RNA interaction motifs) (Collart and Reese, 2014; Villanyi and Collart, 2015) to nascent RNA, which may serve to maintain this complex’s stability and export into the cytoplasm. We further speculate that the association of Ccr4-Not with mRNA from initial transcription to translation may facilitate rapid and timely degradation of mRNAs expressed in rapidly changing transcription programs such as the cell cycle (Eser et al., 2014). Indeed, mutations in other genes (e.g., Xrn1, Dbf2, and Dhh1) that impair mRNA degradation also show morphological and cell cycle phenotypes similar to spt6tSH2Δ cells (Maillet et al., 2000; Manukyan et al., 2008; Traven et al., 2009; Westmoreland et al., 2004). Further, our studies showed that deletion of CCR4 leads to sensitivity to genotoxic agents and a delay in release from G1 arrest, phenotypes that are also possessed by spt6tSH2Δ cells (Supplementary Figure 7).
Although we have discovered a function for Spt6-dependent gene recruitment of Ccr4-Not in the regulation of mRNA stability, it is possible that the stabilization of a significant fraction of mRNAs may also be an effect of a global decrease in transcription (Dahan and Choder, 2013; Goler-Baron et al., 2008; Haimovich et al., 2013; Shalem et al., 2011). Several studies have demonstrated the existence of a feedback loop between mRNA synthesis and decay leading to maintenance of global mRNA levels (i.e., “buffering”) (Sun et al., 2013; Sun et al., 2012). Our analysis of several transcripts (Figure 5B) whose steady state levels increased were, in fact, down-regulated when examining the nascent levels of those RNAs. Thus, a feedback mechanism between the rates of mRNA synthesis and degradation may also contribute to the stability of these mRNAs in addition to our reported connection between Spt6 and the Ccr4-Not complex. Finally, promoter elements are also suggested to control the fate of mRNAs, such that genes that share promoter elements are transcribed and degraded in a similar manner, providing an additional layer of tight control of gene expression (Bregman et al., 2011; Enssle et al., 1993; Trcek et al., 2011). There is no information pointing to a function of Spt6 in recognizing specific promoter elements, which precludes promoters from the regulation of mRNA stability in the spt6tSH2Δ cells in this study.
In conclusion, our studies reveal an important function for Spt6-RNAPII interaction in regulation of the global transcriptome and in mRNA turnover, which is key for proper cell cycle progression. Because of the high evolutionarily conservation of Spt6, we predict that these findings will be mirrored in more complex eukaryotes and critical to the regulation of transcription programs that govern animal development, cell fate specification, and disease progression.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to, and will be fulfilled by, the Lead Contact, Brian D. Strahl (brian_stral@med.unc.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All yeast strains, plasmids, antibodies, and general reagents used in this study are listed in Supplementary Table 2. Deletion and C-terminal tagging were performed using the PCR tool box as described (Gelbart et al., 2001; Janke et al., 2004). All yeast strains used in this study were verified by PCR, Sanger sequencing, and immunoblotting for the respective epitopes.
METHOD DETAILS
SPT6 ANCHOR AWAY
A strain containing an FRB-tagged form of Spt6 was treated with 1 μg/ml of rapamycin to deplete Spt6 from the nucleus (Fan et al., 2011). Cells were collected at indicated time points and fixed in formaldehyde immediately for ChIP assays. The percent Spt6 remaining in the nucleus at indicated time points was calculated using ChIP-qPCR at the PMA1 and TDH3 loci.
SPOTTING ASSAYS
Saturated yeast cultures of the indicated genotypes were diluted five-fold and spotted on plates with or without 200 mM hydroxyurea (HU), 200 μg/ml of 6-azauracil (6-AU), or 20 μg/ml of benomyl. To assess a SPT– phenotype, cells were spotted on either SC or SC-LYS and allowed to grow for 3 days before taking photographs. Strains used for 6-AU assays contained a pRS316 URA3-containing plasmid. Growth was assessed after 3 days at 30°C or 37°C as indicated. All experiments were performed at least three times.
CO-IMMUNOPRECIPITATION OF PROTEIN COMPLEXES
Co-immunoprecipitation was performed using an established method (Moqtaderi et al., 1996). Briefly, an overnight saturated yeast culture was diluted in 100 ml of YPD to an OD600 of 0.2 and grown subsequently for 4 h to an OD600 of ~1.0. Cells were washed once with ice cold water and lysed in 600 μl lysis buffer [450 mM Tris-acetate (pH 7.8), 150 mM potassium acetate, 60% (v/v) glycerol, 3 mM EDTA (pH 8.0), supplemented with fresh 1 mM DTT, 1 mM PMSF, 1X complete EDTA-free protease inhibitors (Roche)] using standard bead-beating procedures as described. Lysates were then clarified by centrifugation for 15 minutes 4°C. One mg of total protein (estimated using Bradford) was incubated with the indicated antibody overnight at 4°C in 1 ml of buffer A [50 mM HEPES-KOH (pH 7.5), 1 mM EDTA (pH 8.0), 20% (v/v) glycerol, 125 mM potassium acetate, 1% (v/v) NP-40, supplemented with fresh 100 mM DTT]. Protein A Agarose beads (Sigma Aldrich) were added and incubated for 2 h at 4°C. The complexes on beads were washed 3 times in buffer A. Beads were heated at 95°C and proteins were separated by SDS-PAGE and subjected to immunoblotting to detect interacting proteins.
IMMUNOBLOTTING
Immunoblotting was performed after extraction of proteins by TCA lysis as described (Keogh et al., 2006a; Keogh et al., 2006b). Lysates were separated by SDS-PAGE and probed using primary antibodies described in Supplementary Table 2. HRP-conjugated anti-rabbit (GE Healthcare, NA934V; 1:10,000) or anti-mouse secondary (GE Healthcare, NA931V; 1:10,000) antibodies were used for detection by ECL Prime or enhanced chemiluminescence ECL (Amersham Biosciences).
RNA-SEQ AND QRT-PCR METHODOLOGY
WT and spt6tSH2Δ strains were cultured in YPD for 4 h after diluting overnight saturated cultures to an OD600 of 0.2. RNA was extracted from the cells using an acid-phenol method as described. Residual DNA was eliminated by DNase treatment (DNAse I, Ambion Cat #AM2222). Library preparation and sequencing were performed at the High-Throughput Sequencing Facility (HTSF) at UNC Chapel Hill. Libraries were prepared using TruSeq Stranded Total RNA with RiboZero Gold Library Prep Kit (Illumina) and were sequenced using Illumina HiSeq 2500 (50 bp, single-end reads). One microgram of total RNA was used to generate cDNA from Random hexamer primers and Superscript Reverse Transcriptase III (Thermo-Fisher Scientific, 108–80044). The cDNA was diluted 1:25 before measuring the relative abundance of the transcripts. The real-time PCR primers employed will be provided upon request. Quantitative real-time PCR was performed using SYBR Green Master mix (Bio-Rad, 1725270), and the relative abundances of the transcripts were calculated using the Livak method (Livak et al., 2013). SCR1 served as a normalization control. Data shown are the replicates of three independent experiments; the significance values were calculated using Student t-test.
RNA-SEQ READ ALIGNMENTS AND ANALYSIS
RNA-seq libraries were sequenced on the Illumina HiSeq 2500. Reads containing Illumina adapter sequences were filtered out via TagDust (v1.12) with an FDR cutoff of 0.001. Reads were then aligned to the sacCer3 genome using Bowtie (v1.1.2) with options -m 1, --seed=123, and --nomaqround. Post-alignment, Samtools (v0.1.9) and bedtools (v2.25.0) were used to interconvert files. Gene counts were calculated using HTSeq (v0.6.1) and the sacCer3 sgdGene table from UCSC Genome Browser, and DESeq2 (v1.6.3) in R (v3.1.1) was used to identify differentially abundant mRNA. Gene expression in reads per kilobase per million mapped reads (RPKM) was calculated using in-house scripts. Only genes that had both a DESeq2 adjusted p-value < 0.05 and a log2(RPKM fold change) of at least 0.5 in either direction were considered for downstream analyses. GO terms were generated using DAVID (v6.8). The accession numbers for the RNA-seq and ATAC-seq datasets reported in this paper are through GEO (accession no. GSE 111815).
CHROMATIN FRACTIONATION AND NASCENT RNA EXTRACTION
Nascent chromatin-bound RNA was extracted form yeast cells as described (Oesterreich et al., 2016). Briefly, 250 ml of wild-type and spt6tSH2Δ strains were grown to an OD600 ~1, collected by centrifugation, and washed twice in PBS. Chromatin fractions were prepared as described by Carrillo et al. The chromatin fractions were resuspended in 250 μl of 50 mM NaAcetate pH 5.0, 50 mM NaCl, 1% SDS, and RNA was extracted from the chromatin fraction using an acid phenol method as described. RNA was subjected to DNAse treatment, and the enrichment of nascent transcripts was calculated using gene specific primers designed for ADH1 as described (Oesterreich et al., 2016). Total and Nascent RNA were subjected to qRT-PCR to detect changes in the levels of different cyclin and histone mRNAs.
mRNA STABILITY MEASUREMENTS
Thiolutin, an inhibitor of all forms of RNA polymerase, was used to measure the mRNA decay rates as described previously (Coller, 2008). Briefly, wild type and spt6tSH2Δ strains were grown to mid log phase in YPD to an OD600 of about 0.6. Thiolutin was added to 5 μg/ml, and cells were collected at indicated time points and flash frozen in liquid nitrogen. RNA was extracted and the relative abundance at each time point was measured by qRT-PCR as described above. To assess a direct role of Spt6 in the regulation of mRNA stability, we depleted Spt6 from the nucleus using 1 μg/ml rapamycin for 90 minutes, after which the cells were treated immediately with 5 μg/ml of Thiolutin. Cells were collected at indicated time intervals and used for RNA extraction and qRT-PCR measurements.
ANALYSIS OF mRNA DECAY RATES
RNA was extracted using acid phenol method as described previously. Poly(A) tail length and rates of mRNA decay were measured by Northern Blotting of RNAs cleaved at the 3’ ends using a gene specific primer only or a combination of gene specific primer and oligo-dT to measure the totally de-adenylated RNA as described (Muhlrad et al., 1994). Briefly, gene specific primer (GSP) and/or oligo-dT12–18 primers of candidate genes were hybridized to total RNA at 68°C for 10 minutes in hybridization buffer (250mM Tris-HCl pH7.5, 1mM EDTA and 50mM NaCl). The reaction was allowed to cool and the mixture was treated with 1U of RNAse H for 60 minutes at 37°C. The reaction was stopped using stop mix (0.04 mg/ml tRNA, 20 mM EDTA and 300 mM sodium acetate). RNA was extracted by phenol chloroform method and resuspended in 10 μL of DEPC-treated water. RNA was mixed with equal volumes of RNA loading buffer and separated in 8% −10% TBE-urea-polyacrylamide gels at constant power (10W). RNA was electroblotted onto ZetaProbe membranes at 250mA for 2h and then at 350mA for a further 2h at 4°C and probed using gamma-32P-labeled oligos, which are available upon request. Dried membranes were exposed to FujiFilm Imaging plate for 24 hrs. The imaging plate was scanned on a Typhoon Trio Variable mode Imager.
ATAC-SEQ LIBRARY PREPARATION
Five million wild-type (S288C) and spt6tSH2Δ cells were collected during their linear phase of growth. Three replicates each were collected for the wild type and mutant (total of 6 replicates). ATAC-seq libraries were made as described (Buenrostro et al., 2015; Schep et al., 2017), with the exception of a double-sided SPRI bead size selection step of each library, using 0.5× and 1× ratio of SPRI beads to obtain a library size range of ~150 bp to ~2 kb. Wild type replicates were index labeled using Ad2.1_TAAGGCGA, Ad2.2_CGTACTAG, and Ad2.3_AGGCAGAA Illumina indexes, whereas the mutant libraries were indexed using Ad2.6_TAGGCATG, Ad2.7_CTCTCTAC, and Ad2.8_CAGAGAGG (Buenrostro 2013). All 6 libraries were combined and sequenced on a single lane of Illumina Hiseq 2500 using v4 chemistry for 50bp paired-end output.
ATAC-SEQ ALIGNMENTS AND INITIAL ANALYSIS
Fastq sequence files were first filtered for high quality reads using trimgalore version 0.4.1, “trim_galore -q 20 --nextera --length 20”, then aligned to the sacCer3 genome using bowtie2, “bowtie2 --threads 6 --very-sensitive --maxins 2000” (Krueger 2015 and Langmead 2012). bowtie2 output files were converted to bam files using Samtools version 1.3.1. Next Bedtools version 2.25.0 “bamtobed” was used to convert bam output files to bed file format to perform the subsequent steps (Quinlan 2010). Using simple custom Awk scripts, exact Tn5 transposase bp insertion sites were created from the bed files by adjusting the insertion sites +4 bp for positive strand sequences, adjusting −5 bp for negative stranded sequences, and adjusting the bed file region to just the 1 bp region at the insertion sites. This bed file was then converted back to bam format using Bedtools “bedtobam” before normalizing the sequence counts using Deeptools’ bamCoverage, “ --binSize 1 --fragmentLength 1 --normalizeTo1× 12100000 --minMappingQuality 20”, to create a bigWig output file for each replicate.
Awk command with For loop:
for i in ./s_*/bowtie2_sacCer3; do
awk ‘BEGIN OFS = “\t”; if ($6 == “+”) print $1, $2 + 4, $2 + 5, $4, $5, $6; else print $1, $3 – 6, $3 – 5, $4, $5, $6}’ $i/sequence.bed > $i/sequence.shifted.exactcut.bed; done
META-ANALYSIS OF ATAC-SEQ SIGNAL
Differential genes determined using DESeq2 were used to create a list of upregulated (567) and downregulated (598) genes in the mutant yeast strain compared with the wild type. A similar number of genes (601) was selected for which the expression levels had little or no change (DESeq2, padj>0.5) but which reflected a range of baseline transcription levels. Cell cycle (800 genes, Spellman 1998) and histone (8 genes) gene lists were compiled. Each set of genes was sorted in decreasing order from highest transcribed to lowest transcribed. Using computematrix.py and plotheatmapper.py programs from Deeptools version 2.2.4, we normalized ATAC-seq signal for a scaled region representing each of the genes in our gene subsets plus/minus 1 kb were compiled and plotted (Borrill et al., 2016).
YEAST CELL CYCLE SYNCHRONIZATION
Saturated cultures of the wild type and spt6tSH2Δ strains were inoculated into YPD at an OD600 of 0.1 and allowed to grow to an OD600 ~0.6. Alpha-factor (25 nM; GenScript USA, RPO1002) was added to cultures to arrest cells in the G1-phase of cell cycle. G1 arrest was confirmed by microscopy. G1-arrested cells were washed three time in water and released into fresh YPD medium. Samples were collected at indicated time points for propidium iodide staining to assess the population distribution across different phases of cell cycle, and additional samples were collected in parallel for RNA extraction and cDNA synthesis. FRB-tagged SPT6 strains were treated with α-factor to induce G1-arrest for 2 h, followed by the addition of 1 μg/ml of rapamycin for 90 minutes. After the treatments, cells were washed and released into fresh YPD medium. At indicated time intervals, cells were collected and flash frozen in liquid nitrogen for RNA extraction and qRT-PCR. Cells were also collected at the same time intervals and fixed in 70% ethanol to measure their cell cycle distribution by flow cytometry of propidium iodide-stained nuclei.
CHROMATIN IMMUNOPRECIPITATION
ChIP was performed as described with modifications (Ahn et al., 2009). DNA was eluted in 100 μl of elution buffer. Two μl of the DNA was subjected to qPCR using SYBR Green (Bio-Rad), and the data were analyzed as described. Data are representative of three independent replicates. Data are represented as the mean percent input values over standard deviations (SD) from three biological replicates with technical triplicates.
Supplementary Material
Table S1. List of genes differentially expressed in the spt6tSH2Δ mutant, Related to Figure 2.
Table S2. List of Oligonucleotides, used for qRT-PCRs and ChIPs, Related to Figures 1, 3, 4, 5 and 6.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-V5 antibody. Polyclonal | Bethyl labs | Catalog #A190–220A |
| Anti-Myc antibody, Monoclonal | EMD Millipore | Catalog # 050419 |
| Anti RNAPII Ser2 monoclonal, (Clone 9E10) | Active Motif | Catalog # 61084 |
| Anti-Spt6, polyclonal | In House | |
| Anti-histone H3 antibody, polyclonal | In House | |
| Anti-histone H2B antibody, polyclonal | Active Motif | Catalog # 39328 |
| Normal rabbit serum | Cell Signaling Technology | Catalog # 2729S |
| Anti-G6PDH antibody, polyclonal | Sigma | Catalog # A9521–1VL |
| ChIP grade RNAPII antibody | BioLegend | Catalog # 664912 |
| HRP-conjugated anti-rabbit | GE Healthcare | Catalog # NA934V |
| HRP-conjugated anti-mouse | GE Healthcare | Catalog # NA931V |
| Chemicals, Peptides, and Recombinant Proteins | ||
| 6-Azauracil | SIGMA (Roche) | Catalog # A1757 |
| Benomyl | SIGMA (Roche) | Catalog # 45339 |
| Hydroxyurea | SIGMA (Roche) | Catalog # H8627 |
| RNAseH | Promega | Catalog # M4281 |
| T4 Polynucleotide Kinase | NEB | Catalog # M0201S |
| ULTRAhyb Oligo Buffer | Thermo Fisher Scientific | Catalog # AM8663 |
| SSIII RT reverse transcriptase | Thermo Fisher Scientific | Catalog # 18080–044 |
| Acid phenol Chloroform | Ambion/Thermo Fisher Scientific | Catalog # AM9722 |
| Zeta Probe Blotting membrane | Biorad | Catalog # 1620153 |
| Rapamycin | SIGMA (Roche) | Catalog # R8781 |
| cOMPLETE, EDTA free protease inhibitor tablets | SIGMA (Roche) | Catalog # 11873580001 |
| PhosSTOP phosphatase inhibitor tablets | SIGMA (Roche) | Catalog # 04906845001 |
| Century TM Plus RNA markers | Thermo Fisher Scientific | Catalog # AM7145 |
| Gel Loading Buffer II | Thermo Fisher Scientific | Catalog # AM8547 |
| iTaq™ Universal SYBR ® Green Supermix | Biorad | Catalog # 172–5124 |
| Protein A Agarose | SIGMA (Roche) | Catalog # 11134515001 |
| Propidium Iodide | Thermo Fisher Scientific | Catalog # P3566 |
| Thiolutin | Abcam | Catalog # ab143556 |
| Dynabeads Protein G | Thermo Fisher Scientific | Catalog # 10009D |
| SPRI Beads (Agencourt AMPure XP) | Beckman Coulter | Catalog # A63881 |
| α-Factor Mating pheromone | Genscript | Catalog # 59804–28–4 |
| Critical Commercial Assays | ||
| TruSeq Stranded Total RNA with RiboZero Gold Library Prep Kit. | Illumina | Catalog # RS-122–2301 |
| Deposited Data | ||
| RNAseq and ATAC seq data | This study | GEO:GSE111815 |
| Raw data for Northern Blots and Immunoblots | This Study | Mendeley:http://dx.doi.org/10.17632/tnx4py69j8 |
| Experimental Models: S.serevisiae strains | ||
| MATa leu2 trp1-Δ63 ura3 his4–912∂ lys2–128∂ | Winston Lab | FY2181 |
| MATa leu2 trp1-Δ63 ura3 his4–912∂ lys2–128∂ spt6–1004 | Winston Lab | FY2180 |
| MATa leu2 trp1-Δ63 ura3 his4–912∂ lys2–128∂ CCR4–9MYC::kanMX | This study | RDY20171 |
| MATa leu2 trp1-Δ63 ura3 his4–912∂ lys2–128∂ spt6tSH2::natMX | This study | RDY20172 |
| MATa leu2 trp1-Δ63 ura3 his4–912∂ lys2–128∂ spt6tSH2::natMX CCR4–9MYC::kanMX | This study | RDY20173 |
| MATa ade2–1 trp1–1can1–100 leu2–3, 112 his3–11, 15 ura3 tor1–1 frp1::NAT RPL13A-2XFKBP12::TRP1 SPT6-FRB::KanMX | This study | yDZ001 |
| Oligonucleotides | ||
| Oligos | This study and other sources | Supplementary Table 2 |
| Recombinant DNA | ||
| NOT1-V5 (plasmid), | DNASU | Catalog # ScCD00102519 |
| NOT2-V5 (plasmid) | DNASU | Catalog # ScCD00103149 |
| DHH1-V5 (plasmid) | DNASU | Catalog # ScCD00102795 |
ACKNOWLEDGMENTS
We sincerely acknowledge the help provided by Joseph B. Bridgers in expressing and purifying the DLD domain of Spt6 for antisera generation, Josh Boyer and Jared Baisden (Qi Zhang lab) and William Marzluff at UNC for all their support and expert advice on Northern Blotting protocols. We thank the UNC high-throughput sequencing facility and the members of B.D.S. and T.M. labs for their support and Howard Fried for editorial help. B.D.S. acknowledges support from NIH grants R35GM126900 and R01GM110058, and I.J.D. acknowledges support from NIH grants CA166447 and CA198482 and the Corn-Hammond Fund for Pediatric Oncology.
Footnotes
DECLARATION OF INTERESTS
The authors declare no conflict of interest.
DATA AND SOFTWARE AVAILABILITY
The accession number for the raw and processed RNA-seq and ATAC-seq data reported in this paper is GEO: GSE111815. The raw data reported in this paper has been deposited into Mendeley: http://dx.doi.org/10.17632/tnx4py69j8
REFERENCES
- Ahn SH, Keogh MC, and Buratowski S (2009). Ctk1 promotes dissociation of basal transcription factors from elongating RNA polymerase II. EMBO J 28, 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardehali MB, Yao J, Adelman K, Fuda NJ, Petesch SJ, Webb WW, and Lis JT (2009). Spt6 enhances the elongation rate of RNA polymerase II in vivo. EMBO J 28, 1067–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler J (2005). Cell-cycle control of gene expression in budding and fission yeast. Annu Rev Genet 39, 69–94. [DOI] [PubMed] [Google Scholar]
- Borrill P, Ramirez-Gonzalez R, and Uauy C (2016). expVIP: a Customizable RNA-seq Data Analysis and Visualization Platform. Plant Physiol 170, 2172–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortvin A, and Winston F (1996). Evidence that Spt6p controls chromatin structure by a direct interaction with histones. Science 272, 1473–1476. [DOI] [PubMed] [Google Scholar]
- Bregman A, Avraham-Kelbert M, Barkai O, Duek L, Guterman A, and Choder M (2011). Promoter elements regulate cytoplasmic mRNA decay. Cell 147, 1473–1483. [DOI] [PubMed] [Google Scholar]
- Buenrostro JD, Wu B, Litzenburger UM, Ruff D, Gonzales ML, Snyder MP, Chang HY, and Greenleaf WJ (2015). Single-cell chromatin accessibility reveals principles of regulatory variation. Nature 523, 486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung V, Chua G, Batada NN, Landry CR, Michnick SW, Hughes TR, and Winston F (2008). Chromatin- and transcription-related factors repress transcription from within coding regions throughout the Saccharomyces cerevisiae genome. PLoS Biol 6, e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang PW, Wang S, Smithivas P, Song WJ, Ramamoorthy S, Hillman J, Puett S, Van Keuren ML, Crombez E, Kumar A, et al. (1996). Identification and analysis of the human and murine putative chromatin structure regulator SUPT6H and Supt6h. Genomics 34, 328–333. [DOI] [PubMed] [Google Scholar]
- Clark-Adams CD, and Winston F (1987). The SPT6 gene is essential for growth and is required for delta-mediated transcription in Saccharomyces cerevisiae. Mol Cell Biol 7, 679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Close D, Johnson SJ, Sdano MA, McDonald SM, Robinson H, Formosa T, and Hill CP (2011). Crystal structures of the S. cerevisiae Spt6 core and C-terminal tandem SH2 domain. J Mol Biol 408, 697–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart MA (2016). The Ccr4-Not complex is a key regulator of eukaryotic gene expression. Wiley Interdiscip Rev RNA 7, 438–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart MA, and Panasenko OO (2012). The Ccr4--not complex. Gene 492, 42–53. [DOI] [PubMed] [Google Scholar]
- Collart MA, and Reese JC (2014). Gene expression as a circular process: cross-talk between transcription and mRNA degradation in eukaryotes; International University of Andalusia (UNIA) Baeza, Spain. RNA Biol 11, 320–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller J (2008). Methods to determine mRNA half-life in Saccharomyces cerevisiae. Methods Enzymol 448, 267–284. [DOI] [PubMed] [Google Scholar]
- Cui P, Jin H, Vutukuru MR, and Kaplan CD (2016). Relationships Between RNA Polymerase II Activity and Spt Elongation Factors to Spt- Phenotype and Growth in Saccharomyces cerevisiae. G3 (Bethesda) 6, 2489–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahan N, and Choder M (2013). The eukaryotic transcriptional machinery regulates mRNA translation and decay in the cytoplasm. Biochim Biophys Acta 1829, 169–173. [DOI] [PubMed] [Google Scholar]
- Das S, Sarkar D, and Das B (2017). The interplay between transcription and mRNA degradation in Saccharomyces cerevisiae. Microb Cell 4, 212–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGennaro CM, Alver BH, Marguerat S, Stepanova E, Davis CP, Bahler J, Park PJ, and Winston F (2013). Spt6 regulates intragenic and antisense transcription, nucleosome positioning, and histone modifications genome-wide in fission yeast. Mol Cell Biol 33, 4779–4792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengl S, Mayer A, Sun M, and Cramer P (2009). Structure and in vivo requirement of the yeast Spt6 SH2 domain. J Mol Biol 389, 211–225. [DOI] [PubMed] [Google Scholar]
- Denis CL, Draper MP, Liu HY, Malvar T, Vallari RC, and Cook WJ (1994). The yeast CCR4 protein is neither regulated by nor associated with the SPT6 and SPT10 proteins and forms a functionally distinct complex from that of the SNF/SWI transcription factors. Genetics 138, 1005–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniz O, Flores O, Aldea M, Soler-Lopez M, and Orozco M (2016). Nucleosome architecture throughout the cell cycle. Sci Rep 6, 19729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold ML, Loeliger E, Koch M, Winston F, Cavarelli J, and Romier C (2010). Noncanonical tandem SH2 enables interaction of elongation factor Spt6 with RNA polymerase II. J Biol Chem 285, 38389–38398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta A, Babbarwal V, Fu J, Brunke-Reese D, Libert DM, Willis J, and Reese JC (2015). Ccr4-Not and TFIIS Function Cooperatively To Rescue Arrested RNA Polymerase II. Mol Cell Biol 35, 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enssle J, Kugler W, Hentze MW, and Kulozik AE (1993). Determination of mRNA fate by different RNA polymerase II promoters. Proc Natl Acad Sci U S A 90, 10091–10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eser P, Demel C, Maier KC, Schwalb B, Pirkl N, Martin DE, Cramer P, and Tresch A (2014). Periodic mRNA synthesis and degradation co-operate during cell cycle gene expression. Mol Syst Biol 10, 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Geisberg JV, Wong KH, and Jin Y (2011). Conditional depletion of nuclear proteins by the Anchor Away system. Curr Protoc Mol Biol Chapter 13, Unit13 10B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores O, Deniz O, Soler-Lopez M, and Orozco M (2014). Fuzziness and noise in nucleosomal architecture. Nucleic Acids Res 42, 4934–4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelbart ME, Rechsteiner T, Richmond TJ, and Tsukiyama T (2001). Interactions of Isw2 chromatin remodeling complex with nucleosomal arrays: analyses using recombinant yeast histones and immobilized templates. Mol Cell Biol 21, 2098–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrist DA, Dos Santos G, Fargo DC, Xie B, Gao Y, Li L, and Adelman K (2010). Pausing of RNA polymerase II disrupts DNA-specified nucleosome organization to enable precise gene regulation. Cell 143, 540–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goler-Baron V, Selitrennik M, Barkai O, Haimovich G, Lotan R, and Choder M (2008). Transcription in the nucleus and mRNA decay in the cytoplasm are coupled processes. Genes Dev 22, 2022–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich G, Medina DA, Causse SZ, Garber M, Millan-Zambrano G, Barkai O, Chavez S, Perez-Ortin JE, Darzacq X, and Choder M (2013). Gene expression is circular: factors for mRNA degradation also foster mRNA synthesis. Cell 153, 1000–1011. [DOI] [PubMed] [Google Scholar]
- Hainer SJ, Pruneski JA, Mitchell RD, Monteverde RM, and Martens JA (2011). Intergenic transcription causes repression by directing nucleosome assembly. Genes Dev 25, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzog GA, Wada T, Handa H, and Winston F (1998). Evidence that Spt4, Spt5, and Spt6 control transcription elongation by RNA polymerase II in Saccharomyces cerevisiae. Genes Dev 12, 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruki H, Nishikawa J, and Laemmli UK (2008). The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol Cell 31, 925–932. [DOI] [PubMed] [Google Scholar]
- Hogan GJ, Lee CK, and Lieb JD (2006). Cell cycle-specified fluctuation of nucleosome occupancy at gene promoters. PLoS Genet 2, e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, and Coller J (2013). Method for measuring mRNA decay rate in Saccharomyces cerevisiae. Methods Enzymol 530, 137–155. [DOI] [PubMed] [Google Scholar]
- Hyle JW, Shaw RJ, and Reines D (2003). Functional distinctions between IMP dehydrogenase genes in providing mycophenolate resistance and guanine prototrophy to yeast. J Biol Chem 278, 28470–28478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanovska I, Jacques PE, Rando OJ, Robert F, and Winston F (2011). Control of chromatin structure by spt6: different consequences in coding and regulatory regions. Mol Cell Biol 31, 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janke C, Magiera MM, Rathfelder N, Taxis C, Reber S, Maekawa H, Moreno-Borchart A, Doenges G, Schwob E, Schiebel E, et al. (2004). A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21, 947–962. [DOI] [PubMed] [Google Scholar]
- Jeronimo C, Watanabe S, Kaplan CD, Peterson CL, and Robert F (2015). The Histone Chaperones FACT and Spt6 Restrict H2A.Z from Intragenic Locations. Mol Cell 58, 1113–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan CD, Holland MJ, and Winston F (2005). Interaction between transcription elongation factors and mRNA 3’-end formation at the Saccharomyces cerevisiae GAL10-GAL7 locus. J Biol Chem 280, 913–922. [DOI] [PubMed] [Google Scholar]
- Kaplan CD, Laprade L, and Winston F (2003). Transcription elongation factors repress transcription initiation from cryptic sites. Science 301, 1096–1099. [DOI] [PubMed] [Google Scholar]
- Kaplan CD, Morris JR, Wu C, and Winston F (2000). Spt5 and spt6 are associated with active transcription and have characteristics of general elongation factors in D. melanogaster. Genes Dev 14, 2623–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Okazaki K, Iida T, Nakayama J, Murakami Y, and Urano T (2013a). Spt6 prevents transcription-coupled loss of posttranslationally modified histone H3. Sci Rep 3, 2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato H, Okazaki K, and Urano T (2013b). Spt6: two fundamentally distinct functions in the regulation of histone modification. Epigenetics 8, 1249–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh MC, Kim JA, Downey M, Fillingham J, Chowdhury D, Harrison JC, Onishi M, Datta N, Galicia S, Emili A, et al. (2006a). A phosphatase complex that dephosphorylates gammaH2AX regulates DNA damage checkpoint recovery. Nature 439, 497–501. [DOI] [PubMed] [Google Scholar]
- Keogh MC, Mennella TA, Sawa C, Berthelet S, Krogan NJ, Wolek A, Podolny V, Carpenter LR, Greenblatt JF, Baetz K, et al. (2006b). The Saccharomyces cerevisiae histone H2A variant Htz1 is acetylated by NuA4. Genes Dev 20, 660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr SC, Azzouz N, Fuchs SM, Collart MA, Strahl BD, Corbett AH, and Laribee RN (2011). The Ccr4-Not complex interacts with the mRNA export machinery. PLoS One 6, e18302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiely CM, Marguerat S, Garcia JF, Madhani HD, Bahler J, and Winston F (2011). Spt6 is required for heterochromatic silencing in the fission yeast Schizosaccharomyces pombe. Mol Cell Biol 31, 4193–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruk JA, Dutta A, Fu J, Gilmour DS, and Reese JC (2011). The multifunctional Ccr4-Not complex directly promotes transcription elongation. Genes Dev 25, 581–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak H, and Lis JT (2013). Control of transcriptional elongation. Annu Rev Genet 47, 483–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Wills QF, Tipping AJ, Datta K, Mittal R, Goldson AJ, Sexton DW, and Holmes CC (2013). Methods for qPCR gene expression profiling applied to 1440 lymphoblastoid single cells. Methods 59, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet L, Tu C, Hong YK, Shuster EO, and Collart MA (2000). The essential function of Not1 lies within the Ccr4-Not complex. J Mol Biol 303, 131–143. [DOI] [PubMed] [Google Scholar]
- Manukyan A, Zhang J, Thippeswamy U, Yang J, Zavala N, Mudannayake MP, Asmussen M, Schneider C, and Schneider BL (2008). Ccr4 alters cell size in yeast by modulating the timing of CLN1 and CLN2 expression. Genetics 179, 345–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Heidemann M, Lidschreiber M, Schreieck A, Sun M, Hintermair C, Kremmer E, Eick D, and Cramer P (2012). CTD tyrosine phosphorylation impairs termination factor recruitment to RNA polymerase II. Science 336, 1723–1725. [DOI] [PubMed] [Google Scholar]
- Mayer A, Lidschreiber M, Siebert M, Leike K, Soding J, and Cramer P (2010). Uniform transitions of the general RNA polymerase II transcription complex. Nat Struct Mol Biol 17, 1272–1278. [DOI] [PubMed] [Google Scholar]
- McCullough L, Connell Z, Petersen C, and Formosa T (2015). The Abundant Histone Chaperones Spt6 and FACT Collaborate to Assemble, Inspect, and Maintain Chromatin Structure in Saccharomyces cerevisiae. Genetics 201, 1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SM, Close D, Xin H, Formosa T, and Hill CP (2010). Structure and biological importance of the Spn1-Spt6 interaction, and its regulatory role in nucleosome binding. Mol Cell 40, 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DA, Jordan-Pla A, Millan-Zambrano G, Chavez S, Choder M, and Perez-Ortin JE (2014). Cytoplasmic 5’−3’ exonuclease Xrn1p is also a genome-wide transcription factor in yeast. Front Genet 5, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JE, and Reese JC (2012). Ccr4-Not complex: the control freak of eukaryotic cells. Crit Rev Biochem Mol Biol 47, 315–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moqtaderi Z, Yale JD, Struhl K, and Buratowski S (1996). Yeast homologues of higher eukaryotic TFIID subunits. Proc Natl Acad Sci U S A 93, 14654–14658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad D, Decker CJ, and Parker R (1994). Deadenylation of the unstable mRNA encoded by the yeast MFA2 gene leads to decapping followed by 5’-->3’ digestion of the transcript. Genes Dev 8, 855–866. [DOI] [PubMed] [Google Scholar]
- Nasmyth K (1993). Control of the yeast cell cycle by the Cdc28 protein kinase. Curr Opin Cell Biol 5, 166–179. [DOI] [PubMed] [Google Scholar]
- Neigeborn L, Celenza JL, and Carlson M (1987). SSN20 is an essential gene with mutant alleles that suppress defects in SUC2 transcription in Saccharomyces cerevisiae. Mol Cell Biol 7, 672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterreich FC, Herzel L, Straube K, Hujer K, Howard J, and Neugebauer KM (2016). Splicing of Nascent RNA Coincides with Intron Exit from RNA Polymerase II. Cell 165, 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos DO, and Parker R (2008). Analysis of cytoplasmic mRNA decay in Saccharomyces cerevisiae. Methods Enzymol 448, 409–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales R, Erickson B, Zhang L, Kim H, Valiquett E, and Bentley D (2013). Gene promoters dictate histone occupancy within genes. EMBO J 32, 2645–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese JC (2013). The control of elongation by the yeast Ccr4-not complex. Biochim Biophys Acta 1829, 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudner AD, Hardwick KG, and Murray AW (2000). Cdc28 activates exit from mitosis in budding yeast. J Cell Biol 149, 1361–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schep AN, Buenrostro JD, Denny SK, Schwartz K, Sherlock G, and Greenleaf WJ (2015). Structured nucleosome fingerprints enable high-resolution mapping of chromatin architecture within regulatory regions. Genome Res 25, 1757–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schep AN, Wu B, Buenrostro JD, and Greenleaf WJ (2017). chromVAR: inferring transcription-factor-associated accessibility from single-cell epigenomic data. Nat Methods 14, 975–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabish MA, and Struhl K (2004). Evidence for eviction and rapid deposition of histones upon transcriptional elongation by RNA polymerase II. Mol Cell Biol 24, 10111–10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sdano MA, Fulcher JM, Palani S, Chandrasekharan MB, Parnell TJ, Whitby FG, Formosa T, and Hill CP (2017). A novel SH2 recognition mechanism recruits Spt6 to the doubly phosphorylated RNA polymerase II linker at sites of transcription. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalem O, Groisman B, Choder M, Dahan O, and Pilpel Y (2011). Transcriptome kinetics is governed by a genome-wide coupling of mRNA production and degradation: a role for RNA Pol II. PLoS Genet 7, e1002273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty A, Kallgren SP, Demel C, Maier KC, Spatt D, Alver BH, Cramer P, Park PJ, and Winston F (2017). Spt5 Plays Vital Roles in the Control of Sense and Antisense Transcription Elongation. Mol Cell 66, 77–88 e75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siwaszek A, Ukleja M, and Dziembowski A (2014). Proteins involved in the degradation of cytoplasmic mRNA in the major eukaryotic model systems. RNA Biol 11, 1122–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman PT, Sherlock G, Zhang MQ, Iyer VR, Anders K, Eisen MB, Brown PO, Botstein D, and Futcher B (1998). Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol Biol Cell 9, 3273–3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Lariviere L, Dengl S, Mayer A, and Cramer P (2010). A tandem SH2 domain in transcription elongation factor Spt6 binds the phosphorylated RNA polymerase II C-terminal repeat domain (CTD). J Biol Chem 285, 41597–41603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Schwalb B, Pirkl N, Maier KC, Schenk A, Failmezger H, Tresch A, and Cramer P (2013). Global analysis of eukaryotic mRNA degradation reveals Xrn1-dependent buffering of transcript levels. Mol Cell 52, 52–62. [DOI] [PubMed] [Google Scholar]
- Sun M, Schwalb B, Schulz D, Pirkl N, Etzold S, Lariviere L, Maier KC, Seizl M, Tresch A, and Cramer P (2012). Comparative dynamic transcriptome analysis (cDTA) reveals mutual feedback between mRNA synthesis and degradation. Genome Res 22, 1350–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MS, Carlson M, and Winston F (1990). SPT6, an essential gene that affects transcription in Saccharomyces cerevisiae, encodes a nuclear protein with an extremely acidic amino terminus. Mol Cell Biol 10, 4935–4941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traven A, Beilharz TH, Lo TL, Lueder F, Preiss T, and Heierhorst J (2009). The Ccr4-Pop2-NOT mRNA deadenylase contributes to septin organization in Saccharomyces cerevisiae. Genetics 182, 955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trcek T, Larson DR, Moldon A, Query CC, and Singer RH (2011). Single-molecule mRNA decay measurements reveal promoter- regulated mRNA stability in yeast. Cell 147, 1484–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M, Staples RR, Valencia-Sanchez MA, Muhlrad D, and Parker R (2002). Ccr4p is the catalytic subunit of a Ccr4p/Pop2p/Notp mRNA deadenylase complex in Saccharomyces cerevisiae. EMBO J 21, 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M, Valencia-Sanchez MA, Staples RR, Chen J, Denis CL, and Parker R (2001). The transcription factor associated Ccr4 and Caf1 proteins are components of the major cytoplasmic mRNA deadenylase in Saccharomyces cerevisiae. Cell 104, 377–386. [DOI] [PubMed] [Google Scholar]
- Villanyi Z, and Collart MA (2015). Ccr4-Not is at the core of the eukaryotic gene expression circuitry. Biochem Soc Trans 43, 1253–1258. [DOI] [PubMed] [Google Scholar]
- Westmoreland TJ, Marks JR, Olson JA Jr., Thompson EM, Resnick MA, and Bennett CB (2004). Cell cycle progression in G1 and S phases is CCR4 dependent following ionizing radiation or replication stress in Saccharomyces cerevisiae. Eukaryot Cell 3, 430–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilusz CJ, Wormington M, and Peltz SW (2001). The cap-to-tail guide to mRNA turnover. Nat Rev Mol Cell Biol 2, 237–246. [DOI] [PubMed] [Google Scholar]
- Yoh SM, Cho H, Pickle L, Evans RM, and Jones KA (2007). The Spt6 SH2 domain binds Ser2-P RNAPII to direct Iws1-dependent mRNA splicing and export. Genes Dev 21, 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Fletcher AG, Cheung V, Winston F, and Stargell LA (2008). Spn1 regulates the recruitment of Spt6 and the Swi/Snf complex during transcriptional activation by RNA polymerase II. Mol Cell Biol 28, 1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobeck KL, Buckley MS, Zipfel WR, and Lis JT (2010). Recruitment timing and dynamics of transcription factors at the Hsp70 loci in living cells. Mol Cell 40, 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of genes differentially expressed in the spt6tSH2Δ mutant, Related to Figure 2.
Table S2. List of Oligonucleotides, used for qRT-PCRs and ChIPs, Related to Figures 1, 3, 4, 5 and 6.