Abstract
Megakaryocytes (MK) are specialized precursor cells committed to producing and proliferating platelets. In a cytoskeletal-driven process, mature MKs generate platelets by releasing thin cytoplasmic extensions, named proplatelets, into the sinusoids. Due to knowledge gaps in this process and mounting clinical demand for non-donor based platelet sources, investigators are successfully developing artificial culture systems to recreate the environment of platelet biogenesis. Nevertheless, drawbacks in current methods entail elaborate procedures for stem cell enrichment, extensive growth periods, low MK yield, and poor proplatelet production. We propose a simple, robust method of primary MK culture that utilizes fetal livers from pregnant mice. Our technique reduces expansion time to 4 days, and generates ~15,000–20,000 MKs per liver. Approximately 20–50% of these MKs produce structurally dense, high quality proplatelets. In this review, we outline our method of MK culture and isolation.
Method
Materials
| 15 mL Centrifuge Tube | - Corning | Catalog #: 430052 |
| 50 mL Centrifuge Tube | - Corning | Catalog #: 352098 |
| 10 cm Tissue Culture Dish | - BD | Catalog #: 353003 |
| 10 mL Syringe | - BD | Catalog #: 309604 |
| 19 Gauge Needle (1–1/2 in) | - BD | Catalog #: 305187 |
| 25 Gauge Needle (1–1/2 in) | - BD | Catalog #: 305122 |
| 40 μm cell strainer | - BD | Catalog #: 325340 |
| 0.45 μm vacuum filter | - Thermo | Catalog #: 168-0045 |
| BSA (1.5% & 3% w/v in PBS) | - Roche | Catalog #: 03117057001 |
| DMEM | - Corning | Catalog #: 10-013-CV |
| FBS (Performance Plus) | - Gibco | Catalog #: 16000-044 |
| Penicillin (50 U/mL) / Streptomycin (10 μg/mL) | - Gibco | Catalog #: 15140-122 |
| PBS | - Corning | Catalog #: 21-040-CV |
| Mouse Thrombopoietin (70 ng/mL, final) |
A). Fetal Liver Cultures (Day 0)
Place DMEM (supplemented with 10% FBS & 1% Pen/Strep) in 37°C water bath.
Pipette complete DMEM into 3 × 10 cm tissue culture dishes (2 × 5 mL and 1 × 10 mL) and 1 × 50 mL centrifuge tube (5 mL DMEM) for each mouse. Leave plates in the 37°C incubator.
Take 50 mL centrifuge tube(s), diaper pad, forceps and dissecting scissors to the animal procedure room.
Euthanize pregnant mice following country & institutionally-accepted guidelines.
Clean dissection tools, prepare diaper pad and the carcass bag during asphyxiation.
Lay the mice on their back and disinfect (wipe or spray form) the stomach to sterilize; use scissors to mat down the fur.
Use forceps and scissors to cut the skin of the lower abdomen, taking care not to penetrate internal organs. Cut up/pull skin back to expose abdominal tissue.
Gently cut open the peritoneum to expose internal organs.
Locate the fetal sacs and remove them from the mouse by cutting away connective tissue. Place the fetal string in a 50 mL conical tube (w/ DMEM).
Place the dead mouse in the carcass bag & repeat for all mice.
Disinfect the tools, tie off and discard the carcass bag, and clean up the area.
Place the 50 mL conical tubes containing the fetal strings into the 37°C incubator.
Retrieve tissue culture dishes and a conical from the incubator, and transfer them to a sterile biosafety cabinet.
In a sterile biosafety cabinet, use ethanol-soaked forceps to transfer the fetal string from the conical into one plate of 5 mL DMEM (Figure 1a).
Use the forceps to dissect the fetuses from their embryonic sacs and place them into the second 5 mL culture dish (Figure 1b).
Be careful to only move fetuses into the second plate, as the red uterine tissue will be confusing for the next step.
.Use the forceps to dissect the liver (large, red, multi-lobed organ) from the fetuses (Figure 1c). Remove all white connective tissue from the liver and discard. Transfer the cleaned livers into a 10 cm dish with 10 mL complete media, and repeat for all fetuses (Figure 1d,e).
Homogenize liver tissue by drawing up media with livers into a 10 mL syringe using a 19 gauge needle. Draw up and purge back into the dish 3 times.
Switch to a 25 gauge needle and repeat the process, making sure to purge only once (Figure 1f).
Filter the homogenate through a 40 μm cell strainer into a new 50 mL tube.
Repeat steps 15–21 for each mouse.
Centrifuge the 50 mL tubes of homogenate at 200 x g for 5 minutes.
During this time filter the DMEM. Pipette 20 mL of DMEM into a vacuum filter for each mouse + 5 mL to account for filtering/pipetting loss.
Add TPO at 70 ng/mL to the filtered media. TPO was derived from tissue culture supernatant from a fibroblast cell line engineered to secrete recombinant human TPO.(1)
Aspirate the supernatant from the centrifuged cells. Aspirate from the ‘elbow’ of the tube as the pellet is very loose.
Suspend each pellet in 2 mL DMEM w/ TPO, and pool together into one tube. If large fibroblasts (floating white tissue, strand-like) are found in suspension, re-filter the pooled homogenate through another 40 μm cell strainer.
Combine all freshly suspended cells in filtered TPO media prepared in step 24, and swirl gently to mix.
Divide pooled resuspension into a new 10 cm culture dishes, 10 mL per dish.
Place culture dishes in 37°C incubator for 4 days. Figure 2 demonstrates the morphology of the cells in culture over days 0 (Figure 2a), 1 (Figure 2b), 2 (Figure 2c), 3 (Figure 2d), and 4 (Figure 2e). By day 4, approximately 3–5% of the entire cell population are MKs.
Figure 1. Murine Fetal Liver Dissection.

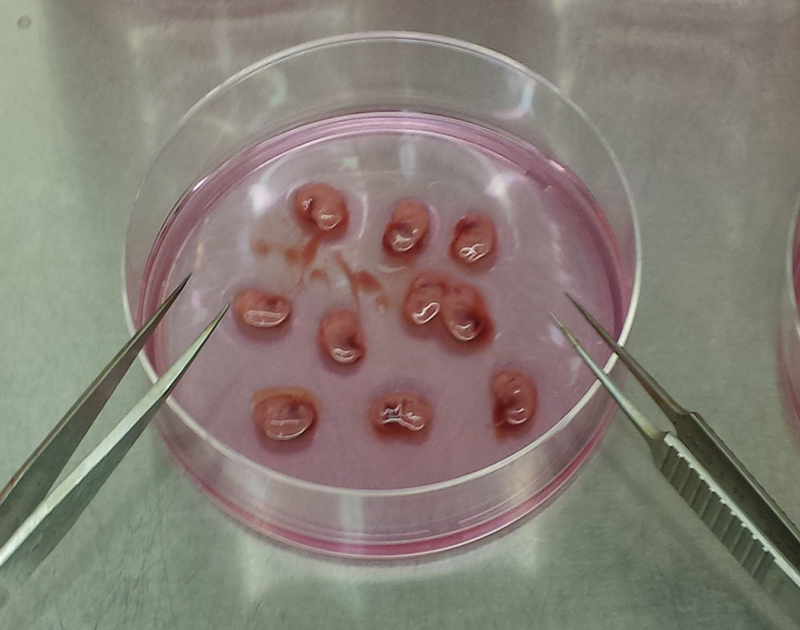
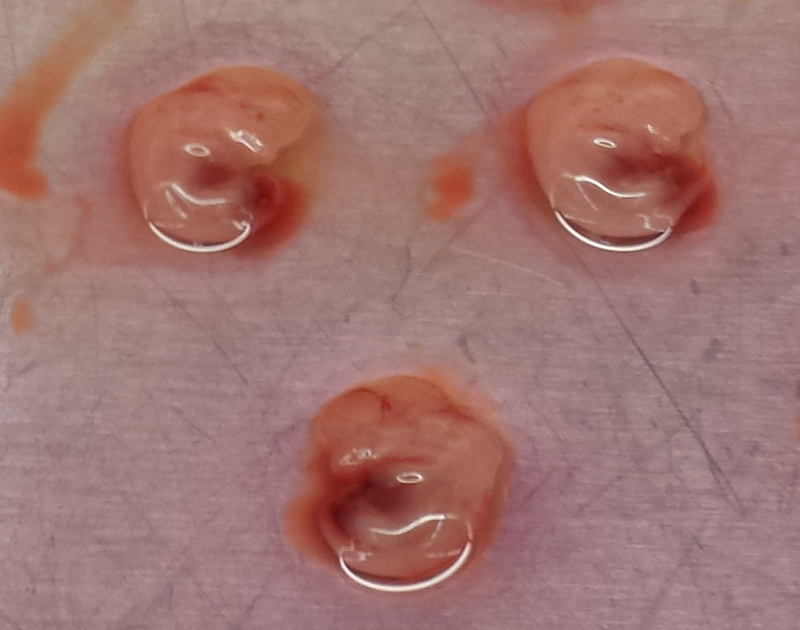
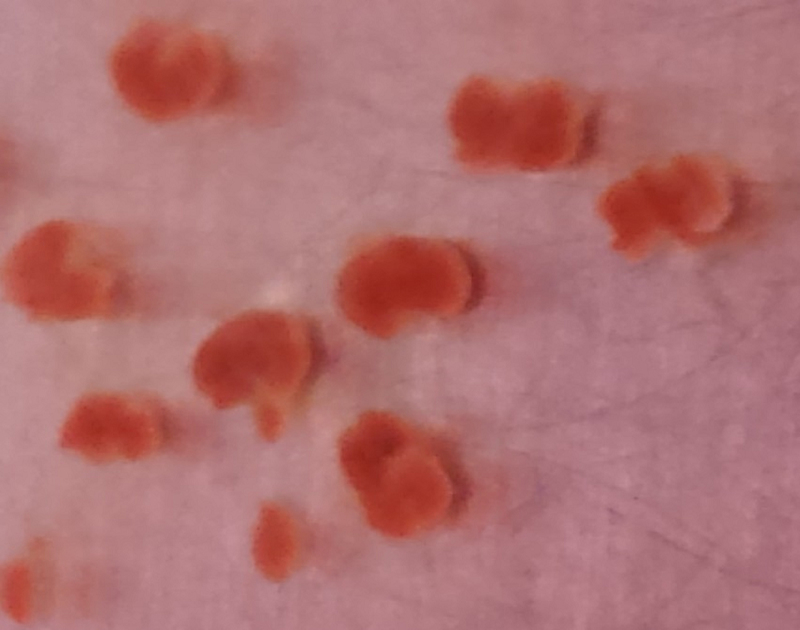

Images of mouse Embryonic Day 13.5 fetal livers derived from pregnant CD-1 mice showing (A) Mouse fetal liver string in first 10 cm dish containing 5 mL DMEM, (B) Intact, isolated fetal sacs in second 10 cm dish containing 5 mL DMEM, (C) Close-up of isolated fetal sacs, with arrowheads pointing to red fetal liver, (D) Isolated fetal livers, (E) Isolated fetal livers in third 10 cm dish containing 10 mL DMEM, and (F) 10 mL of homogenized fetal liver culture.
Figure 2. Megakaryocyte Differentiation Progression.

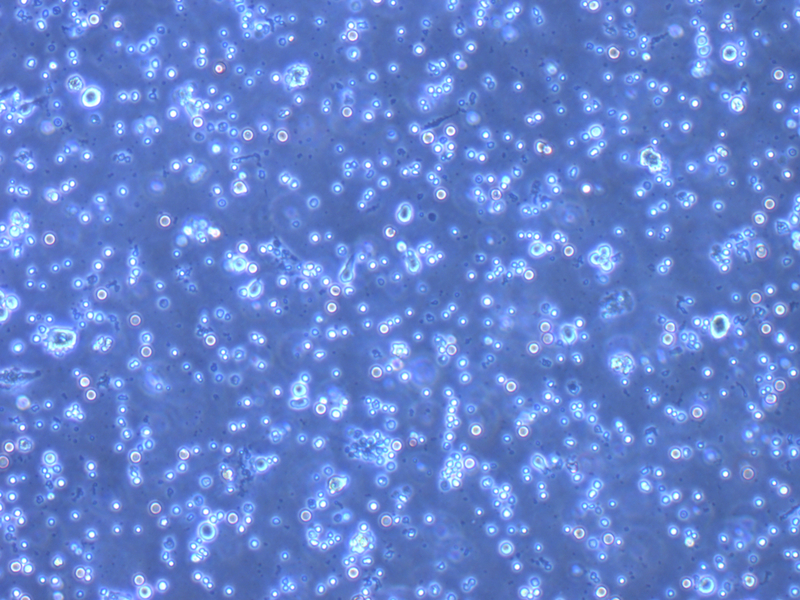

Images were taken on an inverted Olympus CKX41 microscope using a 10x objective, showing megakaryocyte growth progression on (A) Day 0, (B) Day 1, (C) Day 2, (D) Day 3, and (E) Day 4. Scale bars represent 50 µm.
B). BSA Density Gradients (Days 4–5)
Make 1.5% and 3% BSA (weight/volume in PBS), and place with DMEM (no TPO) in 37°C water bath. You will need 1 mL of each (1.5%, 3% BSA, & DMEM) for each culture dish in the incubator.
Working in sterile biosafety cabinet, syringe filter each BSA tube into new tubes, making sure to label accordingly.
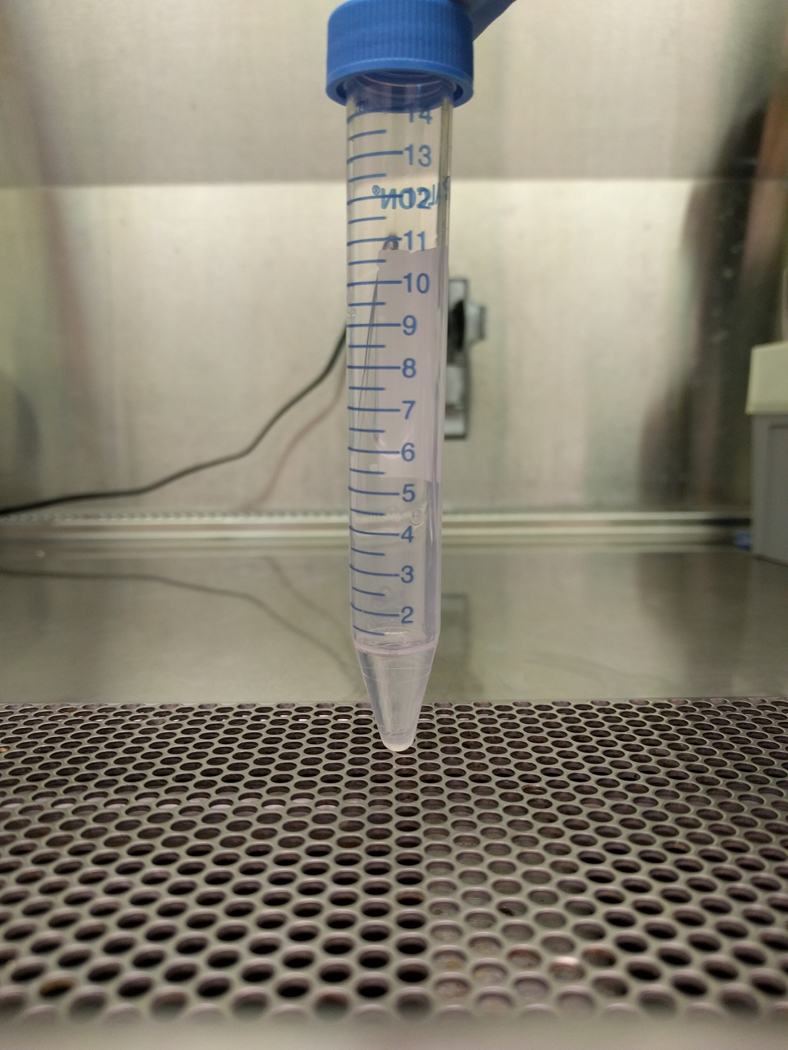
Pipette 1 mL of 3% BSA into a 15 mL conical tubes (1 for every culture, Figure 3a).
Create gradient by carefully tilting tube and pipetting the 1mL of 3% BSA (close to horizontal, Figure 3b). Next pipette 1.5% BSA very slowly, on top of it. Re-orient the tube very slowly, and repeat for all tubes (Figure 3c).
Gently place the gradient tubes aside and retrieve cultures from incubator.
Use a 10 mL pipette to gently resuspend cells on each dish and transfer the contents into individual 15 mL conical tubes. Take care to resuspend all the cells.
Centrifuge the 15 mL tubes at 200 x g for 5 minutes.
Aspirate the supernatant and resuspend each culture in 1 mL DMEM.
Tilt gradient tubes as done in step 4, and carefully pipette 1 mL of resuspended cells over the 1.5% BSA (Figure 3d,e).
Gently place the gradient tubes into the incubator.
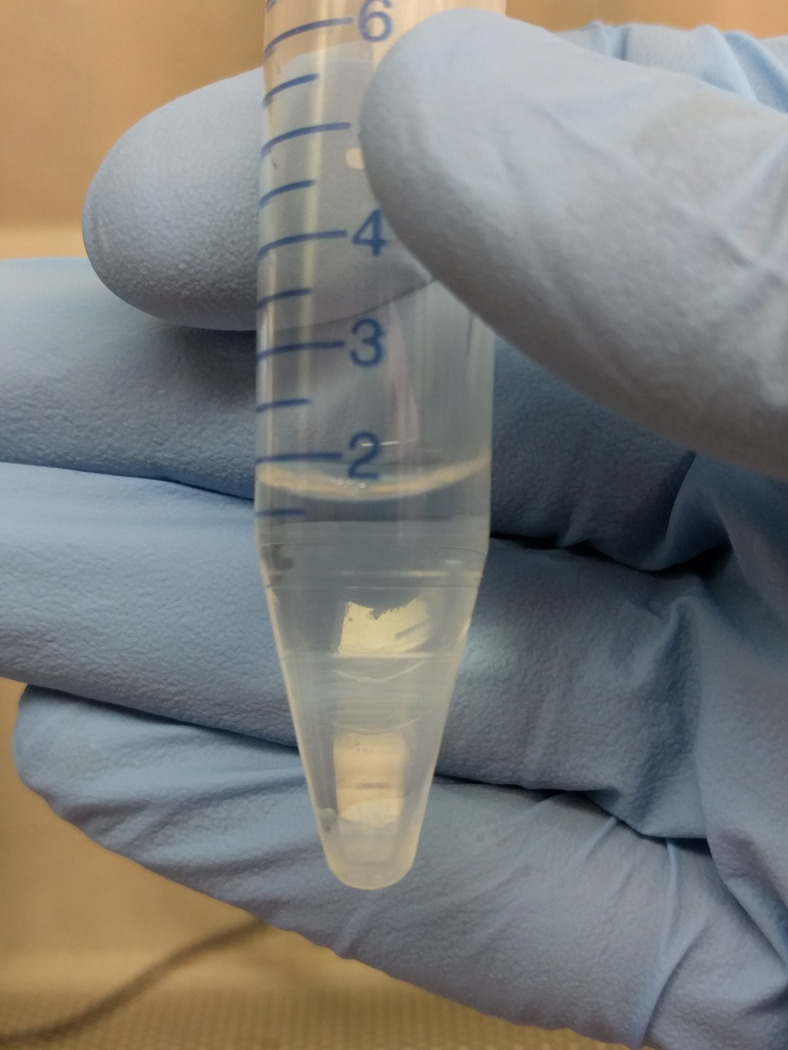
Ensure that each 15 mL conical tube sits for 30 minutes. After that time, the MKs will fall to the bottom in a loose pellet.
Aspirate the supernatant, taking care to not agitate the MK pellet.
Resuspend each MK pellet in 1 mL of DMEM (no TPO) and place the cultures in the 37°C incubator for later use (Figure 4a).
MKs (in DMEM w/o TPO) will produce proplatelets over the next 24 hours (Days 4–5, Figure 4b).
Figure 3. Albumin Step Gradient to Isolate Megakaryocytes.
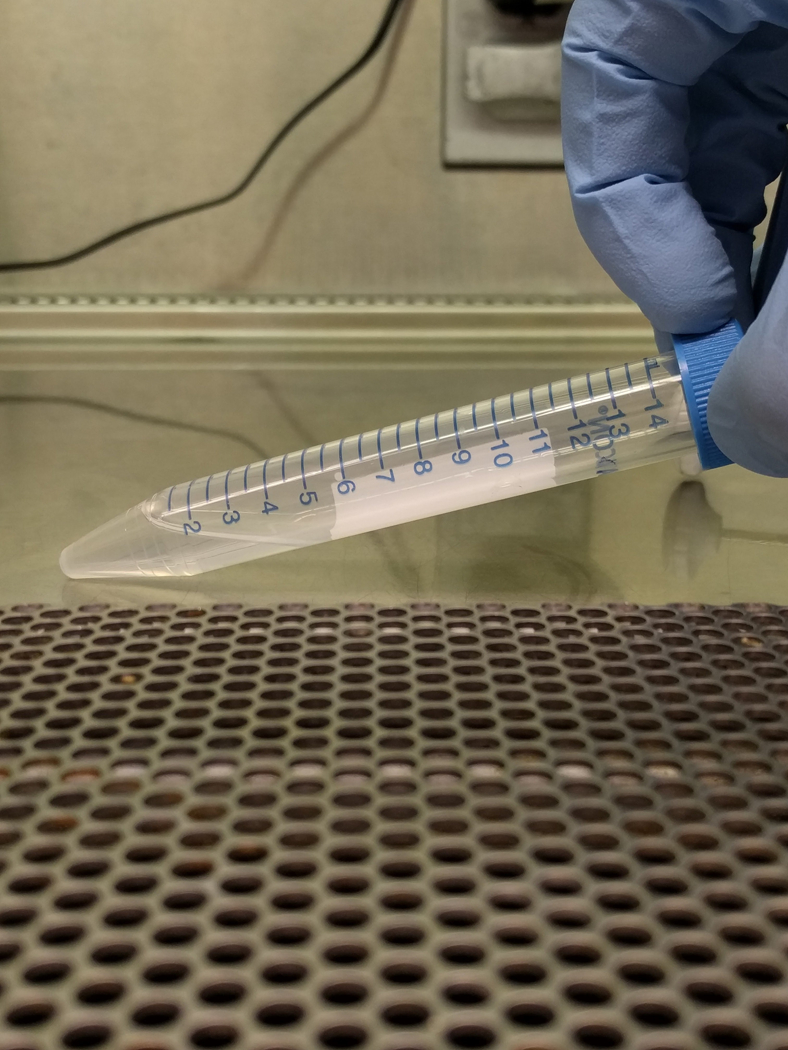

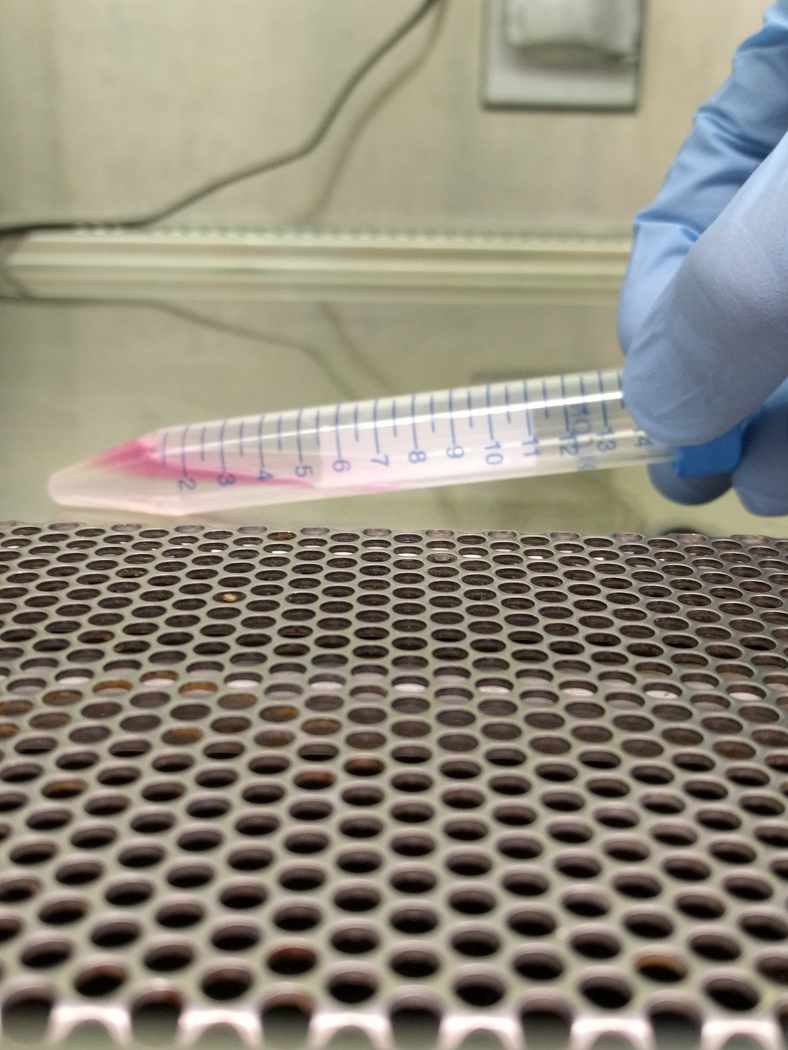
Images show the albumin step gradient setup in a 15 mL conical tube. (A) 1 mL 3% BSA at the bottom of 15 mL conical, (B) 15 mL conical with 3% BSA, tilted to gently layer 1.5% on top, (C) close-up of 1.5 and 3% BSA gradient (arrowheads point to faint lines indicating separation between layers), (D) 15 mL conical with 1.5 and 3% BSA, tilted to gently layer 1 mL of resuspended culture, and (E) completed gradient, pre-incubation.
Figure 4. Post-Gradient Megakaryocytes in Culture.
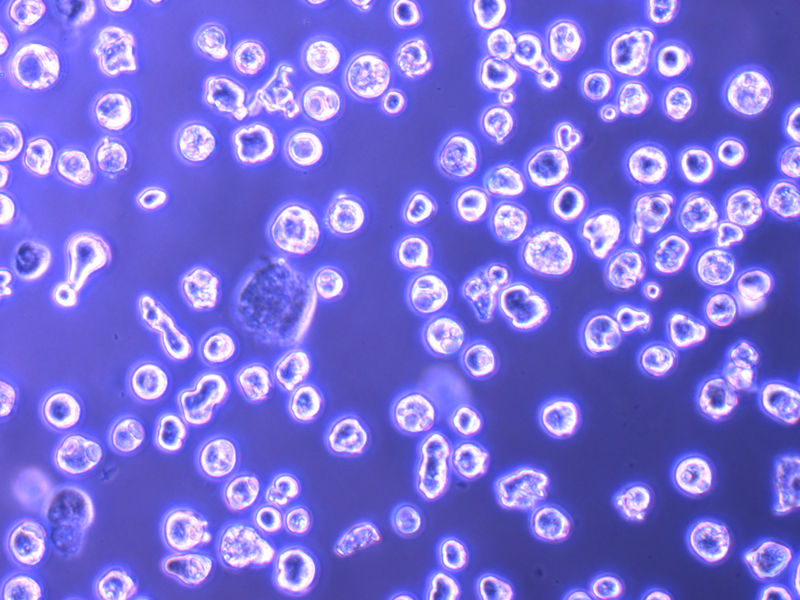

Images taken on an inverted Olympus CKX41 microscope using a 10x objective, showing (A) gradient-isolated megakaryocytes on Day 4 and (B) proplatelet structure on Day 5. Scale bars represent 50 µm.
Discussion
Herein, we outline a method to culture murine fetal liver-derived MKs as a model to study MK maturation and platelet production in vitro. However, the study of these processes is of interest due to the prevalence of human platelet disorders, including thrombocytopenia. In humans, thrombocytopenia (platelet counts < 150×109/L) can be life threatening due to bleeding complications and is a major clinical problem encountered in immune thrombocytopenic purpura (ITP), chemotherapy, surgery, aplastic anemia, human immunodeficiency virus infection, and complications during pregnancy and delivery. It is estimated that approximately 1.5 million platelet transfusions are administered yearly, with each transfusion costing over $600.(2)
The discovery of the c-Mpl ligand, TPO, marked a positive shift in megakaryocyte (MK) and platelet research; for the first time MKs were able to be differentiated and cultured outside the body. (3, 4) Due to the scarcity of MKs within the bone marrow niche and the difficulties in accessing them, investigators have largely moved towards in vitro culture of MKs using TPO to study this microenvironment. The adoption of these differentiation and culture systems has permitted further study of the events leading to megakaryopoiesis and subsequent proplatelet formation.(5–7) In addition, ex vivo MK culture opened the door to the possibility of creating donor-independent platelets for transfusion.
The first human MK culture systems largely utilized CD34+-enriched progenitors originating from mobilized peripheral blood (mPB) or umbilical cord blood (UCB). Researchers tapped these sources to define methods for generating high-ploidy MKs with functional in vivo platelets.(8–11) Such groups exploiting CD34+ cells have concentrated on studying and sustaining MK differentiation and platelet production using various methods, including bioreactors, co-culturing systems, and elegantly bioengineered scaffolding materials to recreate the complex bone marrow microenvironment.(7, 12–17) Stemming from these initial studies, promising techniques such as UCB-derived platelet perfusion systems were established shortly after, detailing production of up to 3.0 −6.0 × 1011 platelets (up to 20 platelets per starting HSC); a clinically acceptable amount for transfusion.(16) Both UCB & mPB MKs have been found to have similar levels of CD34+ expression, indicating comparable rates of MK production. However, the UCB MKs have lower levels of CD41 and ploidy, suggesting that the UCB-derived MKs are immature compared to mPBs.(18) This difference in MKs derived from fetal (i.e. UCB in humans and fetal livers in mice) versus adult progenitors (i.e. mPB in humans and bone marrow in mice) is worth noting and taking into account when planning model systems for experiments. Furthermore, additional studies characterizing differences in the transcriptome of MKs derived from different cell types are warranted.
Nonetheless, preliminary reports of artificial MK culture using these tissue sources have exposed a lack of consistent results owing to heterogenous hematopoietic stem cell (HSC) populations.(17) Ultimately, these elaborate procedures have been bottlenecked by dependencies on viable donor samples, variable effects of cytokines on MK differentiation, and the limited expansion of progenitors. Additionally, the low efficacy of post-transfusion platelets, fixed assembly limits, and tendencies for agonist-devoid activation have provided significant challenges.(19)
More recently, investigators have taken steps towards the directed differentiation and culture of functional MKs from induced pluripotent stem cells (iPSCs) and embryonic stem cells (ESCs).(20) Prior techniques targeting large scale harvesting and differentiation of human-derived ESCs were capable of producing viable MKs, of which approximately 10% produced proplatelets.(21–23) However, the Lu et al. developed a method for ESC culture that propagated 6×108 CD41a+ cells (roughly 6.7 ± 0.4 platelets per starting cell).(20) This shows improvement in MK production, but is still a rate well below accepted in vitro production.
In addition, various groups have had success programming pluripotent stem cells to differentiate into MKs. This was first done by Nishimura et al. using canine iPSCs, with 17% of platelets from their culture supernatant positive for CD41/61, demonstrating the possibility of MK generation and the release of platelets from iPSCs.(24) In addition, Feng et al. generated platelets from human iPSCs in a simplified culture system free of serum and feeder cells; they generated an average of 16 MK progenitors per single iPSC.(25) Due to the promise of these advances, Nakamura sought to establish stable, immortalized MK progenitor cell lines for clinically applicable generation of platelets from iPSCs in vitro.(26) However, this technology currently relies on a relatively sophisticated and expensive reprogramming method, making scale-up to generation of clinically-relevant platelet numbers less feasible.(27)
Although these contemporary isolation methods highlight advancements in in vitro culturing of human cells, they involve time-intensive techniques such as immunomagnetic purification, cell sorting by flow cytometry, and continuous cycling between expensive cytokine and medium combinations.(28–30) As such, murine models have proven to be a practical and ethical approach in researching MK differentiation and platelet production. The culturing of murine MKs eliminates dependencies on human donors and the benefits associated with controlling all facets of the murine growth cycle minimize many of the inconsistencies that accompany human-derived samples.
Lecine and colleagues were the first to successfully culture proplatelet-producing MKs from murine fetal livers in the presence of TPO.(31) Building from this initial protocol, we have established a robust method of culturing murine MKs, employing fetal livers recovered from embryonic day 13.5 fetuses.(32–35) Currently, we and others suggest that murine fetal livers are a suitable choice for high MK yield in comparison to other primary sources like spleen and bone marrow, which are limited in production and often incapable of generating proplatelets.(36)
As outlined above, culturing these cells with recombinant TPO and 10 % w/v Performance-Plus FBS results in high MK differentiation and proliferation by day 3 of culture. The subsequent 1.5% and 3% w/v albumin step gradient is a simple yet effective method of isolating MKs based on density. Although MKs isolated in this gradient are largely unsynchronized, approximately 15,000–20,000 cells are generated per fetal liver, of which 20–50% form proplatelets by day 5, consistent with previous publications.(37, 38) This technique has been used successfully for 20 years, with the advantages being pure, polyploid MKs, capable of generating robust amounts of proplatelets. While studies with murine MKs undoubtedly need to be validated in a human model, our in vitro method of murine culture grants researchers the ability to study the physiological mechanisms of proplatelet formation, with considerably fast turnover.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- 1.Villeval JL, Cohen-Solal K, Tulliez M, Giraudier S, Guichard J, Burstein SA, et al. High thrombopoietin production by hematopoietic cells induces a fatal myeloproliferative syndrome in mice. Blood 1997;90(11):4369–83. [PubMed] [Google Scholar]
- 2.Kaushansky K Historical review: megakaryopoiesis and thrombopoiesis. Blood 2008;111(3):981–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaushansky K, Lok S, Holly RD, Broudy VC, Lin N, Bailey MC, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature 1994;369(6481):568–71. [DOI] [PubMed] [Google Scholar]
- 4.de Sauvage FJ, Hass PE, Spencer SD, Malloy BE, Gurney AL, Spencer SA, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature 1994;369(6481):533–8. [DOI] [PubMed] [Google Scholar]
- 5.Machlus KR, Italiano JE Jr. The incredible journey: From megakaryocyte development to platelet formation. J Cell Biol 2013;201(6):785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Machlus KR, Thon JN, Italiano JE Jr. Interpreting the developmental dance of the megakaryocyte: a review of the cellular and molecular processes mediating platelet formation. Br J Haematol 2014;165(2):227–36. [DOI] [PubMed] [Google Scholar]
- 7.Di Buduo CA, Soprano PM, Tozzi L, Marconi S, Auricchio F, Kaplan DL, et al. Modular flow chamber for engineering bone marrow architecture and function. Biomaterials 2017;146:60–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi ES, Nichol JL, Hokom MM, Hornkohl AC, Hunt P. Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood 1995;85(2):402–13. [PubMed] [Google Scholar]
- 9.Schipper LF, Brand A, Reniers N, Melief CJ, Willemze R, Fibbe WE. Differential maturation of megakaryocyte progenitor cells from cord blood and mobilized peripheral blood. Exp Hematol 2003;31(4):324–30. [DOI] [PubMed] [Google Scholar]
- 10.Matsunaga T, Tanaka I, Kobune M, Kawano Y, Tanaka M, Kuribayashi K, et al. Ex vivo large-scale generation of human platelets from cord blood CD34+ cells. Stem Cells 2006;24(12):2877–87. [DOI] [PubMed] [Google Scholar]
- 11.Mattia G, Vulcano F, Milazzo L, Barca A, Macioce G, Giampaolo A, et al. Different ploidy levels of megakaryocytes generated from peripheral or cord blood CD34+ cells are correlated with different levels of platelet release. Blood 2002;99(3):888–97. [DOI] [PubMed] [Google Scholar]
- 12.Currao M, Malara A, Di Buduo CA, Abbonante V, Tozzi L, Balduini A. Hyaluronan based hydrogels provide an improved model to study megakaryocyte-matrix interactions. Exp Cell Res 2016;346(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Buduo CA, Wray LS, Tozzi L, Malara A, Chen Y, Ghezzi CE, et al. Programmable 3D silk bone marrow niche for platelet generation ex vivo and modeling of megakaryopoiesis pathologies. Blood 2015;125(14):2254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thon JN, Mazutis L, Wu S, Sylman JL, Ehrlicher A, Machlus KR, et al. Platelet bioreactor-on-a-chip. Blood 2014. [DOI] [PMC free article] [PubMed]
- 15.Panuganti S, Papoutsakis ET, Miller WM. Bone marrow niche-inspired, multiphase expansion of megakaryocytic progenitors with high polyploidization potential. Cytotherapy 2010;12(6):767–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sullenbarger B, Bahng JH, Gruner R, Kotov N, Lasky LC. Prolonged continuous in vitro human platelet production using three-dimensional scaffolds. Exp Hematol 2009;37(1):101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Bruyn C, Delforge A, Martiat P, Bron D. Ex vivo expansion of megakaryocyte progenitor cells: cord blood versus mobilized peripheral blood. Stem Cells Dev 2005;14(4):415–24. [DOI] [PubMed] [Google Scholar]
- 18.van den Oudenrijn S, von dem Borne AE, de Haas M. Differences in megakaryocyte expansion potential between CD34(+) stem cells derived from cord blood, peripheral blood, and bone marrow from adults and children. Exp Hematol 2000;28(9):1054–61. [DOI] [PubMed] [Google Scholar]
- 19.Reems JA, Pineault N, Sun S. In vitro megakaryocyte production and platelet biogenesis: state of the art. Transfus Med Rev 2010;24(1):33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu SJ, Li F, Yin H, Feng Q, Kimbrel EA, Hahm E, et al. Platelets generated from human embryonic stem cells are functional in vitro and in the microcirculation of living mice. Cell Res 2011;21(3):530–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takayama N, Nishikii H, Usui J, Tsukui H, Sawaguchi A, Hiroyama T, et al. Generation of functional platelets from human embryonic stem cells in vitro via ES-sacs, VEGF-promoted structures that concentrate hematopoietic progenitors. Blood 2008;111(11):5298–306. [DOI] [PubMed] [Google Scholar]
- 22.Takayama N, Eto K. In vitro generation of megakaryocytes and platelets from human embryonic stem cells and induced pluripotent stem cells. Methods Mol Biol 2012;788:205–17. [DOI] [PubMed] [Google Scholar]
- 23.Eto K, Murphy R, Kerrigan SW, Bertoni A, Stuhlmann H, Nakano T, et al. Megakaryocytes derived from embryonic stem cells implicate CalDAG-GEFI in integrin signaling. Proc Natl Acad Sci U S A 2002;99(20):12819–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishimura T, Hatoya S, Kanegi R, Sugiura K, Wijewardana V, Kuwamura M, et al. Generation of functional platelets from canine induced pluripotent stem cells. Stem Cells Dev 2013;22(14):2026–35. [DOI] [PubMed] [Google Scholar]
- 25.Feng Q, Shabrani N, Thon JN, Huo H, Thiel A, Machlus KR, et al. Scalable generation of universal platelets from human induced pluripotent stem cells. Stem Cell Reports 2014;3(5):817–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura S, Takayama N, Hirata S, Seo H, Endo H, Ochi K, et al. Expandable megakaryocyte cell lines enable clinically applicable generation of platelets from human induced pluripotent stem cells. Cell Stem Cell 2014;14(4):535–48. [DOI] [PubMed] [Google Scholar]
- 27.Moreau T, Evans AL, Vasquez L, Tijssen MR, Yan Y, Trotter MW, et al. Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward programming. Nat Commun 2016;7:11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambert MP, Sullivan SK, Fuentes R, French DL, Poncz M. Challenges and promises for the development of donor-independent platelet transfusions. Blood 2013;121(17):3319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karagiannis P, Eto K. Manipulating megakaryocytes to manufacture platelets ex vivo. J Thromb Haemost 2015;13 Suppl 1:S47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avanzi MP, Chen A, He W, Mitchell WB. Optimizing megakaryocyte polyploidization by targeting multiple pathways of cytokinesis. Transfusion 2012;52(11):2406–13. [DOI] [PubMed] [Google Scholar]
- 31.Lecine P, Villeval JL, Vyas P, Swencki B, Xu Y, Shivdasani RA. Mice lacking transcription factor NF-E2 provide in vivo validation of the proplatelet model of thrombocytopoiesis and show a platelet production defect that is intrinsic to megakaryocytes. Blood 1998;92(5):1608–16. [PubMed] [Google Scholar]
- 32.Machlus KR, Wu SK, Stumpo DJ, Soussou TS, Paul DS, Campbell RA, et al. Synthesis and dephosphorylation of MARCKS in the late stages of megakaryocyte maturation drive proplatelet formation. Blood 2016;127(11):1468–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel SR, Hartwig JH, Italiano JE Jr. The biogenesis of platelets from megakaryocyte proplatelets. J Clin Invest 2005;115(12):3348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Italiano JE Jr., Patel-Hett S, Hartwig JH. Mechanics of proplatelet elaboration. J Thromb Haemost 2007;5 Suppl 1:18–23. [DOI] [PubMed] [Google Scholar]
- 35.Italiano JE Jr., Lecine P, Shivdasani RA, Hartwig JH. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol 1999;147(6):1299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schulze H Culture of murine megakaryocytes and platelets from fetal liver and bone marrow. Methods Mol Biol 2012;788:193–203. [DOI] [PubMed] [Google Scholar]
- 37.Schulze H. Culture, Expansion, and Differentiation of Murine Megakaryocytes from Fetal Liver, Bone Marrow, and Spleen. Curr Protoc Immunol 2016;112:22F 6 1–F 6 15. [DOI] [PubMed] [Google Scholar]
- 38.Shivdasani RA, Schulze H. Culture, expansion, and differentiation of murine megakaryocytes. Curr Protoc Immunol 2005;Chapter 22:Unit 22F 6. [DOI] [PubMed]


