Abstract
Background
Several clinical trials have studied the effects of glucagon-like peptide-1 receptor agonists (GLP-1RAs) on glycometabolism and cardiovascular risk factors since they were identified. Because of their cardiovascular benefits and efficacy in lowering glucose, GLP-1RAs are becoming increasingly important in clinical therapy for patients with or without pathoglycaemia. The aim of this study was to assess the effect of the GLP-1RA liraglutide on blood pressure based on randomised controlled trials (RCTs).
Methods
We searched PubMed for RCTs published from 2009 to 2018 comparing the effect of liraglutide on blood pressure with that of placebo in individuals with or without pathoglycaemia. RCTs in humans that included data describing blood pressure changes from baseline to the end of the trial were selected for inclusion in the meta-analysis.
Results
A total of 18 RCTs that enrolled 7616 individuals in the liraglutide group and 6046 individuals in the control group were included in this meta-analysis. Compared with placebo, liraglutide reduced systolic blood pressure (SBP) by 3.18 mmHg (95% CI -4.32, − 2.05), P < 0.00001, but had no significant effect on diastolic blood pressure (DBP). Subgroup analysis showed that the degree of reduction in SBP was associated with the dose of liraglutide but that significance disappeared when the intervention lasted over 1 year. Liraglutide 3.0 mg/d significantly reduced DBP by 1.46 mmHg (95% CI -2.61, 0.32), P = 0.01, but liraglutide 1.8 mg/d slightly increased DBP by 0.47 mmHg (95% CI 0.11, 0.83), P = 0.01, compared with placebo.
Conclusions
This meta-analysis demonstrated that liraglutide significantly reduced SBP in individuals with or without pathoglycaemia compared with placebo, but the difference was no longer significant when the intervention lasted over 1 year. Moreover, the effect of liraglutide on blood pressure is associated with the dose. This finding may provide additional evidence for cardiovascular protection.
Keywords: Blood pressure, Cardiovascular risk factor, DBP, GLP-1RAs, Liraglutide, SBP
Background
Diabetes, a chronic and progressive metabolic disorder, is becoming a public health issue with a high prevalence and serious complications. The IDF (International Diabetes Federation) has estimated that there will be 59,200,000 patients suffering from diabetes in 2035 [1]. Long-term hyperglycaemia leads to macrovascular and microvascular complications, which places a heavy burden on the health care system [2]. Diabetes, especially type 2 diabetes, is associated with overweight/obesity, hypertension and dyslipidaemia. As a result, the American Diabetes Association (ADA) has recommended providing the components of diabetes care with the cardiovascular risk factors included [3]. Many large clinical studies have confirmed that blood pressure is one of the cardiovascular risk factors associated with diabetes, and strict blood pressure control could improve the cardiovascular prognosis of diabetic patients [4–7]. According to the ADVANCE study, a reduction of 5.6 mmHg in SBP could significantly reduce the relative risk of death from cardiovascular disease by 18% [8].
GLP-1 is an endogenous incretin secreted by the intestines after eating and can promote the secretion of insulin, inhibit the secretion of glucagon, delay gastric emptying, and maintain the stability of blood glucose. Based on this activity, GLP-1RAs, which decrease glucose, the risk of hypoglycaemia and weight, have been developed and used in the treatment of type 2 diabetes patients. GLP-1RAs have been shown to have either superior or noninferior efficacy compared with other hypoglycaemic agents, such as metformin, thiazolidinediones (TZDs), insulin, sulfonylureas, and dipeptidyl peptidase-4 (DPP-4) inhibitors [9–16]. In addition, some studies confirmed that GLP-1RAs could significantly reduce weight, improve insulin sensitivity [17–22], and protect the function of β-cells [23, 24].
In recent years, an increasing number of studies have suggested that GLP-1RAs might produce further benefits with regard to cardiovascular factors [25, 26]. Initially, Viswanathan et al. found that adding exenatide treatment to existing insulin therapy in patients with type 2 diabetes could significantly reduce blood pressure by 9.2 mmHg from baseline and that the reduction in blood pressure was independent of weight loss [27]. Since then, several research teams have conducted clinical studies investigating the efficacy of GLP-1RAs on blood pressure and other cardiovascular risk factors with different conclusions. Most studies concluded that GLP-1RAs could significantly reduce SBP and had a tendency to reduce DBP. Rosso et al. found that SBP significantly decreased by 14.7 mmHg and that DBP significantly decreased by 9 mmHg after 12 months of treatment with liraglutide, while fasting blood glucose, HbA1C, weight, waist circumference, and lipid levels also decreased significantly [28]. A study in nondiabetic obese adults found that SBP decreased by 5.7 mmHg (1.2 mg/day), 5.6 mmHg (1.8 mg/day), 8.8 mmHg (2.4 mg/day), and 6.9 mmHg (3.0 mg/day) compared with baseline after a 20-week treatment with liraglutide and that DBP decreased by 1.2 mmHg, 1.8 mmHg, 1.4 mmHg and 2.9 mmHg, respectively [29]. The LEADER trial found that SBP decreased by 1.2 mmHg and that DBP increased by 0.6 mmHg in the liraglutide group after an intervention of 3.5 years [30, 31]. A study in diabetic patients on peritoneal dialysis found that SBP decreased by 20–30 mmHg after a 12-month treatment with liraglutide, which might be associated with instability of the patients’ volume load [32]. Therefore, exploring the influence of GLP-1RAs on blood pressure in a large population by collecting the data from all relevant trials is necessary.
Liraglutide is one of the long-acting GLP-1RAs marketed in Europe in 2009 and has better efficacy with regard to cardiovascular benefits and hyperglycaemia reduction [11, 33, 34]. This meta-analysis aimed to investigate the effect of liraglutide on blood pressure in individuals with abnormal glucose metabolism or metabolic syndrome by searching randomised controlled trials (RCTs).
Methods
The main objective of this meta-analysis was to assess the influence of liraglutide on blood pressure compared with that of placebo. Outcome measurements included SBP and DBP. We followed the methods specified in the Cochrane Handbook for Reviews on Interventions [35].
Search strategy
Eligible trials were identified by electronic and manual searches. Electronic searches were conducted by searching PubMed for articles dating from 2009 to 2018 using the terms “liraglutide” and “blood pressure”. Manual searches were performed by reading the title, abstract and full text of relevant articles.
Study selection
After searching for candidate articles, further identification of these articles was based on the inclusion and exclusion criteria described below. The process was performed independently by two investigators.
The inclusion criteria were as follows: (a) published studies in humans; (b) randomised, placebo, parallel controlled trials; (c) outcome measurements included blood pressure.
The exclusion criteria were as follows: (a) participants suffered from severe liver or renal insufficiency and required replacement therapy; (b) cross-over control trials; or (c) using diuretics or drinking too much water, which might impact volume load.
Data extraction
The main data were extracted from each study after a full-text reading of each RCT included in the meta-analysis and included the following: (a) general information, such as the first author, title, year of publication, and sample size; (b) baseline characteristics of participants, such as age and duration of diabetes; (c) intervention measures, the duration of intervention and background therapy; and (d) changes in SBP and DBP from baseline to endpoint with the format of the mean (standard deviation).
Quality assessment
The quality assessment of these RCTs included in the meta-analysis was performed according to the Cochrane Collaboration’s risk of bias assessment tools, which included six parts: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data), reporting bias (selective reporting) and other bias [35].
Data analysis
Statistical analysis was conducted by Review Manager (RevMan version 5.3). We assessed the heterogeneity among RCTs by using the Cochrane Q test and I2 statistic. I2 values of less than 25%, 25–50%, 50–75% and more than 75% represent no heterogeneity, mild heterogeneity, moderate heterogeneity and considerable heterogeneity, respectively. We concentrated on the changes in SBP and DBP from baseline to endpoint with the format of the mean (standard deviation). If the article did not provide a calculated standard deviation, we imputed it via sample size, standard error, 95% confidence interval and p value. The results of the meta-analysis were expressed as the weighted mean difference with 95% confidence intervals. To increase the efficacy of the results, even if the heterogeneity was low or there was no heterogeneity, a random-effects model was selected.
Compliance with ethics guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Results
Literature searches and study inclusion
By searching PubMed, a total of 226 articles were screened. After excluding the articles that did not meet our inclusion criteria, 18 RCTs were included in the data analysis. All the included studies were randomised, double-blind, placebo, and parallel controlled trials. The study flow diagram is shown in Fig. 1. The characteristics of the RCTs included in the meta-analysis are shown in Table 1 and Table 2. Liraglutide was given at 0.6 mg once daily in 2 trials [36, 37]. As liraglutide at 0.6 mg/d is rarely used in clinical practice, we removed these data from the comparison of liraglutide with placebo.
Fig. 1.
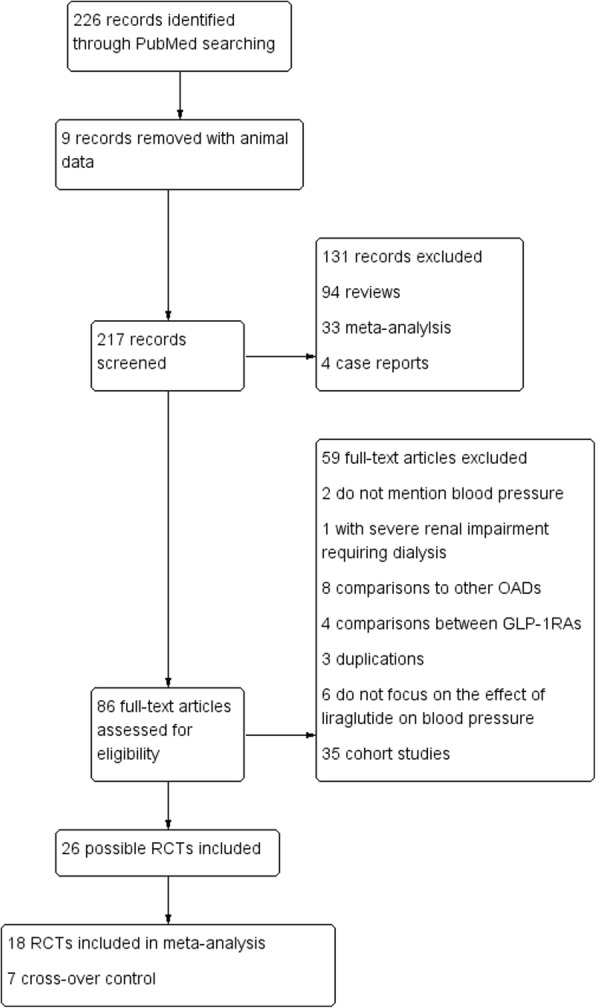
Study flow diagram
Table 1.
| Study | Phase of the study | Duration of intervention | Background therapy | Background disease | Sample size | Intervention group | Measurement of BP | Antihypertensive pharmacological therapy |
|---|---|---|---|---|---|---|---|---|
| A Ahmann 2015 | Phase 3 | 26-week | Insulin, metformin | T2DM | 451 | Liraglutide 1.8 mg/d (n = 225) | Collected at trial visit, without detail | Stable drug dose for at least 8 weeks prior to inclusion and throughout the trialΦ |
| A Astrup 2009 | Phase 2 | 20-week | Diet, exercise | Metabolic syndrome | 564 | Liraglutide 1.2 mg/d (n = 85), 1.8 mg/d (n = 74), 2.4 mg/d (n = 73), 3.0 mg/d (n = 82) | Standardised method [69, 70] | Continued their baseline antihypertensive therapiesΦ |
| A Astrup 2012 | Phase 2 | 1-year | Diet, exercise | Metabolic syndrome | 564 | Liraglutide 1.2 mg/d (n = 85), 1.8 mg/d (n = 74), 2.4 mg/d (n = 73), 3.0 mg/d (n = 82) | Standardised method [69, 70] | Continued their baseline antihypertensive therapiesΦ |
| A Blackman 2016 | Phase 3 | 32-week | Diet, exercise | Metabolic syndrome | 359 | Liraglutide 3.0 mg (n = 178) | Collected at trial visit, without detail | Stable drug dose for at least 3 months prior to inclusion and throughout the trialΦ |
| Christian 2015 | Phase 3 | 12-week | Insulin | T1DM | 40 | Liraglutide 1.2 mg/d (n = 18) | Collected at trial visit, without detail | Continued their baseline antihypertensive therapiesΦ |
| Dejgaard 2016 | Phase 4 | 24-week | Insulin | T1DM | 100 | Liraglutide 1.8 mg/d (n = 46) | Using a portable device (Spacelab Medical Model 90,217, Deerfield, WI, USA) with an appropriately sized cuff around the non-dependent upper arm after excluding between-arm differences in blood pressure > 5 mmHg | Remained unchanged during the study periodΦ |
| LEAD-1 | Phase 3 | 26-week | Glimepiride 2–4 mg/d | T2DM | 1041 | Liraglutide 1.2 mg/d (n = 228) | Standardised method [69] | Continued their baseline antihypertensive therapiesΦ |
| Liraglutide 1.8 mg/d (n = 234) | ||||||||
| LEAD-2 | Phase 3 | 26-week | Metformin 1 g bid | T2DM | 1662 | Liraglutide 1.2 mg/d (n = 241) | Standardised method [69] | Continued their baseline antihypertensive therapiesΦ |
| Liraglutide 1.8 mg/d (n = 242) | ||||||||
| LEAD-4 | Phase 3 | 26-week | Metformin 1 g bid | T2DM | 821 | Liraglutide 1.2 mg/d (n = 178) | Standardised method [69] | Continued their baseline antihypertensive therapiesΦ |
| rosiglitazone 4 mg bid | Liraglutide 1.8 mg/d (n = 178) | |||||||
| LEAD-5 | Phase 3 | 26- 26- Week | Metformin 1 g bid | T2DM | 581 | Liraglutide 1.8 mg/d (n = 232) | Standardised method [69] | Continued their baseline antihypertensive therapiesΦ |
| glimepiride 4 mg/d | ||||||||
| LEADERS trial | Phase 3 | 3.8-year | Diet, exercise, OAH or insulin | T2DM | 9340 | Liraglutide 1.8 mg/d (n = 4668) | Standardised method [69] | Target: 130/80 mmHg; First line: ACE inhibitors or ARBs; Based on individual patient needs: Ca2+ blockers, diuretics, others |
| Mark M. Smits 2016 | Phase 4 | 12-week | Metformin, sulphonylurea | T2DM | 60 | Liraglutide 1.8 mg/d (n = 19) | Standardised method using an automatic oscillometric device (Dinamap, GE Healthcare, Little Chalfont, UK) [71] | Continued their baseline antihypertensive therapiesΦ |
| MDI liraglutide trial | Phase 2 | 24-week | Insulin | T2DM | 124 | Liraglutide 1.8 mg/d (n = 63) | Collected at trial visit, without detail | Remained unchanged during the study periodΦ |
| Nandy 2014 | Phase 3 | 12-week | Lifestyle, metformin | T2DM | 49 | Liraglutide 1.8 mg/d (n = 16) | An arterial catheter was placed in the non-dominant arm in series with a pressure transducer | Stable drug dose for at least 4 weeks prior to inclusion and throughout the trialΦ |
| P. Mensberg MSc 2016 | Phase 4 | 16-week | Exercise | T2DM | 33 | Liraglutide 1.8 mg/d (n = 17) | Measured on the left arm after the patients had rested for 10 min | Remained unchanged during the study periodΦ |
| Robert 2015 | Phase 4 | 12-week | Diet, exercise | Metabolic syndrome | 44 | Liraglutide 1.8 mg/d (n = 21) | Not mentioned | Without hypertension |
| S Frossing 2018 | Phase 4 | 26-week | Lifestyle | Metabolic syndrome | 72 | Liraglutide 1.8 mg/d (n = 48) | Standardised method [69, 72] | Without hypertension |
| Sun H. Kim 2013 | Phase 3 | 14-week | Diet, exercise | Prediabetes | 68 | Liraglutide 1.8 mg/d (n = 24) | Standardised method using a Dinamap automatic blood pressure recorder (GE Healthcare, Tampa, FL) | Without hypertension |
(Φ the number of subjects on antihypertensive pharmacological treatment was not mentioned; BP Blood pressure, DM Diabetes mellitus, OAH Oral antihyperglycaemics, ACE inhibitors Angiotensin-converting enzyme inhibitors, ARBs Angiotensin receptor blockers)
Table 2.
| Study | Age (years) | Duration of diabetes (years) | BMI (kg/m2) | Hypertension (n,%) | SBP (mmHg) | DBP (mmHg) |
|---|---|---|---|---|---|---|
| A Ahmann 2015 | I:59.3(9.2) | I:12.1(7.1) | I:32.3(5.6) | Unavailable | Unavailable | Unavailable |
| P:57.5(11.1) | P:12.1(6.8) | P:32.2(5.7) | ||||
| A Astrup 2009 | I:47.2(9.7)† | NA | I:34.8(2.6)† | Unavailable | I:127(13.1)† | I:79.7(9.1)† |
| 45.5(10.9)‡ | 35.0(2.6)‡ | 123(13.0)‡ | 77.9(7.9)‡ | |||
| 45.0(11.1)§ | 35.0(2.8)§ | 126(13.9)§ | 78.6(8.2)§ | |||
| 45.9(10.7)♭ | 34.8(2.8)♭ | 124(11.3)♭ | 77.8(8.3)♭ | |||
| P:45.9(10.3) | P:34.9(2.8) | P:124(11.1) | P:76.8(8.5) | |||
| A Astrup 2012 | I:47.2(9.7)† | NA | I:34.8(2.6)† | Unavailable | I:127(13.1)† | I:79.7(9.1)† |
| 45.5(10.9)‡ | 35.0(2.6)‡ | 123(13.0)‡ | 77.9(7.9)‡ | |||
| 45.0(11.1)§ | 35.0(2.8)§ | 126(13.9)§ | 78.6(8.2)§ | |||
| 45.9(10.7)♭ | 34.8(2.8)♭ | 124(11.3)♭ | 77.8(8.3)♭ | |||
| P:45.9(10.3) | P:34.9(2.8) | P:124(11.1) | P:76.8(8.5) | |||
| A Blackman 2016 | I:48.6 (9.9) | NA | I:38.9(6.4) | I:75,41.7% | I:125.8(11.5) | I:81.2(7.6) |
| P:48.4(9.5) | P:39.4(7.4) | P:77,43% | P:127.1(12.3) | P:82.2(8.8) | ||
| Christian 2015 | I:39.5(2.7) | I:18.33(2.0) | I:24.17(0.64) | Unavailable | I:129.4(2.5) | I:75.5(1.7) |
| P:36.1(1.6) | P:19.56(1.6) | P:22.75(0.41) | P:127.3(2.2) | P:72.5(1.4) | ||
| Dejgaard 2016 | I:47(13) | I:20(12) | I:30.3(3.5) | Unavailable | I:131(15) | I:82(9) |
| P:49(12) | P:25(12) | P:29.8(3.1) | P:130(16) | P:81(8) | ||
| LEAD-1 | I:57.7(9)† | I:6.7(4.0,10.7)*† | I:29.8(5.1)† | I:155,68%† | I:133(15)† | Unavailable |
| 55.6(10)‡ | 6.5(3.7,10.5)*‡ | 30.0(5.1)‡ | 163,69.7%‡ | 132(16)‡ | ||
| P:54.7(10) | P:6.5(4.5,10.6)* | P:30.3(5.4) | P:74,64.9% | P:131(15.3) | ||
| LEAD-2 | I:57.0(9)† | I:7.0(5)† | I:31.1(4.8)† | Unavailable | I:132(14)† | I:80(10)† |
| 57.0(9)‡ | 8.0(5)‡ | 30.9(4.6)‡ | 131(14)‡ | 79(8)‡ | ||
| P:56.0(9) | P:8.0(6) | P:31.6(4.4) | P:135(16) | P:81(9) | ||
| LEAD-4 | I:55.0(10)† | I:9.0(6)† | I:33.2(5.4)† | Unavailable | I:129(14.8)† | I:75.8(9.0)† |
| 55.0(11)‡ | 9.0(6)‡ | 33.5(5.1)‡ | 126(14.2)‡ | 75.2(8.4)‡ | ||
| P:55.0(10) | P:9.0(6) | P:33.9(5.2) | P:128(14.5) | P:76.2(9.2) | ||
| LEAD-5 | I:57.6(9.5) | I:9.2(5.8) | I:30.4(5.3) | Unavailable | I:135(15.0) | I:80.8(9.1) |
| P:57.5(9.6) | P:9.4(6.2) | P:31.3(5.0) | P:133(14.0) | P:80.4(9.3) | ||
| LEADERS trial | I:64.2(7.2) | I:12.8(8.0) | I:32.5(6.3) | Unavailable | I:135.9(17.8) | I:77.2(10.3) |
| P:64.4(7.2) | P:12.9(8.1) | P:32.5(6.3) | P:135.9(17.7) | P:77.0(10.1) | ||
| Mark M. Smits 2016 | I:62.8(6.9) | Unavailable | I:32.0(30.9–35.9)* | Unavailable | I:136.6(17.0) | I:77.0(5.4) |
| P:137.6(14.9) | P:76.4(6.8) | |||||
| P:62.8(6.9) | ||||||
| P:30.8(28.9–31.5)* | ||||||
| MDI liraglutide trial | I:63.7(8.2) | I:17.3(7.6) | I:33.7(4.3) | Unavailable | I:137.9(16.8) | I:73.5(12.7) |
| P:63.5(7.7) | P:17.0(8.1) | P:33.5(4.0) | P:133.7(13.7) | P:74.9(8.5) | ||
| Nandy 2014 | I:57.7(9) | I:5.3(4.1) | I:32.7(4.5) | Unavailable | Unavailable | Unavailable |
| P:60.3(7.3) | P:8.4(4.6) | P:31.6(4.2) | ||||
| P. Mensberg MSc 2016 | I:56.5(9) | I:6(5.2) | I:32.5(3.7) | Unavailable | I:136.4(11.0) | I:84.1(7.0) |
| P:55.6(12) | P:3.7(3.3) | P:32.4(5.2) | P:136.2(8.9) | P:82.1(7.0) | ||
| Robert 2015 | I:34(9) | NA | I:36.15(3.84) | 0,0% | I:130(15) | I:76(11) |
| P:34(9) | P:35.74(4.55) | P:133(17) | P:78(10) | |||
| S Frossing 2018 | I:29.9(6.1) | NA | I:33.3(5.1) | 0,0% | I:123(9) | I:79(8) |
| P:29.9(6.1) | P:33.3(4.6) | P:124(9) | P:80(7) | |||
| Sun H. Kim 2013 | I:58.0(7) | NA | I:31.9(2.7) | 0,0% | I:127(10) | I:76(9) |
| P:58.0(8) | P:31.9(3.5) | P:119(14) | P:75(8) |
(Abbreviations: BMI Body mass index, SBP Systolic blood pressure, DBP Diastolic blood pressure, NA Not applicable, I Intervention group, P Placebo group, age and the duration of diabetes were expressed as mean(SD) unless otherwise noted, * median (25th and 75th percentile); † liraglutide 1.2 mg/d, ‡ liraglutide 1.8 mg/d, § liraglutide 2.4 mg/d, ♭ liraglutide 3.0 mg/d))
Quality assessment
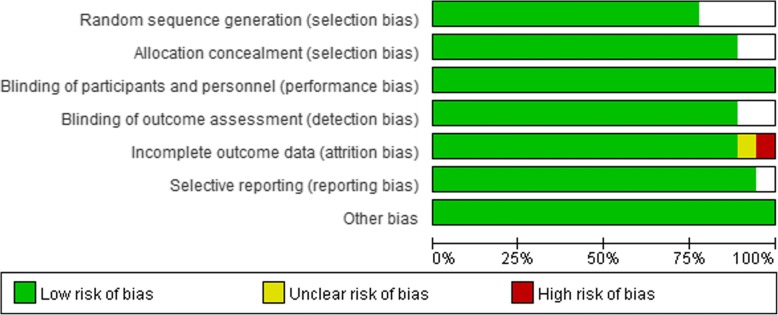
We conducted a quality assessment of the 18 RCTs included in the meta-analysis according to the Cochrane Collaboration’s risk of bias assessment tools. The characteristics at baseline of all 18 RCTs showed no significant difference between the liraglutide group and the placebo group. Four RCTs did not provide clear information on random sequence generation and allocation concealment [38–41]. One RCT did not give the number of people who were lost to follow-up or withdrew and the reason [40]. All 18 RCTs were performed and assessed by blinding researchers and participants [29, 31, 36–51]. The risk of bias is shown in Fig. 2 and Fig. 3.
Fig. 2.
Risk of bias graph
Fig. 3.
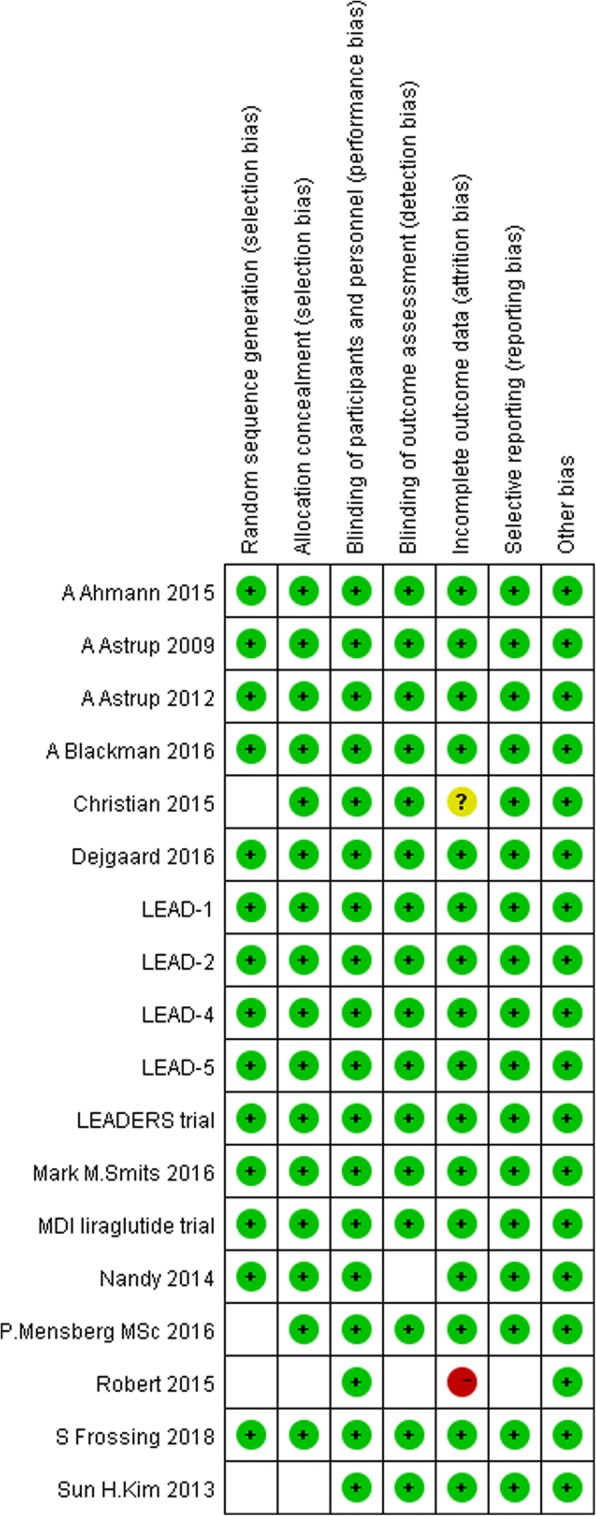
Risk of bias summary (+, low risk of bias; −, high risk of bias;?, unknown risk of bias)
SBP
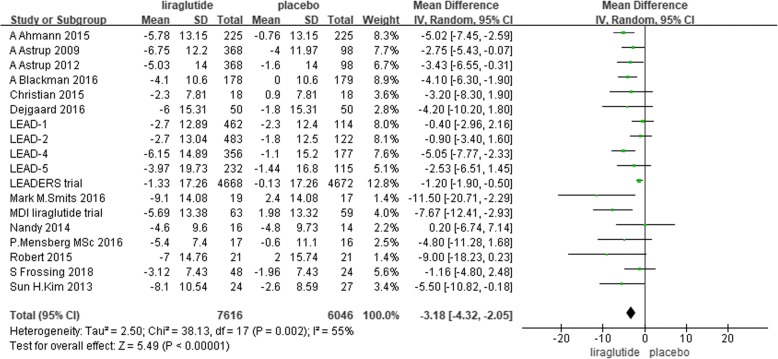
There were 7616 individuals in the liraglutide group and 6046 individuals in the placebo group included in the data analysis. Nine trials reported that liraglutide reduced SBP significantly compared with placebo [29, 31, 41–44, 46, 48, 49]. Eight trials did not show a significant difference in the reduction in SBP between liraglutide and placebo [36–40, 45, 47, 51], and 1 trial reported that liraglutide could slightly increase SBP without a clear significant difference [50]. The random-effects model showed that liraglutide significantly reduced SBP compared with placebo. The mean difference was 3.18 mmHg (− 4.32 to − 2.05, I2 = 55%, P < 0.00001) (Fig. 4). The I2 values suggested moderate heterogeneity, which might be related to the demographic characteristics, background therapy, dose of liraglutide and duration of intervention in each study.
Fig. 4.
The forest plot of the comparison between the effects of liraglutide and placebo on SBP (random-effects model)
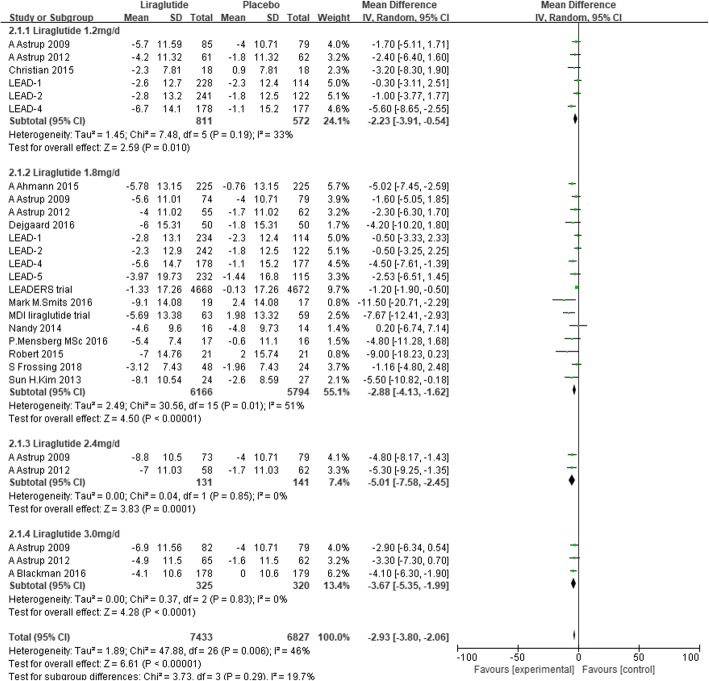
We conducted subgroup analysis defined by the dose of liraglutide. Liraglutide significantly reduced SBP by 2.23 mmHg (− 3.91 to − 0.54, I2 = 33%, P < 0.00001), 2.88 mmHg (− 4.13 to − 1.62, I2 = 51%, P < 0.00001), 5.01 mmHg (− 7.58 to − 2.45, I2 = 0%, P = 0.0001), and 3.67 mmHg (− 5.35 to − 1.99, I2 = 0%, P < 0.0001) compared with placebo in the liraglutide 1.2 mg/d stratification, 1.8 mg/d stratification, 2.4 mg/d stratification and 3.0 mg/d stratification, respectively (Fig. 5).
Fig. 5.
The forest plot of SBP in subgroup analysis defined by the dose of liraglutide (random-effects model)
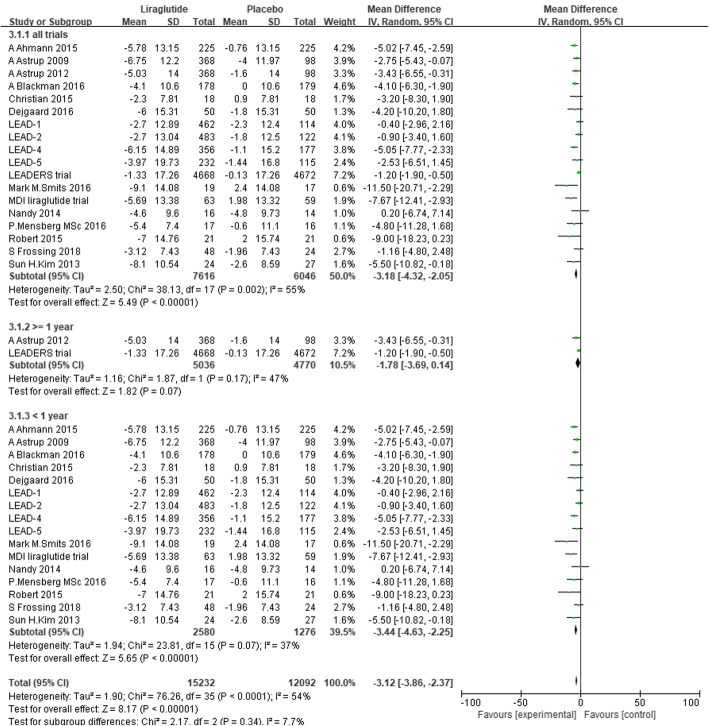
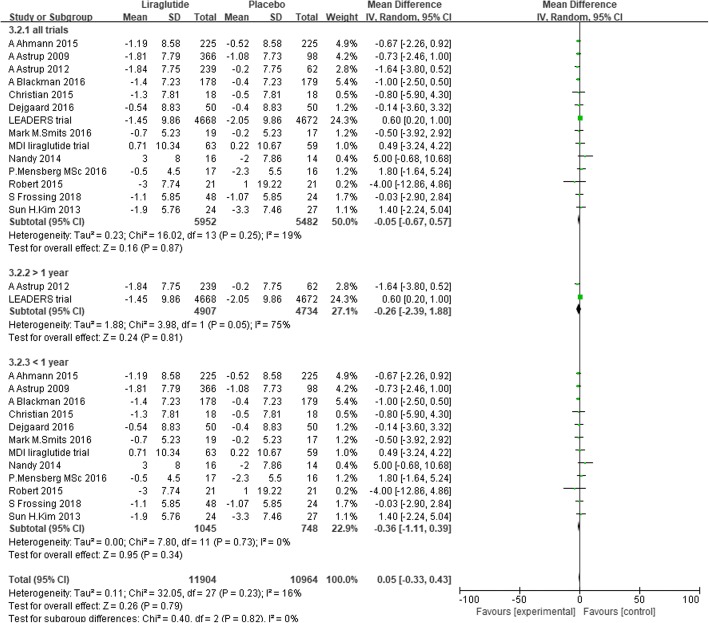
In addition, we conducted subgroup analysis defined by the duration of intervention. Subgroup analysis did not show a significant difference in reduction in SBP between the liraglutide group with a more than 1-year duration of intervention and the placebo group. The mean difference was − 1.78 mmHg (− 3.69 to 0.14, P = 0.07, I2=47%). Compared with the placebo group, the liraglutide group with a less than 1-year duration of intervention showed a significant reduction in SBP of 3.44 mmHg (− 4.63 to − 2.25, P < 0.00001, I2 = 37%) (Fig. 6).
Fig. 6.
The forest plot of SBP in subgroup analysis defined by the duration of intervention (random-effects model)
DBP
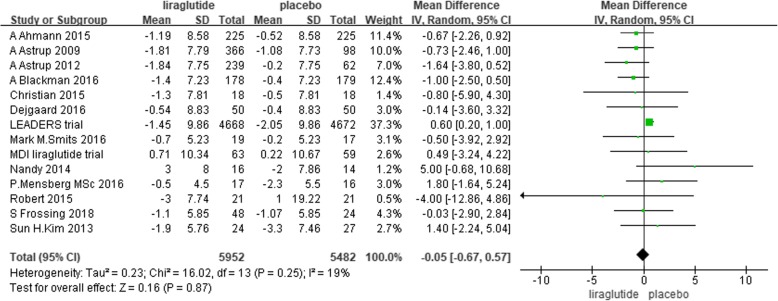
Fourteen trials reported changes in DBP from baseline to endpoint [29, 31, 38–45, 48–51]. We performed a random-effects meta-analysis with 5952 individuals assigned to liraglutide and 5482 individuals assigned to placebo. No significant difference was found in the reduction in DBP between liraglutide and placebo. The mean difference was − 0.05 mmHg (− 0.67 to 0.57, P = 0.87, I2 = 19%) (Fig. 7).
Fig. 7.
The forest plot of the comparison between the effects of liraglutide and placebo on DBP (random-effects model)
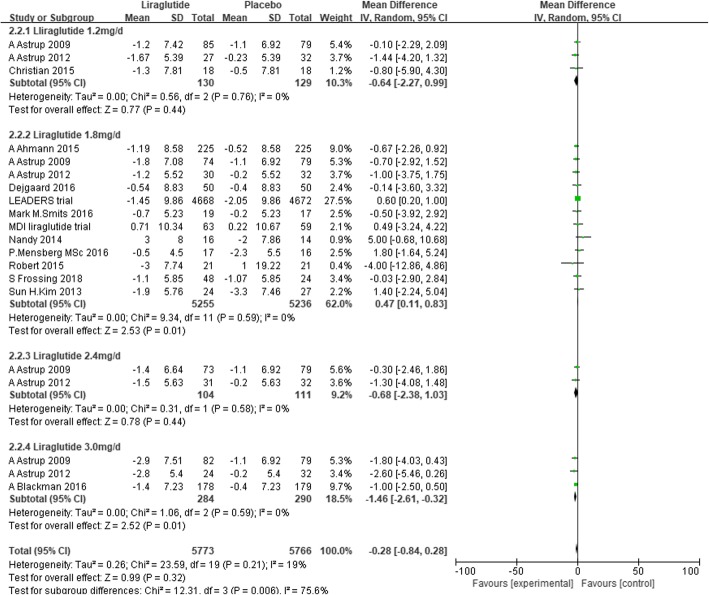
We conducted subgroup analysis defined by the dose of liraglutide. Liraglutide 3.0 mg/d significantly reduced DBP by 1.46 mmHg (− 2.61 to − 0.32, I2 = 0%, P = 0.01) compared with placebo. However, liraglutide 1.8 mg/d slightly increased DBP by 0.47 mmHg (0.11 to 0.83, I2 = 0%, P = 0.01) (Fig. 8). In addition, we conducted subgroup analysis defined by the duration of intervention, which showed that liraglutide did not significantly reduce DBP compared with placebo, whether the duration of intervention was more than or less than 1 year (Fig. 9).
Fig. 8.
The forest plot of DBP in subgroup analysis defined by the dose of liraglutide (random- effects model)
Fig. 9.
The forest plot of DBP in subgroup analysis defined by the duration of intervention (random-effects model)
Discussion
Explanation for findings
As liraglutide 0.6 mg/d subcutaneous injection was well tolerated and rarely used in clinical practice, we eliminated the data on liraglutide 0.6 mg/d. The random-effects model showed a significant difference in reduction in SBP between liraglutide and placebo by − 3.18 mmHg but no significant difference in reduction in DBP. Subgroup analysis showed that the degree of reduction in SBP was associated with the dose of liraglutide and the duration of intervention. The larger the dose of liraglutide was, the greater the reduction in SBP. However, the degree of reduction in SBP declined when the dose of liraglutide was 3.0 mg/d. Subgroup meta-analysis showed that short-term intervention with liraglutide (less than 1 year) could reduce SBP significantly compared with placebo but that the difference in reduction would disappear when the intervention lasted over 1 year. The mechanism underlying this phenomenon was not clear. However, there were only 2 trials with a more than 1-year duration of intervention, so the results might be related to the adherence to the medication, or compliance of the participants. In addition, there were limited trials in the liraglutide 2.4 mg/d stratification and the liraglutide 3.0 mg/d stratification. Thus, additional long-term and large-dosage clinical trials are needed to probe the further efficacy of liraglutide on blood pressure.
Assessment of quality of included studies
This meta-analysis included 18 RCTs. All of the included trials were randomised, double-blind, placebo-controlled, parallel trials. To improve the grade of evidence, we excluded cross-over controlled trials from comparisons between liraglutide and placebo. After quality assessment, the bias risk of the included trials was relatively low.
The GLP-1RA liraglutide, as a new method of antidiabetic therapy, has been shown by a considerable number of trials to demonstrate efficacy in lowering fasting blood glucose, postprandial blood glucose, and weight. Increasing numbers of clinical studies have shown its cardiovascular benefits, providing further evidence for clinical use of liraglutide beyond antihyperglycaemia [8–14, 17–20, 27, 28]. In recent years, some researchers performed meta-analyses to compare liraglutide and other antihyperglycaemic agents, such as sulfonylureas, insulin, TZDs, DPP-4 inhibitors and other GLP-1RAs, demonstrating different effects in lowering HbA1C/fasting plasma glucose/postprandial plasma glucose, adverse events, and improvement in insulin resistance, weight loss and the risk of hypoglycaemia [25, 26, 34, 52–57]. However, the influence of liraglutide on blood pressure was still uncertain.
Hypertension is highly correlated with diabetes but remains underrecognised and undertreated in the diabetic and the general population. The UK prospective diabetes study found that strict blood pressure control in patients with hypertension and type 2 diabetes substantially reduced the risk of death and complications due to diabetes [58]. In the active-treatment arm of the ADVANCE study, a decrease in blood pressure of 5.6/2.2 mmHg in high-risk patients with T2DM reduced the rate of renal adverse events by 21% [59]. Our meta-analysis showed that SBP was reduced by approximately 5 mmHg, which may be a cardioprotective benefit. A study based on healthy adults found that plasma levels of fasting GLP-1 are significantly and positively related to the blood pressure indices assessed [60]. The increase in GLP-1 levels could be a compensatory response to individual BP elevations. The possible mechanisms by which GLP-1 reduces BP are vasodilatory properties [61] and improvement of endothelial function [62, 63]. In addition, there is some evidence that GLP-1RAs mediate sodium excretion and diuresis in order to lower blood pressure [64–66].
Intensive control of glucose levels and blood pressure is currently the mainstay of both prevention and treatment of diabetic nephropathy. The LEADER trial showed that liraglutide-induced benefits on renal outcome could be due to improvements in renal risk factors, such as renal haemodynamics [67]. GLP-1RAs may induce renoprotection by inhibiting renal tubular sodium reabsorption, facilitating water excretion [64–66] and decreasing glomerular pressure. A pooled analysis of four studies showed that DPP-4 inhibitors led to a significant reduction in albuminuria in patients with type 2 diabetes [68].
All trials found that liraglutide significantly reduced body weight compared with placebo [29, 31, 36–51]. The reduction in SBP partly contributes to the reduction in body weight. However, on the basis of the SBP and weight profiles over time, the reduction in SBP may not be fully explained by the reduction in body weight [37]. Based on the time course of SBP and weight reductions, the reduction in SBP occurred before substantial weight loss [47]. A meta-analysis showed that significant reductions in SBP were observed as early as 2 weeks after initiation of liraglutide treatment and could be observed before any significant weight loss occurred [68].
Strengths and limitations
The aim of this meta-analysis was to discuss the influence of liraglutide on blood pressure in individuals with or without abnormal glucose metabolism by searching high-quality RCTs to provide reliable evidence for clinical practice. However, some limitations should be noted. First, Robert SA et al. did not provide the number of people who were lost to follow-up or withdrew and the reasons these participants were lost follow-up or withdrew. Four RCTs did not give a clear method of random sequence and allocation concealment [38–41]. These factors increased the bias risk of the studies included. Second, because of the limitation of sample size in stratifications treated with liraglutide 2.4 mg/d and 3.0 mg/d, the subgroup analysis might be inaccurate. Third, there was a lack of clinical trials on the efficacy of liraglutide on blood pressure in patients with and without hypertension.
Conclusions
In this meta-analysis, 18 RCTs were included to explore the effect of liraglutide on blood pressure. The results showed that compared with placebo, liraglutide significantly reduced SBP. At doses of liraglutide up to 3.0 mg/d, the reduction in DBP was significant. At present, liraglutide is widely recognised to have a beneficial effect on glucose reduction, weight loss and protection of β-cells. With the efficacy on blood pressure, the application of liraglutide in clinical practice may be broadened in the future. More clinical trials are needed to investigate the further effect of liraglutide on blood pressure.
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Availability of data and materials
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Abbreviations
- ACE inhibitors
Angiotensin-converting enzyme inhibitors
- ADA
American Diabetes Association
- ARBs
Angiotensin receptor blockers
- BMI
Body mass index
- BP
Blood pressure
- DBP
Diastolic blood pressure
- DM
Diabetes mellitus
- DPP-4
Dipeptidyl peptidase-4
- GLP-1RAs
Glucagon-like peptide-1 receptor agonists
- IDF
International Diabetes Federation
- NA
Not applicable
- OAH
Oral antihyperglycaemics
- RCTs
Randomised controlled trials
- SBP
Systolic blood pressure
- TZDs
Thiazolidinediones
Authors’ contributions
JD conducted the study. XZ and KH performed the study selection, and MZ performed the process when XZ and Kun Huang had disagreement. XZ analyzed the data and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xu Zhao, Email: soso11019@126.com.
Kun Huang, Email: khuang@bjmu.edu.cn.
Meijie Zheng, Email: moon850617@163.com.
Junting Duan, Phone: +86-18810526954, Email: duanjunting123@sina.com.
References
- 1.International Diabetes Federation Five questions on the IDF diabetes atlas. Diabetes Res Clin Pract. 2013;102(2):147–148. doi: 10.1016/j.diabres.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Bommer C, Sagalova V, Heesemann E, et al. Global economic burden of diabetes in adults: projection from 2015 to 2030. Diabetes Care. 2018;41(5):963–970. doi: 10.2337/dc17-1962. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association 9. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S86–S104. doi: 10.2337/dc18-S009. [DOI] [PubMed] [Google Scholar]
- 4.Hansson L, Zanchetti A, Carruthers SG, et al. Effects of intensive blood pressure lowering and low-dose aspirinin patient with hypertension: principal results of Hypertension Optimal Treatment (HOT) randomised trial. HOT Study Group. Lancet. 1998;351:1755–1762. doi: 10.1016/S0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 5.UK Prospective Diabetes Study Group Tight blood pressure control and rick of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. doi: 10.1136/bmj.317.7160.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner RC, Milns H, Neil HA, et al. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom prospective diabetes study (UKPDS: 23) BMJ. 1998;316:823–828. doi: 10.1136/bmj.316.7134.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estacio RO, Schrier RW. Antihypertensive therapy in type 2 diabetes: implications of the appropriate blood pressure control in diabetes (ABCD) trial. Am J Cardiol. 1998;82:9R–14R. doi: 10.1016/S0002-9149(98)00750-4. [DOI] [PubMed] [Google Scholar]
- 8.Patel A, ADVANCE Collaborative Group. MacMahon S, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): A randomised cotrolled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 9.Brady EM, Davies MJ, Gray LJ, et al. A randomized controlled trial comparing the GLP-1 receptor agonist liraglutide to a sulphonylurea as add on to metformin in patients with established type 2 diabetes during Ramadan: the treat 4 Ramadan Tiral. Diabetes Obes Metab. 2014;16(6):527–536. doi: 10.1111/dom.12249. [DOI] [PubMed] [Google Scholar]
- 10.Nauck M, Frid A, Hermansen K, et al. Long-term efficacy and safety comparison of liraglutide, glimepiride and placebo, all in combination with metformin in type 2diabetes: 2-year results from the LEAD-2 study. Diabetes Obes Metab. 2013;15(3):204–212. doi: 10.1111/dom.12012. [DOI] [PubMed] [Google Scholar]
- 11.Lee WC, Dekoven M, Bouchard J, Massoudi M, Langer J. Improved real-word glycaemic outcome with liraglutide versus other incretin-based therapies in type 2 diabetes. Diabetes Obes Metab. 2014;16(9):819–826. doi: 10.1111/dom.12285. [DOI] [PubMed] [Google Scholar]
- 12.Yang W, Chen L, Ji Q, et al. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: a 16-week, randomized, double-blind, active control trial. Diabetes Obes Metab. 2011;13(1):81–88. doi: 10.1111/j.1463-1326.2010.01323.x. [DOI] [PubMed] [Google Scholar]
- 13.Seino Y, Rasmussen MF, Nishida T, Kaku K. Efficacy and safety of the once-daily human GLP-1 analogue liraglutide vs glibenclamide monotherapy in Japanese patients with type 2 diabetes. Curr Med Res Opin. 2010;26(5):1013–1022. doi: 10.1185/03007991003672551. [DOI] [PubMed] [Google Scholar]
- 14.Abdul-Ghani MA, Williams K, Kanat M, Altuntas Y, DeFronzo RA. Insulin vs GLP-1 analogues in poorly controlled Type 2 diabetic subjects on oral therapy: a meta-analysis. J Endocrinal Invest. 2013;36:168–173. doi: 10.3275/8367. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka K, Saisho Y, Kawai T, et al. Efficacy and safety of liraglutide monotherapy compared with metformin in Japanese overweight/obese patients with type 2 diabetes. Endocr J. 2015;62(5):399–409. doi: 10.1507/endocrj.EJ14-0602. [DOI] [PubMed] [Google Scholar]
- 16.Ma Z, Chen R, Liu Y, Yu P, Chen L. Effect of liraglutide vs. NPH in combination with metformin on blood glucose fluctuations assessed using continuous glucose monitoring in patients with newly diagnosed type 2 diabetes. Int J Clin Pharmacol Ther. 2015;53(11):933–939. doi: 10.5414/CP202415. [DOI] [PubMed] [Google Scholar]
- 17.Garcia Diaz E, Guaqnozzi D, Gutierrez V, et al. Effect of incretin therapies compared to pioglitazone and gliclazide in non-alcoholic fatty liver disease in diabetic patients not controlled on metformin alone: an observational, pilot study. Endocrinol Nutr. 2016;63(5):194–201. doi: 10.1016/j.endonu.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Seino Y, Rasmussen MF, Clasuson P, Kaku K. The once-daily human glucagon-like peptide-1 analog, liraglutide, improves β-cell function in Japanese patients with type 2 diabetes. Diabetes Investig. 2012;3(4):388–395. doi: 10.1111/j.2040-1124.2012.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR, NN2211-1310 International Study Group Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12-week, double-blind, randomized, controlled trial. Diabetes Care. 2004;27(6):1335–1342. doi: 10.2337/diacare.27.6.1335. [DOI] [PubMed] [Google Scholar]
- 20.Bays H, Pi-Sunyer X, Hemminqsson JU, Claudius B, Jensen CB, Van Gaal L. Liraglutide 3.0 mg for weight management: weight-loss dependent and independent effects. Curr Med Res Opin. 2017;33(2):225–229. doi: 10.1080/03007995.2016.1251892. [DOI] [PubMed] [Google Scholar]
- 21.Lane W, Weinrib S, Rappaport J, Hale C. The effect of addition of liraglutide to high-dose intensive insulin therapy: a randomized prospective trial. Diabetes Obes Metab. 2014;16(9):827–832. doi: 10.1111/dom.12286. [DOI] [PubMed] [Google Scholar]
- 22.Davies MJ, Bain SC, Atkin SL, et al. Efficacy and safety of Liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA-RENAL): a randomized clinical trial. Diabetes Care. 2016;39(2):222–230. doi: 10.2337/dc14-2883. [DOI] [PubMed] [Google Scholar]
- 23.Retnakaran R, Kramer CK, Choi H, Swaminathan B, Zinman B. Liraglutide and the preservation of pancreatic β-cell function in early type 2 diabetes: the LIBRA trial. Diabetes Care. 2014;37(12):3270–3278. doi: 10.2337/dc14-0893. [DOI] [PubMed] [Google Scholar]
- 24.Kondo Y, Satoh S, Osada UN, Terauchi Y. Early liraglutide treatment improves β-cell function in patients with type 2 diabetes: a retrospective cohort study. Endocr J. 2015;62(11):971–980. doi: 10.1507/endocrj.EJ15-0206. [DOI] [PubMed] [Google Scholar]
- 25.Sun F, Yu K, Wu S, et al. Cardiovascular safety and glycemic control of glucagon-like peptide-1 receptor agonists for type 2 diabetes mellitus: a pairwise and network meta-analysis. Diabetes Res Clin Pract. 2012;98(3):386–395. doi: 10.1016/j.diabres.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Chudleigh RA, Bain S. Cardiovascular safety of liraglutide for the treatment of type 2 diabetes. Expert Opin Drug Saf. 2017;16(5):627–635. doi: 10.1080/14740338.2017.1313225. [DOI] [PubMed] [Google Scholar]
- 27.Viswanathan P, Chaudhuri A, Bhatia R, Al-Atrash F, Mohanty P, Dandona P. Exentide therapy in obese patients with type 2 diabetes mellitus treated with insulin. Endocr Pract. 2007;13:444–450. doi: 10.4158/EP.13.5.444. [DOI] [PubMed] [Google Scholar]
- 28.Rosso GT, Labate AM, Giandalia A, et al. Twelve-month treatment with Liraglutide ameliorates visceral adiposity index and common cardiovascular risk factors in type 2 diabetes outpatients. J Endocrinol Investig. 2015;38(1):81–89. doi: 10.1007/s40618-014-0163-9. [DOI] [PubMed] [Google Scholar]
- 29.Astrup A, Rossner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomized, double-blind, placebo-controlled study. Lancet. 2009;374:1606–1616. doi: 10.1016/S0140-6736(09)61375-1. [DOI] [PubMed] [Google Scholar]
- 30.Marso SP, Poulter NR, Nisseen SE, et al. Design of the liraglutide effect and action in diabetes: Evaluation of cardiovascular outcome results (LEADER) trial. Am Heart J. 2013;166(5):823–30.e5. doi: 10.1016/j.ahj.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;28:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiramatsu T, Ozeki A, Asai K, Saka M, Hobo A, Furuta S. Liraglutide improves glycemic and blood pressure control and ameliorates progression of left ventricular hypertrophy in patients with type 2 diabetes mellitus on peritoneal dialysis. Ther Apher Dial. 2015;19(6):598–605. doi: 10.1111/1744-9987.12319. [DOI] [PubMed] [Google Scholar]
- 33.Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013;3(1). [DOI] [PMC free article] [PubMed]
- 34.Wang B, Zhong J, Lin H, et al. Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trial. Diabetes Obes Metab. 2013;15(8):737–749. doi: 10.1111/dom.12085. [DOI] [PubMed] [Google Scholar]
- 35.Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (2011). Available: http://handbook-5-1.cochrane.org/
- 36.Marre M, Shaw J, Brandle M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–278. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nauck M, Frid A, Hermansen K, et al. Efficacy and safety comparison of liraglutide, glimepiride, and placebo, all in combination with metformin, in type 2 diabetes: the LEAD (liraglutide effect and action in diabetes)-2 study. Diabetes Care. 2009;32:84–90. doi: 10.2337/dc08-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frandsen CS, Dejgaard TF, Holst JJ, Andersen HU, Thorsteinsson B, Madsbad S. Twelve-week treatment with Liraglutide as add-on to insulin in Normal-weight patients with poorly controlled type 1 diabetes: a randomized, placebo-controlled. Double-Blinded Parallel Study. Diabetes Care. 2015;38(12):2250–7. [DOI] [PubMed]
- 39.Mensberg P, Nyby S, Jorgensen PG, et al. Near-normalization of glycaemic control with glucagon-like peptide-1 receptor agonist treatment combined with exercise in patient with type 2 diabetes. Diabetes Obes Metab. 2017;19(2):172–180. doi: 10.1111/dom.12797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robert SA, Rohana AG, Shah SA, Chinna K, Wan Mohamud WN, Kamaruddin NA. Improvement in binge eating in non-diabetic obese individuals after 3 months of treatment with liraglutide - a pilot study. Obes Res Clin Pract. 2015;9(3):301–304. doi: 10.1016/j.orcp.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Kim SH, Abbasi F, Lamendola C, et al. Benefits of liraglutide treatment in overweight and obese older individual with prediabetes. Diabetes Care. 2013;36(10):3276–3282. doi: 10.2337/dc13-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes: a randomized, placebo-controlled trial. Diabetes Obes Metab. 2015;17:1056–1064. doi: 10.1111/dom.12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Astrup A, Carraro R, Finer N, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int J Obes. 2012;36:843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blackman A, Foster GD, Zammit G, et al. Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE Sleep Apnea randomized clinical trial. Int J Obes. 2016;40:1310–1319. doi: 10.1038/ijo.2016.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dejgaard TF, Frandsen CS, Hansen TS, et al. Efficacy and safety of liraglutide for overweight adult patients with type 1 diabetes and insufficient glycaemic control (lira-1): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocinol. 2016;4(3):221–232. doi: 10.1016/S2213-8587(15)00436-2. [DOI] [PubMed] [Google Scholar]
- 46.Zinman B, Gerich J, Buse JB, et al. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 met+TZD) Diabetes Care. 2009;32(7):1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell-Jones D, Vaag A, Schmitz O, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea trerapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomized controlled trial. Diabetologia. 2009;52(10):2046–2055. doi: 10.1007/s00125-009-1472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smits MM, Tonneijck L, Muskiet MH, et al. GLP-1-based therapies have no microvascular effects in type 2 diabetes mellitus: an acute and 12-week randomized, double-blind. Placebo-Controlled Trial. Arteioscler Thromb Vasc Biol. 2016;36(10):2125–2132. doi: 10.1161/ATVBAHA.116.307930. [DOI] [PubMed] [Google Scholar]
- 49.Lind M, Hirsch IB, Tuomilehto J, et al. Liraglutide in people treated for type 2 diabetes with multiple daily insulin injections: randomized clinical trial (MDI Liraglutide trial) BMJ. 2015;351:h5364. doi: 10.1136/bmj.h5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nandy D, Johnson C, Basu R, et al. The effect of liraglutide on endothelial function in patients with type 2 diabetes. Diab Vasc Dis Res. 2014;11(6):419–430. doi: 10.1177/1479164114547358. [DOI] [PubMed] [Google Scholar]
- 51.Frossing S, Mylander M, Kistorp C, Skouby SO, Faber J. Effect of liraglutide on atrial natriuretic peptide, adrenomedullin, and copeptin in PCOS. Endocr Connect. 2018;7(1):115–123. doi: 10.1530/EC-17-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang T, Gou Z, Wang F, Ma M, Zhai SD. Comparison of GLP-1 analogues versus sitaglipitin in the management of type 2 diabetes: systematic review and meta-analysis of head-to-head studies. PLoS One. 2014;9(8):e103798. doi: 10.1371/journal.pone.0103798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gu J, Menq X, Guo Y, et al. The efficacy and safety of liraglutide added to metformin in patients with diabetes: a meta-analysis of randomized controlled trials. Sci Rep. 2016;6:32714. doi: 10.1038/srep32714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fonseca VA, Devries JH, Henry RR, Donsmark M, Thomsen HF, Plutzky J. Reductions in systolic blood pressure with liraglutide in patients with type 2 diabetes: insights from a patient-level pooled analysis of six randomized clinical trials. J Diabetes Complicat. 2014;28(3):399–405. doi: 10.1016/j.jdiacomp.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun F, Wu S, Guo S, et al. Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: a systematic review and network meta-analysis. Diabetes Res Clin Pract. 2015;110(1):26–37. doi: 10.1016/j.diabres.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 56.Henry RR, Buse JB, Sesti G, et al. Efficacy of antihyperglycemic therapies and the influence of baseline hemoglobin A(1C): a meta-analysis of the liraglutide development program. Endocr Pract. 2011;17(6):906–913. doi: 10.4158/EP.17.6.906. [DOI] [PubMed] [Google Scholar]
- 57.Plutzky J, Garber A, Falahati A, Toft AD, Poulter NR. Reductions in lipids and CV risk markers in patients with type 2 diabetes treated with liraglutide: a meta-analysis. Can J Diabetes. 2009;33(3):209–210. doi: 10.1016/S1499-2671(09)33072-5. [DOI] [PubMed] [Google Scholar]
- 58.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes. BMJ. 1998;317(7160):703–713. doi: 10.1136/bmj.317.7160.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel A, ADVANCE Collaborative Group. MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet. 2007;370(9590):829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 60.Krisai P, Aeschbacher S, Schoen T, Bossard M, et al. Glucagon-like peptide-1 and blood pressure in young and healthy adults from the general population. Hypertension. 2015;65(2):306–312. doi: 10.1161/HYPERTENSIONAHA.114.04718. [DOI] [PubMed] [Google Scholar]
- 61.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, et al. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation. 2008;117(18):2340–2350. doi: 10.1161/CIRCULATIONAHA.107.739938. [DOI] [PubMed] [Google Scholar]
- 62.Basu A, Charkoudian N, Schrage W, Rizza RA, et al. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab. 2007;293(5):E1289–E1295. doi: 10.1152/ajpendo.00373.2007. [DOI] [PubMed] [Google Scholar]
- 63.Gaspari T, Liu H, Welungoda I, Hu Y, et al. A GLP-1 receptor agonist liraglutide inhibits endothelial cell dysfunction and vascular adhesion molecule expression in an ApoE−/− mouse model. Diab Vasc Dis Res. 2011;8(2):117–124. doi: 10.1177/1479164111404257. [DOI] [PubMed] [Google Scholar]
- 64.Thomson SC, Kashkouli A, Singh P. Glucagon-like peptide-1 receptor stimulation increases GFR and suppresses proximal reabsorption in the rat. Am J Physiol Renal Physiol. 2013;304(2):F137–F144. doi: 10.1152/ajprenal.00064.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yu M, Moreno C, Hoagland KM, Dahly A, et al. Antihypertensive effect of glucagon-like peptide 1 in dahl salt-sensitive rats. J Hypertens. 2003;21(6):1125–1135. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 66.Gutzwiller JP, Hruz P, Huber AR, Hamel C, et al. Glucagon-like peptide-1 is involved in sodium and water homeostasis in humans. Digestion. 2006;73(2–3):142–150. doi: 10.1159/000094334. [DOI] [PubMed] [Google Scholar]
- 67.Groop PH, Cooper ME, Perkovic V, Emser A, et al. Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care. 2013;36(11):3460–3468. doi: 10.2337/dc13-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Montanya E, Sesti G. A review of efficacy and safety data regarding the use of liraglutide, a once-daily human glucagon-like peptide 1 analogue, in the treatment of type 2 diabetes mellitus. Clin Ther. 2009;31(11):2472–2488. doi: 10.1016/j.clinthera.2009.11.034. [DOI] [PubMed] [Google Scholar]
- 69.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on high blood pressure research. Hypertension. 2005;45(1):142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 70.Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 71.Smits MM, Tonneijck L, Muskiet MH, et al. Cardiovascular, renal and gastrointestinal effects of incretin-based therapies: an acute and 12-week randomised, double-blind, placebo-controlled, mechanistic intervention trial in type 2 diabetes. BMJ Open. 2015;5(11):e009579. doi: 10.1136/bmjopen-2015-009579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nylander M, Frøssing S, Kistorp C, et al. Liraglutide in polycystic ovary syndrome: a randomized trial, investigating effects on thrombogenic potential. Endocr Connect. 2017;6(2):89–99. doi: 10.1530/EC-16-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study available from the corresponding author on reasonable request.