Abstract
In Nigeria, dogs are the common companions in many households and, harbor wide range of ectoparasites of severe zoonotic potentials. A cross sectional survey was conducted to examine the prevalence, intensity and risk factors of ectoparasite infestation among the owned dogs in Kwara state, Nigeria. A total of 333 dogs were screened for ectoparasites, and questionnaires were applied to obtain information regarding; age, sex, breeds, coat colour and management practices by dog owners. Two hundred and seventy one (81.4%) dogs were infested with at least one species of ectoparasite and multiple infestations recorded mostly among the female dogs. Six species of ectoparasites of three taxa were identified: ticks (Rhipicephalus sanguineus sensu lato, Haemaphysalis leachii and Amblyomma variegatum), fleas (Ctenocephalides canis and C. felis) and louse (Heterodoxus spiniger). R. sanguineus s.l. was the most prevalent (70.3%) followed by C. felis (42.1%) and H. spiniger (30.0%). Intensities of C. felis, H. spiniger and R. sanguineus s.l infestations were significantly higher in female and younger dogs (p < 0.001). Ectoparasites occurrence varied with breed and coat colour of host. A high prevalence of ectoparasite infestation recorded is at variance with the knowledge of dogs' owners in this study area. Therefore, intervention based on provision of veterinary clinics and prevention and management of parasite infestation in endemic area would mitigate the possible health hazard associated with the ectoparasitic infestation of dogs.
Keywords: Ticks, Fleas, Lice, Dog, Epidemiology, Nigeria
1. Introduction
Dogs are common companions in many households, contributing to the physical, social and emotional development of children and the well-being of their owners in both developed and under-developed countries (Alvarado-Esquivel et al., 2015; Robertson et al., 2000). However, the reasons for keeping dogs vary considerably with culture, social and economic activities of individuals (Arong et al., 2011; Pereira et al., 2016). Despite the advantages, pet dogs harbor bewildering number of parasites of zoonotic potentials and thus remain a major threat to public health (Klimpel et al., 2010; Ugbomoiko et al., 2008).
Various species of ticks, fleas, lice and mites infest domestic dogs and cause considerable pathological conditions such as severe allergic dermatitis and non-pruritic skin disorders (Bahrami et al., 2012; González et al., 2004). Besides the direct host damage, some ectoparasites also act as vectors of medically important pathogens such as Babesia spp., Bartonella and Rickettsia species (Alcaíno et al., 2002; Heukelbach et al., 2012; Nuchjangreed and Somprasong, 2007). Previous studies have confirmed that Rhipicephalus sanguineus sensu lato, Ctenocephalides species, Trichodectes canis and Heterodoxus spiniger as well as Linognathus setosus are common ectoparasites of domestic dogs in many tropical countries (Alcaíno et al., 2002; González et al., 2004; Heukelbach et al., 2012).
In Nigeria, the occurrence of ectoparasite infestations have been well documented (Arong et al., 2011; Ugbomoiko et al., 2008) but the accurate epidemiological profiles upon which control measures would be based are still limited. This is necessary for the implementation of integrated control measures. We therefore aimed at bridging the knowledge gap by investigating the prevalence and determinants of common ectoparasites of domestic dogs in Kwara State, Nigeria.
2. Materials and methods
2.1. Study area
The study was conducted in Ilorin and some selected neighboring rural communities (Omaran, Oke-oyi, Ile-Apa, Osin-Tunji, Osin Aremu and Tanke iledu) in Kwara state, Nigeria between November 2016 and June 2017. Ilorin is located within longitude 2°45′ 6°4′ E and latitude 8°10° with a population of about 814,192 (National Population Census, 2006). Majority of inhabitants of our study communities are farmers and hunters with few wage earners. The vegetation features of the communities are similar; it is savannah and the climate is typical with intense rainfalls from April to October and daily temperatures between 23 °C and 37 °C. Living conditions of people in most of our study communities are precarious and greater proportion of inhabitants keep dogs with little or no access to veterinary services; most dogs have never been treated nor screened for any form of parasitic infection prior to the study.
2.2. Study design and sample collection
A door-to-door screening of dogs was conducted in randomly selected 65 households from the list of households in the study area, with average of 5 dogs per household. Overall, a total of 950 dogs were estimated. However, calculation of sampling size revealed that 196 dogs were sufficient for the study considering 80% power at 95% confident interval. A well-structured questionnaire was provided to obtain information on age, breeds, management practices and the vaccination status of dogs from dog owners. To avoid the risk of infection, all examiners were vaccinated with anti-rabis vaccine and dog owners assisted to restrain their dogs with muzzles. The entire body surfaces of each dog were carefully rubbed with a piece of cotton soaked in ether and systematically combed with stainless fine-toothed comb to dislodge ectoparasites onto a clear white cardboard paper. Suspected skin lesions were scrapped into 10% potassium hydroxide. All ectoparasites recovered were preserved in a pre-labelled sample bottle containing 70% alcohol and subsequently transferred to the laboratory for identification. Infested dogs were referred to the University of Ilorin Veterinary Clinic for treatment. The study was approved by university of Ilorin ethical research committee, also an informed consent and agreement was obtained from the owners of the dogs before enrolment.
2.3. Sample analysis
The ectoparasites except ticks collected from each animal were transferred into a petri-dish and counted using an eye lens before they were soaked in 10% potassium hydroxide overnight after which they were dehydrated in ascending strength of alcohol (50%, 70%, 90%, and absolute). The specimens, after dehydration, were cleared in xylene for about 10–15 min, mounted in Canada balsam on a clean glass slide and covered with a cover slip. However ticks were kept at room temperature to thaw, and then washed twice with sterile water to remove excess particulate contamination from animal skin, rinsed once with 70% ethanol. The mounted specimen were allowed to dry before examining under a dissecting microscope (×40 Magnifications) for identification. Species identification was based on diagnostic keys provided by Centre for Disease control Centre, Estrada-Pena &Venzal, Blaine & Bobbi and Walker et al. (Estrada-peña and Venzal, 2007; Mathison and Pritt, 2014; Walker, 2003).
2.4. Statistical analysis
Statistical analysis was performed with IBM version 20; (SPS Inc., Chicago IL). Descriptive statistics intensity of infestation were expressed as means and standard deviations while the student's t-test and Chi square analysis were used for risk analysis of infestation. Proportional test was performed by R console version 3.0.1. The level of significance was set at p value < 0.05.
3. Results
A total of 7710 adult ectoparasites were recovered from 333 dogs (48.9% males and 51.1% females) screened in this study. Of these, 71.2% of the ectoparasites were ticks while 17.3% and 11.5% were fleas and lice respectively. These parasites comprised of 3 species of ticks (R. sanguineus s.l., Haemaphysalis leachii and Amblyomma variegatum), 2 species of fleas (Ctenocephalides canis and C. felis) and 1 species louse (Heterodoxus spiniger).
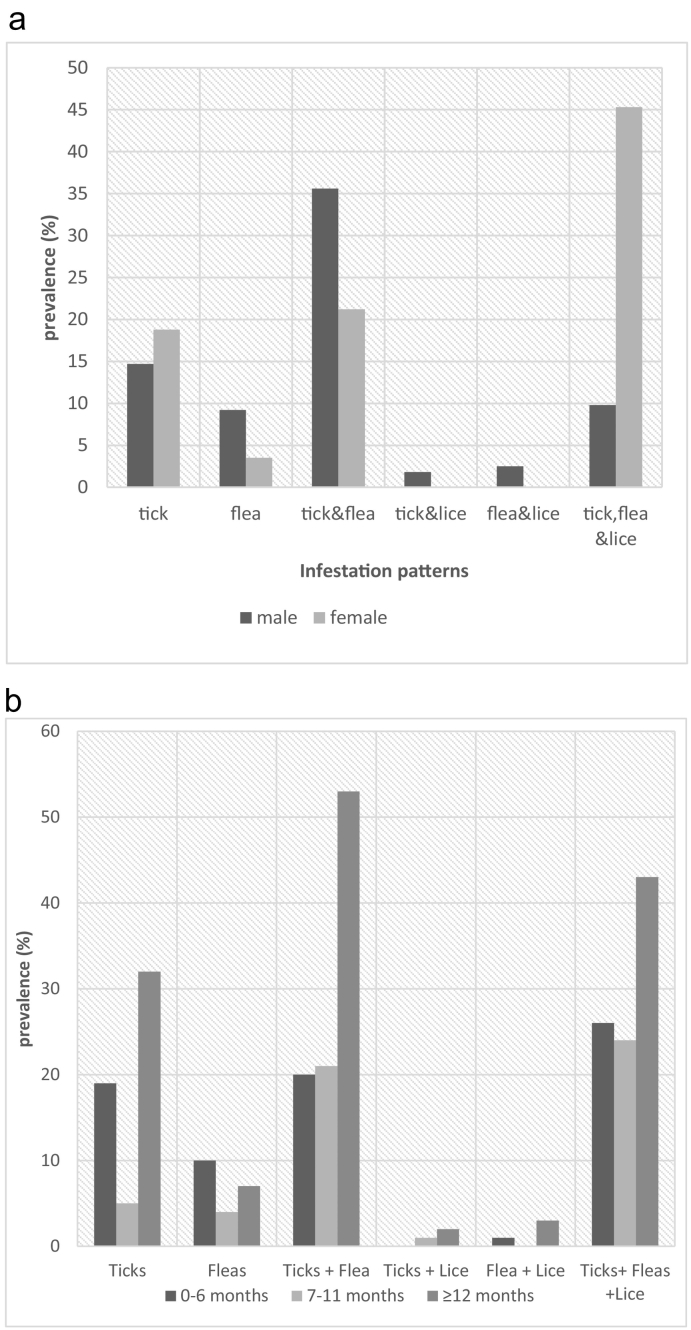
Overall, >80% of dogs were infested by at least one species of ectoparasite, and the prevalence of the infestation was significantly higher (p < 0.001) in female dogs (88.8%) than males but infestation with respect to age was comparable (p = 0.43) (Table 1). Also, 71.6% co-infestation was observed among the examined dogs. The frequencies of co-infestation with respect to age and sex of dogs are presented graphically in Fig. 1a & b.
Table 1.
Demographic characteristics of the dog's population in the study area.
| Variables | No. examined | No. infested | Prevalence [%) |
|---|---|---|---|
| Sex of dog | |||
| Male | 163 | 120 | 73.6 |
| Female | 170 | 151 | 88.8 |
| p value | <0.001 | ||
| Age of dogs [months) | |||
| 0–6 | 89 | 76 | 85.4 |
| 7–11 | 71 | 55 | 77.5 |
| ≥12 | 173 | 140 | 80.9 |
| p value | 0.43 | ||
| Breeds of dogs | |||
| Completely local | 241 | 222 | 92.1 |
| Hybrid | 58 | 46 | 79.3 |
| Exotic | 34 | 3 | 8.8 |
| p value | <0.001 | ||
| Infestation patterns | |||
| Single | 333 | 77 | 28.4 |
| Double | 333 | 101 | 37.3 |
| Three species | 333 | 93 | 34.3 |
Fig. 1.
a. Infestation pattern stratified by sex of the dogs. b. Infestation pattern stratified by age of the dogs.
Table 2 shows that infestation due to R. sanguineus s.l. was the most prevalent (70.3%) followed by C. felis (42.1%) and H. spiniger (45.3%). Age and sex-related pattern of infestation showed that R. sanguineus s.l. was most common in female dogs (80.6%) while H. spiniger and C. canis were comparably higher in young than the ≥12 month's old dogs (Table 3). Similarly, ticks and fleas were more frequent in brown dogs while lice were most frequent on black dogs (Table 4).
Table 2.
Prevalence and intensity of ectoparasite species stratified by sex of dogs.
| Parasites | Total infested dogs (%) | Male prevalence (%) [x ± SD) [95% CI) |
Female prevalence (%) [x ± SD) [95% CI) |
p value |
|---|---|---|---|---|
| R. sanguineus | 234 [70.3) | 59.5 [8.96 ± 13.4) [6.89–11.04) |
80.6 [18.72 ± 26.5) [14.71–22.73) |
<0.001 |
| H. leachii | 98 [29.4) | 26.4 [2.52 ± 7.5) [1.36–3.67) |
32.4 [3.59 ± 8.0) [2.38–4.81) |
0.205 |
| A. variagatum | 15 [4.5) | 4.3 [0.15 ± 0.84) [0.2–0.28) |
4.7 [0.15 ± 0.83) [0.3–0.28) |
0.996 |
| C. canis | 72 [21.6) | 17.2 [1.81 ± 5.02) [0.09–2.98) |
25.9 [3.10 ± 2.63) [2.51–3.89) |
0.037 |
| C. felis | 140 (42.1) | 25.8 (5.13 ± 2.02) (2.95–6.01) |
57.6 (11.41 ± 5.31) (8.17–14.22) |
0.049 |
| H. spiniger | 100 [30.0) | 14.1 [1.02 ± 3.48) [0.49–1.56) |
45.3 [4.22 ± 7.32) [3.11–5.33) |
<0.001 |
[x ± SD) = Mean intensity of the parasite ± standard deviation, CI = confidence interval of the mean, Prevalence are in %.
Table 3.
Prevalence and intensity of ectoparasite species stratified by age of dogs.
| Parasites | 0–6 months Prevalence (%) [x ± SD) [95% CI) |
7–11 months Prevalence (%) [x ± SD) [95% CI) |
≥12 months Prevalence (%) [x ± SD) [95% CI) |
p value |
|---|---|---|---|---|
| R. sanguineus | 70.8 [16.00 ± 25.01) [10.64–21.22) |
64.8 [13.30 ± 23.12) [7.78–18.73) |
72.3 [13.20 ± 19.11) [10.33–16.06) |
0.594 |
| H. leachii | 32.6 [4.34 ± 10.22) [2.18–6.49) |
31.0 [3.10 ± 7.19) [1.38–4.79) |
27.2 [2.40 ± 6.35) [1.45–3.36) |
0.161 |
| A. variagatum | 4.5 [0.19 ± 1.01) [−0.02–0.40) |
4.2 [0.11 ± 0.60) [−0.3–0.25) |
4.6 [0.15 ± 0.81) [0.03–0.27) |
0.838 |
| C. canis | 56.2 [7.14 ± 3.10) [2.06–8.00) |
56.4 [5.38 ± 4.04) [2.04–6.31) |
28.0 [4.18 ± 1.37) [1.83–6.16) |
0.045 |
| C. felis | 28.1 (2.56 ± 1.09) (0.56–3.11) |
28.2 (1.37 ± 0.78) (0.91–1.56) |
15.6 (1.37 ± 0.78) (0.91–1.56) |
0.312 |
| H. spiniger | 30.3 [4.13 ± 8.44) [2.36–5.91) |
35.2 [1.96 ± 3.49) [1.13–2.79) |
27.7 [2.18 ± 5.11) [1.41–2.95) |
0.023 |
[x ± SD) = Mean intensity of the parasite ± standard deviation, CI = confidence interval of the mean, Prevalence are in %.
Table 4.
Stratification of ectoparasite species stratified by coat colour and breed of dogs.
| Parasites | Coat colour of dogs |
Breeds of dogs |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Black | Brown | White | Mixed | p value | Local | Hybrid | Exotic | p value | |
| R. sanguineus | 68.2 | 78.8 | 59.1 | 65.3 | 0.042 | 82.2 | 56.9 | 2.9 | <0.001 |
| H. leachii | 22.7 | 40.7 | 27.3 | 22.7 | 0.221 | 35.7 | 17.2 | 1.9 | 0.002 |
| A. variagatum | 0.0 | 6.2 | 4.5 | 4.0 | 0.542 | 6.2 | 0.0 | 0.0 | 0.115 |
| Ctenocephalides spp. | 59.1 | 67.3 | 59.1 | 62.5 | <0.001 | 75.5 | 46.6 | 6.8 | <0.001 |
| H. spiniger | 59.1 | 25.7 | 4.5 | 32.4 | <0.001 | 37.8 | 10.3 | 8.3 | <0.001 |
All values are in % except p values.
The knowledge of dogs' owners on dog management are presented in Table 5. The majority (88.5%) of the respondents observed ectoparasite infestation in dogs and over 95% identified ectoparasites on their children. However, infestation was lowest (10.0%) in frequently bathed dogs and highest in never treated dogs (100%).
Table 5.
Infestation with respect to owners' understanding and management practices of the infestation.
| Variables | Respondents (%) | No of dogs infested/exam | Percentage | 95% confident interval | p value |
|---|---|---|---|---|---|
| Have you noticed any ectoparasite on your dog? | <0.001 | ||||
| Yes | 106 (67.5) | 253/286 | 88.5 | 84.0–91.8 | |
| No | 51 (32.5) | 18/47 | 38.3 | 24.9–53.6 | |
| Have you found any of these ectoparasites on your children? | <0.025 | ||||
| Yes | 42 (26.0) | 66/69 | 95.7 | 87.0–98.9 | |
| No | 115 (73.2) | 205/264 | 77.7 | 72.0–82.4 | |
| How often do you bath your dog? | <0.031 | ||||
| Once in a week | 23 (14.6) | 44/82 | 53.7 | 42.4–64.6 | |
| Twice in a week | 13 (8.3) | 1/10 | 10.0 | 0.5–45.9 | |
| Monthly | 57 (36.3) | 49/43 | 67.4 | 51.3–80.5 | |
| Never | 64 (40.7) | 197/198 | 99.5 | 96.8–100.0 | |
| How often do you treat your dog? | <0.001 | ||||
| <12 months ago | 34 (21.7) | 33/78 | 42.3 | 31.4–54.0 | |
| ≥12 months ago | 35 (22.3) | 35/52 | 67.3 | 52.7–79.3 | |
| Never | 88 (56.1) | 203/203 | 100 | 97.7–100.0 | |
| Perception of diseases transmitted | <0.001 | ||||
| Serious | 14 (8.9) | 41/68 | 60.3 | 47.7–71.7 | |
| Not serious | 48 (30.6) | 73/89 | 82.0 | 72.1–89.1 | |
| Do not cause any disease | 95 (60.5) | 157/176 | 89.2 | 83.4–93.2 |
4. Discussion
The point prevalence of 81.4% obtained in this current epidemiological survey revealed that ectoparasitic infestation is a serious health problem among the owned dogs in the study area. This is comparably higher than some previous reports (Adamu et al., 2012; Amuta et al., 2010; Ugbomoiko et al., 2008), but consistent with 70.7% obtained in Brazil (Dantas-Torres et al., 2009), 88.6% in Ethiopia (Tesfaye and Chanie, 2011) and 98.5% in southwestern Nigeria (Agbolade et al., 2008). The geographical variation in infestation rates may suggests the importance of several climatic factors (temperature, humidity, light) necessary for development and transmission, host intrinsic resistance (Nayak et al., 1997) as well as the sampling techniques adopted.
Earlier reports showed that Nigerian dogs are commonly infested with Xenopsylla cheopis, Pulex irritans, Tunga penetrans, R. sanguineus s.l., and species of Amblyomma, Ixodes, Otodectes, Demodex, and Sarcoptes scabiei var. canis (Agbolade et al., 2008; Ugbomoiko et al., 2008). In this study, six species of ectoparasites were recorded in dog and A. variegatum, H. leachii and H. spinigeri were reported for the first time in the study area. However, all of these species have been observed in dogs from other geographical regions (Mosallanejad et al., 2012; Nuchjangreed and Somprasong, 2007). Of all the ectoparasites observed in the current study, R. sanguineus s.l. and Ctenocephalides felis were the most prevalent, indicating their wide habitat preference in relation to their hosts. Ectoparasite-transmitted zoonotic pathogens in dogs and related domestic pets have been reported by several authors (Azzag et al., 2015; Bessas et al., 2016; Malheiros et al., 2016; Low et al., 2017; Figueredo et al., 2017). Rhipicephalus sanguineus s.l. is reported to induce allergic inflammation and hypersensitivity reactions in humans (Heukelbach et al., 2012; Kotál et al., 2015; Nuchjangreed and Somprasong, 2007). Also, Dantas-Torres et al. (2010) and Troyo et al. (2012) reported that R. sanguineus s.l. transmits canine leishmaniosis and Ehrlicliosis. Similarly, chewing lice (H. spiniger) in infested dog may cause pruriginous skin reactions and stress while the fleas (Ctenocephalides species) act as an intermediate hosts for Dipylidium caninum, the limited knowledge of dog owners (60.5%) on transmissible diseases of dog is for concern and simply indicates a raising risk of exposure to zoonotic ectoparasites and the associated pathogens.
In this study, multiple infestation is described commonly among the female dogs. This may have been substantially influence by the dogs' living conditions and the number of other associated dogs/pets in the household. This finding, however, largely agreed with the earlier reports from Nigeria (Agbolade et al., 2008; Ugbomoiko et al., 2008) and other endemic communities in tropical countries (Bahrami et al., 2012; Mosallanejad et al., 2012; Nuchjangreed and Somprasong, 2007). Most importantly, prevalence and intensity of the infestation due to R. sanguineus s.l, H. spiniger and C. canis are significantly higher in female dogs which is similar with the findings of Agbolade et al. (2008), Arong et al. (2011), Pereira et al. (2016) and Tesfaye and Chanie (2011). This may probably due to hormonal changes during reproduction and sedentary habits female dogs often engage in while nursing that favour re-infestation as previously reported by Dantas-Torres et al. (2010).
This study also revealed that, the intensity of infestation was higher in puppies (0–6 yrs. old) which may be due to gradual acquisition of immunity and close proximity of the young dogs to the ground. This observation substantiates the report of Mosallanejad et al. (2012). We observed similar high infestation among the local breed dogs, most local breed encountered in the study area were free-ranged and this management practice exposes pets to maximum parasitic infections. This corroborates the views of earlier workers in South Africa (Bryson et al., 2000) and in Nigeria (Nwoke, 2001).
It is observed in this study that most dogs' owners could identify the presence of ectoparasite infestation in dogs but remained unaware of the health implication for both the animal and people around the animals. Therefore, majority of dog owners neither bathed nor applied treatment to control ectoparasite infestation, a behaviour which may partly be responsible for the high prevalence of infestation in dogs in the locality. This study therefore advocates for the increase dogs' owners' awareness on the public health implication of the ectoparasitic infestation in dogs.
This study, however, had limitations that relate to confirmatory evidence of zoonosis. First, none of the ectoparasites encountered was microbiologically confirmed to harbor infectious agents (virus, bacteria and parasites) to validate the zoonotic potential of parasite and the hosts. Secondly, limited number of dogs examined could conceivably affect our data.
5. Conclusions
This study revealed that majority of dogs in the study area habour ectoparasites of public health importance. The necessity for integrated multidisciplinary approach involving veterinary and medical personels as well as public health officials must be prioritized by the Government for the implementation of appropriate intervention measures (through health education and provision of affordable veterinary clinics) to reduce the risk of zoonotic transmission.
References
- Adamu N., Adamu J., Salisu L. Prevalence of ecto-, endo-and haemoparasites in slaughtered dogsin Maiduguri, Nigeria. Rev. Med. Vet. 2012;163(4):178–182. [Google Scholar]
- Agbolade O., Soetan E., Awesu A., Ojo J., Somoye O., Raufu S. Ectoparasites of domestic dogs in some Ijebu communities, Southwest Nigeria. World Appl. Sci. J. 2008;3(6):916–920. [Google Scholar]
- Alcaíno H.A., Gorman T.R., Alcaíno R. Flea species from dogs in three cities of Chile. Vet. Parasitol. 2002;105(3):261–265. doi: 10.1016/s0304-4017(01)00626-4. [DOI] [PubMed] [Google Scholar]
- Alvarado-Esquivel C., Romero-Salas D., Aguilar-Domínguez M., Cruz-Romero A., Ibarra-Priego N., Pérez-de-León A.Á. Epidemiological assessment of intestinal parasitic infections in dogs at animal shelter in Veracruz, Mexico. Asian Pac. J. Trop. Biomed. 2015;5(1):34–39. [Google Scholar]
- Amuta E., Atu B., Houmsou R., Ayashar J. Rhipicephalus sanguineus infestation and Babesia canis infection among domestic dogs in Makurdi, Benue state-Nigeria. Int. J. Acad. Res. 2010;2(3) [Google Scholar]
- Arong G., Shitta K., James-Rugu N., Effanga E. Seasonal variation in the abundance and distribution of ixodid ticks on mongrel, alsatian and mixed breeds of dogs (canis familiaris) in jos, in Plateau state, North-central Nigeria. World J. Sci. Tech. 2011;1(4):24–29. [Google Scholar]
- Azzag N., Petit E., Gandoin C., Bouillin C., Ghalmi F., Haddad N., Boulouis H.J. Prevalence of select vector-borne pathogens in stray andclient-owned dogs from Algiers. Comp. Immunol. Microbiol. Infect. Dis. 2015;38:1–7. doi: 10.1016/j.cimid.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Bahrami A.M., Doosti A., Ahmady_Asbchin S. Cat and dogs ectoparasite infestations in Iran and Iraq boarder line area. World Appl. Sci. J. 2012;18(7):884–889. [Google Scholar]
- Bessas A., Leulmi H., Bitam I., Zaidi S. Molecular evidence of vector-borne pathogens in dogs and cats and their ectoparasites in Algiers, Algeria. Comp. Immunol. Microbiol. Infect. Dis. 2016;45:23–28. doi: 10.1016/j.cimid.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Bryson N., Horak I., Hohn E., Louw J. Ectoparasites of dogs belonging to people in resource-poor communities in North West Province, South Africa. J. S. Afr. Vet. Assoc. 2000;71(3):175–179. doi: 10.4102/jsava.v71i3.709. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Melo M.F., Figueredo L.A., Brandão-Filho S.P. Ectoparasite infestation on rural dogs in the municipality of São Vicente Férrer, Pernambuco, Northeastern Brazil. Rev. Bras. Parasitol. Vet. 2009;18(3):75–77. doi: 10.4322/rbpv.01803014. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Lorusso V., Testini G., de Paiva-Cavalcanti M., Figueredo L.A., Stanneck D., Mencke N., Brandão-Filho S.P., Alves L.C., Otranto D. Detection of Leishmania infantum in Rhipicephalus sanguineus ticks from Brazil and Italy. Parasitol. Res. 2010;106(4):857–860. doi: 10.1007/s00436-010-1722-4. [DOI] [PubMed] [Google Scholar]
- Estrada-peña A., Venzal J.M. Climate niches of tick species in the Mediterranean region: modeling of occurrence data, distributional constraints, and impact of climate change. J. Med. Entomol. 2007;44(6):1130–1138. doi: 10.1603/0022-2585(2007)44[1130:cnotsi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Figueredo L.A., Sales K.G.S., Deuster K., Pollmeier M., Otranto D., Dantas-Torres F. Exposure to vector-borne pathogens in privately owned dogs living in different socioeconomic settings in Brazil. Vet. Parasitol. 2017;243:18–23. doi: 10.1016/j.vetpar.2017.05.020. [DOI] [PubMed] [Google Scholar]
- González A., del C Castro D., González S. Ectoparasitic species from Canis familiaris (Linné) in Buenos Aires province, Argentina. Vet. Parasitol. 2004;120(1–2):123–129. doi: 10.1016/j.vetpar.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Heukelbach J., Frank R., Ariza L., de Sousa Lopes Í., e Silva A.d.A., Borges A.C., Limongi J.E., de Alencar C.H.M., Klimpel S. High prevalence of intestinal infections and ectoparasites in dogs, Minas Gerais State (southeast Brazil) Parasitol. Res. 2012;111(5):1913–1921. doi: 10.1007/s00436-012-3037-0. [DOI] [PubMed] [Google Scholar]
- Klimpel S., Heukelbach J., Pothmann D., Rückert S. Gastrointestinal and ectoparasites from urban stray dogs in Fortaleza (Brazil): high infection risk for humans? Parasitol. Res. 2010;107(3):713–719. doi: 10.1007/s00436-010-1926-7. [DOI] [PubMed] [Google Scholar]
- Kotál J., Langhansová H., Lieskovská J., Andersen J.F., Francischetti I.M., Chavakis T., Kopecký J., Pedra J.H., Kotsyfakis M., Chmelař J. Modulation of host immunity by tick saliva. J. Proteome. 2015;128:58–68. doi: 10.1016/j.jprot.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low V.L., Prakash B.K., Tan T.K., Sofian-Azirun M., Anwar F.H.K., Vinnie-Siow W.Y., AbuBakar S. Pathogens in ectoparasites from free-ranging animals: infection with Rickettsia asembonensis in ticks, and a potentially new species of Dipylidium in fleas and lice. Vet. Parasitol. 2017;245:102–105. doi: 10.1016/j.vetpar.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Malheiros P.S., Cuccovia I.M., Franco B. Inhibition of Listeria monocytogenes in vitro and in goat milk by liposomal nanovesicles containing bacteriocins produced by Lactobacillus sakei subsp sakei 2a. Food Control. 2016;63:158–164. [Google Scholar]
- Mathison B.A., Pritt B.S. Laboratory identification of arthropod ectoparasites. Clin. Microbiol. Rev. 2014;27(1):48–67. doi: 10.1128/CMR.00008-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosallanejad B., Alborzi A., Katvandi N. A survey on ectoparasite infestations in companion dogs of Ahvaz district, south-west of Iran. J. Arthropod. Borne Dis. 2012;6(1):70. [PMC free article] [PubMed] [Google Scholar]
- National Population Census 2006. https://www.nigerianmuse.com/20070820063612zg/sections/important-documents/nigeria-2006-population-census-arranged-by-state
- Nayak D., Tripathy S., Dey P., Ray S., Mohanty D., Parida G., Biswal S., Das M. Prevalence of canine demodicosis in Orissa (India) Vet. Parasitol. 1997;73(3–4):347–352. doi: 10.1016/s0304-4017(97)00125-8. [DOI] [PubMed] [Google Scholar]
- Nuchjangreed C., Somprasong W. Ectoparasite species found on domestic dogs from Pattaya district, Chon Buri province, Thailand. Southeast Asian J. Trop. Med. Public Health. 2007;38:203. [Google Scholar]
- Nwoke B. Urbanization and livestock handling and farming: the public health and parasitological implications. Niger. J. Parasitol. 2001;22(1):121–128. [Google Scholar]
- Pereira A., Martins Â., Brancal H., Vilhena H., Silva P., Pimenta P., Diz-Lopes D., Neves N., Coimbra M., Alves A.C. Parasitic zoonoses associated with dogs and cats: a survey of Portuguese pet owners' awareness and deworming practices. Parasit. Vectors. 2016;9(1):245. doi: 10.1186/s13071-016-1533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson I., Irwin P., Lymbery A., Thompson R. The role of companion animals in the emergence of parasitic zoonoses. Int. J. Parasitol. 2000;30(12−13):1369–1377. doi: 10.1016/s0020-7519(00)00134-x. [DOI] [PubMed] [Google Scholar]
- Tesfaye A., Chanie M. Ectoparasites are major skin diseases of dogs in Gondar, Amhara National Regional State, Ethiopia. Int. J. Anim. Vet. Adv. 2011;3(5):392–396. [Google Scholar]
- Troyo A., Calderon-Arguedas O., Alvarado G., Vargas-Castro L.E., Avendano A. Ectoparasites of dogs in home environments on the Caribbean slope of Costa Rica. Rev. Bras. Parasitol. Vet. 2012;21:179–183. doi: 10.1590/s1984-29612012000200021. [DOI] [PubMed] [Google Scholar]
- Ugbomoiko U.S., Ariza L., Heukelbach J. Parasites of importance for human health in Nigerian dogs: high prevalence and limited knowledge of pet owners. BMC Vet. Res. 2008;4(1):49. doi: 10.1186/1746-6148-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker A.R. Bioscience Reports Edinburgh. 2003. Ticks of domestic animals in Africa: a guide to identification of species. [Google Scholar]