Abstract
The taeniosis/cysticercosis neglected zoonotic disease complex is caused by Taenia solium, and is associated with significant economic and public health impacts. This paper reviews the current knowledge on T. solium in Zambia and the control strategies already studied, covering almost 20 years of research, and explores the way forward. Studies on occurrence of porcine cysticercosis indicated very high prevalences, ranging from 15 to 34% based on detection of circulating antigens, and of 46% to 68% based on full carcass dissection in slaughter age pigs. Taeniosis prevalences have been reported to range from 6.3% to 12% based on copro-Ag-ELISA. Human cysticercosis prevalence results ranged from 5.8% to 13% based on serum Ag-ELISA, and from 34% to 39% based on sero-antibody detection. Later on, a study in people with epilepsy suggested neurocysticercosis to be the single most important cause of epilepsy in this T. solium endemic area, with 57% of the people with active epilepsy diagnosed with probable or definite neurocysticercosis. While the need to reduce the disease burden of T. solium in Zambia is obvious, the exact short and long term goals, and the strategies to achieve these goals, are not clear. We have selected the most promising control/elimination strategies from reviews and assessed these for feasibility via discussions with local stakeholders from both medical and veterinary sectors. The proposed measures were evaluated using the newly developed agent-based disease transmission model, cystiSim and optimised using Zambian demographic and disease data. As a control option, yearly porcine treatments were selected as best option, while the preferred strategy for elimination was determined to be the combination of human and porcine mass drug administration combined with porcine vaccination of all eligible people and pigs, in a schedule of six iterations of four monthly interventions. These interventions are currently being field tested, combined with education. Several other hurdles to control, such as cost and socio-political factors and the need for an improved advocacy and awareness creation are discussed.
Keywords: Taenia solium, Control, Elimination, Zambia, CystiSim
1. Taenia solium
Taeniosis/cysticercosis is a neglected zoonotic disease complex caused by Taenia solium. This parasite is associated with significant economic and public health impacts and was ranked first on the global scale of foodborne parasites by FAO (Food and Agriculture Organisation of the United Nations, FAO/WHO, 2014). It affects mostly developing countries in Latin America, sub-Saharan Africa, South and South East Asia where poverty, lack of sanitation and free-range pig management is prevalent (Murrell et al., 2005).
As the definitive host, man is the carrier of the adult tapeworm (taeniosis, TS, in the human intestines), and releases infective eggs via the stool into the environment. When the normal intermediate pig host ingests these eggs via its coprophagic behaviour or contaminated food/water, the metacestode larval stage (cysticercus) develops in muscle, subcutaneous, and other tissues, resulting in cysticercosis (Murrell et al., 2005). Porcine cysticercosis (PCC) is responsible for meat deterioration and carcass condemnation, and human consumption of undercooked infected pork leads to the development of the tapeworm (TS). PCC has been long described as asymptomatic, though recent research has highlighted the occurrence of seizures in infected pigs (Trevisan et al., 2016). Taeniosis usually leads to no or mild symptoms, primarily abdominal complaints (Murrell et al., 2005). However, people can also act as accidental dead-end intermediate hosts and develop cysticercosis (HCC). In humans, the cysticerci may lodge in the central nervous tissue (neurocysticercosis, NCC), causing various neurological symptoms, the most common are seizures, epilepsy and chronic headache (Murrell et al., 2005). NCC is estimated to be responsible for 30% of cases of acquired epilepsy in endemic areas (Ndimubanzi et al., 2010).
Lack of good quality data on T. solium infections has hindered estimating the exact public health and economic impacts of this zoonosis. Recently, the global disease burden of T. solium has been estimated at 2.8 million Disability-Adjusted Life Years (DALYs), with a mortality rate of approximately 28,000 deaths per year (Havelaar et al., 2015; Torgerson et al., 2015). An increasing interest, especially from the research community, has led to more data becoming available, indeed revealing striking results on occurrence and impact of this parasite, calling for urgent action to control this neglected zoonosis. In the WHO roadmap on neglected tropical diseases, scaling up of interventions to control T. solium by 2020 is emphasised (World Health Organisation, 2012). This need was also recognised and re-confirmed by the World Health Assembly with the adoption of resolution WHA66.12 on May 23, 2013 (World Health Organisation, 2015).
This paper reviews the current knowledge on T. solium in Zambia and the control strategies already studied, covering almost 20 years of research, and explores the way forward.
2. Taenia solium in Zambia: what do we know?
Zambia is a landlocked country located in the southern part of Africa bordered by eight countries and divided into ten provinces. According to the Food and Agricultural Organization (www.fao.org), Zambia's pig population rose from 308,872 in the year 2000 to 715,000 in 2010, and 1,374,137 in 2016. The Eastern and Southern Provinces account for over 50% of this increase in pig numbers. Most of these pigs are kept under smallholder conditions in rural areas, with free roaming pigs. This management system is preferred by the local communities, primarily for socio-economic reasons. Pigs are often kept as a transitory activity, when financial needs occur. Pig keeping is considered as a flexible, low input, fast output system (as pigs are fast growing, multiparous animals and easy to rear), which does not allow for the costs related to feed, corral construction and time which enclosing pigs would entail (Thys et al., 2016).
Despite efforts of large scale sanitation programmes, most rural households do not (always) use latrines, for varying reasons. Especially men, demonstrated reluctance to build and use latrines explained by privacy issues and toilet associated taboos (Thys et al., 2015). The lack of proper sanitation combined with free roaming pigs, offers the animals free access to human stool, potentially contaminated with tapeworm eggs.
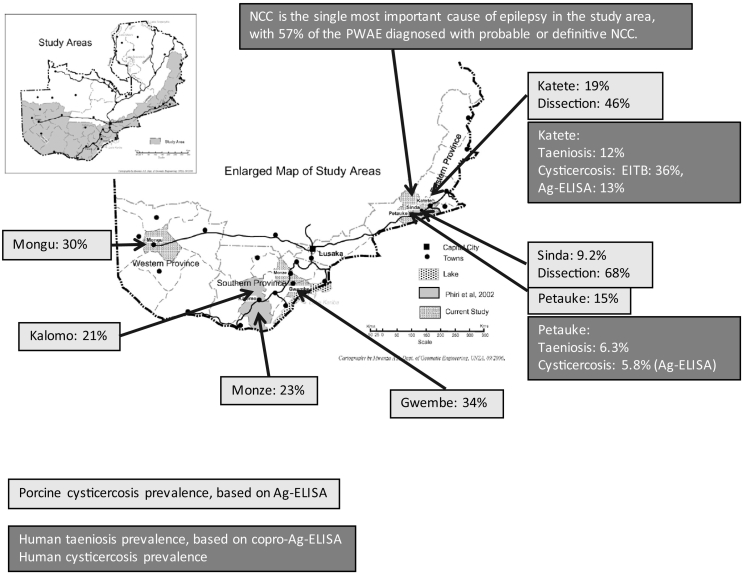
With the increase in smallholder pig keeping came reports of cysticerci being observed in pigs slaughtered in backyards without controlled meat inspection (Phiri et al., 2003). Subsequent studies carried out in pigs in six districts of three provinces of the country namely, Eastern, Southern and Western Provinces (Fig. 1) indicated very high prevalences of porcine cysticercosis, ranging from 15 to 34% based on detection of circulating antigens (Ag-ELISA, B158/B60 monoclonal based antigen detecting ELISA) (Phiri et al., 2002; Sikasunge et al., 2008a) while in Katete and Sinda districts (Eastern Province) prevalences in slaughter age pigs of 46% and 68%, respectively were detected based on full carcass dissection, the generally accepted gold standard detection technique (Chembensofu et al., 2017). A study conducted at a slaughter slab in Lusaka, determined a prevalence of porcine cysticercosis of 64%, based on a Bayesian approach including multiple diagnostic techniques (Dorny et al., 2004).
Fig. 1.
Map of Zambia showing districts were human/porcine cysticercosis occurrence has been determined (adapted from Sikasunge et al., 2008a, Sikasunge et al., 2008b, with additional information based on Mwape et al., 2012, Mwape et al., 2013, Mwape et al., 2015a, Mwape et al., 2015b; Phiri et al., 2002; Chembensofu et al., 2017). NCC: neurocysticercosis, PWAE: people with active epilepsy, EITB: enzyme-linked immunoelectrotransfer blot, Ag-ELISA: antigen detecting enzyme-linked immunosorbent assay.
Following the striking results on occurrence of PCC, studies investigating the occurrence in people were conducted, mostly in the Eastern Province, in which the high endemicity was again confirmed (Fig. 1). Taeniosis prevalences have been reported to range from 6.3% (45/718) to 12% (27/226) based on copro-Ag-ELISA, while human cysticercosis prevalence results ranged from 5.8% (41/708) to 13% (141/1129) based on serum Ag-ELISA (Mwape et al., 2012; Mwape et al., 2013). Human cysticercosis seroprevalences based on sero-antibody (Ab) detection (with the Lentil Lectin bound Glycoprotein enzyme-linked immunoelectrotransfer blot) ranged from 34% (55/161) to 39% (63/161), as determined in a longitudinal study conducted in the Eastern Province. In that study, HCC incidence rates of 6300 (sero-Ag, per 100,000 persons-year) and 23,600 (sero-Ab) were obtained, with many people becoming re-infected during the study period, indicating a high environmental contamination (Mwape et al., 2013). Subsequently, a study examining the role of NCC as a cause of epilepsy was conducted. In that study, test results including computed tomography (CT), specific antibody and antigen detection in serum, in-depth interviews and neurological examinations from 49 people with active epilepsy (PWAE) were compared to a group of 40 CT scan-negative controls. Based on the Del Brutto diagnostic criteria (Del Brutto et al., 2001), 52% of the PWAE were diagnosed with probable or definite NCC. When applying the adapted criteria as proposed by Gabriël et al. (2012), including serum Ag-ELISA results, a proportion of 57% was obtained (Mwape et al., 2015a). That study suggested NCC to be the single most important cause of epilepsy in this T. solium endemic area.
Studies on risk factors associated with T. solium infections in both pigs and people have also been carried out in Zambia and have highlighted the importance of free-range pigs and absence of latrines in maintaining the infection in pigs (Sikasunge et al., 2007). Another study used non-parametric methods (classification and regression tree model, CART) to rank the risk factors in order of importance and highlighted the fact that a risk factor determined to be very important in one area might not be ranked as high in another area. The authors therefore suggested that control options may have to be area specific (Mwape et al., 2015b). That study further identified risk factors, such as the number of household members, not determined to be significant in more conventional risk factor studies, while vice versa, commonly mentioned factors, such as open defecation and free roaming pigs did not appear important in the CART analyses. While there are a lot of similarities in occurrence of some risk factors such as open defecation and free roaming pigs (Phiri et al., 2003), research conducted in Zambia characterised the high endemicity, with often very high prevalences, compared for example to Kenya (with sero-Ag prevalence of human cysticercosis of 8.07%) or Burkina Faso (with sero-Ag prevalence of human cysticercosis of 6.13%) as reviewed by Coral -Almeida and colleagues (Coral-Almeida et al., 2015).
The actual disease burden and economic impact of T. solium in Zambia still needs to be determined. A recent study by Hobbs et al. (2018a) has highlighted some preliminary data based on human health questionnaires (n = 267) and pig socio economic questionnaires (n = 271) implemented in the Eastern Province. Sixty-two percent of the respondents of the human health questionnaire had reportedly experienced some of the surveyed symptoms typically associated with NCC (e.g. seizures-like episodes 12%, severe chronic headaches 36%, vision problems 23% of respondents) during the last five years, resulting in 147 health care visits and 17 hospitalisations. The productivity losses were estimated at 608 working days per year for the 62 questionnaire respondents with symptoms. The value of infected pigs decreased with 55%, and when the animal was determined infected before the sales by tongue palpation, it could not be sold in 95% of the cases. Though preliminary, these data indicate a potentially substantial impact of T. solium on the area.
3. Need for control: which potholed road to choose?
3.1. Control versus elimination
While the need to reduce the disease burden of T. solium in Zambia is obvious, the exact short and long term goals, and the strategies to achieve these goals, are not clear. Whether the goal is to control (defined as: the reduction of disease incidence, prevalence, morbidity or mortality to a locally acceptable level as a result of deliberate efforts (Dowdle, 1999)) or eliminate the disease (defined as: the reduction to zero of the incidence of a specified disease in a defined geographical area as a result of deliberate efforts (Dowdle, 1999)) needs to be decided. Elimination and eradication are the final goals of public health, evolving from disease control. One of the basic questions is whether these goals are to be achieved in the short or long term (future generation(s)) (Dowdle, 1999). To achieve elimination, three important criteria need to be present: the availability of an effective intervention to interrupt transmission, the availability of diagnostic tools that are capable of identifying levels of infection leading to transmission (high sensitivity and specificity), and the fact that humans are essential for the life cycle of the pathogen, with no other vertebrate reservoir or environmental amplification. For T. solium the first criterion is fulfilled, the second is debatable, while the last criterion is clearly not fulfilled, as there is a pig intermediate host and an important role for environmental contamination. Still, T. solium cysticercosis has been considered a potentially eradicable disease (Schantz et al., 1993). Indeed, T. solium disappeared in most European countries as hygienic and sanitary conditions improved, and (indoor) pig husbandry and rigorous meat inspection were implemented (Schantz, 2006). Recent results from endemic areas in Peru and Ecuador further indicate that (active) elimination can be achieved there as well, with interruption of disease transmission (Garcia et al., 2016; Del Brutto et al., 2018). In more resource poor countries, however, the situation remains complicated. These countries often lack the necessary financial resources and political, social and economic structures to set up and maintain an elimination program (Assana et al., 2013), moreover in many of these countries, T. solium is not a priority disease. So although technically possible, the question is whether governments would be able to dedicate sufficient resources to actually implement T. solium control. For sub-Saharan Africa, the higher level of endemicity is an additional complicating factor. Aiming for control and reduction of disease burden might therefore be a more feasible approach.
3.2. Which intervention to choose?
Control options for T. solium can be broadly grouped in strategies targeting the definitive host (human) and strategies targeting the pig intermediate host. Measures targeting the definitive host include anthelmintic treatment of human tapeworm carriers, health education and improved sanitation. Treatment of human carriers can be implemented as a mass drug administration (MDA) or based on a selective or targeted treatment scheme (TT).
Measures aimed at the intermediate host include improved standards of meat inspection, including tongue palpation, changes in pig management, and anthelmintic treatment and/or vaccination of pigs.
A number of reviews have been conducted to determine which tools are currently available and suitable for T. solium control in sub Saharan Africa (SSA) (Gabriël et al., 2017; Thomas, 2015; de Coster et al., 2018), these will as such not be repeated in depth here.
In Zambia, a first study aiming to control T. solium infections was based on the Community Led Total Sanitation (CLTS, www.communityledtotalsanitation.org) approach, in which communities are triggered to construct latrines and stop open defecation, without subsidies. Improved sanitation would have an additional beneficial impact on many other sanitation-related pathogens, making this approach a very appealing option. While the implementation of CLTS indeed led to the start of latrine construction in the 11 intervention villages in the Eastern Province of Zambia, variations between villages were high and complete latrine coverage was not observed. This was corroborated by a lack of significant reduction in disease occurrence in pigs in intervention villages and a failure to improve the behavioural practices of village inhabitants after eight months post-implementation (Bulaya et al., 2015). Factors contributing to households not constructing latrines were primarily socio-economic, such as lack of financial means and cultural taboos related to toilet use.
In general, the few reports available describing the evaluation of control programmes in SSA have been focussed on individual control options. It is becoming clear that these stand-alone options have the potential to reduce the occurrence of the parasite, however either long term or more integrated efforts, aiming at both hosts, seem to be required to reach an elimination status (de Coster et al., 2018). Globally, there has been only one large scale T. solium elimination study, carried out by the Cysticercosis Working Group in Peru, in which all main control possibilities were included (MDA in the human definitive host, mass treatment and vaccination of the pig intermediate host, health education, sanitation), and in which elimination was achieved (Garcia et al., 2016) (evaluated by the lack of infective pigs in 105 of the 107 intervention villages). Recent epidemiological insights indicate a situation of low endemicity in South America, characterised by low infection incidences and prevalences and less severe clinical disease. In SSA, the situation is very different. The higher endemicity is characterised by higher infection prevalence (both in definitive and intermediate hosts) and more severe disease outcomes (Coral-Almeida et al., 2014). This should be taken into consideration when designing strategies suitable for SSA.
3.3. Application of cystiSim
Specifically for Zambia, we have selected the most promising strategies from the reviews and assessed these for feasibility via discussions with local stakeholders from both medical and veterinary sectors. The proposed measures were evaluated using the newly developed agent-based disease transmission model, cystiSim (http://cran.r-project.org/package=cystiSim), developed in the statistical programme language R as described by Braae et al. (2016a), and optimised using Zambian demographic and disease data (Fig. 1). In short, cystiSim calculates the transmission parameters that correspond to an observed endemic situation of T. solium, and evaluates the potential impact of intervention tools. It consists of human and pig entities, and includes parasite maturation, host immunity, and environmental contamination. Outputs of the model include prevalence of taeniosis, porcine cysticercosis and proportion of resistant pigs (due to vaccination, or assumed three months' resistance of previously infected pigs after treatment (Gonzalez et al., 2001)). With the model, the most optimal type of strategy, number of interventions needed and intervals between interventions were determined. Options targeting disease control, envisaging a lower input/investment spread over multiple years, aiming for long term results; were compared to options targeting elimination of transmission, based on high input, short term, intensive interventions with results expected in the short term. For example, the interventions aimed at control should be a low investment system (financial, labour etc.), less demanding to the system, meaning that although six-monthly interventions would achieve better and faster outcomes, it is not an appropriate strategy for that system, and as such not a feasible control option.
The modelled elimination and control interventions were ranked based on the highest probability that elimination would be achieved (on the short term for elimination, on the long term for control) (Table 1). Health education was not assessed in the model, as we had no data to define its efficacy. Improving meat inspection at official slaughterhouses was not considered either, as it is rather ineffective as firstly the sensitivity of the technique is very poor, especially for light infections (Dorny et al., 2004), and secondly, in rural areas most pigs are not slaughtered in these places (Gilman et al., 2012). Improving pig husbandry by raising pigs in confinement (Lekule and Kyvsgaard, 2003; Mkupasi et al., 2013) has had a mixed success of uptake, especially in rural resource-poor communities where scavenging behaviour is a desirable nutritional benefit of keeping pigs under free-range conditions (Thys et al., 2016). It was therefore also discarded as an intervention strategy.
Table 1.
Description of intervention options, eligible groups, expected coverage and efficacy and probability of elimination.
| Intervention | Age group |
Coveragea |
Efficacy |
Prelim PCC, HT | |||
|---|---|---|---|---|---|---|---|
| Human | Pig | Human | Pig | Human | Pig | ||
| Control | |||||||
| TT 12q12 | ≥5 years | NA | 85% | NA | 95% | NA | 0.00 |
| MDA 12q12 | ≥5 years | NA | 85% | NA | 95% | NA | 0.01 |
| OXF 12q12 | NA | ≥2 months | NA | 90% | NA | 100% | 0.75 |
| MDA 24q6 | ≥5 years | NA | 85% | NA | 95% | NA | 0.86 |
| OXF 24q6 | NA | ≥2 months | NA | 90% | NA | 100% | 1.00 |
| Elimination | |||||||
| OXF 6q4 | NA | ≥2 months | NA | 90% | NA | 100% | 0.31 |
| OXF + VACC 6q4 | NA | ≥2 months | NA | 90% | NA | 100% | 0.46 |
| TT + OXF + VAC 6q4 | ≥5 years | ≥2 months | 85% | 90% | 65% | 100% | 0.86 |
| MDA + OXF 6q4 | ≥5 years | ≥2 months | 85% | 90% | 85% | 100% | 0.91 |
| MDA + OXF + VAC 6q4 | ≥5 years | ≥2 months | 85% | 90% | 95% | 100% | 0.97 |
Prelim: probability of elimination of PCC (porcine cysticercosis) and HT (human taeniosis), MDA: human mass drug administration (praziquantel), TT: selective treatment based on detection with copro-Ag ELISA, OXF: porcine mass drug administration (oxfendazole), VAC: porcine vaccination (TSOL 18), XqY: a total of X iterations of the intervention given at intervals of Y months.
Coverage of eligible population.
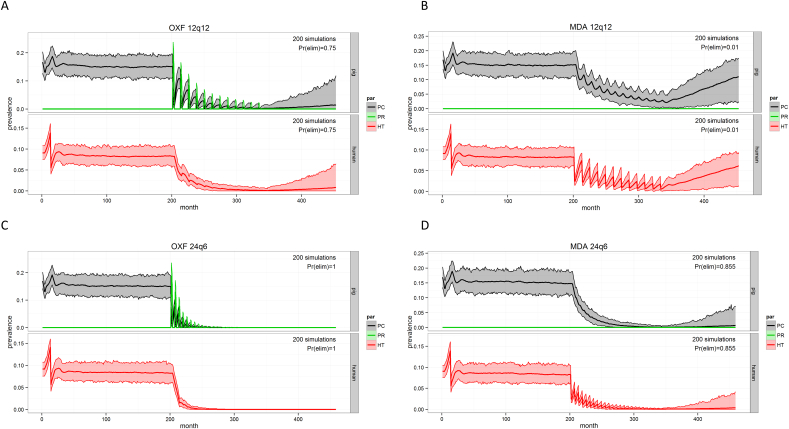
3.3.1. Interventions aiming for control (Fig. 2)
Fig. 2.
CystiSim output for different intervention scenarios aiming for control of Taenia solium in Zambia.
Pr(elim): probability of elimination of PCC (porcine cysticercosis) and HT (human taeniosis), MDA: human mass drug administration (praziquantel), OXF: porcine mass drug administration (oxfendazole), XqY: a total of X iterations of the intervention given at intervals of Y months. Interventions are introduced after 200 months.
The options mainly investigated as control strategies were either treatment of people or pigs, as MDA or selective human treatments (TT). Anthelmintic treatment of pigs, with the benzimidazole drug oxfendazole (OFZ) is generally considered safe, inexpensive, easy to administer and very effective against muscle cysticerci (Mkupasi et al., 2013), as well as gastrointestinal helminths. However, there is a withdrawal period of 17 days post-treatment for drug residues and it takes 3 to 6 months for the cysts to resolve and the meat to become aesthetically suitable for human consumption (Gonzales et al., 1998; Sikasunge et al., 2008b). Porcine vaccination was not taken into account, as this was not an option within a yearly treatment system considering the two-doses needed with a maximum interval of 4 months (Lightowlers et al., 2016). Human MDA has shown its relative effectiveness, though less as a sustainable system (Thomas, 2015). It has the advantage nevertheless of potentially being included in the currently ongoing MDA campaigns (Braae et al., 2016b); furthermore, the anthelmintics are cheap or subsidized by pharmaceutical companies, and communities are used to MDAs, which improves compliance.
Considering the low (slow decrease) and non-sustainable impact of yearly human MDA (Probability of elimination, Prelim = 0.01) or human targeted treatment (based on detection by copro-Ag ELISA) (Prelim = 0.00) in the simulations, the preference of yearly porcine treatments (Prelim = 0.75) was obvious. The latter show a faster decrease in PCC, though with increases simulated between treatment periods. Fig. 2 also shows simulations of six-monthly interventions, either human or porcine MDA, and the potential impact of these. Though results seem promising, especially for porcine MDA (every 6 months oxfendazole for 24 times would achieve elimination), this was not considered to be a low intensive/investment strategy and therefore not a feasible control option. Thus, the most optimal control strategy based on the transmission model was determined to be the yearly treatment of all available and eligible pigs (all pigs ≥2 months of age, with an expected coverage of eligible pigs of 90% and a 100% efficacy of the treatment).
3.3.2. Interventions aiming for elimination
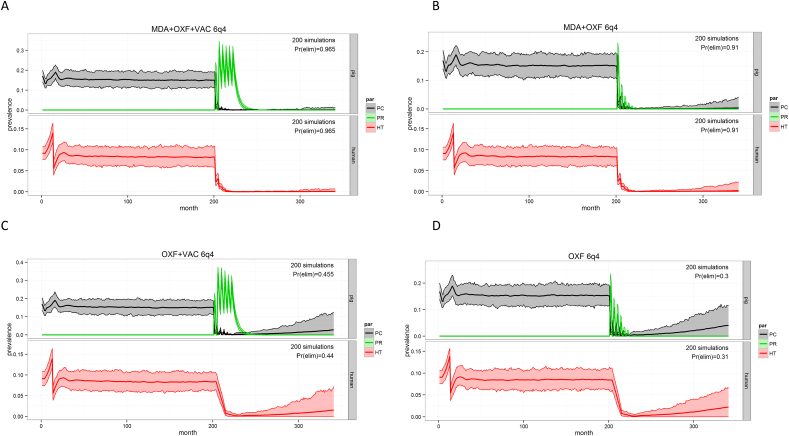
The most promising porcine vaccine, TSOL18 has been successful in experimental vaccine trials and field studies (Lightowlers, 2010). A disadvantage of the TSOL18 vaccination is the need to give animals two to three immunisations, at fairly short intervals, as well as the need for a strict cold chain for the vaccine, rendering the implementation cumbersome. Nevertheless, in an intensive system, with multiple interventions planned within a year, these difficulties can be tackled. Based on previous outcomes from both field and simulation studies, achieving elimination would only be possible when applying an integrated approach, tackling both human and animal hosts. Multiple simulations were conducted in the cystiSim model (Fig. 3, Table 1), starting from a very intensive three monthly intervention system including human and porcine MDA as well as porcine vaccination. Downscaling to four monthly schedules still reached satisfactory probabilities of elimination, while six monthly intervals could not achieve this within a two-year intervention period (see control above, 24 iterations needed).
Fig. 3.
CystiSim output for different intervention scenarios aiming for elimination of Taenia solium in Zambia.
Pr(elim): probability of elimination of PCC (porcine cysticercosis) and HT (human taeniosis), MDA: human mass drug administration (praziquantel), OXF: porcine mass drug administration (oxfendazole), VAC: porcine vaccination (TSOL 18), XqY: a total of X iterations of the intervention given at intervals of Y months. Interventions are introduced after 200 months.
The preferred strategy for elimination was determined to be the combination of human and porcine MDA joined with porcine vaccination of all eligible people and pigs, in a schedule of six iterations of four monthly interventions. Adding the porcine vaccine to the interventions, increased the probability of elimination with 5.5% (from 91% to 96.5%). Replacing the human MDA by a track and treat system did not yield the intended results (Prelim = 0.87), probably because of the lack of sensitivity of the currently available diagnostic tools to detect taeniosis cases, leading to an insufficient coverage of the actual tapeworm carriers, while only few carriers are needed to maintain the life cycle.
The selected strategies have been field tested with the addition of health education in the Eastern Province of Zambia, and final results analysis is ongoing. While the effects of health education was not included in the model as these were difficult to quantify, it is expected that it will provide additional benefits to the selected intervention strategies. Health education of the community on T. solium disease transmission, disease prevention, pig-management and sanitation knowledge (Ngowi et al., 2008; Carabin et al., 2018) has been shown to play an important role by helping to change eating habits (boiling meat, washing vegetables), promote the self-diagnosis of infection, and improve sanitation (e.g. use of closed latrines or toilets, good personal hygiene) and pig husbandry systems (the confinement of pigs). The recent evaluation of the computerised educational tool ‘The Vicious Worm’ in school aged children did indicate a good uptake of knowledge and retention of this knowledge even after one year. While the primary school children had captured the preventive measures well, the complexity of disease transmission was less well understood and retained, highlighting the importance of simplifying certain educational messages, especially when focussing on children as change agents (Hobbs et al., 2018b). Nevertheless, improvements in knowledge do not systematically translate into the attitudinal and practical changes necessary to make an impact on disease occurrence (Sarti and Rajshekhar, 2003). Further, a number of cultural taboos and beliefs will have to countered for communities to change their risk behaviours (Thys et al., 2015).
4. The potholed road to success?
Available data on occurrence of T. solium in Zambia leave no doubt whether this neglected zoonoses should receive more attention and whether control/elimination programmes should be implemented. While results from the sanitation-related intervention were rather disappointing (Bulaya et al., 2015), probably mostly due to a lack of full coverage of latrine use and the biological need for only a few number of tapeworm carriers contaminating the environment, combined chemotherapeutic interventions focussing on both human and pig hosts seem much more promising. Indeed, results indicate that elimination is actually an achievable option in terms of available tools (Garcia et al., 2016) however, whether it is feasible to implement all these tools in SSA setting is a totally different question, especially from a governmental perspective (see further).
The use of the recently developed agent-based disease transmission models (Dixon et al., submitted), aids in selecting the most optimal control strategies and in defining their frequency and interval of use. Of course, theoretically and technically feasible intervention and elimination strategies, demonstrated to be highly effective in simulation models, should always be field-tested for confirmation. We assumed high coverages in our models (85% for human interventions, 90% for pig oriented interventions), which, if not feasible in the field, will potentially have a big impact on the outcome.
Under Zambian conditions, high levels of African Swine fever lead to a high pig turnover in the pig population, causing an insufficient proportion of the pig population that is actually protected on the one hand, and reluctance of the pig owners to apply any intervention via injections on their pigs (Hobbs et al., 2018a, Hobbs et al., 2018b, submitted); porcine vaccination is therefore not considered a feasible and effective strategy. This is corroborated by simulations in the transmission models (Fig. 3), indicating that, even under ideal conditions (i.e., no African Swine fever), vaccination offers only a minimal added value on elimination probability compared to human and pig treatment alone.
The development and evaluations of new educational tools such as ‘The Vicious Worm’ are encouraging and will hopefully lead to an increased use as improved knowledge is key in sustaining the achievements acquired using chemotherapy. After all, an absence of behavioural change (sanitation, culinary and pig management oriented) once chemotherapeutic interventions have stopped, will lead to a rapid resurgence of infection.
However, when designing control or elimination programmes, other important factors, besides the biological and technical criteria, need to be taken into consideration, such as, whether resources should be used for elimination or rather continued control, or whether the limited available resources should be used for T. solium control at all. This decision should be based on scientifically sound evaluations of the costs versus the benefits (monetary value) or utility (health value) of both options, preferably by cost-effectiveness analyses. However, calculating the latter is not an easy task, as the costs and effectiveness on the long term are hard to estimate. The benefits of control or elimination are obvious in terms of health problems that are prevented or reduced, but the economic benefits extend beyond the benefits to the individuals that are protected. As well as the increased income for the pig-raising households, due to higher prices obtained from non-infected or condemned pork, the economic benefits can include savings in health-care costs for the treatment of illness, the economic productivity of having a healthy workforce (or of not having parents removed from the workforce to care for an ill child) and coincident benefits that result from the interventions (such as an improved health-care infrastructure). A recent study assessing the cost-effectiveness of T. solium control in human and pigs in northern Lao PDR determined that controlling only T. solium would not be beneficial (USD 3662 per zoonotic DALY averted), while tackling multiple pathogens including T. solium, soil transmitted helminths and Classical Swine Fever would be (USD 234 per zoonotic DALY averted) (Okello et al., 2018).
Also social and political factors should be taken into account. It is obvious that the success of any intervention is largely dependent on the level of societal and political acceptance and commitment. Though the significance of social and behavioural influences on the spread of human cysticercosis is known (Ngowi et al., 2008), culturally adapted control measures have not yet been implemented in endemic areas such as Zambia. The findings of a recent anthropological study (Thys et al., 2015) indicate that the reluctance to latrine use as opposed to open defecation is grounded in toilet-associated taboos with in-laws and grown-up children of the opposite gender, and sociocultural barriers related to privacy, dignity and comfort, all mainly explained by the matrilineal descent of the society. Also, the free roaming pig management finds its resilience in socio-economic and culturally determined factors (Thys et al., 2016), confirming the need to include socio-anthropological sciences in the design of intervention studies.
A fundamental step that is currently lacking in Zambia is government and local NGOs' awareness and motivation to invest in this neglected zoonosis. Advocacy and awareness creation, through efficient communication/dissemination to stakeholders are often not the strongest points of the scientists evaluating the control possibilities in the field. Liaison with experts in this field should therefore be encouraged. Of course, burden and cost effectiveness estimates, which are underway for Zambia, will provide essential information for governmental decision making. Whether to aim for control or elimination is indeed one of the big questions. Nevertheless, elimination in a limited area should be followed by scaling up in the province, country and region. If this cannot be ascertained, long term elimination will not be achievable and efforts invested in vain. Therefore, at this stage of regional interest, control might be the most realistic aim, following a ‘potholed’ path which is not without hurdles to tackle, but which can eventually be negotiated with perseverance and effort.
Acknowledgements
This work was based on projects by the Flemish Inter University Council, Research Initiative Programme, Zambia (VLIR-UOS.ZIUS2008RIP-8961), with the Belgian Directorate for Development Co-operation through the 3rd framework agreement with the Institute of Tropical Medicine, Antwerp; by the European Union's Seventh Framework Programme (FP7/2007–2013) under the grant agreement no. 221948 (ICONZ) (The contents of this publication are the sole responsibility of the authors and don't necessarily reflect the views of the European Commission.); by the Institute of Tropical Medicine, Antwerp via the Flemish Ministry of Research (www.ewi-vlaanderen.be/en/science-technology-and-innovation-sti-flanders/funding-rd) and via the Belgian Cooperation in the framework of the collaboration between the ITM and the University of Pretoria, South Africa.
The publication is based on the keynote presentation (S. Gabriël) at ICOPA 2018, South Korea.
References
- Assana E., Lightowlers M.W., Zoli A.P., Geerts S. Taenia solium taeniosis/cysticercosis in Africa: risk factors, epidemiology and prospects for control using vaccination. Vet. Parasitol. 2013;195(1–2):14–23. doi: 10.1016/j.vetpar.2012.12.022. [DOI] [PubMed] [Google Scholar]
- Braae U.C., Devleesschauwer B., Gabriël S., Dorny P., Speybroeck N., Magnussen P. CystiSim - an agent-based model for Taenia solium transmission and control. PLoS Negl. Trop. Dis. 2016;10(12) doi: 10.1371/journal.pntd.0005184. (16. eCollection 2016 Dec) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braae U.C., Magnussen P., Harrison W., Ndawi B., Lekule F., Johansen M.V. Effect of National Schistosomiasis Control Programme on Taenia solium taeniosis and porcine cysticercosis in rural communities of Tanzania. Parasite Epidemiol. Control. 2016;1(3):245–251. doi: 10.1016/j.parepi.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulaya C., Mwape K.E., Michelo C., Sikasunge C.S., Makungu C., Gabriel S. Preliminary evaluation of community-led total sanitation for the control of Taenia solium cysticercosis in Katete District of Zambia. Vet. Parasitol. 2015;207:241–248. doi: 10.1016/j.vetpar.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Carabin H., Millogo A., Ngowi H.A., Bauer C., Dermauw V., Koné A.C. Effectiveness of a community-based educational programme in reducing the cumulative incidence and prevalence of human Taenia solium cysticercosis in Burkina Faso in 2011-14 (EFECAB): a cluster-randomised controlled trial. Lancet Glob. Health. 2018;6(4):e411–e425. doi: 10.1016/S2214-109X(18)30027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chembensofu M., Mwape K.E., Van Damme I., Hobbs E., Phiri I.K., Masuku M., Zulu G. Re-visiting the detection of porcine cysticercosis based on full carcass dissections of naturally Taenia solium infected pigs. Parasit. Vectors. 2017;10(1):572. doi: 10.1186/s13071-017-2520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coral-Almeida M., Rodriguez-Hidalgo R., Celi-Erazo M., Garcia H.H., Rodriguez S., Devleesschauwer B. Incidence of human Taenia solium larval infections in an Ecuadorian endemic area: implications for disease burden assessment and control. PLoS Negl. Trop. Dis. 2014;8(5) doi: 10.1371/journal.pntd.0002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coral-Almeida M., Gabriël S., Abatih E.N., Praet N., Benitez W., Dorny P. Taenia solium human cysticercosis: a systematic review of sero-epidemiological data from endemic zones around the world. PLoS Negl. Trop. Dis. 2015;9(7) doi: 10.1371/journal.pntd.0003919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Coster T., Van Damme I., Baauw J., Gabriël S. Recent advancements in the control of Taenia solium: a systematic review. Food Waterborne Parasitol. 2018;12 doi: 10.1016/j.fawpar.2018.e00030. (Available online 13 November) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Brutto O.H., Rajshekhar V., White A.C., Tsang V.C., Nash T.E., Takayanagui O.M. Proposed diagnostic criteria for neurocysticercosis. Neurology. 2001;57(2):177–183. doi: 10.1212/wnl.57.2.177. 24. (Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Brutto O.H., O'Neal S.E., Dorny P., García H.H. Spontaneously arrested transmission of cysticercosis in a highly endemic village with a very low migration rate. Am. J. Trop. Med. Hyg. 2018;98(3):776–778. doi: 10.4269/ajtmh.17-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorny P., Phiri I.K., Vercruysse J., Gabriel S., Willingham A.L., Brandt J. A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. Int. J. Parasitol. 2004;34(5):569–576. doi: 10.1016/j.ijpara.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Dowdle W.R. The principles of disease elimination and eradication. MMWR Suppl. 1999;48(SU01):23–27. (December 31) [PMC free article] [PubMed] [Google Scholar]
- FAO, WHO . 2014. Multicriteria-Based Ranking for Risk Management of Food-Borne Parasites. Rome. [Google Scholar]
- Gabriël S., Blocher J., Dorny P., Abatih E.N., Schmutzhard E., Ombay M. Added value of antigen ELISA in the diagnosis of neurocysticercosis in resource poor settings. PLoS Negl. Trop. Dis. 2012;6(10) doi: 10.1371/journal.pntd.0001851. (Epub 2012 Oct 18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriël S., Dorny P., Mwape K.E., Trevisan C., Braae U.C., Magnussen P. Control of Taenia solium taeniasis/cysticercosis: the best way forward for sub-Saharan Africa? Acta Trop. 2017;165:252–260. doi: 10.1016/j.actatropica.2016.04.010. [DOI] [PubMed] [Google Scholar]
- Garcia H.H., Gonzalez A.E., Tsang V.C., O'Neal S.E., Llanos-Zavalaga F., Gonzalvez G., Cysticercosis Working Group in Peru Elimination of Taenia solium transmission in Northern Peru. N. Engl. J. Med. 2016;374(24):2335–2344. doi: 10.1056/NEJMoa1515520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman R.H., Gonzalez A.E., Llanos-Zavalaga F., Tsang V.C., Garcia H.H., Cysticercosis Working Group in Peru, P Prevention and control of Taenia solium taeniasis/cysticercosis in Peru. Pathog. Glob. Health. 2012;106(5):312–318. doi: 10.1179/2047773212Y.0000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales A.E., Falcon N., Gavidia C., Garcia H.H., Tsang V.C.W., Bernal T. Time-response curve of oxfendazole in the treatment of swine cysticercosis. Am. J. Trop. Med. Hyg. 1998;59(5):832–836. doi: 10.4269/ajtmh.1998.59.832. [DOI] [PubMed] [Google Scholar]
- Gonzalez A.E., Gavidia C., Falcon N., Bernal T., Verastegui M. Protection of pigs with cysticercosis from further infections after treatment with oxfendazole. Am. J. Trop. Med. Hyg. 2001;65:15–18. doi: 10.4269/ajtmh.2001.65.15. (pmid:11504400) [DOI] [PubMed] [Google Scholar]
- Havelaar A.H., Kirk M.D., Torgerson P.R., Gibb H.J., Hald T., Lake R.J., World Health Organization Foodborne Disease Burden Epidemiology Reference Group World Health Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015;12(12) doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs E.C., Mwape K.E., Devleesschauwer B., Gabriël S., Chembensofu M., Mambwe M. Taenia solium from a community perspective: preliminary costing data in the Katete and Sinda districts in Eastern Zambia. Vet. Parasitol. 2018;251:63–67. doi: 10.1016/j.vetpar.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Hobbs E.C., Mwape K.E., Van Damme I., Berkvens D., Zulu G., Mambwe M. Preliminary assessment of the computer-based Taenia solium educational program 'The Vicious Worm' on knowledge uptake in primary school students in rural areas in eastern Zambia. Tropical Med. Int. Health. 2018;23(3):306–314. doi: 10.1111/tmi.13029. (Epub 2018 Jan 29) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekule F.P., Kyvsgaard N.C. Improving pig husbandry in tropical resource-poor communities and its potential to reduce risk of porcine cysticercosis. Acta Trop. 2003;87(1):111–117. doi: 10.1016/s0001-706x(03)00026-3. [DOI] [PubMed] [Google Scholar]
- Lightowlers M.W. Eradication of Taenia solium cysticercosis: a role for vaccination of pigs. Int. J. Parasitol. 2010;40(10):1183–1192. doi: 10.1016/j.ijpara.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Lightowlers M.W., Donadeu M., Elaiyaraja M., Maithal K., Kumar K.A., Gauci C.G., Firestone S.M. Anamnestic responses in pigs to the Taenia solium TSOL18 vaccine and implications for control strategies. Parasitology. 2016;143(4):416–420. doi: 10.1017/S0031182016000202. [DOI] [PubMed] [Google Scholar]
- Mkupasi E.M., Sikasunge C.S., Ngowi H.A., Johansen M.V. Efficacy and safety of anthelmintics tested against Taenia solium cysticercosis in pigs. PLoS Negl. Trop. Dis. 2013;7(7) doi: 10.1371/journal.pntd.0002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrell K.D., Dorny P., Flisser A., Geerts S., Kyvsgaard N.C., McManus D. In: WHO/FAO/OIE Guidelines for the Surveillance, Prevention & Control of Taeniosis/Cysticercosis. Murrell K.D., editor. 2005. http://www.oie.int/doc/ged/d11245.pdf Retrieved from. [Google Scholar]
- Mwape K.E., Phiri I.K., Praet N., Muma J.B., Zulu G., Van den Bossche P. Taenia solium infections in a rural area of eastern Zambia-a community based study. PLoS Negl. Trop. Dis. 2012;6(3) doi: 10.1371/journal.pntd.0001594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwape K.E., Phiri I.K., Praet N., Speybroeck N., Muma J.B., Dorny P., Gabriël S. The incidence of human cysticercosis in a rural community of Eastern Zambia. PLoS Negl. Trop. Dis. 2013;7(3) doi: 10.1371/journal.pntd.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwape K.E., Blocher J., Wiefek J., Schmidt K., Dorny P., Praet N. Prevalence of neurocysticercosis in people with epilepsy in the Eastern Province of Zambia. PLoS Negl. Trop. Dis. 2015;9(8) doi: 10.1371/journal.pntd.0003972. (eCollection 2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mwape K.E., Phiri I.K., Praet N., Dorny P., Muma J.B., Zulu G. Study and ranking of determinants of Taenia solium infections by classification tree models. Am. J. Trop. Med. Hyg. 2015;92(1):56–63. doi: 10.4269/ajtmh.13-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndimubanzi P.C., Carabin H., Budke C.M., Nguyen H., Qian Y.J., Rainwater E. A systematic review of the frequency of neurocysticercosis with a focus on people with epilepsy. PLoS Negl. Trop. Dis. 2010;4(11) doi: 10.1371/journal.pntd.0000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngowi H.A., Carabin H., Kassuku A.A., Mlozi M.R.S., Mlangwa J.E.D., Willingham A.L. A health-education intervention trial to reduce porcine cysticercosis in Mbulu District, Tanzania. Prev. Vet. Med. 2008;85:52–67. doi: 10.1016/j.prevetmed.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Okello W.O., Okello A.L., Inthavong P., Tiemann T., Phengsivalouk A., Devleesschauwer B. Improved methods to capture the total societal benefits of zoonotic disease control: demonstrating the cost-effectiveness of an integrated control programme for Taenia solium, soil transmitted helminths and classical swine fever in northern Lao PDR. PLoS Negl. Trop. Dis. 2018;12(9) doi: 10.1371/journal.pntd.0006782. (eCollection 2018 Sep) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phiri I.K., Dorny P., Gabriel S., Willingham A.L., Speybroeck N., Vercruysse J. The prevalence of porcine cysticercosis in Eastern and Southern provinces of Zambia. Vet. Parasitol. 2002;108(1):31–39. doi: 10.1016/s0304-4017(02)00165-6. 30. [DOI] [PubMed] [Google Scholar]
- Phiri I.K., Ngowi H., Afonso S., Matenga E., Boa M., Mukaratirwa S. The emergence of Taenia solium cysticercosis in Eastern and Southern Africa as a serious agricultural problem and public health risk. Acta Trop. 2003;87(1):13–23. doi: 10.1016/s0001-706x(03)00051-2. [DOI] [PubMed] [Google Scholar]
- Sarti E., Rajshekhar V. Measures for the prevention and control of Taenia solium taeniosis and cysticercosis. Acta Trop. 2003;87(1):137–143. doi: 10.1016/s0001-706x(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Schantz P.M. Progress in diagnosis, treatment and elimination of echinococcosis and cysticercosis. Parasitol. Int. 2006;55(Supplement):S7–S13. doi: 10.1016/j.parint.2005.11.050. [DOI] [PubMed] [Google Scholar]
- Schantz P.M., Cruz M., Sarti E., Pawlowski Z. Potential eradicability of taeniasis and cysticercosis. Bull. Pan Am. Health Organ. 1993;27(4):397–403. [PubMed] [Google Scholar]
- Sikasunge C.S., Phiri I.K., Phiri A.M., Dorny P., Siziya S., Willingham A.L. Risk factors associated with porcine cysticercosis in selected districts of Eastern and Southern provinces of Zambia. Vet. Parasitol. 2007;143(1):59–66. doi: 10.1016/j.vetpar.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Sikasunge C.S., Phiri I.K., Phiri A.M., Siziya S., Dorny P., Willingham A.L. Prevalence of Taenia solium porcine cysticercosis in the Eastern, Southern and Western provinces of Zambia. Vet. J. 2008;176(2):240–244. doi: 10.1016/j.tvjl.2007.02.030. [DOI] [PubMed] [Google Scholar]
- Sikasunge C.S., Johansen M.V., Willingham A.L., Leifsson P.S., Phiri I.K. Taenia solium porcine cysticercosis: viability of cysticerci and persistency of antibodies and cysticercal antigens after treatment with oxfendazole. Vet. Parasitol. 2008;158:57–66. doi: 10.1016/j.vetpar.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Thomas L.F. World Health Organization; 2015. Landscape Analysis: Control of Taenia Solium. [Google Scholar]
- Thys S., Mwape K.E., Lefevre P., Dorny P., Marcotty T., Phiri A.M. Why latrines are not used: communities' perceptions and practices regarding latrines in a Taenia solium endemic rural area in Eastern Zambia. PLoS Negl. Trop. Dis. 2015 doi: 10.1371/journal.pntd.0003570. (March 4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thys S., Mwape K.E., Lefèvre P., Dorny P., Phiri A.M., Marcotty T. Why pigs are free-roaming: communities' perceptions, knowledge and practices regarding pig management and taeniosis/cysticercosis in a Taenia solium endemic rural area in Eastern Zambia. Vet. Parasitol. 2016;30(225):33–42. doi: 10.1016/j.vetpar.2016.05.029. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R., Devleesschauwer B., Praet N., Speybroeck N., Willingham A.L., Kasuga F. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLoS Med. 2015;12(12) doi: 10.1371/journal.pmed.1001920. (eCollection 2015 Dec. Review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevisan C., Mkupasi E.M., Ngowi H.A., Forkman B., Johansen M.V. Severe seizures in pigs naturally infected with Taenia solium in Tanzania. Vet. Parasitol. 2016;220:67–71. doi: 10.1016/j.vetpar.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation . WHO; Geneva: 2012. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases: A Roadmap for Implementation. [Google Scholar]
- World Health Organisation . World Health Organization; Geneva, Switzerland: 2015. Investing to Overcome the Global Impact of Neglected Tropical Diseases: Third who Report on Neglected Tropical Diseases; p. 211. [Google Scholar]