Abstract
A 55-year-old lady developed a corneal ring infiltrate following trauma with a wooden stick. 10% KOH mount of corneal scrapings revealed septate hyaline fungal filaments. White feathery colonies with shiny black dots grew on potato dextrose agar. Characteristic features of Pestalotiopsis spores were seen on Lactophenol cotton blue mount. DNA sequencing showed 99% similarity with Pseudopestalotiopsis theae. Complete resolution was noted with topical and oral antifungals. To the best of our knowledge, this is the first report of Pseudopestalotiopsis keratitis following trauma with vegetative matter highlighting the role of DNA sequencing in identification of rare fungi.
Keywords: Fungal keratitis, phylogenetic analysis, Pseudopestalotiopsis theae, trauma
There are millions of fungi worldwide, however, few are pathogenic causing diseases in humans. Difficulty in identification of fungi causing keratitis arises owing to absence of specific spores in culture or previously unknown type of sporulation.[1] For rapid and accurate identification, DNA sequencing technology and matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS) are being used.[2] The genus Pestalotiopsis has undergone considerable revisions recently.[3] To the best of our knowledge, this is the first case of keratitis caused by Pseudopestalotiopsis theae in the healthy cornea of an immunocompetent patient.
Case Report
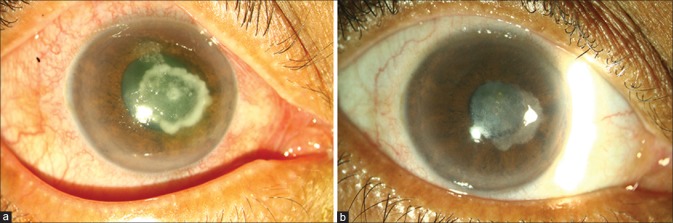
A 55-year-old lady from a rural area presented with pain, redness, and decreased vision in the right eye following injury with a small wooden stick 3 days earlier. Visual acuity in the right eye was counting fingers close to face. The cornea had an epithelial defect with an underlying anterior to mid-stromal ring-shaped infiltrate with hyphate edges measuring 5 × 4 mm (Haag Streit, BM 900, USA) and a smaller dense central infiltrate within [Fig. 1a].
Figure 1.
(a) Slit lamp photograph of the right eye in diffuse illumination showing a central ring-shaped anterior to mid stromal dry infiltrate (4 mm × 5 mm) with dense focus of infiltrate within the ring. (b) Slit lamp photograph of the right eye with sclerotic scatter highlighting the central scar
A microbiological work up was done.[1] Direct microscopy of potassium hydroxide with calcofluor white mount showed septate hyaline fungal filaments. Natamycin (5%) eye drops half hourly, tapered fortnightly to 8 times a day and continued 2 weeks beyond complete resolution with oral ketoconazole 200 mg twice daily for 6 weeks cured her infection in 4 months [Fig. 1b], At the last follow-up, her unaided visual acuity in the right eye was 20/200 with no further improvement owing to the central scar. The options of contact lens trial and deep anterior lamellar keratoplasty were discussed which she declined.
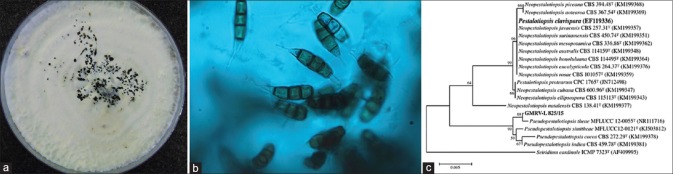
White, feathery fungal colonies grew on blood agar, chocolate agar, and potato dextrose agar (PDA) in 48 h. Further, incubation of PDA showed black shiny dots in the center with irregular surface [Fig. 2a], Lactophenol cotton blue mount showed brown septate hyphae and characteristic 5-celled brown spores with fine appendages at the tip [Fig. 2b], resembling spores of Pestalotiopsis. Anti-fungal susceptibility testing was not done because this facility was not available in our laboratory. DNA was isolated from the culture using QIAamp DNA kit (Qiagen, Germany), and ITS1-5.8S-ITS2 regions of the rRNA gene were amplified by PCR. The purified PCR products were sequenced with the same primers using Sanger dideoxy sequencing technology through an automatic DNA sequencer (Applied Biosystems, USA).[4]
Figure 2.
(a) Pseudopestalotiopsis theae conidiomata sporulating on potato dextrose agar at day 20. (b) Lactophenol cotton blue mount (1000x magnification) showing fusoid 4-septate conidia with slight constrictions at the septae and conical basal cell with truncated base. The 3 median cells are concolorous and brown with darker colored septae. The conical apical cells have filiform branched appendages. (c) ITS sequence analysis generated a phylogram which shows that our sample GMRV-L 825/15 has 99% similarity with Pseudopestalotiopsis theae
The internal transcribed spacer (ITS) sequence data were analyzed by obtaining consensus with SeqMan Pro (DNASTAR, Inc., Madison, WI, USA). Calculations of pairwise similarities of ITS region and the identification of phylogenetic neighbors were achieved using the NCBI (BLASTn 2.2.26) and phylogenetic tree construction, respectively. The closely related phylogenetic neighbors were retrieved and phylogenetically compared using the neighbor-joining algorithm of Mega software version 7.0 (Pennsylvania State University, USA) at 1,000 bootstrap replications to assess the confidence limits of the branching.[5] The same sequence was deposited in the Genbank database under KX216781 accession number. NCBI-BLAST analysis of ITS sequence of our strain (GMRV-L 825/15) showed 99% similarity with Pseudopestalotiopsis theae. Phylogenetic analysis [Fig. 2c], with the type strains of three closely related genera Pseudopestalotiopsis, Neopestalotiopsis, and Pestalotiopsis within the family Amphisphaeriaceae showed the clustering of all isolates into 2 distinct clades. All species of the genus Pseudopestalotiopsis formed separate clade, the members of Neopestalotiopsis were divided into 3 sub-clades. Our patient isolate (GMRV-L 825/15) clustered with Pseudopestalotiopsis theae.
Discussion
Pestalotiopsis species are important phytopathogens that cause a variety of diseases in plants, namely canker lesions, shoot dieback, leaf spots, and needle blight.[3] Pestalotiopsis species are commonly isolated as endophytes, and numerous compounds of medicinal, agricultural, and industrial importance are produced from these endophytes.[6]
Recent analysis of 28S rRNA gene (LSU) of several strains of Pestalotiopsis species described 2 new genera Neopestalotiopsis and Pseudopestalotiopsis depending on the morphology and phylogenetic analysis.[7]
Pseudopestalotiopsis species causes gray blight in tea plants and leaf spot in oil palm.[3] Despite being a common pathogen in plants, infection in humans and animals is extremely rare. On extensive Pubmed literature search, there were no reports of Pseudopestalotiopsis infections in humans. A single report of Pestalotiopsis clavispora keratitis was reported from Japan in a gardener after sweeping up leaves and twigs.[8] The affected eye was more susceptible to infection owing to chronic use of topical steroids for multiple ocular surgeries and recurrent Herpes simplex virus keratitis. Concomitant bullous keratopathy resulted in a poor ocular surface. Initial treatment included topical voriconazole eye drops and natamycin eye ointment for 8 months followed by topical and intravenous micafungin according to the anti-fungal sensitivity report. Duration of therapy was 15 months. The sequence (EF119336) of the isolate of Pestalotiopsis clavispora[8] was included in the phylogenetic analysis and it grouped with members of the genus Neopestalotiopsis.
In our case, the predisposing factor was vegetative material injury similar to the above case. There were no associated ocular co-morbidities. It is known that fungus is a weak opportunistic pathogen and remains as an endophyte on vegetative matter. These spores may have gained entry into the cornea following trauma. Although we did not perform a sensitivity profile to various antifungals, the infection responded to our routine first line therapy of topical 5% natamycin and oral ketoconazole. A compromised ocular surface owing to bullous keratopathy, prolonged prior use of topical steroids, and less frequent dosing of topical antifungals may have caused the slow and suboptimal clinical response seen with Pestalotiopsis clavispora keratitis.[8]
The fungus grew in culture, but morphological identification was difficult, hence, DNA sequencing was done for our patient's isolate which clustered with Pseudopestalotiopsis theae. Molecular diagnosis is increasingly being used to identify rare species of pathogenic fungi.[2]
Conclusion
In conclusion, Pseudopestalotiopsis keratitis is extremely rare and occurs following trauma with vegetative matter. Good response to topical antifungals was noted in the absence of other ocular pathology. DNA sequencing is an important tool in identification of rare fungi.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial disclosure
None.
Financial support and sponsorship
This work was supported by Hyderabad Eye Research Foundation.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
Authors thank Dr. R. Jayasudha for her help with the phylogenetic analysis.
References
- 1.Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: Experience of over a decade. Indian J Ophthalmol. 2009;57:273–9. doi: 10.4103/0301-4738.53051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kozel TR, Wickes B. Fungal diagnostics. Cold Spring Harb Prospect Med. 2014;4:a109299. doi: 10.1101/cshperspect.a019299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maharachchikumbura SSN, Guo LD, Chukeatirote E, Bahkali AH, Hyder KD. Pestalotiopsis-morphology, phylogeny, diversity and biochemistry. Fungal Diversity. 2011;50:167–87. [Google Scholar]
- 4.Tarai B, Gupta A, Ray P, Shivaprakash MR, Chakrabarti A. Polymerase chain reaction for early diagnosis of post operative fungal endophthalmitis. Indian J Med Res. 2006;123:671–8. [PubMed] [Google Scholar]
- 5.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR Protocols: A Guide to Methods and Applications. NewYork: Academic Press, Inc; 1990. pp. 315–22. [Google Scholar]
- 6.Yang XL, Zhang JZ, Luo DQ. The taxonomy, biology and chemistry of the fungal Pestalotiopsis genus. Nat Prod Rep. 2012;29:622–41. doi: 10.1039/c2np00073c. [DOI] [PubMed] [Google Scholar]
- 7.Maharachchikumbura SSN, Hyde KD, Gorenewald JZ, Xu J, Crous PW. Pestalotiopsis revisited. Stud Mycol. 2014;79:121–86. doi: 10.1016/j.simyco.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monden Y, Yamamoto S, Yamakawa R, Sunada A, Asari S, Makimura K, et al. First case of fungal keratitis caused by Pestalotiopsis clavispora. Clin Ophthalmol. 2013;7:2261–4. doi: 10.2147/OPTH.S48732. [DOI] [PMC free article] [PubMed] [Google Scholar]