Abstract
Purpose:
To report the distribution and trends of types of organisms and antibiotic susceptibility of the bacterial isolates obtained from patients with microbial keratitis.
Methods:
Microbiology records of culture-positive microbial keratitis that underwent a diagnostic corneal scraping and cultures were reviewed. Fungal, bacterial, and parasitic culture results and antibiotic susceptibility profile of bacteria were analyzed and comparisons were made between two halves of the study period (2007–2010 vs. 2011–2014).
Results:
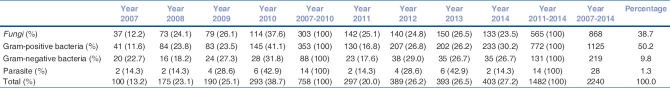
A total of 3981 corneal scrapings were processed during the 8-year study period. Pathogen was recovered in culture in 1914 (48.1%) samples. Fungi, bacteria, and parasites constituted 38.7%, 60%, and 1.3% of the total isolates, respectively. The common fungal isolates were Aspergillus spp. (224/868, 25.8%) and Fusarium spp. (200/868, 23.0%), while common Gram-positive bacteria were Streptococcus pneumoniae (217/1125, 19.3%) and Staphylococcus aureus (185/1125, 16.4%), and common Gram-negative bacteria was Pseudomonas spp. (99/219, 45.2%). There was no significant difference in proportion of bacterial (P = 0.225) and fungal (P = 0.421) keratitis between the first half and second half of the study period. There was a significant increase in proportion of Gram-positive isolates (P = 0.015) [353/758 (46.6%) vs. 772/1482 (52.1%)] and decrease in proportion of Gram-negative organisms (P = 0.044) [88/758 (11.6%) vs. 131/1482 (8.8%)] in the recent years. In-vitro antibiotic susceptibility testing showed decrease in susceptibility to moxifloxacin for Pseudomonas spp. (P = 0.016) in recent years.
Conclusion:
Prevalence of fungal and bacterial keratitis has remained unchanged over the years. This study shows a significant increase in Gram-positive bacterial infection and decrease in Gram-negative bacterial infection of the cornea in the recent years.
Keywords: Antibiotics, bacteria, fungi, microbial keratitis, susceptibility
Microbial keratitis is an ophthalmic emergency which requires urgent attention. It is one of the leading causes of blindness.[1] It is commonly associated with contact lens wear, ocular trauma, surgery, and ocular surface diseases, but can also occur without any predisposing factor.[2,3,4] Accurate and rapid identification of the microorganism is required for successful treatment of the disease.[5,6,7,8] Smear and culture are considered to be the gold standards to identify the offending organisms and guide appropriate treatment.[9] The common organisms are bacteria, viruses, and fungi. The prevalence of different organisms varies in different geographical locations.[3,4]
With widespread use of broad-spectrum antibiotics, a corresponding change in the microbial spectrum and antibiotic susceptibility may occur.[10] Regional differences exist in terms of the organism isolated, their susceptibility, and resistance pattern.[11] Therefore, local epidemiological studies are required to provide evidence-based management of microbial keratitis. This study reviews the distribution and trends in types of organisms isolated and antibiotic susceptibility pattern of bacterial isolates from microbial keratitis during a period of 8 years (i.e. 2007–2014).
Methods
A retrospective review of microbiology records of all patients with microbial keratitis from a tertiary eye care center in eastern India who underwent a diagnostic corneal scraping for direct microscopy and cultures from January 2007 to December 2014 was conducted. Corneal scrapings with positive culture were included for analysis. The collected data included patient profile (age and gender), culture results, and antibiotic susceptibility profile of bacterial isolates. Culture results and antibiotic susceptibility profiles were analyzed. Cases of viral keratitis were not included in the analysis. Trend analysis of the data was done year-wise and in two blocks of 4 years each (i.e. 2007–2010 and 2011–2014).
As a part of the institute protocol, patients presenting with clinical features of microbial keratitis underwent slit lamp examination and corneal scrapings. Microbiological processing of the corneal scrapings included smear preparation for microscopy after Gram stain and potassium hydroxide with calcofluor white (KOH + CFW) mount. Corneal scrapings were also inoculated on appropriate media (5% sheep blood agar, chocolate agar, Sabouraud dextrose agar, potato dextrose agar, non-nutrient agar with Escherichia coli, thioglycolate broth, and brain heart infusion broth). All media were incubated aerobically at 37°C except chocolate agar (incubated in 5% CO2 at 37°C). The media were observed for 14 days for any growth. Conventional Ziehl-Neelsen (ZN) stain and modified ZN stain using 1% H2 SO4 were done whenever indicated. A culture was considered positive when there was growth of the same organism on two or more media, or confluent growth at the site of inoculation on one solid medium, or growth in one medium with consistent direct microscopy findings, or growth of the same organism on repeated corneal scrapings.
Cultured bacterial isolates were subjected to antimicrobial susceptibility testing to a range of antibiotics commonly used in the treatment of corneal ulcer. Antibiotic susceptibility was done by disc diffusion Kirby-Bauer method as per the Clinical and Laboratory Standards Institute (CLSI) guidelines, which classify organisms as susceptible, resistant, or intermediately susceptible to antibiotics. For data analysis in this study, organisms with intermediate susceptibility were grouped as susceptible.
The Chi-square test was used for the comparison of two proportions. A P value of ≤ 0.05 was regarded as evidence of significance.
Results
A total of 3981 corneal scrapings were taken during the 8 years of the study period. Pathogen was recovered in culture in 1914/3981 (48%) samples. More than one pathogen were isolated in 306/1914 (16%) samples. Mixed infections were reported in 165 cases (56 in 2007–2010 and 109 in 2011–2014). There was no significant difference between the two periods (P = 0.86). Results of direct microscopy were considered to determine the significance of culture and all instances of smear-positive, but culture-negative were excluded from the analysis. Corneal scrapings of patients of microsporidial keratitis with smear-positive for microsporidial spores were not included, as it does not grow in routine culture media.
Prevalence of distribution of different organisms
Distribution of fungi, bacteria, and Acanthamoeba (38.7%, 60%, and 1.3%, respectively) is shown in Table 1. The common isolates among fungi were Aspergillus spp. (224/868, 25.8%)and Fusarium spp. (200/868, 23%); in Gram-positive bacteria were Streptococcus pneumoniae (217/1125, 19.3%) and other Streptococcus spp. (55/1125, 4.9%), and Staphylococcus aureus (185/1125, 16.4%) and other Staphylococcus spp. (413/1125, 36.7%); and in Gram-negative bacteria was Pseudomonas spp. (99/219, 45.2%).
Table 1.
Year-wise distribution of microorganisms
There was no significant difference in the proportion of bacterial [441/758 (58.2%) vs. 903/1482 (60.9%)] (P = 0.225) and fungal [303/758 (40.0%) vs. 565/1482 (38.1%)] (P = 0.421) keratitis between the first and the last 4 years. There was a significant increase in proportion of Gram-positive isolates [353/758 (46.6%) vs. 772/1482 (52.1%)] (P = 0.015) and decrease in proportion of Gram-negative organisms [88/758 (11.6%) vs. 131/1482 (8.8%)] (P = 0.044) in the recent years.
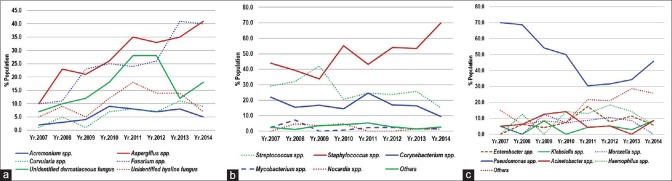
Overall the proportion of fungi (868/2240, 38.7%) and Gram-positive bacteria (1125/2240, 50.2%) was more compared to Gram-negative bacteria (219/2250, 9.8%) and Acanthamoeba (28/2240, 1.3%). The proportion of Aspergillus spp. and Fusarium spp. in the distribution of fungi shows an increasing trend [Fig. 1a]. Similarly, in Gram-positive bacteria, Staphylococcus spp. shows an increasing trend [Fig. 1b]. In Gram-negative bacteria, Pseudomonas spp. shows a decreasing trend during 2007–2011, while it is increasing steadily during 2012–2014 [Fig. 1c].
Figure 1.
(a) Year-wise distribution of fungi. (b) Year-wise distribution of Gram-positive bacteria. (c) Year-wise distribution of Gram-negative bacteria
Susceptibility to antibiotics: Gram-positive organisms
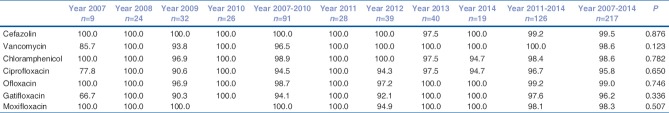
The overall susceptibility of S. pneumoniae was > 90% to all the tested antibiotics, highest being to cefazolin (100%) and lowest to ciprofloxacin (204/213, 95.8%) [Table 2]. While the susceptibility of S. aureus to vancomycin was 100% it was the least to ciprofloxacin (96/180, 53.3%) [Table 3]. Higher proportion of S. aureus was susceptible to chloramphenicol compared to fluroquinolone. Methicillin resistance was found in 26/173 isolates (15%) of S. aureus (MRSA) by disc diffusion testing with cefoxitin. Although susceptibility to fourth generation fluroquinolone (gatiofloxacin and moxifloxacin) has decreased in the last 4 years, there was no significant difference in the susceptibility pattern of both the Gram-positive organisms (S. pneumoniae and S. aureus) in the last 4 years compared to the first 4 years.
Table 2.
Year-wise susceptibility (%) of Streptococcus pneumoniae
Table 3.
Year-wise susceptibility (%) of Staphylococcus aureus
Susceptibility to antibiotics: Gram-negative organisms
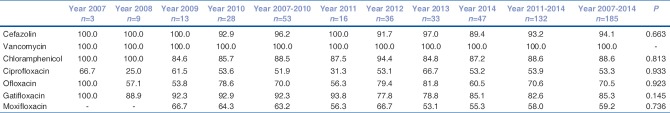
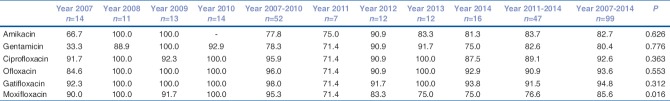
Overall, Pseudomonas spp. constitutes the largest proportion (99/219, 45.2%) of all Gram-negative isolates. The susceptibility tested against ciprofloxacin, ofloxacin, gatifloxacin, and moxifloxacin was: 92.6% (88/95), 93.6% (88/94), 94.8% (92/97), and 85.6% (77/90), respectively [Table 4]. Its overall susceptibility to gentamicin (74/92) and amikacin (44/53) was > 80%. There is a significant decrease in susceptibility to fourth generation fluroquinolone (moxifloxacin) in recent years (P = 0.016).
Table 4.
Year-wise susceptibility (%) of Pseudomonas spp.
Discussion
The phenomenon of increasing antibiotic resistance is a matter of concern worldwide. The excessive and inappropriate systemic use of antibiotics is thought to be a leading factor for the emergence of antibiotic resistance. Literature has demonstrated that increasing use of antibiotics leads to development of resistant strains.[12,13] Therefore, periodic susceptibility surveys are important to detect emerging resistance patterns.
Prophylactic use of antibiotic has been associated with bacterial resistance. In ophthalmology, numerous reports have warned about indiscriminate use of ophthalmic antibiotics because it has been found to promote the emergence of antibiotic resistance.[14,15] Fluroquinolone is used widely as a monotherapy for presumed bacterial keratitis due to its broad-spectrum of action. However, increasing use of antimicrobial agents is responsible for development of resistance. Increasing in-vitro resistance of systemic and ocular isolates to fluroquinolones have been reported.[12,16]
We have included only culture-positive cases for analysis. Bacterial and parasitic keratitis constituted largest and least proportion, respectively. Our result showed 48.1% culture positivity. This is slightly lower compared to other studies.[17,18] This might be due to inclusion of microsporidial keratitis, which represents a major group of culture-negative microbial keratitis in our institute.[19] Microsporidial spores were confirmed by smear examination and they do not grow in conventional culture media. During the study period, 464 microsporidia cases were present. The lower rate of positive culture also might be due to culture of corneal scraping in all cases irrespective of prior medication and size.
The etiological agents for microbial keratitis vary in different geographical locations. Fungal keratitis is common in tropical and subtropical region. It accounts for 30–50% cases of microbial keratitis in developing countries.[20,21,22,23] Filamentous fungus is most commonly reported from patients in the tropical region.[3,20,21] Our findings correlate with the earlier studies published from India and other tropical countries.[3,20,21,24,25]
Gram-positive organism has been reported to be commoner etiological agent of microbial keratitis compared to Gram-negative organism,[2,17,26] Staphylococcus spp. and Stereptococcus spp. being most commonly isolated pathogens, which is similar to our study. In our study, Gram-positive organisms have in fact increased significantly in recent years. Correspondingly, Gram-negative organisms have decreased. Amongst Gram-positive organisms, Staphylococcus spp. have increased significantly (P = 0.0003) in the last 4 years. In the same period, Pseudomonas spp. have decreased significantly (P = 0.001), whereas other Gram-negative organisms are more or less stable. Pandita et al. have reported non-significant rise of Gram-positive organism in their 10 years’ experience.[17] Alexndrakis et al. have described increased incidence of keratitis due to S. aureus and decreasing trend with Pseudomonas aeruginosa.[27] In contrast, Lichtinger et al. have reported an increase in Gram-negative and decrease in Gram-positive organism.[28] They have attributed this to increased use of contact lens. The increased trend of Gram-positive organisms in our study may be attributable to increasing number of patients with ocular surface problems and keratoplasties.
There are reports mentioning about rise in MRSA from different parts of the world.[26,28,29,30] Asbell et al. have reported an increase in the proportion of MRSA among S. aureus ocular infection from 29.5 to 41.6% in 5 years’ time.[29] Lichtinger et al. have described a trend toward increasing laboratory resistance to methicillin from 28% during first 4 years to 38.8% in last 3 years.[28] In our study, MRSA is less compared to other reported studies. In the past, we had reported methicillin resistance in 7.8% cases with S. aureus ocular infection.[31]
Gradual increase of resistance of S. aureus to fluroquinolone has been reported.[27] In our series, susceptibility to cefazolin and vancomycin was better compared to fluoroquinolones [Table 3]. Although, there is decrease in susceptibility to gatifloxacin and moxifloxcacin for S. aureus, the pattern is stable for S. pneumoniae. An interesting observation was resistance of Pseudomonas spp. to moxifloxacin that has increased in recent years [Table 4]. This might be due to widespread use of moxifloxacin for various ocular infections as well as prophylactic use.
This study shows a significant increase in Gram-positive bacterial infection and decrease in Gram-negative bacterial infection of the cornea in the recent years. The Gram-positive organisms did not show a significant shift in their susceptibility to fluoroquinolones over the 8-year study period.
Conclusion
This study shows a significant increase in Gram-positive and decrease in Gram-negative bacterial infection of the cornea in the recent years. The Gram-positive organisms did not show a significant shift in their susceptibility to fluoroquinolones.
Financial support and sponsorship
Hyderabad Eye Research Foundation, Hyderabad.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Whitcher JP. Corneal ulceration. Int Ophthalmol Clin. 1990;30:30–2. [PubMed] [Google Scholar]
- 2.Keay L, Edwards K, Naduvilath T, Taylor HR, Snibson GR, Forde K, et al. Microbial keratitis predisposing factors and morbidity. Ophthalmology. 2006;113:109–16. doi: 10.1016/j.ophtha.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 3.Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: Experience of over a decade. Indian J Ophthalmol. 2009;57:273–9. doi: 10.4103/0301-4738.53051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: Predisposing factors, clinical and microbiological review of 300 cases. Br J Ophthalmol. 2003;87:834–8. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allan BD, Dart JK. Strategies for the management of microbial keratitis. Br J Ophthalmol. 1995;79:777–86. doi: 10.1136/bjo.79.8.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asbell P, Stenson S. Ulcerative keratitis. Survey of 30 years’ laboratory experience. Arch Ophthalmol. 1982;100:77–80. doi: 10.1001/archopht.1982.01030030079005. [DOI] [PubMed] [Google Scholar]
- 7.Jones DB. Initial therapy of suspected microbial corneal ulcers. II. Specific antibiotic therapy based on corneal smears. Surv Ophthalmol. 1979;24(97):105–16. doi: 10.1016/0039-6257(79)90128-0. [DOI] [PubMed] [Google Scholar]
- 8.McLeod SD, Kolahdouz-Isfahani A, Rostamian K, Flowers CW, Lee PP, McDonnell PJ, et al. The role of smears, cultures, and antibiotic sensitivity testing in the management of suspected infectious keratitis. Ophthalmology. 1996;103:23–8. doi: 10.1016/s0161-6420(96)30738-0. [DOI] [PubMed] [Google Scholar]
- 9.Jones DB. Decision-making in the management of microbial keratitis. Ophthalmology. 1981;88:814–20. doi: 10.1016/s0161-6420(81)34943-4. [DOI] [PubMed] [Google Scholar]
- 10.Odonkor ST, Addo KK. Bacteria resistance to antibiotics: Recent trends and challenges. Int J Biol Med Res. 2011;2:1204–10. [Google Scholar]
- 11.Dalhoff A. Global fluoroquinolone resistance epidemiology and implictions for clinical use. Interdiscip Perspect Infect Dis 2012. 2012:976273. doi: 10.1155/2012/976273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein MH, Kowalski RP, Gordon YJ. Emerging fluoroquinolone resistance in bacterial keratitis: A 5-year review. Ophthalmology. 1999;106:1313–8. [PubMed] [Google Scholar]
- 13.Kunimoto DY, Sharma S, Garg P, Rao GN. In vitro susceptibility of bacterial keratitis pathogens to ciprofloxacin. Emerging resistance. Ophthalmology. 1999;106:80–5. doi: 10.1016/S0161-6420(99)90008-8. [DOI] [PubMed] [Google Scholar]
- 14.Dave SB, Toma HS, Kim SJ. Ophthalmic antibiotic use and multidrug-resistant staphylococcus epidermidis: A controlled, longitudinal study. Ophthalmology. 2011;118:2035–40. doi: 10.1016/j.ophtha.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 15.Milder E, Vander J, Shah C, Garg S. Changes in antibiotic resistance patterns of conjunctival flora due to repeated use of topical antibiotics after intravitreal injection. Ophthalmology. 2012;119:1420–4. doi: 10.1016/j.ophtha.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 16.Garg P, Sharma S, Rao GN. Ciprofloxacin-resistant pseudomonas keratitis. Ophthalmology. 1999;106:1319–23. doi: 10.1016/S0161-6420(99)00717-4. [DOI] [PubMed] [Google Scholar]
- 17.Pandita A, Murphy C. Microbial keratitis in Waikato, New Zealand. Clin Exp Ophthalmol. 2011;39:393–7. doi: 10.1111/j.1442-9071.2010.02480.x. [DOI] [PubMed] [Google Scholar]
- 18.Gebauer A, McGhee CN, Crawford GJ. Severe microbial keratitis in temperate and tropical Western Australia. Eye (Lond) 1996;10(Pt 5):575–80. doi: 10.1038/eye.1996.133. [DOI] [PubMed] [Google Scholar]
- 19.Das S, Sharma S, Sahu SK, Nayak SS, Kar S. Diagnosis, clinical features and treatment outcome of microsporidial keratoconjunctivitis. Br J Ophthalmol. 2012;96:793–5. doi: 10.1136/bjophthalmol-2011-301227. [DOI] [PubMed] [Google Scholar]
- 20.Srinivasan M, Gonzales CA, George C, Cevallos V, Mascarenhas JM, Asokan B, et al. Epidemiology and aetiological diagnosis of corneal ulceration in Madurai, South India. Br J Ophthalmol. 1997;81:965–71. doi: 10.1136/bjo.81.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopinathan U, Garg P, Fernandes M, Sharma S, Athmanathan S, Rao GN, et al. The epidemiological features and laboratory results of fungal keratitis: A 10-year review at a referral eye care center in South India. Cornea. 2002;21:555–9. doi: 10.1097/00003226-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Dunlop AA, Wright ED, Howlader SA, Nazrul I, Husain R, McClellan K, et al. Suppurative corneal ulceration in Bangladesh. A study of 142 cases examining the microbiological diagnosis, clinical and epidemiological features of bacterial and fungal keratitis. Aust N Z J Ophthalmol. 1994;22:105–10. doi: 10.1111/j.1442-9071.1994.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 23.Hagan M, Wright E, Newman M, Dolin P, Johnson G. Causes of suppurative keratitis in Ghana. Br J Ophthalmol. 1995;79:1024–8. doi: 10.1136/bjo.79.11.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Upadhyay MP, Karmacharya PC, Koirala S, Tuladhar NR, Bryan LE, Smolin G, et al. Epidemiologic characteristics, predisposing factors, and etiologic diagnosis of corneal ulceration in Nepal. Am J Ophthalmol. 1991;111:92–9. doi: 10.1016/s0002-9394(14)76903-x. [DOI] [PubMed] [Google Scholar]
- 25.Leck AK, Thomas PA, Hagan M, Kaliamurthy J, Ackuaku E, John M, et al. Aetiology of suppurative corneal ulcers in Ghana and South India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86:1211–5. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez-Camarena JC, Graue-Hernandez EO, Ortiz-Casas M, Ramirez-Miranda A, Navas A, Pedro-Aguilar L, et al. Trends in microbiological and antibiotic sensitivity patterns in infectious keratitis: 10-year experience in Mexico city. Cornea. 2015;34:778–85. doi: 10.1097/ICO.0000000000000428. [DOI] [PubMed] [Google Scholar]
- 27.Alexandrakis G, Alfonso EC, Miller D. Shifting trends in bacterial keratitis in South Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107:1497–502. doi: 10.1016/s0161-6420(00)00179-2. [DOI] [PubMed] [Google Scholar]
- 28.Lichtinger A, Yeung SN, Kim P, Amiran MD, Iovieno A, Elbaz U, et al. Shifting trends in bacterial keratitis in Toronto: An 11-year review. Ophthalmology. 2012;119:1785–90. doi: 10.1016/j.ophtha.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 29.Asbell PA, Colby KA, Deng S, McDonnell P, Meisler DM, Raizman MB, et al. Ocular TRUST: Nationwide antimicrobial susceptibility patterns in ocular isolates. Am J Ophthalmol. 2008;145:951–8. doi: 10.1016/j.ajo.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 30.McDonald LC, Lauderdale TL, Shiau YR, Chen PC, Lai JF, Wang HY, et al. The status of antimicrobial resistance in Taiwan among Gram-positive pathogens: The Taiwan Surveillance of Antimicrobial Resistance (TSAR) programme, 2000. Int J Antimicrob Agents. 2004;23:362–70. doi: 10.1016/j.ijantimicag.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 31.Kar S, Panda S, Sharma S, Singh DV, Das S, Sahu SK, et al. Comparison of type of species and antibacterial susceptibility profile of staphylococci isolated from normal healthy conjunctiva and ocular infections. Asia Pac J Ophthalmol (Phila) 2013;2:365–71. doi: 10.1097/APO.0b013e31829c022a. [DOI] [PubMed] [Google Scholar]