Abstract
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) is a distinct Hodgkin lymphoma subtype composed of few neoplastic lymphocyte-predominant (LP) cells in a back-ground of reactive small B and T cells. We have seen occasional NLPHL cases that contain background T cells with prominent cytologic atypia, raising the differential diagnosis of peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) or a composite lymphoma. We sought to characterize the clinicopathologic features of such cases. Eleven NLPHL cases with atypical T cells diagnosed from 1977 to 2010 were identified at 2 institutions and compared with 24 control NLPHL cases lacking atypical T cells. All 9 male patients and 2 female patients presented with localized peripheral lymphadenopathy. In comparison with control patients, they were younger (median age, 13.8 vs. 36.1 y; P = 0.015), with more frequent cervical lymph node involvement (54.5% vs. 8.3%, P = 0.015). In all 11 cases, areas of NLPHL with typical B-cell-rich nodules containing LP cells were present. Nine cases contained sheets of atypical T cells surrounding primary and secondary follicles in a pattern mimicking the T-zone pattern of PTCL-NOS; the remaining 2 cases contained atypical T cells presented as large clusters at the periphery of B-cell-rich nodules. In all cases, the atypical T-cell-rich areas contained rare scattered LP cells, which were IgD+ in 5 of 7 cases (71.4%). The atypical T cells showed no pan-T-cell antigen loss or aberrant T-cell antigen expression in any case, and polymerase chain reaction or Southern blot analysis showed no evidence of T-cell clonality in 6 cases tested. The atypical T cells exhibited a variable immunophenotype with respect to germinal center, follicular T-helper, T-regulatory, and cytotoxic T-cell markers. Among 8 patients with clinical follow-up (median follow-up: 6.4 y), 5 patients had recurrent NLPHL at 6 months to 12 years after diagnosis and 6 patients are alive without disease at 9 months to 18 years after diagnosis. In comparison with control patients, NLPHL patients with atypical T cells were more likely to develop recurrent NLPHL (71.4% vs. 13.6%, P = 0.008) and to have a shorter time to relapse (P = 0.04). Our findings suggest that some cases of NLPHL, occurring predominantly in younger patients, contain prominent populations of morphologically atypical T cells that may raise the possibility of concurrent nodal involvement by PTCL-NOS, a rare diagnosis in children. The clinical behavior of these cases appears similar to that of NLPHL with T-cell-rich diffuse areas, with a higher risk of disease recurrence and no difference in overall survival; however, this finding warrants confirmation in studies of larger numbers of patients.
Keywords: nodular lymphocyte-predominant Hodgkin lymphoma, LP cells, atypical T cells, peripheral T-cell lymphoma, IgD
Nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) accounts for approximately 5% of cases of Hodgkin lymphoma.29,38,42 It commonly presents with localized peripheral lymphadenopathy and generally follows an indolent clinical course with frequent late relapses.29 The tumor is composed of few neoplastic lymphocyte-predominant (LP) Reed-Sternberg cell variants, also known as “popcorn” cells, in a background of non-neoplastic small lymphocytes, histiocytes, and follicular dendritic cells, indicating a relationship to B-cell follicles.12,29,45 Unlike the Reed-Sternberg cells in classical Hodgkin lymphoma, LP cells in NLPHL are positive for CD20, CD45, and bcl-6, usually negative for CD30, and negative for CD15.29 They retain the B-cell program, with positivity for CD79a, PAX5 (BSAP), OCT2, and BOB.1 and express monoclonal immunoglobulin heavy and light chains and activation-induced cytidine deaminase.9,16,23,29,41
Several immunoarchitectural patterns of nodal involvement have been described in which the proportions and patterns of neoplastic LP cells in relation to non-neoplastic cells vary.12 Typically, NLPHL exhibits a B-cell-rich nodular or follicular growth pattern, in which LP cells reside within expanded nodules of small B cells encompassed by follicular dendritic cell meshworks.12 Nodules contain increased numbers of follicular T-helper cells (CD4+, CD57+, PD-1+) that often form rosettes around LP cells, whereas interfollicular areas contain reactive T cells lacking significant CD57 or PD-1 expression.3,8,12,24,26,45 Occasional cases may contain prominent extrafollicular LP cells in a T-cell-rich back-ground, and most NLPHL with IgD+ LP cells exhibit this growth pattern.12,30 Less commonly, NLPHL contains T-cell-rich nodular or diffuse areas resembling T-cell/ histiocyte-rich large B-cell lymphoma.7,12 The T-cell-rich diffuse pattern is associated with a higher rate of disease recurrence, but there is no difference in overall survival.4,12 In all of the morphologic variants described and in IgD+ NLPHL, the predominant background T lymphocytes are small, lack morphologic atypia, and are considered to represent a non-neoplastic reactive cell population.12,30 We and others have reported that CD4+, CD8dim+ T cells are commonly detected by flow cytometry in NLPHL; however, these cells presumably represent an activated or reactive T-cell population, as similar populations have been described in progressive transformation of germinal centers and in peripheral blood in the setting of current or recent viral infection; such cases also lack morphologically atypical T cells.25,27,32–33
We have seen occasional cases of NLPHL that contain background T cells with prominent cytomorphologic atypia.18 In some of these cases, the cytology and growth pattern of the atypical T cells raised the possibility of concurrent nodal involvement by a peripheral T-cell lymphoma, particularly as some T-cell lymphomas may also contain scattered large atypical B cells.12,18,19,31,34,48 In this study, we sought to characterize the morphologic and clinical features of these rare cases of NLPHL, to characterize the nature of atypical T cells by immunohistochemistry, flow cytometry, and molecular genetic analysis, and to compare the clinical features of such cases with conventional NLPHL lacking morphologically atypical T cells.
MATERIALS AND METHODS
Selection of Cases and Controls
Computer-assisted searches of the pathology files and hematopathology consultation files of 2 institutions [the Massachusetts General Hospital (MGH) and the National Cancer Institute (NCI) of the National Institutes of Health] were made for cases of NLPHL diagnosed between 1977 and 2010. The Institutional Review Boards of Partners HealthCare and the NCI approved the study before its initiation. Eleven cases were identified in which atypical T cells were reported: 2 cases originated from the MGH, one of which had been reported previously,18 and the other 9 cases originated from the consultation files of the authors of the study (E.S.J., J.A.F., N.L.H.). None of the cases originating from the NCI had been included in a previous study of IgD+ NLPHL by Prakash et al.30 Pathology reports, hematoxylin and eosin-stained and immunohistochemical-stained slides, and reports of flow cytometry and molecular genetic analysis performed at the time of diagnosis were retrieved and reviewed by at least 3 of the authors. Cases were evaluated for immunoarchitectural patterns of NLPHL involvement as described by Fan et al.12 Clinical information was obtained from medical records or from referring physicians. Information about age, sex, clinical presentation, and site(s) of involvement was available in all cases. Information regarding stage, treatment and clinical outcome, including time to first NLPHL relapse (defined as time from the date of diagnostic biopsy to the date of biopsy showing relapsed NLPHL) and overall survival (defined as time from the date of diagnostic biopsy to the date of death or last follow-up) was retrieved when available. As a control group, 24 consecutive cases of NLPHL lacking morphologically atypical T cells diagnosed at the MGH from 1998 to 2009 were identified. Information about age at earliest diagnosis, sex, clinical presentation, site(s) of involvement, relapse of NLPHL if any, and overall survival was collected for the control cases.
Immunohistochemical Studies
Formalin, B-plus or B5-fixed, paraffin-embedded tissue of 7 NLPHL cases with atypical T cells was available for additional immunohistochemistry to characterize the T cells for expression of germinal center, follicular T-helper, T-regulatory, Th1-related, and cytotoxic T-cell markers and to characterize LP cells for expression of various antigens, including IgD. Stains for IgD and Ki67 were performed on a subset of the control NLPHL cases for the purpose of comparing staining patterns of these antigens between the 2 groups. Two-micron-thick tissue sections were prepared, deparaffinized, and rehydrated according to standard laboratory protocols. Immunohistochemistry was performed using an avidin-biotin peroxidase complex with a peroxidase-labeled detection system on a Benchmark XT automated immunostainer (Ventana Medical Systems, Tucson, AZ) using validated staining protocols. A combination of ethylenediaminetetraacetic acid and boric acid in Tris buffer (CC1 reagent, Ventana) was used for antigen retrieval before primary antibody incubation. Antibodies, the type of antigen represented, manufacturers, and dilutions are listed in Table 1. Areas of atypical T cells were assessed for the proportion of cells expressing various antigens as follows: rare, <10% of cells; occasional, 10% to 30% of cells; many, 30% to 50% of cells; and most, >50% of cells.
TABLE 1.
Antibodies Used for Immunohistochemical Analysis
| Antibody | Type of Antigen | Manufacturer | Dilution |
|---|---|---|---|
| CD2 | Pan-T cell | Novocastra Laboratories, Newcastle Upon Tyne, UK | 1:20 |
| CD3 | Pan-T cell | Ventana Medical Systems, Tucson, AZ | Prediluted |
| CD4 | T helper | Novocastra Laboratories | 1:20 |
| CD5 | Pan-T cell | Ventana Medical Systems | Prediluted |
| CD7 | Pan-T cell | Dako, Carpinteria, CA | Prediluted |
| CD8 | Cytotoxic | Ventana Medical Systems | Prediluted |
| CD10 | Germinal center | Ventana Medical Systems | Prediluted |
| CD15 | CHL | Ventana Medical Systems | Prediluted |
| CD20 (L26) | B cell | Ventana Medical Systems | Prediluted |
| CD25 | T-regulatory | Novocastra Laboratories | 1:150 |
| CD30 | Classical Hodgkin lymphoma | Ventana Medical Systems | Prediluted |
| CD43 | Pan-T cell | BioGenex, San Ramon, CA | 1:40 |
| CD45RA | Naïve T cell | Dako | 1:30 |
| CD45RO | Pan-T cell, memory T cell | Ventana Medical Systems | Prediluted |
| CD57 | Follicular T-helper | Ventana Medical Systems | Prediluted |
| Ki67 | Proliferation | Ventana Medical Systems | Prediluted |
| bcl-6 | Germinal center | Dako | 1:10 |
| IgD | Immunoglobulin heavy chain subclass | Cell Marque, Rocklin, CA | Prediluted |
| Granzyme B | Cytotoxic | Chemicon International, Chemicon, CA | 1:10 |
| Perforin | Cytotoxic | Lab Vision Products, Freemont, CA | 1:20 |
| FOXP3 | T-regulatory | Abcam, Cambridge, MA | 1:800 |
| T-bet | T-helper 1 | Santa Cruz Biotechnology, Santa Cruz, CA | 1:100 |
| PD-1 | Follicular T-helper | Cell Marque | 1:50 |
Molecular Genetic Studies
In situ hybridization for Epstein-Barr virus-encoded ribonucleic acid (INFORM EBER PROBE, Ventana) was performed in 8 cases using an HX Automatic System Benchmark (Ventana) according to the manufacturer’s instructions. Signals were detected using the Alkaline Phosphatase Enhanced Detection Kit (Ventana). In 6 cases, DNA was isolated from paraffin-embedded tissue using xylene, followed by 100% ethanol rinse; deparaffinized tissue was then processed with QIAGEN QIAamp DNA micro Kits (QIAGEN, Valencia, CA). T-cell clonality assays were performed using polymerase chain reaction (PCR) according to the manufacturer’s instructions with reagents purchased from InVivoScribe Technologies LLC, San Diego, CA (TCRγ Gene Clonality Assay for ABI Fluorescence Detection). The kits used the primer sequences published by BIOMED-2 46 and had all the necessary reagents except for sample DNA and Taq polymerase (AmpliTAQ Gold, Applied Biosystems, Foster City, CA). PCR products were analyzed by capillary gel electrophoresis (Applied Biosystems 3100xl). In 4 cases, Southern blot analysis was performed on DNA extracted from frozen tissue using 32P-labeled probes to the T-cell receptor (TCR) β (cases 5, 9, 10, and 11)and γ (cases 9 and 11) constant regions (Dako, Carpinteria, CA).
Statistical Analysis
Statistical analysis was performed using Prism 5 and GraphPad QuickCalcs (GraphPad Software Inc., La Jolla, CA). The 2-tailed Fisher exact test was used to compare categorical variables between cases and controls, and the Student unpaired t test was used to compare continuous variables between the 2 groups. Analysis of time to first relapse of NLPHL and overall survival was performed using the method of Kaplan and Meier, and the stratified log-rank test was used to assess differences between the 2 groups. P values < 0.05 were considered statistically significant.
RESULTS
Clinical Data
Clinical features are summarized in Table 2. The 11 patients included 9 male and 2 female patients with a median age of 13.8 years (range, 6 to 66 y). All presented with localized peripheral lymphadenopathy, and staging information available for 6 patients confirmed Ann Arbor stage I or II disease.15
TABLE 2.
Clinical and Morphologic Findings of NLPHL With Atypical T Cells
| Case | Age/Sex | Clinical Presentation | Site(s) of Involvement | Pattern in Typical NLPHL Areas* | Architecture of Atypical T-Cell Infiltrate | T-Cell Cytomorphology |
|---|---|---|---|---|---|---|
| 1 | 29/M | Localized LAD (stage I) | Right groin lymph node | B-cell-rich nodular | Interfollicular surrounding primary and secondary reactive follicles | Enlarged nuclei, vesicular chromatin, abundant cytoplasm and occasional mitoses |
| 2 | 12/M | Localized LAD that developed post pharyngitis | Right cervical LN, recurrence at the same site at 20 mo | B-cell-rich nodular | Interfollicular surrounding primary follicles | Mostly medium-sized cells with irregular nuclei and abundant pale cytoplasm |
| 3 | 32/M | Localized LAD | Submandibular LN | B-cell-rich nodular, prominent extranodular LP cells | Interfollicular surrounding primary follicles | Small slightly atypical cells with dispersed chromatin, frequent mitotic activity, and scattered apoptotic cells |
| 4 | 12/M | Localized LAD, no B symptoms, (stage I) | Axillary LN | B-cell-rich nodular with fibrosis, T-cell-rich diffuse | Interfollicular surrounding primary follicles | Medium-sized cells with dispersed chromatin, irregular nuclei, abundant pale cytoplasm and increased mitotic activity, associated with fine compartmentalizing fibrosis |
| 5 | 9/M | Localized LAD for 2 y with recent enlargement (stage II) | Left cervical LN, recurrence in left side of the neck at 6 mo | B-cell-rich nodular with fibrosis, T-cell-rich diffuse, T-cell-rich nodular | Interfollicular surrounding primary follicles | Small-to-medium-sized cells with irregular nuclei, dispersed chromatin, small prominent nucleoli, abundant pale cytoplasm, and increased mitotic activity |
| 6 | 6/M | Localized LAD for 1 y, no B symptoms | Left groin LN | B-cell-rich nodular with fibrosis, T-cell-rich nodular (focal) | Interfollicular surrounding primary and secondary reactive follicles | Small-to-medium-sized cells with oval or irregular nuclei, dispersed chromatin, and increased mitotic activity |
| 7 | 17/M | Localized LAD | Submental LN | B-cell-rich nodular with fibrosis | Interfollicular surrounding primary follicles | Small-to-medium-sized cells with irregular nuclei, dispersed chromatin, abundant eosinophilic cytoplasm, and increased mitoses |
| 8 | 17/M | Localized LAD (stage I) | Cervical LN | B-cell-rich nodular, T-cell-rich nodular, T-cell-rich diffuse | Interfollicular surrounding primary follicles | Small-to-medium-sized cells with oval nuclei, vesicular chromatin, small prominent nucleoli, abundant eosinophilic cytoplasm, and increased mitotic activity |
| 9 | 8/M | Localized LAD (stage II) | Posterior cervical and parotid LN, recurrence in posterior cervical LN (7 mo) | B-cell-rich nodular with fibrosis, T-cell-rich nodular, T-cell-rich diffuse | Interfollicular surrounding primary follicles; vaguely nodular to diffuse on biopsy at relapse | Small-to-medium-sized cells with oval-to- irregular nuclei, vesicular chromatin, and occasional small prominent nucleoli |
| 10 | 66/F | Localized LAD (stage I) | Cervical LN; relapse in supraclavicular LN (6 y). | B-cell-rich nodular | At periphery of and in between nodules | Medium-sized cells with irregular nuclei, abundant pale cytoplasm, and increased mitotic activity |
| 11 | 13/F | Localized LAD | Left cervical LN; relapses in left side of the neck (12 y), and right axillary LN and BM (14 y) | B-cell-rich nodular with fibrosis, T-cell-rich diffuse | At periphery of and in between nodules | Medium-sized and larger cells with round nuclei, prominent nucleoli, and abundant pale cytoplasm with scattered mitoses |
BM indicates bone marrow; F, female; LAD, lymphadenopathy; LN, lymph node; M, male.
As described by Fan et al.12
Further follow-up information was available for 8 patients (median follow-up: 6.4 y). Five patients relapsed with NLPHL: 3 relapsed within 5 years of diagnosis (patients 2, 5, and 9), and 2 relapsed after 5 years (patients 10 and 11). The right cervical lymph node biopsy from patient 2 was diagnosed initially as an atypical lymphoid infiltrate, and he received no treatment. He had recurrent lymphadenopathy at 20 months involving the same area in the right side of the neck; a biopsy showed NLPHL with focal areas containing atypical T cells. A rereview of his previous lymph node biopsy revealed a focal area of NLPHL. No further follow-up information is available. Patient 5 had recurrent NLPHL at 6 months while undergoing chemotherapy (regimen not known) and low-dose radiation therapy; he is alive and in remission 18 years after his original diagnosis. Patient 9 relapsed with NLPHL at 7 months after completion of 4 cycles of vincristine, adriamycin, and methylprednisolone. He was subsequently retreated with 2 cycles of a modified BEACOPP regimen with adriamycin omitted because of his previous therapy (bleomycin, etoposide, cyclophosphamide, vincristine, procarbazine, and prednisone) and low-dose involved-field radiation therapy. He is alive and in remission 6 years after his original diagnosis. Patient 10 declined therapy for her initial tumor and relapsed at 6 years with NLPHL, at which time she again declined therapy; no further follow-up information is known. Patient 11 relapsed at the same lymph node site 12 years after diagnosis and was treated with 6 cycles of adriamycin, bleomycin, vinblastine, and dacarbazine. She experienced a second nodal relapse at a different site 2 years later, at which time a bone marrow biopsy demonstrated involvement by NLPHL and an atypical T-cell infiltrate. She underwent an allogeneic bone marrow transplant for refractory disease 16 years after her original diagnosis and achieved a sustained remission. However, she died of complications related to a cerebrovascular accident 22 years after diagnosis. The remaining 3 patients had no known NLPHL recurrences and are alive and well: patient 1 was treated with radiation alone and is alive without evidence of disease 8 years after diagnosis; treatment was not known in patient 3, but he is alive and in remission 6.6 years after diagnosis; patient 8 was treated with 3 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone and is in remission 9 months after diagnosis.
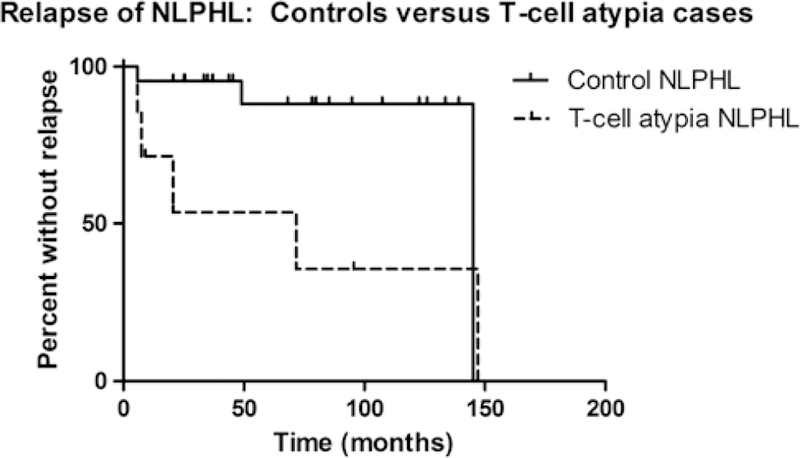
Table 3 compares the clinical features of NLPHL patients with and without morphologically atypical T cells. Both groups showed a similar male predominance. In comparison with control patients, patients with morphologically atypical T cells presented at a younger median age (13.8 vs. 36.1 y, P = 0.015) and had more frequent cervical lymph node involvement (54.5% vs. 8.3% of cases, P = 0.015). The age difference between the 2 groups was more striking when the analysis was restricted to male patients (12.2 vs. 35.2 y, P = 0.002). Patients with morphologically atypical T cells were more likely to develop recurrent NLPHL [5 of 7 (71.4%) vs. 3 of 22 (13.6%) patients with available follow-up information, P = 0.008] and had a shorter time to relapse compared with patients without atypical T cells (P = 0.04) (Fig. 1). No patient in either group had a recurrence of any other type of lymphoma. As described above, 1 patient (patient 11) in the atypical T-cell group died. Two patients in the group lacking atypical T cells died: one due to progressive NLPHL and the other due to acute graft-versus-host disease after an allogeneic stem cell transplant. There was no difference in overall survival between the 2 groups (data not shown).
TABLE 3.
Comparison of Clinical Features Between NLPHL Patients With and Without Atypical T cells
| Characteristic | NLPHL With T-Cell Atypia |
NLPHL Without T-Cell Atypia |
p |
|---|---|---|---|
| N | 11 | 24 | — |
| Sex | |||
| Male | 9 (81.8%) | 19 (79.2%) | NS |
| Female | 2 (18.2%) | 5 (20.8%) | |
| Male:female ratio | 4.5 | 3.8 | |
| Median age (range) | |||
| All patients | 13.8 (6–66) | 36.1 (12–73) | 0.015 |
| Male | 12.2 (6–32) | 35.2 (12–59) | 0.002 |
| Female | 39.9 (13–66) | 37.1 (18–73) | NS |
| Predominant site of nodal involvement | 0.015* | ||
| Cervical | 6 (54.5%) | 2 (8.3%) | |
| Submental/ | 2 (18.2%) | 3 (12.5%) | |
| submandibular | |||
| Inguinal | 2 (18.2%) | 7 (29.2%) | |
| Axillary | 1 (9.1%) | 5 (20.8%) | |
| Parotid | 0 (0%) | 4 (16.7%) | |
| Retroperitoneal | 0 (0%) | 2 (8.3%) | |
| Abdominal | 0 (0%) | 1 (4.2%) | |
| Relapse of NLPHL | 5/7 (71.4%) | 3/22 (13.6%) | 0.008 |
NS, not statistically significant.
P value reflects frequency of cervical involvement vs. all other nodal sites of disease.
FIGURE 1.
Kaplan-Meier analysis of time to first relapse following the initial diagnosis of NLPHL. Patients with morphologically atypical T cells had a shorter time to relapse compared with control patients lacking morphologically atypical T cells (P = 0.04). Broken line indicates NLPHL patients with morphologically atypical T cells and solid line indicates control patients. Vertical lines represent censored patients.
Morphology
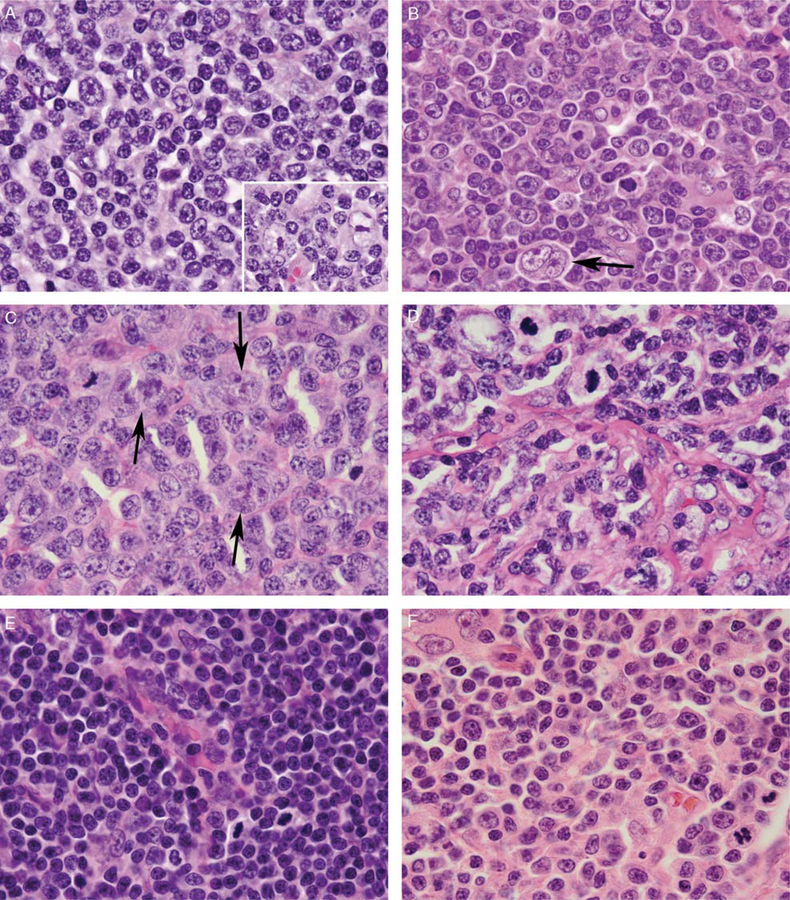
The overall morphologic findings are summarized in Table 2. The atypical interfollicular and parafollicular T cells exhibited a morphologic spectrum across cases; however, they were enlarged (larger than small B cells within primary follicles or mantle zones) in 10 cases, had moderately abundant pale or eosinophilic cytoplasm in 8 cases, highly irregular nuclear contours in 7 cases, dispersed chromatin in 5 cases, vesicular chromatin in 3 cases, and prominent nucleoli in 4 cases (Fig. 2). Mitotic figures were visibly increased (2 to 10 per high-power field) in 9 cases (Fig. 2). Scattered apoptotic cells were seen in 1 case; however, no case contained areas of geographic necrosis. In all 11 cases, rare large cells with irregular, multilobated nuclei, pale chromatin, and prominent nucleoli, consistent with LP cells, were identified within the atypical T-cell-rich areas (Fig. 2A–C and Fig. 4B).
FIGURE 2.
Cytologic features of NLPHL with atypical T cells. Atypical T cells demonstrated a morphologic spectrum but were typically larger than the small lymphocytes within primary follicles (A, case 1; B, case 5; C, case 7; D, case 4; E, case 6; F, case 11), with vesicular (A) or dispersed (B-E) chromatin, irregular nuclei (B-D), occasional prominent nucleoli (B, F), moderately abundant pale eosinophilic cytoplasm (B-C, F), and increased mitotic activity (B-F). Rare scattered LP cells with multilobated nuclei, pale chromatin, and prominent nucleoli were visible within the T-cell-rich areas, both interspersed among the atypical T cells and at the periphery of primary follicles (A inset; B-C, arrows). In case 4, the T cells were associated with fine compartmentalizing background fibrosis (D).
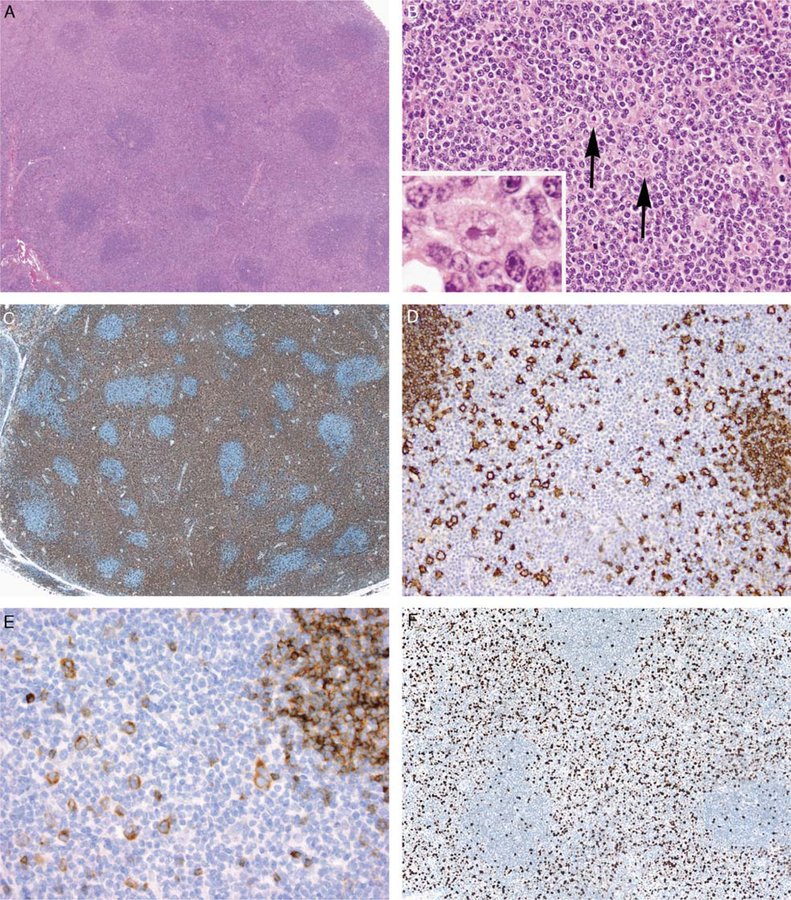
FIGURE 4.
Immunohistochemical features of NLPHL with atypical T cells. A, Low-power view of case 3 showing the T-cell-rich area with an interfollicular growth pattern. B, On higher magnification, the T cells were relatively small and only slightly atypical with dispersed chromatin and occasional apoptotic cells; scattered LP cells were present in these areas (arrows and inset). C, A CD3 stain highlights T-cell-rich areas. D, A CD20 stain highlights scattered large cells within T-cell-rich areas, consistent with LP cells. Small primary follicles at the left-hand and right-hand sides of the image also stain for CD20. E, LP cells were positive for IgD in this case. F, The T-cell-rich areas demonstrated an elevated proliferation index by Ki67 staining (approximately 30% in this case). Note the contrast with the much lower proliferation index in the B cells of the adjacent primary follicles.
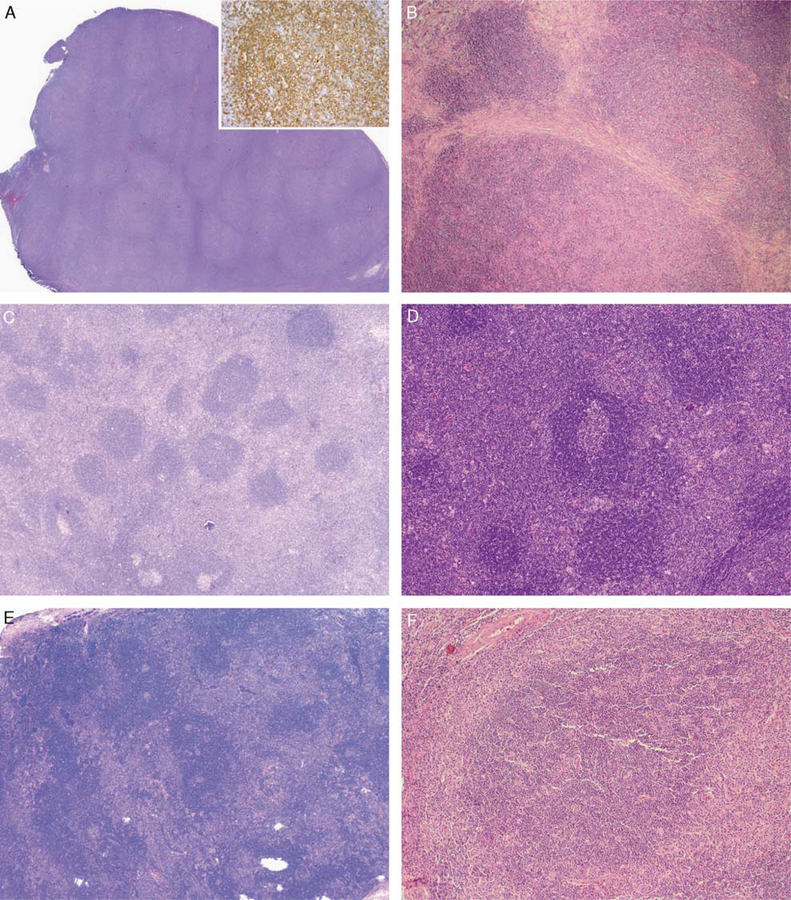
In all cases, areas of NLPHL with typical B-cell-rich nodules were present at least focally (Figs. 3A, B). In 6 cases, conspicuous bands of fibrosis partially or completely surrounded the expanded B-cell-rich nodules (Fig. 3B, F), whereas in 1 case (case 4) there was fine compartmentalizing fibrosis in the diffuse T-cell-rich areas (Fig. 2D). Additional immunoarchitectural patterns as described by Fan et al12 were present as follows: T-cell-rich nodular and T-cell-rich diffuse in each of 4 cases and nodular with prominent extrafollicular LP cells in 1 case.
FIGURE 3.
Architectural features of NLPHL with atypical T cells. A, All cases contained at least focal areas of NLPHL with typical B-cell-rich nodules (inset: CD20 stain), as illustrated in this representative image from case 1. B, In some cases, such as case 4, conspicuous fibrotic bands surrounded expanded B-cell-rich nodules. C, Case 1 also contained areas with a distinct interfollicular growth pattern, seen in the majority of cases, in which sheets of atypical T cells were present around mostly primary follicles and some secondary follicles. D, Case 6 with interfollicular atypical T cells, surrounding primary and secondary follicles; secondary follicles were prominent in this case. E, In a minority of cases, the atypical T cells formed large aggregates at the periphery of and in between expanded B-cell-rich nodules of NLPHL, as shown in this representative image from case 10. F, Case 11 also showed the less common parafollicular growth pattern, with atypical T cells surrounding an enlarged B-cell-rich NLPHL nodule. Note the narrow band of fibrosis partially encircling the atypical T-cell-rich area on the left.
In 9 cases (cases 1 to 9, all male patients), atypical T cells effaced the nodal architecture and formed prominent interfollicular sheets surrounding primary and secondary reactive follicles in a pattern mimicking the T-zone pattern of peripheral T-cell lymphoma, not other-wise specified (PTCL-NOS); expanded B-cell-rich nodules were not present in these areas (Fig. 3C, D and Fig. 4A).10,43
In case 2, this interfollicular growth pattern predominated on the initial biopsy, and the diagnosis of NLPHL was made retrospectively when recurrent lymphadenopathy developed 2 years later, with involvement by NLPHL containing a predominance of B-cell-rich no-dules. In case 5, areas containing atypical T cells comprised a greater proportion of the biopsied tissue at relapse. In case 9, the biopsy taken at the time of relapse showed atypical T cells to have a vaguely nodular to diffuse growth pattern lacking primary and secondary follicles.
In 2 cases (cases 10 and 11, both female patients), atypical T cells formed large clusters or aggregates at the periphery of and in between expanded B-cell-rich nodules (Fig. 3E, F). At relapse, NLPHL in patient 10 contained only B-cell-rich nodules and lacked any atypical T cells. From patient 11, only lymph node biopsies from the relapses at 12 and 14 years after her original diagnosis were available for review. The later biopsy demonstrated coalescence of the atypical T-cell aggregates with effacement of the nodal architecture and replacement of the NLPHL pattern in some areas, raising the possibility of concurrent nodal involvement by PTCL-NOS.18
Immunophenotype
Results of immunohistochemical stains are summarized in Table 4. Nine cases contained CD20+, CD15–, and CD30– LP cells within areas of typical NLPHL; LP cells showed weak coexpression of CD30– in 2 cases.40 The LP cells within T-cell-rich areas expressed the same immunophenotype (Fig. 4D). The LP cells were positive for IgD in 5 of 7 cases tested (71.4%) (Fig. 4E). Among 13 control cases stained for IgD, 3 (23.1%) were IgD+, a difference that did not reach statistical significance (P = 0.06).
TABLE 4.
Immunohistochemical Features of NLPHL With Atypical T Cells
| LP Cell Immunophenotype |
T-Cell Immunophenotype |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | CD20 | CD30 | CD15 | IgD | Pan- T* |
CD4 vs. CD8 pre-dominance † | CD45RA vs. CD45RO Pre-dominance | CD10 | bcl-6 | CD57 | PD-1 | T-bet | CD25 | FOXP3 | Perforin | Gran-zyme B |
Ki67 PI |
| 1 | + | – | – | ND | + | Equal | CD45RO | ND | ND | ND | ND | ND | ND | ND | ND | ND | 40% |
| 2 | + | – | – | + | + | CD8 | Equal | – | Rare | Rare | – | Many | Many | Rare | Occ | Occ | 40% |
| + | + | + | + | + | + | + | |||||||||||
| 3 | + | – | – | + | + | CD4 | CD45RO | – | Rare | Occ | Occ | Many | Many | Rare + | Rare + | Occ | 30% |
| + | + | + | + | + | + | ||||||||||||
| 4 | + | – | – | ND | + | ND | CD45RO | ND | ND | – | ND | ND | ND | ND | ND | ND | ND |
| 5 | + | – | – | ND | + | CD4 | CD45RO | ND | ND | – | ND | ND | ND | ND | ND | ND | ND |
| 6 | + | – | – | + | + | CD4 | CD45RO | – | Rare | – | – | ND | ND | Rare + | Rare + | Rare | 20% |
| + | + | ||||||||||||||||
| 7 | + | – | – | + | + | CD4 | CD45RO | – | – | – | Occ | ND | Rare | Rare + | – | Rare | ND |
| + | + | + | |||||||||||||||
| 8 | + | +‡ | – | – | + | CD4 | ND | Rare | Many | ND | Many | ND | ND | ND | ND | ND | 50% |
| + | + | + | |||||||||||||||
| 9 | + | +‡ | – | + | + | CD4 | ND | ND | Many | Rare | ND | ND | ND | ND | ND | ND | 40%§ |
| + | + | ||||||||||||||||
| 10 | + | – | – | – | + | CD4 | Equal | – | ND | Occ | Occ | Rare | – | – | Rare + | Rare | 30% |
| + | + | + | + | ||||||||||||||
| 11 | + | – | – | + | + | ND | CD45RA | ND | ND | ND | ND | ND | ND | ND | ND | ND | 25% |
indicates positive; –, negative; IgD, immunoglobulin D heavy chain; ND, not done; Occ, occasional; PI, proliferation index. The proportion of T cells staining positively for CD10, bcl-6, CD57, PD-1, T-bet, CD25, FOXP3, perforin, and granzyme B was quantified as follows: rare, < 10% of cells; occasional, 10% to 30% of cells; many, 30% to 50% of cells; most, >50% of cells.
Pan-T-cell antigens tested in each case: cases 1, 2, 3, 6, and 7: CD2, CD3, CD5, CD7, and CD43; case 4: CD43; cases 5 and 11: CD3 and CD43; case 8: CD3 and CD5; case 9: CD2, CD3, CD5, and CD7; case 10: CD2, CD3, CD5, and CD43.
Determined by flow cytometry and immunohistochemistry in cases 8 and 10, by flow cytometry alone in case 9, and by immunohistochemistry alone in all other cases tested.
A small subset of LP cells showed weak staining for CD30 in these cases, but LP cells were strongly CD20+ and positive for bcl-6, OCT2, and BOB.1.
Performed on the patient’s second lymph node biopsy showing relapsed disease.
Ki67 staining performed in 8 cases showed the T-cell-rich areas to have a moderately elevated proliferation index, with positivity ranging from 20% to 50% of all cells (median, 35%) (Fig. 4F). This was significantly higher than the median proliferation index of 10% (range, 5% to 15%) encountered in T-cell-rich nodular and diffuse areas of 5 control cases that were stained with Ki67 for comparison (P = 0.0003). No case showed any pan-T-cell antigen loss. The atypical T cells exhibited variable CD4:CD8 and CD45RA:CD45RO ratios among cases and a variable immunophenotype with respect to germinal center (CD10, bcl-6), follicular T-helper (CD57, PD-1), T-regulatory (CD25, FOXP3), cytotoxic (granzyme B, perforin), and Th1-related (T-bet) T-cell markers, with only rare-to-occasional T-cell staining positively for these antigens in most cases. However, in 2 cases (cases 2 and 3), many T cells were CD25+ and T-bet+, and in 2 cases (cases 8 and 9), many T cells were bcl-6+; one of the latter cases (case 8) also had many PD-1+ cells. Flow cytometry performed in 3 cases (cases 8, 9, and 10) showed polytypic B cells and no evidence of CD4/ CD8 coexpressing cells.
Molecular Genetic Analysis
Results of molecular genetic studies are summarized in Table 5. In situ hybridization for Epstein-Barr virus-encoded ribonucleic acid was negative in both the LP cells and the atypical T cells of all 8 cases tested. TCR gene rearrangement studies were performed by Southern blot or PCR in 9 cases. All 6 cases with sufficient material for analysis showed no evidence of a clonal T-cell population. In the remaining 3 cases, PCR for the TCRγ gene showed no detectable amplification, which may have been related to the age of the paraffin-embedded tissue samples and type of fixative used.
TABLE 5.
Molecular Genetic Features of NLPHL With Atypical T Cells
|
TCR Gene |
||||
|---|---|---|---|---|
| Case | EBER | β | γ | Method(s) for TCR Clonality Assessment |
| 1 | ND | ND | ND | — |
| 2 | Negative | ND | Polyclonal | PCR* |
| 3 | Negative | ND | Polyclonal | PCR |
| 4 | Negative | ND | No amplification | PCR |
| 5 | Negative | Polyclonal | ND | Southern blot |
| 6 | Negative | ND | No amplification | PCR |
| 7 | Negative | ND | No amplification | PCR |
| 8 | Negative | ND | ND | — |
| 9 | Negative | Polyclonal | Polyclonal | Southern blot† |
| 10 | ND | Polyclonal | ND | Southern blot* |
| 11 | ND | Polyclonal | Polyclonal | PCR (γ) and Southern blot (β and γ)‡ |
EBER, Epstein-Barr virus encoded RNA; ND, not done.
Performed on the patient’s first lymph node biopsy showing a predominance of atypical T cells.
Performed on the patient’s second lymph node biopsy showing relapsed disease.
Performed on both the patient’s second and third lymph node biopsies showing relapsed disease
DISCUSSION
We present clinical and pathologic features of a rare morphologic variant of NLPHL characterized by prominent populations of atypical T cells that may mimic PTCL-NOS. Unlike other previously described T-cell-rich variants of NLPHL, the background non-neoplastic T cells in the cases in this series have conspicuous cytomorphologic atypia: enlarged and irregular nuclei, dispersed or vesicular chromatin, frequent mitoses, increased proliferative activity, and/or prominent nucleoli (Fig. 2). The interfollicular growth pattern characterizing most cases, in which LP cells and associated atypical T cells surround primary and secondary reactive follicles, has not been described previously in NLPHL to our knowledge. Although reactive germinal centers may be present in lymph nodes involved by NLPHL, they are typically present only focally, usually at the periphery of the involved node; it is unusual to see intact primary and secondary follicles completely surrounded by lymphomatous growth.12,17 From 1998 to 2009, 25 cases of NLPHL were diagnosed in patients presenting at the MGH, of which a single case contained atypical T cells (case 9 in this series). Therefore, we estimate the incidence of T-cell atypia to occur in approximately 4% of NLPHL cases.
Patients with NLPHL containing atypical T cells share certain clinical characteristics, including a younger age at onset (particularly in male patients) and more frequent cervical lymph node involvement, when compared with NLPHL cases lacking atypical T cells. Similar clinical features have been described in IgD+ NLPHL, and LP cells were IgD+ in the majority of cases in this series, raising the possibility of a relationship between these cases and IgD+ NLPHL.30 However, significant T-cell atypia was not observed in the previously studied series of cases of IgD+ NLPHL (S. Pittaluga, personal communication). Moreover, IgD positivity was detected with relative frequency (3 of 13, 23.1%) in control cases lacking T-cell atypia, at a level similar to that seen in the larger series by Prakash et al.30 Given that cases of IgD+ NLPHL have a tendency to be rich in background T cells,30 the high rate of IgD expression in our cases may reflect their prominent T-cell-rich background. Finally, NLPHL patients with morphologically atypical T cells were more likely to develop recurrent NLPHL and had a shorter time to relapse but similar overall survival, compared with patients lacking atypical T cells. A similar higher rate of relapse has been noted in NLPHL with T-cell-rich diffuse areas.4,12
Awareness of this unusual variant of NLPHL is important because the pattern of infiltration, cytology, and moderately elevated proliferation index of the back-ground T cells may raise the possibility of PTCL-NOS. Rare cases of composite NLPHL and PTCL-NOS have been reported.2,11 In all such cases, the neoplastic T-cell component exhibited morphologic or immunophenotypic features that were strongly suggestive of malignancy (such as diffuse infiltration by large, malignant-appearing cells, necrosis, pan-T-cell antigen loss, and/or a markedly elevated Ki67 proliferation index), and a clonal T-cell population was confirmed by molecular genetic analysis.2,11 All patients were adults and the majority presented with advanced-stage disease and/or had progressive disease despite receiving combination chemotherapy.2,11 In contrast, the atypical T cells in our NLPHL cases lack evidence of pan-T-cell antigen loss or T-cell clonality by gene rearrangement studies, supporting them to be non-neoplastic in nature, albeit morphologically atypical. In addition, although only limited clinical follow-up was available in our patients, all presented with localized lymphadenopathy, a clinical presentation more in keeping with NLPHL and distinct from the usually advanced clinical presentation of PTCL-NOS.13,20,35,36,47
The distinction between NLPHL with atypical T cells versus a composite lymphoma is clinically relevant because PTCL-NOS is generally aggressive with a poor prognosis and requires a different therapeutic approach from NLPHL.6,13,20,28,29,35,36,47 Indeed, patient 2 was initially suspected to have PTCL-NOS; however, given his young age and the absence of an aberrant T-cell immunophenotype or T-cell clonality by gene rearrangement studies, a definitive diagnosis of lymphoma was not rendered. Twenty months later, NLPHL developed in the same nodal site, and a retrospective review of his initial lymph node biopsy confirmed the presence of NLPHL with a predominant T-cell-rich interfollicular growth pattern and a focal B-cell-rich follicular growth pattern. In this case, awareness of this unusual pattern of NLPHL could have suggested the correct diagnosis at the time of initial biopsy. Patient 11 was suspected to have a diagnosis of composite NLPHL and PTCL-NOS, particularly at the time of her third biopsy, demonstrating a more prominent growth of the T-cell aggregates with areas of architectural effacement.18 However, no clonal T-cell population could be demonstrated in either of her relapse biopsies by PCR and Southern blot of the TCRγ and TCRβ genes (Table 4). Ultimately, the patient underwent allogeneic bone marrow transplant for refractory NLPHL and developed a sustained remission; however, the patient died 6 years later of an unrelated cause. Although it is not possible to exclude the diagnosis of PTCL-NOS in this case, the clinical and molecular genetic features are consistent with an unusual reactive T-cell proliferation. Knowledge that rare cases of NLPHL may contain significant numbers of atypical T cells should prompt pathologists to maintain a high threshold for making the diagnosis of composite NLPHL and PTCL-NOS, particularly in younger patients in whom PTCL-NOS is uncommon, and to rely on a combination of morphologic, immunophenotypic, and molecular genetic evidence to render such a diagnosis.2,11
Various interactions between the neoplastic cells in Hodgkin lymphoma and the tumor microenvironment have been described and are thought to play an important role in the pathogenesis of this neoplasm and in fostering immune privilege of the neoplastic cells.1,3,14,21,39 More-over, T cells in NLPHL have previously been shown to have a distinct activation profile by immunohistochemistry, flow cytometry, and gene expression profiling, particularly in cases containing T-cell-rich diffuse areas.3,22,32 In an attempt to delineate more clearly the nature of the atypical T cells in this unusual variant of NLPHL, we performed extensive immunohistochemical analysis (Table 4). These studies failed to reveal a consistent immunophenotype among the atypical T cells, with only rare-to-occasional cells demonstrating positivity for germinal center, T-regulatory, cytotoxic, or Th1-related antigens in most cases. Although follicular T-helper cells are increased within the B-cell-rich nodules of NLPHL,24,26 the atypical parafollicular and interfollicular T cells in these cases were generally negative or only focally positive for bcl-6, PD-1, and CD57, with the exception of case 8, in which areas of T-cell atypia were in close proximity to areas of conventional NLPHL with expanded B-cell-rich nodules. Cases also demonstrated variable CD4:CD8 and CD45RA:CD45RO ratios. These findings suggest that the morphologic appearance of T cells in these cases may reflect a heterogeneous phenomenon of T-cell activation rather than a specific T-cell immune response. Interestingly, cases of PTCL-NOS arising after a diagnosis of NLPHL have been reported, and some studies suggest an increased incidence of T-cell lymphoma after a diagnosis of NLPHL.2,5,37,44 Any relationship between cases of NLPHL with morphologically atypical T cells described here, cases of composite NLPHL and PTCL-NOS,2,11 and cases of PTCL-NOS occurring after a diagnosis of NLPHL2,5,37,44 remains to be determined. It is possible that these entities represent different forms of T-cell activation and proliferation in NLPHL that span a spectrum ranging from reactive atypia in some cases to overt malignancy in others.
In summary, we describe a rare and unusual clinicopathologic variant of NLPHL with morphologically atypical T cells in a growth pattern that may mimic nodal involvement by PTCL-NOS. In comparison with NLPHL patients lacking atypical T cells, affected patients are younger, with frequent cervical lymph node involvement. NLPHL patients with morphologically atypical T cells have a similar clinical presentation to conventional NLPHL with localized lymphadenopathy. Clinical follow-up was limited in this small series but suggests a prognosis analogous to that of NLPHL containing T-cell-rich diffuse areas, with an increased risk of recurrence but no difference in overall survival. Awareness of the distinct clinicopathologic features of this rare NLPHL variant is important in avoiding a misdiagnosis of concomitant PTCL-NOS and in suggesting the correct diagnosis of NLPHL with morphologically atypical T cells when this growth pattern predominates. Further studies are needed to determine the factors that drive the atypical T-cell proliferation and to confirm its impact on prognosis.
ACKNOWLEDGMENTS
The authors thank Dr Janina A. Longtine of the Center for Advanced Molecular Diagnostics, Brigham and Women’s Hospital, Boston, MA for technical assistance with gene rearrangement studies; Dr Robert B. Colvin; Ms Tricia Della Pelle; and Ms Guilin (Linda) Wang of the Department of Pathology, Massachusetts General Hospital for technical assistance with certain immunohistochemical studies; Mr Stephen Conley and Ms Michelle Lee of the MGH Pathology Photography Laboratory for technical assistance with certain illustrations; and the following pathologists and oncologists for providing case materials and clinical follow-up information: Dr Anthony F. D’Aguillo, Somerset Medical Center, Somerville, NJ; Dr Damion L. Kistler, Mercy Health Center, Oklahoma City, OK; Dr Charles F. Luyrink, Harrington Memorial Hospital, Southbridge, MA; Dr Rene´ McNall, Children’s Hospital, University of Oklahoma Health Sciences Center, Oklahoma City, OK; and Dr Caroline Meunier, Hoˆpital Maisonneuve-Rosemont, Boucherville, QC, Canada.
Footnotes
Conflicts of Interest and Source of Funding: The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
REFERENCES
- 1.Alvaro T, Lejeune M, Salvado MT, et al. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res 2005;11: 1467–1473. [DOI] [PubMed] [Google Scholar]
- 2.Arevalo A, Caponetti GC, Hu Q, et al. Cytotoxic peripheral T cell lymphoma arising in a patient with nodular lymphocyte pre-dominant Hodgkin lymphoma: a case report. J Hematop 2010;3: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atayar C, Poppema S, Visser L, et al. Cytokine gene expression profile distinguishes CD4+/CD57+ T cells of the nodular lymphocyte predominance type of Hodgkin’s lymphoma from their tonsillar counterparts. J Pathol 2006;208:423–430. [DOI] [PubMed] [Google Scholar]
- 4.Bakshi N, Aljabry M, Akhter S, et al. Tumor microenvironment in nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) influences occurrence of relapses and progression to large cell lymphoma (abstract). Blood 2010;116:2684.20610818 [Google Scholar]
- 5.Bennett MH, MacLennan KA, Vaughan Hudson G, et al. Non-Hodgkin’s lymphoma arising in patients treated for Hodgkin’s disease in the BNLI: a 20-year experience. British National Lymphoma Investigation. Ann Oncol 1991;2(Suppl 2):83–92. [DOI] [PubMed] [Google Scholar]
- 6.Biasoli I, Stamatoullas A, Meignin V, et al. Nodular, lymphocyte-predominant Hodgkin lymphoma: a long-term study and analysis of transformation to diffuse large B-cell lymphoma in a cohort of 164 patients from the Adult Lymphoma Study Group. Cancer 2010;116:631–639. [DOI] [PubMed] [Google Scholar]
- 7.Boudova L, Torlakovic E, Delabie J, et al. Nodular lymphocyte-predominant Hodgkin lymphoma with nodules resembling T-cell/ histiocyte-rich B-cell lymphoma: differential diagnosis between nodular lymphocyte-predominant Hodgkin lymphoma and T-cell/ histiocyte-rich B-cell lymphoma. Blood 2003;102:3753–3758. [DOI] [PubMed] [Google Scholar]
- 8.Bowen MB, Butch AW, Parvin CA, et al. Germinal center T cells are distinct helper-inducer T cells. Hum Immunol 1991;31:67–75. [DOI] [PubMed] [Google Scholar]
- 9.Browne P, Petrosyan K, Hernandez A, et al. The B-cell transcription factors BSAP, Oct-2, and BOB.1 and the pan-B-cell markers CD20, CD22, and CD79a are useful in the differential diagnosis of classic Hodgkin lymphoma. Am J Clin Pathol 2003;120: 767–777. [DOI] [PubMed] [Google Scholar]
- 10.de Leval L, Ralfkiaer E, Jaffe ES. Peripheral T-cell lymphoma, not otherwise specified. In: Jaffe ES, Harris NL, Vardiman JW, et al. , eds. Hematopathology St. Louis MO: Elsevier; 2011:545–546. [Google Scholar]
- 11.Delabie J, Greiner TC, Chan WC, et al. Concurrent lymphocyte predominance Hodgkin’s disease and T-cell lymphoma: a report of three cases. Am J Surg Pathol 1996;20:355–362. [DOI] [PubMed] [Google Scholar]
- 12.Fan Z, Natkunam Y, Bair E, et al. Characterization of variant patterns of nodular lymphocyte predominant Hodgkin lymphoma with immunohistologic and clinical correlation. Am J Surg Pathol 2003;27:1346–1356. [DOI] [PubMed] [Google Scholar]
- 13.Gallamini A, Stelitano C, Calvi R, et al. Peripheral T-cell lymphoma unspecified (PTCL-U): a new prognostic model from a retrospective multicentric clinical study. Blood 2004;103:2474–2479. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi MK, Lambley E, Duraiswamy J, et al. Expression of LAG-3 by tumor-infiltrating lymphocytes is coincident with the suppression of latent membrane antigen-specific CD8+ T-cell function in Hodgkin lymphoma patients. Blood 2006;108:2280–2289. [DOI] [PubMed] [Google Scholar]
- 15.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manual New York, NY: Springer; 2002. [Google Scholar]
- 16.Greiner A, Tobollik S, Buettner M, et al. Differential expression of activation-induced cytidine deaminase (AID) in nodular lymphocyte-predominant and classical Hodgkin lymphoma. J Pathol 2005;205:541–547. [DOI] [PubMed] [Google Scholar]
- 17.Harris NL. The relationship between Hodgkin’s disease and non-Hodgkin’s lymphoma. Semin Diagn Pathol 1992;9:304–310. [PubMed] [Google Scholar]
- 18.Harris NL. Hodgkin’s disease: classification and differential diagnosis. Mod Pathol 1999;12:159–175. [PubMed] [Google Scholar]
- 19.Higgins JP, Van de Rijn M, Jones CD, et al. Peripheral T-cell lymphoma complicated by a proliferation of large B cells. Am J Clin Pathol 2000;114:236–247. [DOI] [PubMed] [Google Scholar]
- 20.Illes A, Simon Z, Toth E, et al. Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL): clinicopathological features based on the data of two Hungarian lymphoma centres. Pathol Oncol Res 2008;14:411–421. [DOI] [PubMed] [Google Scholar]
- 21.Ishida T, Ishii T, Inagaki A, et al. Specific recruitment of CC chemokine receptor 4-positive regulatory T cells in Hodgkin lymphoma fosters immune privilege. Cancer Res 2006;66: 5716–5722. [DOI] [PubMed] [Google Scholar]
- 22.Lin P, Medeiros LJ, Wilder RB, et al. The activation profile of tumour-associated reactive T-cells differs in the nodular and diffuse patterns of lymphocyte predominant Hodgkin’s disease. Histo-pathology 2004;44:561–569. [DOI] [PubMed] [Google Scholar]
- 23.Loddenkemper C, Anagnostopoulos I, Hummel M, et al. Differential Emu enhancer activity and expression of BOB.1/OBF.1, Oct2, PU.1, and immunoglobulin in reactive B-cell populations, B-cell non-Hodgkin lymphomas, and Hodgkin lymphomas. J Pathol 2004;202:60–69. [DOI] [PubMed] [Google Scholar]
- 24.Nam-Cha SH, Roncador G, Sanchez-Verde L, et al. PD-1, a follicular T-cell marker useful for recognizing nodular lymphocyte-predominant Hodgkin lymphoma. Am J Surg Pathol 2008;32:1252–1257. [DOI] [PubMed] [Google Scholar]
- 25.Nascimbeni M, Shin EC, Chiriboga L, et al. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood 2004;104:478–486. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen PL, Ferry JA, Harris NL. Progressive transformation of germinal centers and nodular lymphocyte predominance Hodgkin’s disease: a comparative immunohistochemical study. Am J Surg Pathol 1999;23:27–33. [DOI] [PubMed] [Google Scholar]
- 27.Oh YH, Weng A, Connors J, et al. CD4/CD8-double positive T cells in nodular lymphocyte predominance Hodgkin lymphoma (abstract). Haematologica 2007;92(s5):3. [Google Scholar]
- 28.Pileri SA, Weisenburger DD, Sng I, et al. Peripheral T-cell lymphoma, not otherwise specified. In: Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues Lyon, France: IARC Press; 2008:306–308. [Google Scholar]
- 29.Poppema S, Delsol G, Pileri SA, et al. Nodular lymphocyte predominant Hodgkin lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al. , eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues Lyon, France: IARC Press; 2008:323–325. [Google Scholar]
- 30.Prakash S, Fountaine T, Raffeld M, et al. IgD positive L&H cells identify a unique subset of nodular lymphocyte predominant Hodgkin lymphoma. Am J Surg Pathol 2006;30:585–592. [DOI] [PubMed] [Google Scholar]
- 31.Quintanilla-Martinez L, Fend F, Moguel LR, et al. Peripheral T-cell lymphoma with Reed-Sternberg-like cells of B-cell phenotype and genotype associated with Epstein-Barr virus infection. Am J Surg Pathol 1999;23:1233–1240. [DOI] [PubMed] [Google Scholar]
- 32.Rahemtullah A, Reichard KK, Preffer FI, et al. A double-positive CD4+CD8+ T-cell population is commonly found in nodular lymphocyte predominant Hodgkin lymphoma. Am J Clin Pathol 2006;126:805–814. [DOI] [PubMed] [Google Scholar]
- 33.Rahemtullah A, Harris NL, Dorn ME, et al. Beyond the lymphocyte predominant cell: CD4+CD8+ T-cells in nodular lymphocyte predominant Hodgkin lymphoma. Leuk Lymphoma 2008;49: 1870–1878. [DOI] [PubMed] [Google Scholar]
- 34.Reichard KK, Schwartz EJ, Higgins JP, et al. CD10 expression in peripheral T-cell lymphomas complicated by a proliferation of large B-cells. Mod Pathol 2006;19:337–343. [DOI] [PubMed] [Google Scholar]
- 35.Reiser M, Josting A, Soltani M, et al. T-cell non-Hodgkin’s lymphoma in adults: clinicopathological characteristics, response to treatment and prognostic factors. Leuk Lymphoma 2002;43:805–811. [DOI] [PubMed] [Google Scholar]
- 36.Rizvi MA, Evens AM, Tallman MS, et al. T-cell non-Hodgkin lymphoma. Blood 2006;107:1255–1264. [DOI] [PubMed] [Google Scholar]
- 37.Rysenga E, Linden MD, Carey JL, et al. Peripheral T-cell non-Hodgkin’s lymphoma following treatment of nodular lymphocyte predominance Hodgkin’s disease. Arch Pathol Lab Med 1995;119: 88–91. [PubMed] [Google Scholar]
- 38.Schmid C, Sargent C, Isaacson PG. Land H cells of nodular lymphocyte predominant Hodgkin’s disease show immunoglobulin light-chain restriction. Am J Pathol 1991;139:1281–1289. [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz R, Stanelle J, Hansmann ML, et al. Pathogenesis of classical and lymphocyte-predominant Hodgkin lymphoma. Annu Rev Pathol 2009;4:151–174. [DOI] [PubMed] [Google Scholar]
- 40.Seliem RM, Wang WL, Hasserjian RP, et al. Nodular lymphocyte predominant Hodgkin lymphoma (NLPHL) with CD30 positive lymphocyte predominant (LP) cells. J Hematop 2011. (in press). [DOI] [PMC free article] [PubMed]
- 41.Stein H, Marafioti T, Foss HD, et al. Down-regulation of BOB.1/ OBF.1 and Oct2 in classical Hodgkin disease but not in lymphocyte predominant Hodgkin disease correlates with immunoglobulin transcription. Blood 2001;97:496–501. [DOI] [PubMed] [Google Scholar]
- 42.Stoler MH, Nichols GE, Symbula M, et al. Lymphocyte predominance Hodgkin’s disease: evidence for a kappa light chain-restricted monotypic B-cell neoplasm. Am J Pathol 1995;146:812–818. [PMC free article] [PubMed] [Google Scholar]
- 43.Suchi T, Lennert K, Tu LY, et al. Histopathology and immunohistochemistry of peripheral T cell lymphomas: a proposal for their classification. J Clin Pathol 1987;40:995–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tefferi A, Wiltsie JC, Kurtin PJ. Secondary T-cell lymphoma in the setting of nodular lymphocyte predominance Hodgkin’s disease. Am J Hematol 1992;40:232–233. [DOI] [PubMed] [Google Scholar]
- 45.Timens W, Visser L, Poppema S. Nodular lymphocyte predominance type of Hodgkin’s disease is a germinal center lymphoma. Lab Invest 1986;54:457–461. [PubMed] [Google Scholar]
- 46.van Dongen JJ, Langerak AW, Bruggemann M, et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98–3936. Leukemia 2003;17:2257–2317. [DOI] [PubMed] [Google Scholar]
- 47.Weisenburger DD, Savage KJ, Harris NL, et al. Peripheral T-cell lymphoma, not otherwise specified: a report of 340 cases from the International Peripheral T-cell Lymphoma Project. Blood 2011;117: 3402–3408. [DOI] [PubMed] [Google Scholar]
- 48.Zettl A, Lee SS, Rudiger T, et al. Epstein-Barr virus-associated B-cell lymphoproliferative disorders in angioimmunoblastic T-cell lymphoma and peripheral T-cell lymphoma, unspecified. Am J Clin Pathol 2002;117:368–379. [DOI] [PubMed] [Google Scholar]