Abstract
Background:
Current guidelines recommend adjuvant chemotherapy for resected pancreatic adeno-carcinoma (PDAC). However, no studies have addressed its survival benefit for stage I patients as they comprise <10% of PDAC.
Methods:
Using the NCDB 2006–2012, resected PDAC patients with stage I disease who received adjuvant therapy (chemotherapy or chemoradiation) were analyzed. Factors associated with overall survival (OS) were identified.
Results:
3909 patients with resected stage IA or IB PDAC were identified. Median OS was 60.3 months (mo) for stage IA and 36.9 mo for IB. 45.5% received adjuvant chemotherapy; 19.9% received adjuvant chemoradiation. There was OS benefit for both stage IA/IB patients with adjuvant chemotherapy (HR = 0.73 and 0.76 for IA and IB, respectively, p = 0.002 and <0.001). For patients with Stage IA disease (n = 1,477, 37.8%), age ≥70 (p < 0.001), higher grade (p < 0.001), ≤10 lymph nodes examined (p = 0.008), positive margins (p < 0.001), and receipt of adjuvant chemoradiation (p = 0.002) were associated with worse OS. For stage IB patients (n = 2,432, 62.2%), similar associations were observed with the exception of adjuvant chemoradiation whereby there was no significant association (p = 0.35).
Conclusion:
Adjuvant chemotherapy was associated with an OS benefit for patients with stage I PDAC; adjuvant chemoradiation was either of no benefit or associated with worse OS.
Introduction
Annually over 43,000 people are diagnosed with pancreatic adenocarcinoma (PDAC) in the United States.1 Approximately 15% of patients who present with PDAC are resectable at the time of diagnosis. Of those that do present with resectable disease, stage IA and IB patients comprise less than 10–15% of the population. For all patients with resectable disease, even after resection, 5-year overall survival (OS) is still less than 20%.2–4 Due to the poor OS and high recurrence rate, adjuvant chemo-therapy is recommended by current National Comprehensive Cancer Network guidelines (NCCN).5 These recommendations are based on prospective randomized trials such as CONKO 001 and ESPAC 1–3 which showed a survival benefit with adjuvant therapy over surgery alone.6–9
However, when these studies are analyzed for the percentage of patients with early stage pancreatic cancer, patients with stage I pancreatic cancer compromise less than 20% of these studies.7,8 This results in global recommendations based on fewer than 200 patients. Furthermore, these studies are not powered to determine if early stage patients benefit from adjuvant therapy as compared to the more advanced stages (node positive and advanced T stage patients), which comprise the majority of the patients studied. Stage I patients tend to have an improved survival that may not be impacted by the administration of adjuvant therapy.
Given the rarity of stage I disease, it would be challenging to accrue a study that could demonstrate a survival benefit for these patients. Therefore, this study analyzes the impact of adjuvant chemotherapy and chemoradiation therapy on OS for stage 1 PDAC patients using a large national cancer database.
Methods
Patients
Patients were selected from the National Cancer Data Base (NCDB) between 2006 and 2012. At the time of this study, vital status was available until 2012. This database compromises approximately 70% of newly diagnosed cancer cases identified in the United States. Data were collected on patients with adenocarcinoma histologies including ICD-0–3 codes 8140, 8211, 8261, 8262, 8310, 8323, 8430, 8323, 8430, 8440, 8450, 8452, 8471, 8480, 8410, 8490, 8500, 8510, 8550, 8570, 8560. Patients were included in the study if they had invasive histology, analytic stage I disease, and underwent surgical resection. Patient staging was performed utilizing the AJCC 6th and 7th editions. Based on each of these editions, stage 1A was defined as T1 N0 M0. Stage 1B was defined as T2 N0 M0. Surgical resection was defined using codes 25, 30, 35–37, 70, 40, 60,70 and 80 which include (local excision, partial pancreatectomy, local/partial pancreatectomy and duodenectomy with or without distal gastrectomy, pancreaticoduodenectomy, pancreatectomy with or without subtotal gastrectomy, extended pancreatectomy or pancreatectomy NOS). Lymph node (LN) harvest was defined as the number of lymph nodes reviewed by pathology in the surgical resection. We used as our “standard” lymph node harvest as n = 11 given that the current NCCN guidelines recommend a minimum of 11–17 lymph nodes be reviewed for adequate staging.5 Patients were excluded if they did not have pathologic stage I disease or underwent neoadjuvant chemotherapy or neoadjuvant radiation therapy.
Statistical analysis
Patient characteristics were reported as the mean and standard deviation for continuous data; and as frequencies and relative frequencies for categorical data. Comparisons were made using the Mann–Whitney U or Kruskal–Wallis test for continuous variables; and the Pearson chi-square test for categorical data, respectively. Univariate associations with OS were reported using standard Kaplan–Meier methods and the log-rank test. To determine which clinical and pathological factors may have an independent association with OS, multivariable analysis was performed using Cox regression. Factors included in the final model were selected a priori by the research team. All analyses were conducted in SAS v9.4 (Cary, NC) at a significance level of 0.05.
Results
Patient characteristics
During the study period, 3909 patients were identified with resected pathologic stage I PDAC (Table 1). Of these patients 1477 patients had stage IA disease (37.8%) and 2432 patients had stage IB disease (62.2%). The mean age was 65.6 years old and46.2% of the patients were male. There was no difference with regard to the Charlson–Deyo comorbidity score. The median number of resected LN was 11 (1–72). Adjuvant chemotherapy and adjuvant chemoradiation therapy was administered to45.5% and 19.9% of patients, respectively.
Table 1.
Patient Demographics of overall cohort and summarized by stage IA and IB
| Variable | IA | IB | Overall | p-value |
|---|---|---|---|---|
| Age (mean, standard deviation) | 66.5 ± 11.2 | 65.1 ± 13.2 | 65.6 ± 12.5 | 0.043 |
| Gender (n, % male) | 675 (45.7) | 1130 (46.5) | 1805 (46.2) | 0.64 |
| Race (n, %) | ||||
| White | 1234 (83.5) | 2019 (83.0) | 3253 (83.2) | 0.14 |
| Black | 155 (10.5) | 297 (12.2) | 452 (11.6) | |
| Asian | 18 (1.2) | 19 (0.8) | 37 (0.9) | |
| Other | 70 (4.7) | 97 (4.0) | 167 (4.3) | |
| Charlson-Deyo score | ||||
| 0 | 942 (63.8) | 1582 (65.0) | 2524 (64.6) | 0.55 |
| 1 | 424 (28.7) | 659 (27.1) | 1083 (27.7) | |
| 2 | 111 (7.5) | 191 (7.9) | 302 (7.7) | |
| Primary site | ||||
| Head | 840 (56.9) | 1314 (54.0) | 2154 (55.1) | <0.001 |
| Body/Tail | 377 (25.5) | 767 (31.5) | 1144 (29.3) | |
| Other/Overlapping | 260 (17.6) | 351 (14.5) | 611 (15.6) | |
| Grade | ||||
| Well differentiated | 327 (22.1) | 356 (14.6) | 683 (17.5) | <0.001 |
| Moderately differentiated | 638 (43.2) | 1105 (45.4) | 1743 (44.6) | |
| Poorly differentiated | 242 (16.4) | 587 (24.1) | 829 (21.2) | |
| Undifferentiated | 270 (18.3) | 384 (15.8) | 654 (16.7) | |
| Lymph nodes resected (mean, standard deviation) | 12.9 ± 9.4 | 12.2 ± 8.5 | 12.5 ± 8.9 | 0.08 |
| Margins | ||||
| Positive | 81 (5.6) | 214 (8.9) | 295 (7.6) | <0.001 |
| Negative | 1375 (94.4) | 2191 (91.1) | 3566 (92.4) | |
| Adjuvant chemotherapy | 592 (40.1) | 1185 (48.7) | 1777 (45.5) | <0.001 |
| Adjuvant chemoradiation | 223 (15.1) | 556 (22.9) | 779 (19.9) | <0.001 |
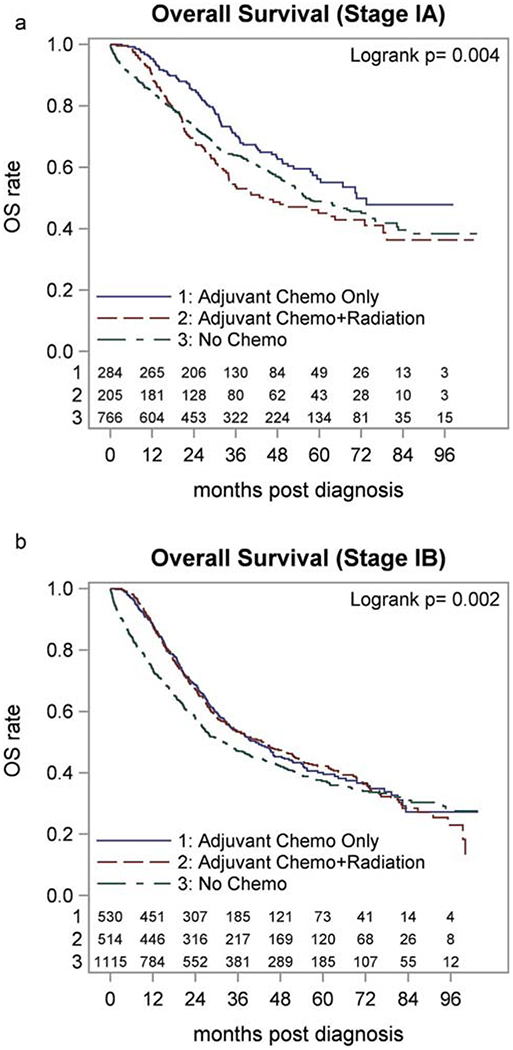
Fig. 1 shows the Kaplan–Meier curve for stage IA and IB patients, stratified by receipt of adjuvant therapy (no adjuvant treatment, chemotherapy alone, and chemoradiation). Median follow up was 53.6 mo (95% CI 0.2–107.7 mo). Median OS for all stage I patients was 45.9 mo (95% CI 42.6–49.1 mo): 60.3 mo (95% CI 54.6–72.0 mo) for stage IA and 36.9 mo (95% CI33.5–41.1 mo) for stage IB patients.
Figure 1.
Kaplan–Meier curves for patients with stage IA (a) and IB (b) PDAC, derived from the NCDB, 2006–2012 stratified by no adjuvant therapy, adjuvant chemotherapy, adjuvant chemoradiation
Differences in stage I patients who received adjuvant chemotherapy and chemoradiation
We sought to identify potential factors that could explain why patients did not receive adjuvant chemotherapy or chemo-radiation (Table 2). Patients with stage IA PDAC who received adjuvant chemotherapy/chemoradiation were more likely to be younger (p < 0.001), have disease within the pancreatic head (p = 0.014), have higher grade tumors (p < 0.001), and have positive margins (p < 0.001). Similarities were found for patients with stage IB PDAC with regard to age, grade, and margin status.
Table 2.
Patient characteristics stratified by receipt of adjuvant chemotherapy
| No adjuvant therapy | Adjuvant chemotherapy | Adjuvant chemoradiation | Overall | p-value | |
|---|---|---|---|---|---|
| Stage IA (n, %) | 885 (59.9) | 223 (15.1) | 369 (25.0) | 1477 (100%) | |
| Age (mean, standard deviation) | 68.1 ± 11.7 | 63.0 ± 8.9 | 64.7 ± 10.4 | 66.5 ± 11.2 | <0.001 |
| Gender (n, % male) | 399 (45.1%) | 102 (45.7%) | 174 (47.2%) | 675 (45.7%) | 0.80 |
| Race (n, %) | |||||
| White | 740 (83.6%) | 187 (83.9%) | 307 (83.2%) | 1234 (83.5%) | 0.31 |
| Black | 97 (11.0%) | 26 (11.7%) | 32 (8.7%) | 155 (10.5%) | |
| Asian | 8 (0.9%) | 2 (0.9%) | 8 (2.2%) | 18 (1.2%) | |
| Other | 40 (4.5%) | 8 (3.6%) | 22 (6.0%) | 70 (4.7%) | |
| Charlson-Deyo score | |||||
| 0 | 546 (61.7%) | 159 (71.3%) | 237 (64.2%) | 942 (63.8%) | 0.09 |
| 1 | 273 (30.8%) | 50 (22.4%) | 101 (27.4%) | 424 (28.7%) | |
| 2 | 66 (7.5%) | 14 (6.3%) | 31 (8.4%) | 111 (7.5%) | |
| Primary site | |||||
| Head | 480 (54.2%) | 147 (65.9%) | 213 (57.7%) | 840 (56.9%) | 0.02 |
| Body/Tail | 227 (25.6%) | 49 (22.0%) | 101 (27.4%) | 377 (25.5%) | |
| Other/Overlapping | 178 (20.2%) | 27 (12.1%) | 55 (14.9%) | 260 (17.3%) | |
| Grade | |||||
| Well differentiated | 209 (23.6%) | 43 (19.3%) | 75 (20.3%) | 327 (22.1%) | 0.003 |
| Moderately differentiated | 365 (41.2%) | 105 (47.1%) | 168 (45.5%) | 638 (43.2%) | |
| Poorly differentiated | 125 (14.1%) | 44 (19.7%) | 73 (19.8%) | 242 (16.4%) | |
| Undifferentiated | 186 (21.0%) | 31 (13.9%) | 53 (14.4%) | 270 (18.3%) | |
| Lymph nodes resected (mean, standard deviation) | 12.7 ± 9.5 | 12.2 ± 9.3 | 14.0 ± 9.2 | 12.9 ± 9.4 | 0.005 |
| Margins | |||||
| Positive | 40 (4.6%) | 24 (10.9%) | 17 (4.7%) | 81 (5.6%) | <0.001 |
| Negative | 832 (95.4%) | 197 (89.1%) | 346 (95.3%) | 1375 (94.4%) | |
| Stage IB (n, %) | 1247 (51.4) | 556 (22.9) | 624 (25.7) | 2427 (100%) | |
| Age (mean, standard deviation) | 65.5 ± 15.3 | 63.6 ± 10.0 | 65.5 ± 10.9 | 65.1 ± 13.2 | <0.001 |
| Gender (n, % male) | 546 (43.8%) | 294 (52.9%) | 286 (45.8%) | 1130 (46.5%) | 0.002 |
| Race (n, %) | |||||
| White | 1014 (81.3%) | 472 (84.9%) | 528 (84.6%) | 2019 (83.0%) | 0.44 |
| Black | 170 (13.6%) | 60 (10.8%) | 67 (10.7%) | 297 (12.2%) | |
| Asian | 10 (0.8%) | 5 (0.9%) | 4 (0.6%) | 19 (0.8%) | |
| Other | 53 (4.3%) | 19 (3.4%) | 25 (4.0%) | 97 (4.0%) | |
| Charlson-Deyo score | |||||
| 0 | 799 (64.1%) | 376 (67.6%) | 403 (64.6%) | 1582 (65.0%) | 0.34 |
| 1 | 340 (27.3%) | 147 (26.4%) | 171 (27.4%) | 659 (27.1%) | |
| 2 | 108 (8.7%) | 33 (5.9%) | 50 (8.0%) | 191 (7.9%) | |
| Primary site | |||||
| Head | 669 (53.6%) | 314 (56.5%) | 328 (52.6%) | 1314 (54.0%) | 0.81 |
| Body/Tail | 400 (32.1%) | 160 (28.8%) | 206 (33.0%) | 767 (31.5%) | |
| Other/Overlapping | 178 (14.3%) | 82 (14.7%) | 90 (14.5%) | 351 (14.4%) | |
| Grade | |||||
| Well differentiated | 207 (16.6%) | 66 (11.9%) | 81 (13.0%) | 356 (14.6%) | <0.001 |
| Moderately differentiated | 514 (41.2%) | 281 (50.5%) | 307 (49.2%) | 1105 (45.4%) | |
| Poorly differentiated | 260 (20.9%) | 155 (27.9%) | 172 (27.6%) | 587 (24.1%) | |
| Undifferentiated | 266 (21.3%) | 54 (9.7%) | 64 (10.3%) | 384 (15.8%) | |
| Lymph nodes resected (mean, standard deviation) | 11.5 ± 8.3 | 12.1 ± 8.3 | 13.8 ± 9.1 | 12.2 ± 8.5 | <0.001 |
| Margins | |||||
| Positive | 100 (8.1%) | 83 (15.0%) | 31 (5.1%) | 214 (8.9%) | <0.001 |
| Negative | 1135 (91.9%) | 470 (85.0%) | 581 (94.9%) | 2191 (91.1%) |
Univariate analysis
Univariate analysis of factors associated with OS for stage IA and IB patients are summarized in Table 3. Age (p < 0.001), CD score (p = 0.032), site of disease (p < 0.001), grade (p < 0.001), extent of LN harvest (p = 0.004), and margins (p < 0.001) were all associated with OS for stage IA patients. Of note, gender (p = 0.31), race (p = 0.13), adjuvant chemotherapy (p = 0.17), and adjuvant chemoradiation (p = 0.061) were not associated with OS.
Table 3.
Univariate analysis of patient clinical and pathologic factors on median OS by stage (IA or IB)
| Stage IA patients | Stage IB patients | |||||
|---|---|---|---|---|---|---|
| OS (mo) | 95% CI | P value | OS (mo) | 95% CI | p-value | |
| Age | ||||||
| <70 | 85.3 | 70.7–NRa | <0.001 | 50.2 | 44.3–58.0 | <0.001 |
| ≥70 | 45.8 | 26.7 | 24.4–29.3 | |||
| Grade | ||||||
| Well differentiated | NR | 76.0–NR | <0.001 | NR | 78.9–NR | <0.001 |
| Moderately differentiated | 73.9 | 70.7–NR | 66.1 | 51.5–84.9 | ||
| Poorly differentiated | 54.2 | 45.7–63.7 | 32.2 | 29.1–36.1 | ||
| Undifferentiated | 31.3 | 26.0–36.7 | 23.0 | 20.0–25.3 | ||
| LN harvest | ||||||
| 1–10 | 53.0 | 46.7–63.7 | 0.004 | 30.3 | 27.3–34.0 | <0.001 |
| >10 | 73.5 | 58.3–NR | 45.3 | 39.1–52.0 | ||
| Margin | ||||||
| Negative | 64.5 | 56.0–73.9 | <0.001 | 39.4 | 35.2–45.0 | <0.001 |
| Positive | 30.2 | 21.3–48.7 | 20.5 | 15.2–25.0 | ||
| Adjuvant chemotherapy | ||||||
| No | 56.1 | 51.6–72.0 | 0.17 | 32.0 | 27.4–37.3 | <0.001 |
| Yes | 64.5 | 50.7–79.3 | 42.6 | 36.8–47.4 | ||
| Adjuvant chemoradiation | ||||||
| No | 66.3 | 55.1–73.9 | 0.061 | 34.8 | 31.7–39.3 | 0.035 |
| Yes | 43.8 | 34.1–64.5 | 44.5 | 35.4–52.3 | ||
NR; not reached.
In comparison, for patients with stage IB disease, age (p < 0.001), gender (p = 0.003), CD score (p < 0.001), site of disease (p < 0.001), grade (p < 0.001) extent of LN harvest (p < 0.001), margin status (p < 0.001), adjuvant chemotherapy (p < 0.001), and adjuvant chemoradiation (p = 0.035) were all associated with OS.
Multivariable analysis
On multivariate analysis (Table 4), age (p < 0.001), grade (p < 0.001), LN harvest (p = 0.008), margins (p < 0.001), adjuvant chemotherapy (p = 0.004), and adjuvant chemo-radiation (p = 0.002) were all associated with OS for patients with stage IA disease. For stage IB patients, each of these factors were also associated with OS with the exception of adjuvant radiation (p = 0.35). Of note, use of adjuvant chemoradiation was associated with a worse OS for stage 1A patients, while no survival difference was detected for stage 1B patients receiving adjuvant chemoradiotherapy.
Table 4.
Multivariable analysis of patient clinical and pathologic factors on OS by stage (IA or IB)
| Stage IA | Stage IB | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | p-value | |
| Age: ≥70 vs <70 | 1.63 | 1.36–1.95 | <0.001 | 1.45 | 1.29–1.63 | <0.001 |
| Grade: | ||||||
| Moderate vs well | 1.86 | 1.42–2.42 | <0.001 | 1.78 | 1.47–2.16 | <0.001 |
| Poor vs well | 3.10 | 2.3–4.18 | 2.24 | 1.91–2.87 | ||
| Undifferentiated vs well | 1.25 | 0.89–1.75 | 0.81 | 0.63–1.04 | ||
| LN: >10 vs 1–10 | 0.79 | 0.66–0.94 | 0.008 | 0.80 | 0.71–0.90 | <0.001 |
| Margins: Positive vs Negative | 1.95 | 1.43–2.67 | <0.001 | 1.72 | 1.43–2.07 | <0.001 |
| Adjuvant chemotherapy | 0.73 | 0.58–0.91 | 0.004 | 0.76 | 0.66–0.87 | <0.001 |
| Adjuvant ohemoradiation | 1.52 | 1.18–1.97 | 0.002 | 0.93 | 0.80–1.09 | 0.35 |
Discussion
We analyzed patients with stage I PDAC to determine if there was an OS benefit for adjuvant therapy because this patient population is poorly represented within clinical trials that support its use. Patients were excluded if they received neoadjuvant chemotherapy or neoadjuvant radiation therapy. Because this study is based on a large national database, it represents the largest collection of resected patients with stage IA and stage IB disease in the literature. National trends in PDAC treatment suggest that the rates of adjuvant chemotherapy and chemoradiotherapy are increasing.10 According to our study, 40.1% of stage IA PDAC patients and 48.7% of stage IB PDAC underwent adjuvant chemotherapy (Table 1). Regarding adjuvant chemo-radiation therapy, 15.1% of stage IA and 22.9% of stage IB PDAC patients had this treatment (Table 1). Thus, the implications of our results apply to a large proportion of PDAC patients receiving adjuvant treatment.
For patients with either stage IA or IB PDAC, adjuvant chemotherapy was associated with improved OS, supporting its use and recommendation by the NCCN. Thus, our results which were derived using a large nationwide database, supported the use of adjuvant chemotherapy for this otherwise small subset of patients with stage I PDAC. In contrast, adjuvant chemoradiation was associated with worse OS for patients with stage IA PDAC (HR = 1.52, 95% CI 1.17–1.97, p = 0.002) and had no significant association with stage IB PDAC patients (HR = 0.93, 95% CI0.80–1.09, p = 0.35) on multivariable analysis. Our results pertaining to adjuvant chemoradiation were also consistent with existing studies which overall showed no benefit or even detriment to survival when adjuvant chemoradiation was administered.11,12 However, similar to the studies investigating adjuvant chemotherapy, these studies investigating adjuvant chemo-radiation also had small numbers of stage I patients with the pT1–2 status ranging from 4 to 25% totaling 26 patients. Therefore, biases in selection for receipt of adjuvant chemo-radiation or significant toxicities from this treatment could account for the findings of our group and others. Some of the potential biases were analyzed (Table 2) in our study, while others such as patient access or preference for adjuvant therapy, were unable to assessed using the NCDB. While our study offered a larger number of stage I patients to assess the results of these previous prospective trials with regard to adjuvant chemo-radiation as well adjuvant chemotherapy, the proportion of patients who received adjuvant chemoradiation were less that those who received adjuvant chemotherapy alone (223/592 or 37.7% and 556/1185 or 46.9% for stage IA and IB, respectively). Thus, the smaller sample size of patients receiving adjuvant chemo-radiation could also have affected our analysis.
For patients with stage I PDAC, other factors related to tumor biology (such as poor differentiation) and the quality of operation and pathologic analysis (such as margin status and number of LN examined) were independently associated with OS (Table 4). Positive margins are thought to represent more aggressive tumor biology,13 and other studies have demonstrated that an extended margin resection approach to avoid a R1 resection may not improve survival, which further supports it as a marker of poor biology.14 For stage I PDAC patients, the retrieval of >10 LN was also associated with an improved OS and was consistent with other studies.15 The number of LN resected was likely a reflection of adequate staging through appropriate oncologic surgery and emphasized the point that good operative technique and pathologic review was associated with a significant impact on OS for stage I PDAC patients.
Given that our study was based on a nationally collected data, there were limitations to the conclusions that can be made. The type of chemotherapy administered to these patients was not available. Although during this time period, standard of care was gemcitabine alone or 5-Fluorouracil based regimens, newer agents have been approved for patients with stage III and IV PDAC such as Nab-paclitaxel and FOLFIRINOX, which may have a potential benefit in the adjuvant setting.16,17 Gemcitabine/Nab-paclitaxel is currently under investigation as an adjuvant regimen and may have a more significant impact on survival. Furthermore, the NCDB does not provide information on whether patients received adjuvant chemotherapy followed by adjuvant chemoradiation, which may also have impact on outcomes. There was also limited data available regarding the reasons many patients did not get chemotherapy within this cohort, introducing an important source of uncontrolled bias within the study. In our patient population, of the 1777 patients (45.5%) who did not receive adjuvant chemotherapy, the rationale for why they did not receive chemotherapy was unavailable for the vast majority of patients. This posed difficulty in determining the reasons as to why patients did not receive adjuvant therapy. Potential reasons included patient related factors such as medical comorbidities, complications related to resection, or unspecified clinician judgment. Studies have suggested that 50–60% of patients undergo adjuvant chemotherapy and that receipt of therapy was more common in those who experience fewer postoperative complications.18 Patients who experienced peri-operative complications have been shown to have worse OS compared to those patients who did not have a complication.19 Consequently, these unknown variables may have been a source of bias within our study. Such bias may also provide explanation for why adjuvant chemotherapy was not significantly associated with OS on the univariate analysis for stage IA patients. Biases in selecting which patients receive adjuvant chemotherapy (Table 2) may skew the OS results. Whereas the univariate comparison does not adjust for these factors, the multivariable analysis provides for a more balanced comparison, which accounts for the significant association of adjuvant chemotherapy and OS on the multivariable analysis for stage IA patients. Lastly, because the NCDB does not provide data regarding disease-free survival and local recurrence, we were unable to comment on the effects of adjuvant therapy on stage I patients in this regard.
Despite these limitations, with robust numbers available in our study, our results support the conclusion that there is an association of OS benefit and adjuvant chemotherapy for patients with resected stage I pancreatic cancer. Its inclusion in treatment management appears to be supported by this nationwide analysis. However, the decision to offer adjuvant chemotherapy for these patients should take into account not only the potential benefit in OS, but also the short-term toxicities that many of these regimens may have. Therefore, while this analysis in consistent with recommendations by the NCCN, it is important to stress the individualized approach to adjuvant chemotherapy decision making. Further study is warranted to assess biologic factors to determine what molecular phenotype may derive the most OS benefit from adjuvant chemotherapy, such as is currently being done with Oncotype in early stage breast cancer and colon cancer.
In conclusion, for patients with stage I PDAC, improved OS was associated with the receipt of adjuvant chemotherapy as well as by the quality of operation and staging (suggested by lymph node yield, margin negativity), patient age and tumor biology (such as poor differentiation). Conversely, the role of adjuvant chemoradiation for stage I PDAC was much less clearly defined from our investigation but was consistent with other studies, suggesting that chemoradiation may potentially be omitted as adjuvant treatment for early stage PDAC.
Acknowledgements
We thank the Commission on Cancer of the American College of Surgeons for access to the NCDB Participant User File. The statistical analysis was supported by Roswell Park Cancer Institute and National Cancer Institute (NCI) grant P30CA016056.
Footnotes
Disclosures
There are no financial disclosures, conflicts of interest, and/or no funding sources for this manuscript for any of the listed authors. The American College of Surgeons Committee on Cancer provided the Participant User File from the National Cancer Data Base, but has not reviewed or validated the results or conclusions of our study.
References
- 1.Siegel RL, Miller KD, Jemal A. (2015) Cancer statistics, 2015. CA Cancer J Clin 65:5–29. [DOI] [PubMed] [Google Scholar]
- 2.Sohn TA, Yeo CJ, Cameron JL, Koniaris L, Kaushal S, Abrams RA et al. (2000) Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg 4:567–579. [DOI] [PubMed] [Google Scholar]
- 3.Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A et al. (2008) Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 299:1019–1026. [DOI] [PubMed] [Google Scholar]
- 4.Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J et al. (2006) 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg 10: 1199–1210. discussion 1210–1191. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network. Pancreatic adenocarcinoma (Version 2.2016). http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. (accessed 5 August 2016).
- 6.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K et al. (2013) Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 310:1473–1481. [DOI] [PubMed] [Google Scholar]
- 7.Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K et al. (2007) Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297:267–277. [DOI] [PubMed] [Google Scholar]
- 8.Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D et al. (2010) Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 304:1073–1081. [DOI] [PubMed] [Google Scholar]
- 9.Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H et al. (2004) A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 350:1200–1210. [DOI] [PubMed] [Google Scholar]
- 10.Mayo SC, Gilson MM, Herman JM, Cameron JL, Nathan H, Edil BH et al. (2012) Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg 214:33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Laethem JL, Hammel P, Mornex F, Azria D, Van Tienhoven G, Vergauwe P et al. (2010) Adjuvant gemcitabine alone versus gemcitabine-based chemoradiotherapy after curative resection for pancreatic cancer: a randomized EORTC-40013–22012/FFCD-9203/GERCOR phase II study. J Clin Oncol 28:4450–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt J, Abel U, Debus J, Harig S, Hoffmann K, Herrmann T et al. (2012) Open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J Clin Oncol 30:4077–4083. [DOI] [PubMed] [Google Scholar]
- 13.Mathur A, Ross SB, Luberice K, Kurian T, Vice M, Toomey P et al. (2014) Margin status impacts survival after pancreaticoduodenectomy but negative margins should not be pursued. Am Surg 80:353–360. [PubMed] [Google Scholar]
- 14.Hernandez J, Mullinax J, Clark W, Toomey P, Villadolid D, Morton C et al. (2009) Survival after pancreaticoduodenectomy is not improved by extending resections to achieve negative margins. Ann Surg 250:76–80. [DOI] [PubMed] [Google Scholar]
- 15.Huebner M, Kendrick M, Reid-Lombardo KM, Que F, Therneau T, Qin R et al. (2012) Number of lymph nodes evaluated: prognostic value in pancreatic adenocarcinoma. J Gastrointest Surg 16:920–926. [DOI] [PubMed] [Google Scholar]
- 16.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y et al. (2011) FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364:1817–1825. [DOI] [PubMed] [Google Scholar]
- 17.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M et al. (2013) Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369:1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merkow RP, Bilimoria KY, Tomlinson JS, Paruch JL, Fleming JB, Talamonti MS et al. (2014) Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 260: 372–377. [DOI] [PubMed] [Google Scholar]
- 19.Petermann D, Demartines N, Schafer M. (2013) Severe postoperative complications adversely affect long-term survival after R1 resection for pancreatic head adenocarcinoma. World J Surg 37:1901–1908. [DOI] [PubMed] [Google Scholar]