Abstract
Recent evidence suggests that paternal age at birth influences myriad developmental outcomes among children, but few studies have examined the possibility for father’s age to influence children’s intellectual development among a sample of high-risk families. The current study utilizes data from the Fragile Families and Child Wellbeing Study (FFCWS) to examine the association between paternal age at birth among 480 male and 449 female children’s verbal IQ scores, as assessed with a version of the Peabody Picture Vocabulary Test (PPVT) at age 9. The nonlinear association between paternal age and children’s verbal intelligence was also examined. Paternal age at birth appears to have a marginally significant nonlinear relationship with male children’s verbal IQ scores, despite controlling for a number of possible confounders associated with both young and advanced paternal age.
Introduction
A considerable amount of attention has been placed on the role paternal age plays in the development of children’s cognitive, emotional, and psychosocial outcomes (D’Onofrio et al., 2014; Malaspina et al., 2001; McGrath et al., 2014; Menezes et al., 2010). Within this body of literature, support has been found for the detrimental effects of both young and advanced paternal ages on offspring development. Father’s age at the time of birth appears to be salient for children’s birth outcomes, such that children born to fathers under the age of 25 are more likely to develop spina bifida (Yang et al., 2007) and Down syndrome (McIntosh, Olshan, & Baird, 1995). Additionally, advanced paternal age has been linked to increased risks for heart malformation and other musculoskeletal abnormalities at birth (Yang et al., 2007).
Paternal age also appears to influence psychological and behavioral outcomes among offspring. Advanced paternal age, for instance, has been linked to the development of bipolar affective disorders (Menezes et al., 2010), autism (D’Onofrio et al., 2014), and externalizing behavior problems (Saha, Barnett, Buka, & McGrath, 2009). Some evidence even suggests a nonlinear relationship between paternal age and psychosocial development in children (Janecka et al., 2017a), where children of younger and older fathers display decreased social abilities and an increased risk for developing major depressive disorder (Buizer-Voskamp et al., 2011; Weiser et al., 2008).
Giving the extent of the empirical support surrounding the influence of paternal age on myriad developmental outcomes, the potential for father’s age to influence children’s intellectual development appears plausible as well. Although most research has focused on the influence of parental age on offspring psychiatric and behavioral development, the relationship between fathers’ age and children’s intelligence has recently gained attention. Children born to older fathers, for instance, have been found to be at an increased risk for low educational attainment and to receive failing grades (D’Onofrio et al., 2014). Saha and colleagues (2009) also reported an association between advanced paternal age and offspring performance on a variety of neurocognitive measures during infancy and childhood. Advanced paternal age was reported to be significantly associated with decreased scores on the Bayley Scales for Infant Development (motor), Graham-Ernhart Block Sort Test, and the Wechsler Intelligence Scale for Children (WISC). Moreover, evidence of an inverted U-shaped relationship between offspring’s nonverbal performance IQ and paternal age has been found among a sample of adolescents (Malaspina et al., 2005). Adolescents born to fathers younger than age 20, as well as those born to fathers older than age 50, displayed the lowest scores on measures of nonverbal IQ. However, other research suggests that the inverse U-shaped relationship between paternal age and scores of general intelligence may become attenuated once parental education, parental socioeconomic status (SES), and the number of siblings within the household are accounted for (Whitley, Deary, Der, Batty, & Benzeval, 2012). Additional studies also find support for an attenuated relationship once other potential confounders, such as mother’s age, mother’s education, SES, birth complications, and birth year are included in the analyses (Arslan, Penke, Johnson, Iacono, & McGue, 2014; Edwards & Roff, 2010; Myrskylä, Silventoinen, Tynelius, & Rasmussen, 2013; Svensson, Abel, Dalman, & Magnusson, 2011).
The link between paternal age and offspring outcomes is relatively well established, yet the mechanisms by which paternal age influences offspring intelligence remains elusive. Among the few explanations that have been advanced, the adverse effects of paternal age are hypothesized to operate through biological and/or social mechanisms. The biological argument posits that older fathers are at an increased risk for copy error mutations in the spermatogonia during cell division (Penrose, 1955; Risch, Reich, Wishnick, & McCarthy, 1987). Once fathers reach a certain age, the efficiency in DNA proof-reading and repair enzyme activity dramatically declines (Malaspina et al., 2001). The increased number of cell mutations in the sperm of older fathers, in turn, may impact children’s neurodevelopment and contribute to intelligence variability (Malaspina et al., 2005). The social mechanisms by which paternal age impacts offspring development are hypothesized to operate through the social and economic resources that parents present to their children. Parents who delay parenthood are typically characterized as having a higher SES status and more education. Older fathers, therefore, may be more adept in providing optimal environments for offspring development. Children of younger fathers, in contrast, are more likely to be raised in a low income household and miss out on quality parental interactions while their younger parents work to make ends meet. This argument has been hypothesized as a social-influence effect, where the social, economic, and familial consequences of young parenthood can work to adversely affect offspring outcomes (Jaffee, Caspi, Moffitt, Belsky, & Silva, 2001).
Paternal age may not be a causal factor in determining children’s intelligence, but rather the relationship between the two could be confounded by the social and/or personal characteristics associated with young parenthood (Jaffee et al., 2001). Additionally, as more individuals are beginning to delay childbearing into later life, the investigation of whether advanced paternal age influences children’s intelligence is warranted. The literature suggests that paternal age may exert a nonlinear effect on children’s IQ scores, such that children of younger and older fathers may be at an increased risk for poor cognitive development. Against this backdrop, the purpose of the current study is twofold. First, in order to determine whether social factors and/or other parental characteristics associated with paternal age at birth may account for the relationship between paternal age and children’s intelligence, a number of father-level variables were examined, such as father-child interactions, fathers’ IQ, and father’s social status. Mother’s race, education, household income, marital status, and age at the time of the child’s birth were also included to limit potential confounding effects. Second, the possibility of a nonlinear relationship between paternal age and child IQ was examined with the inclusion of a quadratic term for father’s age. To our knowledge, this is the first study to examine the nonlinear relationship between paternal age and offspring verbal intelligence in a U.S.-based population of at-risk families.
Method
Data
Data for this study were drawn from the Fragile Families and Child Wellbeing Study (FFCWS). The FFCWS is a cohort study of approximately 5,000 families, which were identified as “fragile families” due to the number of risk factors associated with non-marital childbearing (Reichman, Teitler, Garfinkel, & McLanahan, 2001). To date, the FFCWS includes five waves of data. The first wave of data was collected approximately 48 hours after the birth of the focal child. The next three waves of data were collected when the children were ages 1, 3, and 5. The fifth wave of data were collected during the focal child’s 9th birthday. Additionally, in-home interviews were conducted with the primary caregiver and child during waves three, four, and five.
The FFCWS is unique in that both mothers and fathers were interviewed at the birth of their child, thereby providing much needed comprehensive data on fathers, especially unwed fathers (Reichman et al., 2001). In order to assess the longitudinal impact of paternal age at birth on child verbal intelligence at age 9, cases with nonmissing values on the variables of interest were retained in the sample, yielding a final analytical sample of 929 families.1
Measures
Intelligence (verbal IQ)
Children’s intelligence was assessed by using scores obtained from the Peabody Picture Vocabulary Test-Revised (PPVT-R) during the wave five in-home interview when the children were age 9. The PPVT provides a standardized assessment of children’s receptive vocabulary and variation in verbal ability. Variants of the PPVT have been used as measures of verbal IQ in previous research (Beaver et al., 2013; Rowe, Jacobson, & Van den Oord, Edwin JCG, 1999).
Fathers’ age
During the baseline interview, fathers reported their age during or shortly after the birth of their child. The ages of the fathers included in the sample spanned from 17–53, with a mean age of approximately 28 years old.
Father incarceration
At baseline, both mothers and fathers were asked if the father was incarcerated during the time the child was born. A constructed measure of father incarceration was created from both mother and father reports. Responses were coded such that 1 = yes and 0 = no.
Father intelligence
During the wave three interview, fathers’ intelligence was assessed with a subtest of the Wechsler Adult Intelligence Subscale-Revised (WAIS-R). Fathers were asked to identify the comparability of objects and concepts, thereby providing an overall assessment of verbal concept formation and reasoning skills. The values of the WAIS-R subscale ranged from 0 to 15, with higher scores indicating greater verbal intelligence.
Father low self-control
Fathers’ levels of self-control were assessed with an abbreviated version of Dickman’s (1990) impulsivity scale at wave two. Fathers were asked about their tendencies to say or do things without considering the consequences and to act before thinking. Responses were coded such that higher scores indicated greater impulsivity. The questions were combined to create an overall low self-control scale (α = .84).
Father involvement
Fathers were asked about their overall involvement with their child during the wave five interview. Fathers reported whether they and their child completed chores together, played outside, watched TV together, read books, and talked about current events. Fathers were also asked how often they asked about their child’s day and helped him/her with their homework. Responses were compiled into an overall involvement scale and coded so that higher scores indicated greater paternal involvement (α = .84).
Number of children living within the household
To illuminate the potential influences of parental resources on child IQ, the number of children living within the home was assessed. Previous research suggests that as the number of children within the home increases, parental inputs may have less of an impact on child outcomes due to the divvying up of resources (Becker, 1991). At the wave five interview, mothers were asked to report the number of additional people living with her and the child. Child household members were identified as belonging to one of the following categories: biological child, stepchild, adopted child, foster child, non-related child, and grandchild. An additive scale of the number of children living within the household was then compiled from the information provided by the mother. The coding scheme for the additive scale was as follows: 3 = seven or more children, 2 = four to six children, 1 = two to three children, and 0 = no other children (besides the focal child).
Covariates
Separate measures of father’s and mother’s race (1 = nonwhite, 0 = white), father’s and mother’s highest level of education (1 = high school or more, 0 = less than high school), father’s and mother’s total household annual income (1 = $20,000 or more, 0 = less than $20,000), father’s and mother’s marital status (1 = married, 0 = not married), and mother’s age at the time of the birth were included as controls in every model. Additionally, the models were adjusted for child sex (1 = male, 0 = female) and the number of children living within the household. The means, standard deviations, along with maximum and minimum values for each of the predictor variables are presented in Table 1. A correlation matrix for all study variables is presented in Table 2.
Table 1.
Descriptive Statistics
| Variable | M | SD | Min | Max |
|---|---|---|---|---|
| PPVT | 97.75 | 15.09 | 44 | 148 |
| PPVT (male scores) | 98.51 | 15.39 | 44 | 148 |
| PPVT (female scores) | 96.94 | 14.73 | 48 | 143 |
| Father age at birth | 28.98 | 7.02 | 17 | 53 |
| Father incarceration | 0.01 | 0.10 | 0 | 1 |
| Father WAIS-R score | 6.94 | 2.70 | 0 | 15 |
| Father low self-control | 1.93 | 0.64 | 1 | 4 |
| Father involvement | 0.09 | 0.60 | −1.65 | 1.42 |
| # Children in household | 1.53 | 0.57 | 0 | 3 |
| Father race | 0.59 | 0.49 | 0 | 1 |
| Father education | 0.79 | 0.41 | 0 | 1 |
| Father income | 0.76 | 0.43 | 0 | 1 |
| Father married | 0.43 | 0.49 | 0 | 1 |
| Mother race | 0.65 | 0.48 | 0 | 1 |
| Mother education | 0.77 | 0.42 | 0 | 1 |
| Mother income | 0.67 | 0.47 | 0 | 1 |
| Mother married | 0.43 | 0.49 | 0 | 1 |
| Mother age at birth | 26.73 | 6.07 | 17 | 43 |
| Child sex | 0.52 | 0.50 | 0 | 1 |
Table 2.
Correlation Matrix
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | PPVT | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 2 | Father age at birth | 0.19 | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 3 | Father incarceration | 0.01 | −0.01 | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 4 | Father WAIS-R score | 0.27 | 0.12 | 0.02 | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 5 | Father low self-control | −0.10 | −0.14 | 0.05 | −0.22 | 1.00 | - | - | - | - | - | - | - | - | - | - | - | - |
| 6 | Father involvement | 0.08 | 0.06 | −0.06 | 0.05 | −0.16 | 1.00 | - | - | - | - | - | - | - | - | - | - | - |
| 7 | # children in household | −0.17 | −0.10 | −0.01 | −0.05 | 0.00 | −0.01 | 1.00 | - | - | - | - | - | - | - | - | - | - |
| 8 | Father race | −0.31 | −0.11 | 0.06 | −0.24 | 0.07 | −0.09 | 0.04 | 1.00 | - | - | - | - | - | - | - | - | - |
| 9 | Mother race | −0.32 | −0.17 | −0.02 | −0.25 | 0.05 | −0.07 | −0.01 | 0.70 | 1.00 | - | - | - | - | - | - | - | - |
| 10 | Father education | 0.27 | 0.24 | −0.03 | 0.25 | −0.21 | 0.14 | −0.12 | −0.15 | −0.17 | 1.00 | - | - | - | - | - | - | - |
| 11 | Mother education | 0.25 | 0.22 | −0.05 | 0.21 | −0.18 | 0.06 | −0.15 | −0.16 | −0.15 | 0.40 | 1.00 | - | - | - | - | - | - |
| 12 | Married father | 0.34 | 0.36 | −0.04 | 0.16 | −0.13 | 0.11 | −0.02 | −0.37 | −0.34 | 0.25 | 0.26 | 1.00 | - | - | - | - | - |
| 13 | Married mother | 0.34 | 0.37 | −0.04 | 0.16 | −0.14 | 0.11 | −0.02 | −0.38 | −0.34 | 0.23 | 0.26 | 0.99 | 1.00 | - | - | - | - |
| 14 | Father income | 0.28 | 0.18 | −0.10 | 0.21 | −0.17 | 0.17 | −0.05 | −0.24 | −0.18 | 0.32 | 0.28 | 0.32 | 0.33 | 1.00 | - | - | - |
| 15 | Mother income | 0.33 | 0.20 | −0.05 | 0.25 | −0.14 | 0.10 | −0.14 | −0.21 | −0.22 | 0.34 | 0.39 | 0.40 | 0.40 | 0.46 | 1.00 | - | - |
| 16 | Mother age at birth | 0.23 | 0.78 | −0.04 | 0.16 | −0.17 | 0.08 | −0.09 | −0.18 | −0.18 | 0.22 | 0.32 | 0.44 | 0.43 | 0.24 | 0.28 | 1.00 | - |
| 17 | Child sex | 0.05 | 0.01 | −0.01 | 0.02 | 0.03 | 0.10 | −0.02 | 0.01 | 0.03 | −0.04 | −0.04 | 0.08 | 0.08 | 0.03 | 0.04 | 0.01 | 1.00 |
Data Analysis
The analysis for the current study was carried out in a sequence of steps. First, in order to examine whether a nonlinear relationship exists between paternal age at birth and children’s verbal IQ scores, an age squared term (age2) and an age cubed term (age3) were created for fathers’ age. Hierarchical polynomial regression models were then conducted to assess whether any of the higher-order polynomials retained significance in the models. After progressively entering each polynomial into the model, the highest order polynomial to remain significant was the quadratic term for fathers’ age, or age2. After retaining the quadratic term for fathers’ age in the polynomial regression model, each predictor variable was then progressively entered into each model, yielding a total of seven estimated polynomial regression models (see Table 3). Additionally, each of the models were corrected for child sex and the number of children living in the home.
Table 3.
Nonlinear Ordinary Least Squares (OLS) Regression Models Predicting Child PPVT Scores with Father’s Age†
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
Model 6 |
Model 7 |
||
|---|---|---|---|---|---|---|---|---|
| b/ Beta | b/ Beta | b/ Beta | b/ Beta | b/ Beta | b/ Beta | b/ Beta | ||
| Father age | .38/.18*** | 2.16/1.00*** | .52/.24 | .50/.23 | .55/.25 | .55/.26 | .55/.26 | |
| SE | .07 | .51 | .49 | .49 | .49 | .49 | .49 | |
| Father age2 | −.03/−.83*** | −.01/−.25 | −.01/−.23 | −.01/−.26 | −.01/−.26 | −.01/−.26 | ||
| SE | .01 | .01 | .01 | .01 | .01 | .01 | ||
| Paternal Measures | ||||||||
| Father incarceration | 4.72/.03 | 3.85/.03 | 3.81/.02 | 3.81/.02 | ||||
| SE | 4.47 | 4.44 | 4.45 | 4.45 | ||||
| Father WAIS-R score | .68/.12*** | .69/.12*** | .69/.12*** | |||||
| SE | .17 | .17 | .17 | |||||
| Father low self-control | .21/.01 | .21/.01 | ||||||
| SE | .71 | .72 | ||||||
| Father involvement | −.01/−.00 | |||||||
| SE | .74 | |||||||
| Father race | −2.35/−.08* | −2.46/−.08* | −2.06/−.07 | −2.06/−.07 | −2.06/−.07 | |||
| SE | 1.28 | 1.29 | 1.28 | 1.28 | 1.28 | |||
| Father education | 3.27/.09*** | 3.27/.09*** | 2.66/.07** | 2.70/.07** | 2.70/.07** | |||
| SE | 1.24 | 1.24 | 1.24 | 1.25 | 1.26 | |||
| Father income | 2.69/.08** | 2.79/.08** | 2.53/.07** | 2.55/.07** | 2.55/.07** | |||
| SE | 1.20 | 1.20 | 1.20 | 1.20 | 1.21 | |||
| Father married | .43/.01 | .48/.02 | −.13/−.00 | −.20/−.01 | −.19/−.01 | |||
| SE | 5.49 | 5.49 | 5.45 | 5.46 | 5.46 | |||
| Maternal Measures | ||||||||
| Mother race | −4.94/−.16*** | −4.81/−.15*** | −4.36/−.14*** | −4.35/−.14*** | −4.35/−.14*** | |||
| SE | 1.30 | 1.31 | 1.30 | 1.30 | 1.30 | |||
| Mother education | 1.90/.05 | 1.93/.05 | 1.66/.05 | 1.68/.05 | 1.68/.05 | |||
| SE | 1.21 | 1.21 | 1.20 | 1.20 | 1.20 | |||
| Mother income | 3.63/.11*** | 3.63/.11*** | 3.20/.10*** | 3.20/.10*** | 3.20/.10*** | |||
| SE | 1.15 | 1.15 | 1.15 | 1.15 | 1.15 | |||
| Mother married | 3.30/.11 | 3.26/.11 | 4.04/.13 | 4.12/.14 | 4.12/.14 | |||
| SE | 5.50 | 5.50 | 5.46 | 5.47 | 5.48 | |||
| Mother age | .10/.04 | .10/.04 | .08/.03 | .08/.03 | .08/.03 | |||
| SE | .12 | .12 | .12 | .12 | .12 | |||
| n | 929 | 929 | 929 | 929 | 929 | 929 | 929 | |
| R2 | .06 | .07 | .25 | .25 | .26 | .26 | .26 | |
p< 0.10,
p< 0.05,
p<0.01;
= All analyses corrected for child sex and number of children living in the home
While each of the models for the full sample were adjusted for child sex, we also split the sample between male and female children in order to assess whether children’s gender moderated the nonlinear relationship between paternal age and child verbal IQ scores. The male sample included 480 children (Table 4), and the female sample included 449 children (Table 5). A series of polynomial regression models were then estimated for the male and female samples, just as they were for the full analytical sample. Finally, because a number of maternal characteristics have been identified as influential for a wide range of childhood outcomes (Fergusson & Woodward, 1999; Moffitt, 2002; Sandin et al., 2012; Tearne, 2015)—and could thereby potentially confound the association between paternal age and child verbal IQ—maternal race, education, income, marital status, and age at the time of birth were controlled for in each of the models across the full, male, and female samples.
Table 4.
Nonlinear Ordinary Least Squares (OLS) Regression Models Predicting Male Children’s PPVT Scores with Father’s Age†
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
Model 6 |
Model 7 |
|
|---|---|---|---|---|---|---|---|
| b/ Beta | b/ Beta | b/ Beta | b/ Beta | b/ Beta | b/ Beta | b/ Beta | |
| Father age | .28/.13*** | 2.78/1.28*** | 1.04/.48 | 1.04/.48 | 1.14/.53 | 1.15/.53 | 1.18/.54* |
| SE | .10 | .70 | .71 | .71 | .70 | .70 | .70 |
| Father age² | −.04/−1.16*** | −.02/−.51 | −.02/−.51 | −.02/−.54* | −.02/−.54* | −.02/−.56* | |
| SE | .01 | .01 | .01 | .01 | .01 | .01 | |
| Paternal Measures | |||||||
| Father incarceration | .51/.00 | −.30/−.00 | −.31/−.00 | −.95/−.01 | |||
| SE | 7.02 | 6.95 | 6.96 | 6.99 | |||
| Father WAIS-R score | .87/.16*** | .87/.16*** | .85/.15*** | ||||
| SE | .25 | .26 | .26 | ||||
| Father low self-control | −.01/−.00 | −.16/−.01 | |||||
| SE | 1.01 | 1.03 | |||||
| Father involvement | −1.10/−.04 | ||||||
| SE | 1.11 | ||||||
| Father race | −3.76/−.12* | −3.77/−.12* | −3.62/−.12* | −3.61/−.12* | −3.77/−.12* | ||
| SE | 1.95 | 1.96 | 1.94 | 1.94 | 1.95 | ||
| Father education | 2.63/.07 | 2.62/.07 | 1.63/.04 | 1.63/.04 | 1.75/.05 | ||
| SE | 1.86 | 1.87 | 1.87 | 1.88 | 1.88 | ||
| Father income | 2.75/.07 | 2.75/.08 | 1.99/.05 | 1.99/.05 | 2.18/.06 | ||
| SE | 1.87 | 1.87 | 1.86 | 1.87 | 1.88 | ||
| Father married | −5.42/−.18 | −5.42/−.18 | −7.57/−.25 | −7.56/−.25 | −6.97/−.23 | ||
| SE | 8.19 | 8.20 | 8.13 | 8.15 | 8.17 | ||
| Maternal Measures | |||||||
| Mother race | −1.78/−.05 | −1.77/−.05 | −.78/−.02 | −.78/−.02 | −.73/−.02 | ||
| SE | 1.96 | 1.97 | 1.97 | 1.97 | 1.97 | ||
| Mother education | 1.66/.05 | 1.67/.05 | 1.26/.04 | 1.26/.04 | 1.22/.03 | ||
| SE | 1.77 | 1.77 | 1.75 | 1.76 | 1.76 | ||
| Mother income | 3.33/.10* | 3.33/.10* | 3.00/.09* | 3.00/.09* | 2.96/.09* | ||
| SE | 1.73 | 1.74 | 1.72 | 1.72 | 1.72 | ||
| Mother married | 10.14/.33 | 10.14/.33 | 12.53/.41 | 12.53/.41 | 11.95/.39 | ||
| SE | 8.21 | 8.21 | 8.15 | 8.18 | 8.20 | ||
| Mother age | .01/.01 | .01/.01 | −.04/−.02 | −.04/−.02 | −.04/−.02 | ||
| SE | .17 | .17 | .17 | .17 | .17 | ||
| n | 480 | 480 | 480 | 480 | 480 | 480 | 480 |
| R² | .04 | .06 | .21 | .21 | .23 | .23 | .23 |
p< 0.10,
p< 0.05,
p<0.01;
= All analyses corrected for number of children living in the home
Table 5.
Nonlinear Ordinary Least Squares (OLS) Regression Models Predicting Female Children’s PPVT Scores with Father’s Age†
| Model 1 |
Model 2 |
Model 3 |
Model 4 |
Model 5 |
Model 6 |
Model 7 |
|
|---|---|---|---|---|---|---|---|
| b/ Beta | b/ Beta | b/ Beta | b/ Beta | b/ Beta | b/ Beta | b/ Beta | |
| Father age | .49/.23*** | 1.41/.66* | −.14/−.06 | −.20/−.09 | −.18/−.09 | −.17/−.08 | −.17/−.08 |
| SE | .10 | .73 | .69 | .69 | .69 | .69 | .69 |
| Father age² | −.01/−.43 | .00/.07 | .00/.10 | .00/.08 | .00/.08 | .00/.08 | |
| SE | .01 | .01 | .01 | .01 | .01 | .01 | |
| Paternal Measures | |||||||
| Father incarceration | 9.35/.07 | 8.65/.06 | 8.47/.06 | 8.44/.06 | |||
| SE | 5.75 | 5.71 | 5.75 | 5.75 | |||
| Father WAIS-R score | .61/.11*** | .62/.11*** | .62/.11*** | ||||
| SE | .23 | .24 | .24 | ||||
| Father low self-control | .31/.01 | .43/.02 | |||||
| SE | 1.00 | 1.01 | |||||
| Father involvement | .99/.04 | ||||||
| SE | .98 | ||||||
| Father race | −1.37/−.05 | −1.67/−.06 | −1.10/−.04 | −1.10/−.04 | −1.15/−.04 | ||
| SE | 1.69 | 1.69 | 1.70 | 1.70 | 1.70 | ||
| Father education | 4.10/.11** | 4.26/.11** | 3.88/.10** | 3.92/.11** | 3.77/.10** | ||
| SE | 1.67 | 1.67 | 1.66 | 1.67 | 1.67 | ||
| Father income | 2.25/.07 | 2.59/.08* | 2.59/.08* | 2.61/.08* | 2.46/.07 | ||
| SE | 1.55 | 1.56 | 1.55 | 1.55 | 1.56 | ||
| Father married | 6.51/.22 | 6.50/.21 | 6.54/.22 | 6.47/.21 | 6.86/.23 | ||
| SE | 7.45 | 7.43 | 7.38 | 7.39 | 7.40 | ||
| Maternal Measures | |||||||
| Mother race | −7.83/−.26*** | −7.52/−.25*** | −7.42/−.24*** | −7.40/−.24*** | −7.31/−.24*** | ||
| SE | 1.75 | 1.75 | 1.74 | 1.74 | 1.75 | ||
| Mother education | 2.94/.08* | 2.99/.08* | 2.90/.08* | 2.93/.08* | 3.01/.08* | ||
| SE | 1.66 | 1.66 | 1.65 | 1.65 | 1.66 | ||
| Mother income | 3.84/.12** | 3.84/.12** | 3.28/.11** | 3.30/.11** | 3.29/.11** | ||
| SE | 1.51 | 1.51 | 1.51 | 1.52 | 1.52 | ||
| Mother married | −3.93/−.13 | −3.88/−.13 | −3.78/−.12 | −3.70/−.12 | −4.11/−.14 | ||
| SE | 7.44 | 7.42 | 7.37 | 7.39 | 7.40 | ||
| Mother age | .25/.10 | 2.61/.10 | .27/.11 | .27/.11 | .27/.11 | ||
| SE | .18 | .18 | .17 | .18 | .18 | ||
| n | 449 | 449 | 449 | 449 | 449 | 449 | 449 |
| R² | .09 | .09 | .31 | .31 | .32 | .32 | .32 |
p<0 .10,
p<0 .05,
p<0.01;
= All analyses corrected for number of children living in the home
Results
The first step in the analysis was to estimate a series of nonlinear ordinary least squares (OLS) regression models for the full analytic sample. A total of seven models were estimated and include the unstandardized and standardized coefficient estimates, the standard errors, the proportion of variance explained by each model (R2), and sample size. The findings are presented in Table 3. Recall that each predictor variable was entered into the models one at a time, thus allowing for an estimation of whether any of the predictor variables attenuated the relationship between father’s age and child verbal IQ scores. Moreover, models 1 through 7 were all adjusted for child sex and the number of children living in the home. As shown in model 1, father’s age was significantly associated with child PPVT scores (p < 0.01). Model 2 represents the baseline model for the higher-order polynomial regression equation, and includes the higher-order polynomial for father’s age (age2). Once the quadratic term was entered into the model, father’s age maintained a significant relationship with child verbal IQ, while father’s age2 was also significantly related to child PPVT scores (p < 0.01). These findings suggest a nonlinear relationship between father’s age and child PPVT scores. A graphical depiction of the nonlinear baseline model (e.g., Model 2) is presented in Figure 1.
Figure 1.
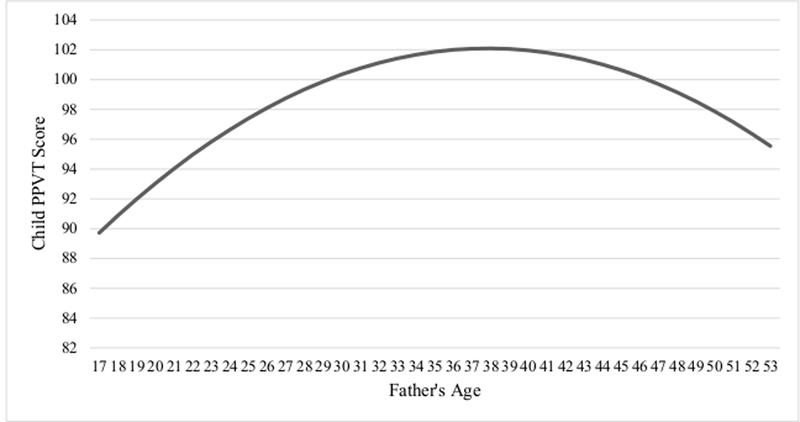
The Effect of Father’s Age at Birth on Child PPVT Score* *Baseline model
Model 7 represents the full polynomial regression model, which regressed child verbal IQ on father’s age and the squared term, father’s incarceration at baseline, father’s WAIS-R score, father’s low self-control, father involvement, as well as on each of the paternal and maternal control variables. Father’s WAIS-R scores, father’s education, and father’s income were among the strongest paternal associations with child verbal IQ scores, and were statistically significant (p < 0.01 to p < 0.05). Moreover, father’s age was no longer significantly associated with child verbal IQ scores and evidence for the nonlinear relationship between father’s age and child verbal IQ was no longer supported. Among the maternal measures, mother’s education and mother’s reported total household annual income was significantly associated with child IQ (p < 0.01). The full model explained 26% of the variance in child verbal IQ scores and is graphically displayed in Figure 2.
Figure 2.
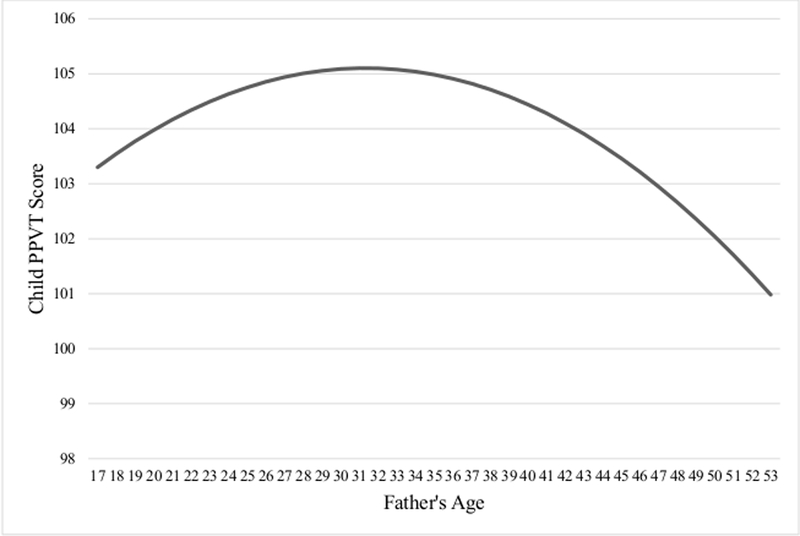
The Etfect of Father’s Age at Birth on Child PPVT Score* *Full model
The next step in the analysis estimated the nonlinear relationship between father’s age and child PPVT scores among the male- and female-only samples. Utilizing a split-sample procedure allowed for an estimation of the conditional effect of child gender on the nonlinear relationship between father’s age and child verbal IQ. The results for the male sample are presented in Table 4. As shown in model 1, father’s age was significantly associated with male children’s PPVT scores, while adjusting for the number of children living in the home (p < 0.01). The baseline polynomial regression model is represented in model 2, and also displays a significant nonlinear relationship between father’s age and male children’s verbal IQ scores (p < 0.01). The nonlinear relationship is graphically depicted in Figure 3.
Figure 3.
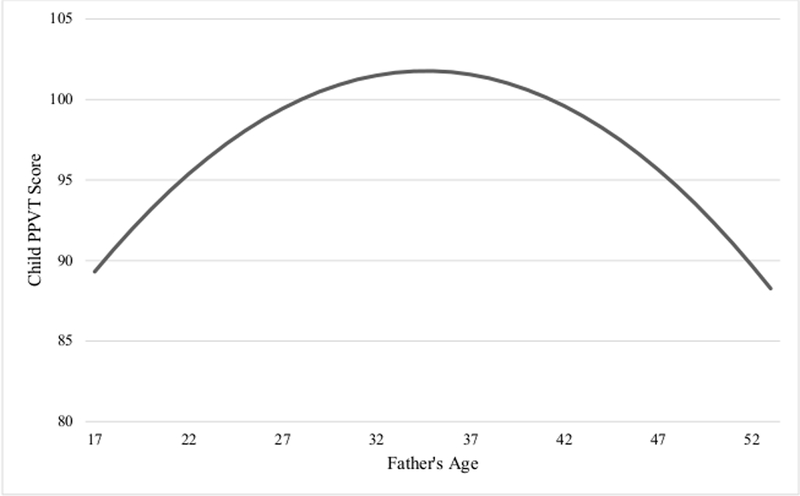
The Etfect of Father’s Age at Birth on Male Children’s PPVT Score* *Baseline model
Once all of the predictor variables were entered into the models, the nonlinear relationship between father’s age and male children’s verbal IQ scores remained marginally significant (p < 0.10). Model 7 represents the full polynomial regression model, and indicates that along with father’s age, father’s WAIS-R scores, father’s race, and mother’s total household annual income were significantly associated with male children’s verbal IQ scores. The full polynomial regression model accounted for 23% of the variance in verbal IQ scores among the male children in the sample.
The findings for the female sample are presented in Table 5. Model 1 shows father’s age at birth to be significantly related to female children’s PPVT scores (p < 0.01), while adjusting for the number of children in the home. However, once the quadratic term for father’s age was entered into the baseline polynomial regression model (model 2), the influence of father’s age was attenuated (but retained significant), while father’s age2 was not a significantly related to female children’s verbal IQ. These findings indicate the absence of a nonlinear relationship between father’s age and female’s PPVT scores. The baseline polynomial model for female children is represented graphically in Figure 4.
Figure 4.
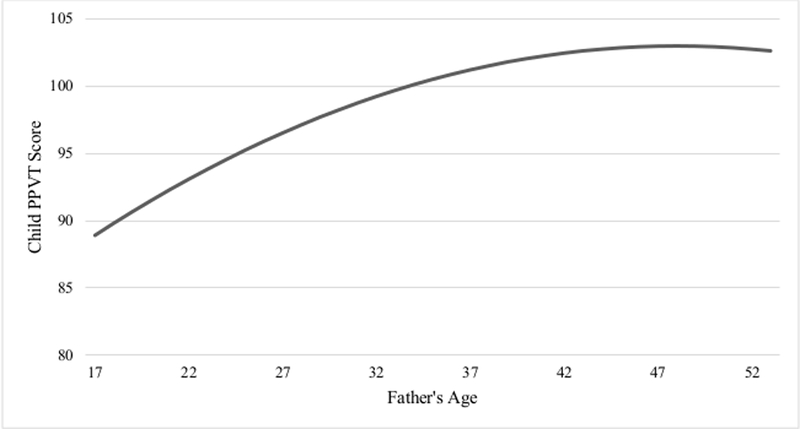
The Etfect of Father’s Age at Birth on Female Children’s PPVT Score* *Baseline model
Once all of the predictor variables were entered into the full model (model 7), father’s WAIS-R scores remained significantly associated with female PPVT scores, along with father’s education, mother’s race, mother’s education, and mother’s total household annual income. The full polynomial regression model accounted for 32% of the variance in verbal IQ scores among the female children in the sample. Across each of the models, no support was found for a nonlinear relationship between father’s age and female children’s verbal IQ scores.
Discussion
The relationship between paternal age at birth and children’s verbal IQ may be difficult to examine, due to a number of potential confounders linked to paternal age. The analyses sought to control for many paternal characteristics, social factors, and maternal characteristics that could act as confounders. Once the analyses controlled for these potential confounders, father’s age at birth no was longer significantly associated with children’s verbal IQ at age 9 among the full sample and female sample. Nevertheless, the results suggest that the nonsignificant relationship between paternal age and children’s intelligence cannot be solely accounted for by the social-influence argument. We speak to the findings of the nonlinear relationship between paternal age and male children’s verbal IQ scores and consider possible explanations for the moderating role of children’s gender on the relationship between paternal age and verbal IQ.
While a significant inverted U-shaped relationship between father’s age at birth and children’s verbal IQ was supported within each of the baseline models (albeit for the female sample), many of the individual-level and social risk factors associated with both young and advanced paternal age had a limited impact on children’s verbal IQ. The association between young paternal age and male children’s lower verbal IQ scores—despite controlling for a number of important paternal characteristics (e.g., fathers’ low self-control, educational attainment, and income status)—contrasts with previous research, which suggests that environmental risk factors associated with young parenthood operate independently on children’s behavioral outcomes (D’Onofrio et al., 2009). Additionally, advanced paternal age continued to be marginally associated with male offspring intelligence while controlling for a number of factors associated with delayed fatherhood, such as education, household income, marital status, and father’s own intelligence. Nonetheless, it is important to note that among the paternal characteristics examined, paternal intelligence maintained one of the strongest associations with offspring intelligence across the full, male, and female samples. Indeed, due to the high heritability of traits such as intelligence (Vinkhuyzen, van der Sluis, Maes, & Posthuma, 2012), these findings are not surprising. Previous research, moreover, suggests that the association between paternal age and offspring intelligence may be attenuated once measures of parental traits (i.e., intelligence) are accounted for (Arslan et al., 2014).2
In regards to maternal characteristics, age at the time of birth, educational level, and marital status also did not attenuate the observed relationship between male children’s IQ scores and paternal age. These findings suggest that fathers’ age may be an important biological risk factor in determining male children’s verbal IQ scores later in life. For instance, one potential mechanism linking advanced paternal age to children’s verbal IQ may be related to biological processes. Recall the possibility for copy error mutations in the spermatogonia during cell division with an increase in paternal age (Penrose, 1955). These mutations in the male gamete have been associated with adverse offspring outcomes and may contribute to intelligence variability (D’Onofrio et al., 2014; Malaspina et al., 2005). Recent research also highlights the importance of genetic factors for contributing to advanced paternal age effects (Gratten et al., 2016), where genetic influences on offspring developmental outcomes have been reported to be stronger among offspring born to older fathers rather than younger fathers (Janecka et al., 2017a). Future research will need to decipher the underlying mechanisms linking both advanced and young paternal age to offspring outcomes.
Support for the significant nonlinear association between paternal age and male children’s verbal IQ—but not for female children’s verbal IQ—is worth noting. To our knowledge, very few studies have considered the moderating role of gender on the relationship between paternal age and children’s cognitive outcomes (Janecka, Rijsdijk, Rai, Modabbemia, & Reichenberg, 2017b; Svensson et al., 2011), although many studies include child’s sex as a covariate in their models (Arslan et al., 2014; D’Onofrio et al., 2014; Edwards & Roff, 2010; Saha et al., 2009). One explanation for why girls may be more resilient to the effects of paternal age aligns with the male disadvantage hypothesis, which suggests that males are more vulnerable to biosocial stressors during gestation, infancy, and childhood (Kirchengast & Hartmann, 2009). Newborn males display higher morbidity and mortality rates than do female newborns (Elsmén, Steen, & Hellström-Westas, 2004), are more likely to be born prematurely (Cooperstock & Campbell, 1996), and show an increased vulnerability for low birth weight (Kirchengast & Hartmann, 2009). Furthermore, males are more likely to experience adverse neonatal outcomes and obstetric risks, such as hypoglycemia, cesarean sections, and neurological deficits (Moffitt, Caspi, Rutter, & Silva, 2001; Simchen et al., 2014).
In turn, a strong male disadvantage emerges due to a number of adversities arising during pregnancy and after birth. Such an explanation suggests that the biological feature of being male puts them at this disadvantage (Naeye, Burt, Wright, Blanc, & Tatter, 1971). Support has been found for the male vulnerability hypothesis, as males are much more vulnerable to maternal stresses during pregnancy (Rueness, Vatten, & Eskild, 2012). In this context, paternal age at birth could serve as a risk factor for male offspring, thereby influencing intellectual development later in life.
A number of limitations and recommendations for future research should be addressed before the relationship between paternal age and child IQ can be fully understood. First, replication is needed in order to illuminate the mechanisms responsible for reducing the nonlinear association of paternal age to nonsignificance among the full and female sample once potential confounders were included in the present analyses. Indeed, the case may be that our models were overly saturated, thereby underestimating the association between paternal age and children’s verbal IQ scores. Tests of multicollinearity revealed high variance inflation factor (VIF) values for the paternal and maternal marital status measures. A series of analyses (available upon request) revealed that once these two variables were removed from the models, the nonlinear relationship between paternal age and child IQ remained statistically significant for the full sample within the full estimation model (i.e., with all other potential confounders included in the models). Second, because listwise deletion was utilized within the present study, the risk of a Type II error was inherit due to the resulting smaller sample size once all of the additional confounders were included in the models. The small sample size likely reduced the ability to detect significant results within the full estimation models.3 Indeed, previous research has acknowledged the limitation to detect a true relationship between paternal age and offspring outcomes due to small sample sizes (Carslake, Tynelius, van den Berg, Smith, & Rasmussen, 2017; Gratten et al., 2016).4
Third, because the FFCWS is not representative of a typical sample of children and their families, the results may not align with those from a more representative population (Janecka et al., 2017a; Janecka et al., 2017b). In addition, future research utilizing samples of high-risk families should strive to examine the role of parental age with a sibling comparison approach. Such an approach helps to account for any unmeasured confounders (e.g., genetic and/or environmental factors) shared among family members that could rule the observed relationship between paternal age and offspring outcomes as spurious (D’Onofrio et al., 2014; Fieder & Huber, 2015). Unfortunately, measures of offspring intelligence were only assessed among the focal child within the FFCWS, thereby not allowing such a quasi-experimental approach to be utilized in the current study. Fourth, only the verbal abilities of IQ were assessed among the sample. It would be interesting to determine if these findings held for other measures of IQ, such as numerical reasoning and reaction time. Previous research has found support for the nonlinear relationship between paternal age and scores of general intelligence, but not for offspring’s reaction times (Whitley et al., 2012). Finally, future research should examine whether the influence of paternal age on children’s verbal IQ persists into adolescence. Unfortunately, the last available measure for children’s verbal IQ was assessed at age 9 within the FFCWS.5
In conclusion, fathers’ age at birth may have a significant impact on children’s verbal IQ scores, especially among male children. The nonlinear nature of this relationship offers implications for the provision of health care resources. Most often, resources are geared towards younger fathers and their families, but older fathers may also benefit from interventional guidance. While intervention efforts typically target parenting behaviors, our findings suggest that biological mechanisms might be important for explaining the nonlinear relationship between paternal age and male children’s IQ. Intervention efforts, therefore, may benefit the most from targeting parental health outcomes among both young and older males who are planning on becoming fathers.
Acknowledgments:
Preparation of this article was supported by the Prevention and Methodology Training Program (T32 DA017629; PI: L. M. Collins) and award P50 DA039838 (PI L. M. Collins). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Drug Abuse or the National Institutes of Health.
Footnotes
There are a number of reasons for why complete case analysis (i.e., listwise deletion) was utilized for the present analyses. Many of the respondents were missing from the analysis by definition of the study population (e.g., many respondents were not administered surveys by design). Likewise, there were issues surrounding the expected number of fathers to be included in the analyses before losing cases due to non-item response (e.g., the number of fathers included in the FFCWS subsequently reduced in size across each of the waves). Therefore, we chose to retain only cases with complete data, due in part to the sampling design of the FFCWS.
To test the robustness of our findings, we also re-estimated the analyses by controlling for a measure of maternal IQ (i.e., the same measure used to assess paternal IQ [WAIS-R]). The findings remained robust, where paternal age was found to have a significant, albeit marginally, nonlinear association with male offspring IQ (results available upon request).
Although listwise deletion was utilized in the present analyses (for reasons discussed previously), we nonetheless utilized a number of other missing data strategies (e.g., mean substitution, multiple imputation). The results of these analyses did not find support for a significant nonlinear relationship between paternal age and child IQ within the full estimation models (results available upon request).
An anonymous reviewer suggested that issues surrounding multiple testing may be a concern in the current study. Nonetheless, we point out that a risk of a Type II error is probably more likely, given the study’s limited power to detect significant results (e.g., due to small sample sizes) and the issues surrounding multicollinearity. In order to avoid further limiting our ability to detect significant results by employing a smaller p-value, we chose to keep p < 0.10 as a reference, but note that the significance of these results is characterized as marginally significant.
We chose to examine the relationship between paternal age at birth and children’s verbal IQ at age 9 as a conservative test of this relationship. Although measures of children’s verbal IQ were available as early as age 3, we wanted to examine whether the observed relationship would hold until mid-to-late childhood
References
- Arslan RC, Penke L, Johnson W, Iacono WG, & McGue M (2014). The effect of paternal age on offspring intelligence and personality when controlling for parental trait levels. PloS One, 9, e90097. doi: 10.1371/journal.pone.0090097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver KM, Schwartz JA, Nedelec JL, Connolly EJ, Boutwell BB, & Barnes J (2013). Intelligence is associated with criminal justice processing: Arrest through incarceration. Intelligence, 41, 277–288. [Google Scholar]
- Becker GS (1991). A treatise on the family. Cambridge: Harvard University Press. [Google Scholar]
- Buizer-Voskamp JE, Laan W, Staal WG, Hennekam EA, Aukes MF, Termorshuizen F, . . . Ophoff RA (2011). Paternal age and psychiatric disorders: Findings from a Dutch population registry. Schizophrenia Research, 129, 128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carslake D, Tynelius P, van den Berg G, Smith GD, & Rasmussen F (2017). Associations of parental age with health and social factors in adult offspring. Methodological pitfalls and possibilities. Scientific Reports, 7, 45278. doi: 10.1038/srep45278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperstock M, & Campbell J (1996). Excess males in preterm birth: Interactions with gestational age, race, and multiple birth. Obstetrics & Gynecology, 88, 189–193. [DOI] [PubMed] [Google Scholar]
- D’Onofrio BM, Goodnight JA, Van Hulle CA, Rodgers JL, Rathouz PJ, Waldman ID, & Lahey BB (2009). Maternal age at childbirth and offspring disruptive behaviors: Testing the causal hypothesis. Journal of Child Psychology and Psychiatry, 50, 1018–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Onofrio BM, Rickert ME, Frans E, Kuja-Halkola R, Almqvist C, Sjölander A, . . . Lichtenstein P (2014). Paternal age at childbearing and offspring psychiatric and academic morbidity. JAMA Psychiatry, 71, 432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman SJ (1990). Functional and dysfunctional impulsivity: Personality and cognitive correlates. Journal of Personality and Social Psychology, 58, 95–102. [DOI] [PubMed] [Google Scholar]
- Edwards RD, & Roff J (2010). Negative effects of paternal age on children’s neurocognitive outcomes can be explained by maternal education and number of siblings. PLoS One, 5, e12157. doi: 10.1371/journal.pone.0012157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsmén E, Steen M, & Hellström-Westas L (2004). Sex and gender differences in newborn infants: Why are boys at increased risk? The Journal of Men’s Health & Gender, 1, 303–311. [Google Scholar]
- Fergusson DM, & Woodward LJ (1999). Maternal age and educational and psychosocial outcomes in early adulthood. Journal of Child Psychology and Psychiatry, 40(3), 479–489. [PubMed] [Google Scholar]
- Fieder M, & Huber S (2015). Paternal age predicts offspring chances of marriage and reproduction. American Journal of Human Biology, 27, 339–343. [DOI] [PubMed] [Google Scholar]
- Gratten J, Wray NR, Peyrot WJ, McGrath JJ, Visscher PM, & Goddard ME (2016). Risk of psychiatric illness from advanced paternal age is not predominantly from de novo mutations. Nature Genetics, 48, 718–724. [DOI] [PubMed] [Google Scholar]
- Jaffee S, Caspi A, Moffitt TE, Belsky J, & Silva P (2001). Why are children born to teen mothers at risk for adverse outcomes in young adulthood? results from a 20-year longitudinal study. Development and Psychopathology, 13, 377–397. [DOI] [PubMed] [Google Scholar]
- Janecka M, Haworth CMA, Ronald A, Krapohl E, Happé F, Mill J, ... & Rijsdijk F (2017a). Paternal age alters social development in offspring. Journal of the American Academy of Child & Adolescent Psychiatry, 56, 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janecka M, Rijsdijk F, Rai D, Modabbernia A, & Reichenberg A (2017b). Advantageous developmental outcomes of advancing paternal age. Translational Psychiatry, 7, e1156. doi: 10.1038/tp.2017.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchengast S, & Hartmann B (2009). The male disadvantage hypothesis reconsidered: Is there really a weaker sex? an analysis of gender differences in newborn somatometrics and vital parameters. J Life Sci, 1, 63–71. [Google Scholar]
- Malaspina D, Harlap S, Fennig S, Heiman D, Nahon D, Feldman D, & Susser ES (2001). Advancing paternal age and the risk of schizophrenia. Archives of General Psychiatry, 58, 361–367. [DOI] [PubMed] [Google Scholar]
- Malaspina D, Reichenberg A, Weiser M, Fennig S, Davidson M, Harlap S, . . . Knobler HY (2005). Paternal age and intelligence: Implications for age-related genomic changes in male germ cells. Psychiatric Genetics, 15, 117–125. doi:00041444-200506000-00008 [pii] [DOI] [PubMed] [Google Scholar]
- McGrath JJ, Petersen L, Agerbo E, Mors O, Mortensen PB, & Pedersen CB (2014). A comprehensive assessment of parental age and psychiatric disorders. JAMA Psychiatry, 71, 301–309. [DOI] [PubMed] [Google Scholar]
- McIntosh GC, Olshan AF, & Baird PA (1995). Paternal age and the risk of birth defects in offspring. Epidemiology, 6, 282–288. [DOI] [PubMed] [Google Scholar]
- Menezes P, Lewis G, Rasmussen F, Zammit S, Sipos A, Harrison G, . . . Gunnell D (2010). Paternal and maternal ages at conception and risk of bipolar affective disorder in their offspring. Psychological Medicine, 40, 477–485. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Rutter M, & Silva PA (2001). Sex differences in antisocial behavior: Conduct disorder, delinquency, and violence in the dunedin longitudinal study. Cambridge, United Kingdom: Cambridge University Press. [Google Scholar]
- Moffitt TE (2002). Teen‐aged mothers in contemporary britain. Journal of Child Psychology and Psychiatry, 43, 727–742. [DOI] [PubMed] [Google Scholar]
- Myrskylä M, Silventoinen K, Tynelius P, & Rasmussen F (2013). Is later better or worse? association of advanced parental age with offspring cognitive ability among half a million young swedish men. American Journal of Epidemiology, 177, 649–655. doi: 10.1093/aje/kws237 [DOI] [PubMed] [Google Scholar]
- Naeye RL, Burt LS, Wright DL, Blanc WA, & Tatter D (1971). Neonatal mortality, the male disadvantage. Pediatrics, 48, 902–906. [PubMed] [Google Scholar]
- Penrose L (1955). Parental age and mutation. The Lancet, 266, 312–313. [DOI] [PubMed] [Google Scholar]
- Reichman NE, Teitler JO, Garfinkel I, & McLanahan SS (2001). Fragile families: Sample and design. Children and Youth Services Review, 23, 303–326. [Google Scholar]
- Risch N, Reich EW, Wishnick MM, & McCarthy JG (1987). Spontaneous mutation and parental age in humans. American Journal of Human Genetics, 41, 218–248. [PMC free article] [PubMed] [Google Scholar]
- Rowe DC, Jacobson KC, & Van den Oord, Edwin JCG (1999). Genetic and environmental influences on vocabulary IQ: Parental education level as moderator. Child Development, 70, 1151–1162. [DOI] [PubMed] [Google Scholar]
- Rueness J, Vatten L, & Eskild A (2012). The human sex ratio: Effects of maternal age. Human Reproduction, 27, 283–287. doi: 10.1093/humrep/der347 [doi] [DOI] [PubMed] [Google Scholar]
- Saha S, Barnett AG, Buka SL, & McGrath JJ (2009). Maternal age and paternal age are associated with distinct childhood behavioural outcomes in a general population birth cohort. Schizophrenia Research, 115, 130–135. [DOI] [PubMed] [Google Scholar]
- Saha S, Barnett AG, Foldi C, Burne TH, Eyles DW, Buka SL, & McGrath JJ (2009). Advanced paternal age is associated with impaired neurocognitive outcomes during infancy and childhood. PLoS Medicine, 6, e1000040. doi: 10.1371/journal.pmed.1000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandin S, Hultman CM, Kolevzon A, Gross R, MacCabe JH, & Reichenberg A (2012). Advancing maternal age is associated with increasing risk for autism: A review and meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 51, 477–486. [DOI] [PubMed] [Google Scholar]
- Simchen MJ, Weisz B, Zilberberg E, Morag I, Weissmann-Brenner A, Sivan E, & Dulitzki M (2014). Male disadvantage for neonatal complications of term infants, especially in small-for-gestational age neonates. The Journal of Maternal-Fetal & Neonatal Medicine, 27, 839–843. [DOI] [PubMed] [Google Scholar]
- Svensson AC, Abel K, Dalman C, & Magnusson C (2011). Implications of advancing paternal age: Does it affect offspring school performance? PloS One, 6, e24771. doi: 10.1371/journal.pone.0024771 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tearne JE (2015). Older maternal age and child behavioral and cognitive outcomes: A review of the literature. Fertility and Sterility, 103, 1381–1391. [DOI] [PubMed] [Google Scholar]
- Vinkhuyzen AA, van der Sluis S, Maes HH, & Posthuma D (2012). Reconsidering the heritability of intelligence in adulthood: Taking assortative mating and cultural transmission into account. Behavior Genetics, 42, 187–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M, Reichenberg A, Werbeloff N, Kleinhaus K, Lubin G, Shmushkevitch M, . . . Davidson M (2008). Advanced parental age at birth is associated with poorer social functioning in adolescent males: Shedding light on a core symptom of schizophrenia and autism. Schizophrenia Bulletin, 34, 1042–1046. doi: 10.1093/schbul/sbn109 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitley E, Deary IJ, Der G, Batty GD, & Benzeval M (2012). Paternal age in relation to offspring intelligence in the west of scotland twenty-07 prospective cohort study. PloS One, 7, e52112. doi: 10.1371/journal.pone.0052112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Wen SW, Leader A, Chen XK, Lipson J, & Walker M (2007). Paternal age and birth defects: How strong is the association? Human Reproduction (Oxford, England), 22, 696–701. doi:del453 [pii] [DOI] [PubMed] [Google Scholar]


