Summary
The ventral tegmental area (VTA) receives phenotypically distinct innervations from the pedunculopontine tegmental nucleus (PPTg). While PPTg-to-VTA inputs are thought to play a critical role in stimulus-reward learning, direct evidence linking PPTg-to-VTA phenotypically distinct inputs in the learning process remains lacking. Here, we used optogenetic approaches to investigate the functional contribution of PPTg excitatory and inhibitory inputs to the VTA in appetitive Pavlovian conditioning. We show that photoinhibition of PPTg-to-VTA cholinergic or glutamatergic inputs during cue presentation dampens the development of anticipatory approach responding to the food receptacle during the cue. Furthermore, we employed in vivo optetrode recordings to show that photoinhibition of PPTg cholinergic or glutamatergic inputs significantly decreases VTA non-dopamine (non-DA) neural activity. Consistently, photoinhibition of VTA non-DA neurons disrupts the development of cue-elicited anticipatory approach responding. Taken together, our study reveals a crucial regulatory mechanism by PPTg excitatory inputs onto VTA non-DA neurons during appetitive Pavlovian conditioning.
Keywords: pedunculopontine tegmental nucleus, PPTg, ventral tegmental area, VTA, appetitive Pavlovian conditioning, VTA non-DA neurons, cholinergic input, glutamatergic input
Graphical Abstract

Introduction
The dopamine (DA) neurons in the ventral tegmental area (VTA) play a key role in appetitive reward associative learning (Fields et al., 2007, Steinberg et al., 2013, Wise, 2004). Although the VTA contains phenotypically distinct non-DA neurons (Taylor et al., 2014), previous studies have mainly implicated their functions through local regulation of DA neurons (Fields et al., 2007, van Zessen et al., 2012, Wang et al., 2015). The roles of VTA non-DA efferents in appetitive reward learning remain poorly understood.
During appetitive Pavlovian learning, midbrain DA neurons exhibit phasic burst firing activity to unconditioned appetitive stimuli (Ljungberg et al., 1991). After being repeatedly paired with unconditioned appetitive stimuli, the neutral stimuli become predictive of reward and are able to elicit DA burst activity (Ljungberg et al., 1991, Mirenowicz and Schultz, 1996, Schultz and Romo, 1990). The roles of DA burst activity in acquiring conditioned responding depend on whether predictive values or incentive values are assigned to reward-predicting stimuli through Pavlovian conditioning (Flagel et al., 2011). It is believed that DA neuron burst activity is not intrinsic but depends on afferent inputs (Grace et al., 2007). In order to evolve the capability to drive DA neurons burst firing to the conditioned stimuli, the afferent inputs must have the ability to recruit midbrain VTA circuits, as unconditioned appetitive rewards do (Wise, 2004, Zellner and Ranaldi, 2010). Thus, the afferent innervations to the VTA have been mapped to help understand how the inputs regulate the firing pattern of midbrain DA neurons and what information is conveyed to the VTA (Coizet et al., 2003, Geisler and Wise, 2008, Geisler and Zahm, 2005, Lammel et al., 2012, Liu et al., 2012, Matsui et al., 2014, Watabe-Uchida et al., 2012). Several lines of evidence led us to study the regulatory roles of the pedunculopontine tegmental nucleus (PPTg) in VTA circuits and its role in modulating behaviors. Anatomically, PPTg provides ascending cholinergic and non-cholinergic inputs to the VTA (Holmstrand and Sesack, 2011). Acetylcholine (ACh) released from PPTg cholinergic inputs can act on nicotinic or muscarinic ACh receptors to regulate VTA DA and non-DA neural activity (Grillner et al., 2000, yin and French, 2000, Zhang et al., 2005). Besides, inactivation of PPTg decreases VTA DA spontaneous and conditioned stimuli-elicited burst activity (Floresco et al., 2003, Pan and Hyland, 2005). Electrophysiological studies show that PPTg neurons respond to visual and auditory stimuli earlier than DA cells’ activation (Pan and Hyland, 2005). In line with this evidence, PPTg lesions impair stimulus-reward learning (Inglis et al., 2000). These studies raise the possibility that PPTg relays sensory information onto VTA and thereafter modulates behaviors through regulating firing pattern of midbrain DA neurons.
Despite the relevance of these studies, direct evidence linking PPTg-to-VTA inputs in the acquisition of stimulus-reward association is lacking. Given that PPTg projects to several brain regions (Hamani et al., 2007, Holmstrand and Sesack, 2011, Oakman et al., 1995), conventional methods of pharmacological inactivation in PPTg cannot conclusively establish a causal link between specific PPTg outputs and observed behavioral or cellular deficits. Moreover, PPTg contains heterogeneous neuronal populations (Wang and Morales, 2009), thus lesions or pharmacological manipulations in PPTg cannot distinguish the relative contributions of different PPTg neuronal populations. To overcome limitations associated with pharmacological manipulations, we employed an optogenetic approach to selectively inhibit PPTg cholinergic, glutamatergic, or GABAergic inputs to the VTA during cue-reward pairings in order to determine the contribution of these inputs in Pavlovian appetitive conditioning. Furthermore, we performed in vivo optetrode recording in freely moving mice as they performed a Pavlovian task to examine the regulation of VTA neural activity by PPTg afferents. Here, we report that PPTg-to-VTA excitatory inputs are necessary in the acquisition of stimulus-reward associations through the regulation onto VTA non-DA neurons.
Results
The Development of Anticipatory Approach Responding during Pavlovian Conditioning
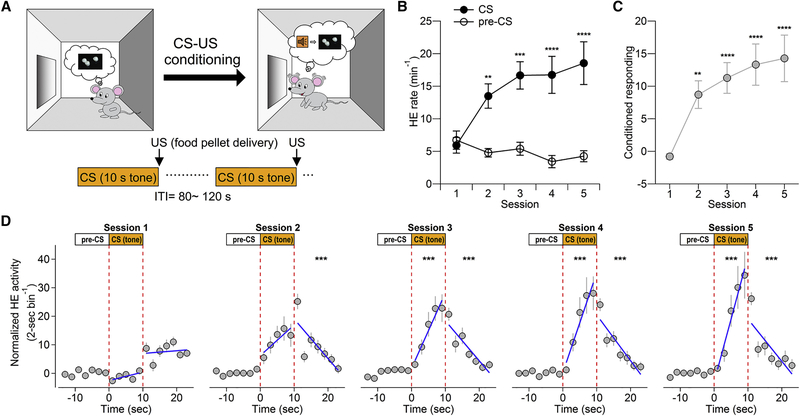
We used an appetitive Pavlovian conditioning paradigm to study the functional contribution of PPTg-to-VTA innervations. Mice were trained in daily cue-reward conditioning, where a food pellet reward, an unconditioned stimulus (US), was delivered to the food receptacle immediately after a 10-s tone presentation, a conditioned stimulus (CS) (Figure 1A). After repeated CS-US pairings, the mice increased head entries (HEs) into the food receptacle during tone presentation across training sessions (Figure 1B), indicating that the mice learned to anticipate food pellet delivery based on tone presentation (CS) (Parker et al., 2010). As a consequence of the evolved ability to predict US from CS presentation, the mice gradually withheld their HEs into the food receptacle during the tone-absent inter-trial intervals (ITIs) across training sessions (Figure 1B, estimated by the HE rate during a 10-s period prior the CS [pre-CS]). We quantified each mouse’s acquisition of anticipatory conditioned responding by subtracting HE rate during pre-CS from the HE rate during the CS (Stuber et al., 2008) (Figure 1C). The analysis indicated that the mice started to display significantly elevated anticipatory approach responses on day 2 (Figure 1C).
Figure 1.
Cue-Reward Association in Pavlovian Appetitive Conditioning Paradigm
(A)Schematic of Pavlovian appetitive conditioning. The behavioral paradigm consists of five consecutive sessions and each training session consists of 40 pairings of 10-s tone-food pellet at variable intervals averaging 100 s.
(B)Plot illustrates significantly elevated head entry (HE) rate during the CS relative to pre-CS period across training sessions (n = 14 mice, two-way ANOVA, CS versus pre-CS; F(1, 26) = 24.11, p < 0.0001).
(c)Plot illustrates elevated conditioned responding to the CS across training sessions (one-way ANOVA, F = 11.36, p < 0.0001). Post hoc pairwise comparisons between CS and pre-CS (B) or with the first session (C) were conducted with Bonferroni adjustments. Error bars represent SEM. Asterisks denote post hoc statistical significance (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).
(D)The distribution of HEs during the CS and after food pellet delivery across training sessions. Data points represent normalized HEs in 2-s bins by subtracting the average number of HEs during a 10-s pre-CS. Mixed-effects linear regression is used to estimate the change of HE rate (slope, β) during the CS and after food pellet delivery. The comparisons of slopes of regression lines were made between the first session and other sessions during the CS and after food pellet delivery. During the CS: session 1, β = 0.30 ±0.14; session 2, β = 1.08 ± 0.33, z = 1.43, p = 0.152; session 3, β = 2.65 ± 0.35, z = 4.34, p < 0.001; session 4, β = 3.51 ± 0.42, z = 5.91, p < 0.001; session 5, β = 4.42 ± 0.56, z = 7.58, p < 0.001. After food pellet delivery: session 1, β = 0.095 ±0.15; session 2, β = −1.38 ± 0.21, z = −5.33, p < 0.001; session 3, β = −1.40 ± 0.20, z = −5.39, p < 0.001; session 4, β = −1.60 ± 0.18, z = −6.12, p < 0.001; session 5, β = −1.48 ± 0.23, z = −5.68, p < 0.001.
Asterisks denote significant difference in rate of HE change in the respective time windows compared to the first session (*p < 0.05; **p < 0.01; ***p < 0.001).
To better understand how the mice responded to the CS, we conducted a detailed analysis of temporal distribution of HEs in 2-s bins around CS presentation. We used mixed-effects linear regression to estimate the temporal distribution of HEs during and after the CS for each mouse (Figure S1; Figure 1D). We found that, starting from day 2, compared with pre-CS baseline, mice increased anticipatory approach responses following CS onset and made most approach responses around CS offset/food pellet delivery, then decreased approach responses in a time-dependent manner (Figure 1D). When compared to the fairly even distribution of CS-elicited anticipatory approach responses on day 1, the temporal distribution of anticipatory approach responses during the CS was significantly biased toward CS offset starting from day 3 (Figure 1D), suggesting that mice learn to use the discrete temporal relationship between CS presentation and pellet drop to predict reward delivery and make anticipatory approach responses.
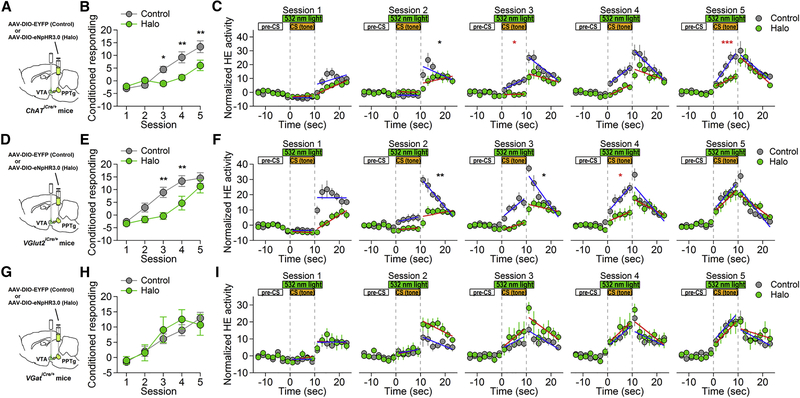
Photoinhibition of PPTg-to-VTA Specific Afferent Inputs during Pavlovian Conditioning
To determine which PPTg afferent inputs to the VTA are required for acquisition of cue-reward association, we used an inhibitory optogenetic approach. We bilaterally injected Cre-dependent viral vector encoding eNpHR3.0-EYFP (Halo) into PPTg and implanted fiber optics in the VTA of ChATiCre/+, VGlut2iCre/+, and VGatiCre/+ mice, respectively (Figures 2A, 2D, and 2G; Figures S2-S4). Littermates of the respective mouse lines were bilaterally injected with Cre-dependent virus encoding EYFP alone into PPTg and implanted with fiber optics in the VTA to serve as the control, to rule out confounding variables such as surgery procedures or intra-VTA light exposure. Following recovery from surgery and allowing for robust expression of EYFP in the VTA, these mice were trained on the appetitive Pavlovian conditioning paradigm as described above. Photoinhibition was achieved by bilaterally delivering a pulse of 532-nm green light (10 mW) into the VTA via fiber optics, to hyperpolarize Halo-expressing terminals during each tone presentation (Figures 2A, 2D, and 2G). This would selectively inhibit PPTg cholinergic, glutamatergic or GABAergic inputs onto VTA neurons.
Figure 2.
Photoinhibition of PPTg-to-VTA Cholinergic or Glutamatergic Inputs Dampens Cue-Reward Association
(A, D, and G) Schematic illustrations of experimental designs for targeting PPTg-to-VTA cholinergic (A), glutamatergic (D), and GABAergic (G) afferent inputs, respectively.
(B, E, and H) Plots illustrate the development of conditioned responding to the CS in the control and Halo groups of PPTg cholinergic (B), glutamatergic (E), and GABAergic (H) inhibition experiments, respectively. (B) A two-way ANOVA (group × session) demonstrated a significant interaction (F(4,88) = 6.79, p < 0.0001) and significant main effects of group (F(1, 22) = 7.17, p = 0.0138) and session (F(4, 88) = 30.69, p < 0.0001) (control group, n = 13; Halo group, n = 11). (E) A two-way ANOVA (group × session) demonstrated a significant interaction (F(4,120) = 2.9, p = 0.0247) and significant main effects of group (F(1, 30) = 8.31, p = 0.0072) and session (F(4, 120) = 35.88, p < 0.0001) (control group, n = 15; Halo group, n = 17). (H) A two-way ANOVA (group × session) revealed neither interaction (F(4,60) = 0.82, p = 0.5167) nor main effect of group (F(1, 15) = 0.16, p = 0.6922) (control group, n = 10; Halo group, n = 7). However, there was a significant main effect of session ((F(4, 60) = 18.35, p < 0.0001).
(C, F, and I) The distribution of head entries during the CS and after food pellet delivery across training sessions in PPTg cholinergic (C), glutamatergic (f), and GABAergic (I) inhibition experiments, respectively. Data points represent normalized head entries (HEs) in 2-s bins by subtracting the averaged head entries during a 10-s pre-CS. The changes of head entries distribution over training sessions are estimated by mixed-effects linear regression models. Error bars represent SEM. Asterisks denote significant difference in change of HE rate in the respective time windows between control and Halo groups, referenced to the first session.
*p < 0.05; **p < 0.01; ***p < 0.001.
Photoinhibition of PPTg cholinergic terminals in the VTA significantly decreased conditioned anticipatory responding to the CS (Figure 2B). Further examination of the temporal distribution of HEs around CS indicated that mice in the control group exhibited time-dependent increase in HEs with respect to CS presentation starting on day 3, whereas mice in the Halo group failed to exhibit this behavior until day 4 (Figure 2C, sessions 3 and 4; Table 1). Moreover, mice in the Halo group showed significantly lower rate of HE increase during CS than control group mice on day 3 and day 5 (Figure 2C, sessions 3 and 5; Table 1). These results suggest that PPT-to-VTA cholinergic input plays a critical role in cue-reward associative learning.
Table 1.
Summary Statistics for Distribution of Head Entries during the CS across Training Sessions in PPTg-to-VTA Manipulation Experiments
| Control | Halo | Comparison between Control and Halo |
|||||
|---|---|---|---|---|---|---|---|
| Session | Slope Mean (SEM) |
p Value of Comparison to Session 1 |
Slope Mean (SEM) |
p Value of Comparison to Session 1 |
Z Value | p Value | |
| PPTg-to-VTA cholinergic manipulation |
1 | 0.004 (0.12) | — | 0.10 (0.12) | — | — | — |
| 2 | −0.02 (0.14) | 0.936 | −0.06 (0.16) | 0.578 | −0.29 | 0.775 | |
| 3 | 0.85 (0.22) | 0.012 | 0.04 (0.22) | 0.832 | −2.03 | 0.042 | |
| 4 | 1.38(0.28) | <0.001 | 0.83 (0.19) | 0.008 | −1.45 | 0.148 | |
| 5 | 2.55 (0.35) | <0.001 | 0.89 (0.26) | 0.004 | −3.95 | < 0.001 | |
| PPTg-to-VTA glutamatergic manipulation |
1 | 0.02 (0.07) | — | −0.09 (0.08) | — | — | — |
| 2 | 0.36 (0.15) | 0.329 | 0.13 (0.12) | 0.413 | −0.29 | 0.773 | |
| 3 | 1.37 (0.24) | <0.001 | 0.52 (0.18) | 0.023 | −1.71 | 0.087 | |
| 4 | 1.82 (0.35) | <0.001 | 0.71 (0.22) | 0.003 | −2.31 | 0.021 | |
| 5 | 1.87 (0.32) | <0.001 | 1.58 (0.27) | <0.001 | −0.45 | 0.654 | |
| PPTg-to-VTA GABAergic manipulation |
1 | 0.01 (0.12) | — | 0.26 (0.16) | — | — | — |
| 2 | 0.25 (0.18) | 0.459 | 0.31 (0.23) | 0.908 | −0.36 | 0.720 | |
| 3 | 0.76 (0.24) | 0.021 | 1.21 (0.34) | 0.028 | 0.38 | 0.706 | |
| 4 | 1.25 (0.26) | <0.001 | 1.34 (0.40) | 0.012 | −0.29 | 0.773 | |
| 5 | 1.68 (0.31) | <0.001 | 1.53 (0.36) | 0.004 | −0.74 | 0.462 | |
Similarly, we observed that photoinhibition of PPTg glutamatergic input to the VTA also dampened the development of conditioned approach responding to CS (Figures 2D and 2E). On days 3 and 4, mice in the Halo group failed to elevate conditioned responses as compared to the control. However, the performance of the Halo group became comparable to that of the control group by the end of training sessions. In fact, conditioned responding between the control and Halo groups was not significantly different on day 5 (Figure 2E). From the analysis of HE distribution around CS across training sessions, we found that the Halo group showed significantly less steep time-dependent decrease of HEs following food delivery on days 2 and 3 (Figure 2F, sessions 2 and 3; Table S1). Moreover, although both the control and Halo groups started to show biased temporal distribution of HEs toward CS offset during CS presentation from day 3, mice in the Halo group showed a significantly lower increase of HEs following CS onset than mice in the control group (Figure 2F, session 4; Table 1).
In sharp contrast, photoinhibition of PPTg GABAergic input to the VTA did not affect the acquisition of conditioned responding (Figures Figures 2G and 2H). No difference in acquisition curve was found between the control and Halo groups at any stage of training (Figure 2H). Regarding the distribution pattern of HEs during CS, both control and Halo groups displayed similar temporal distribution of HEs following CS onset and CS offset/food delivery across the training sessions (Figure 2I; Table 1; Table S1).
Together, these results indicate that the excitatory cholinergic and glutamatergic afferent inputs from PPTg to VTA are required to develop temporally coupled conditioned responding to CS during Pavlovian conditioning.
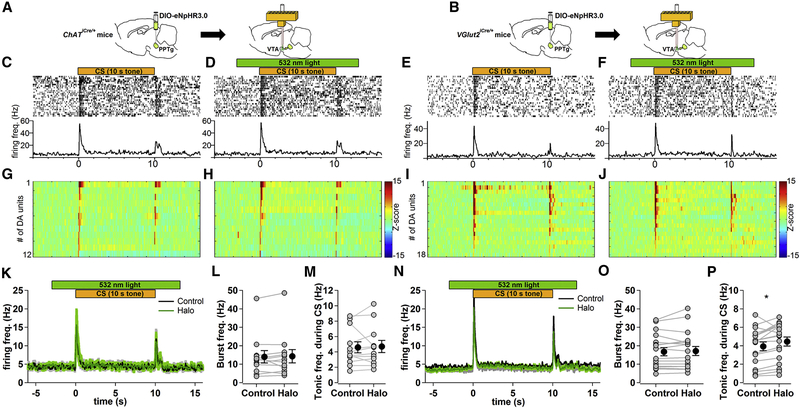
Changes in VTA Neural Activity by Photoinhibition of PPTg-to-VTA Excitatory Inputs
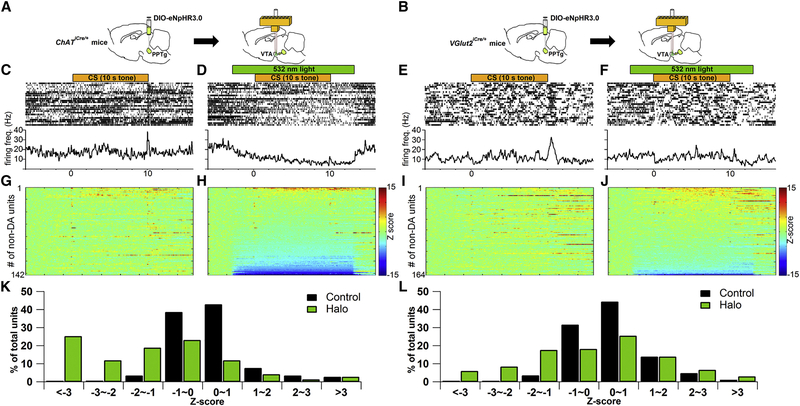
To further examine how optical inhibition of PPTg-to-VTA excitatory inputs results in the observed behavioral deficits, we investigated how PPTg excitatory inputs regulate VTA neuronal activity during CS-US pairings with in vivo optetrode recording experiments in freely moving mice. Cre-inducible eNpHR3.0-EYFP was first unilaterally expressed in PPTg of either ChATiCre/+ or VGlut2iCre/+ mice (Figures 3A and 3B). Following recovery from surgery, the mice were trained in the same Pavlovian task described above (Figure 1A). After the mice learned to increase conditioned approach responses to the tone, a bundle of eight tetrodes surrounding a fiber optic was targeted to the ipsilateral VTA to record VTA neuronal activity and to selectively photoinhibit cholinergic or glutamatergic innervating terminals from PPTg (Figures 3A and 3B; Figure S5). In each recording session, 50 trials of tone-food pellet pairings were presented to the mice. Half of these trials were randomly assigned as Halo trials, in which the mice received green light illumination during tone presentation to optically activate eNpHR3.0-expressing terminals in the VTA (Figures 3D and 3F). The other half of trials was control trials, in which the mice did not receive light illumination (Figures 3C and 3E). Putative DA cells were determined by a significant increase in firing between 50 and 200 ms after the onset of the conditioned tone, low baseline firing rate (< 10 Hz), and long-lasting suppression in firing rate by a D2 receptor agonist quinpirole (0.1~0.5 mg/kg, intraperitoneally; Figure S5). We found that the tone-elicited phasic firing of putative DA neurons was not affected by photoinhibition of PPTg cholinergic (Figures 3G, 3H, 3K, and 3L) or glutamatergic inputs in the VTA (Figures 3I, 3J, 3N, and 30). The average firing rate of DA neurons between 0.25 and 10 s after tone onset was slightly increased by photoinhibition of PPTg glutamatergic, but not cholinergic, inputs in the VTA (Figures 3M and 3P). On the other hand, in the neurons that did not meet the criteria of putative DA cells, photoinhibition of PPTg cholinergic (Figures 4A, 4D, and 4H) and glutamatergic inputs (Figures 4B, 4F, and 4J) significantly decreased the firing rate in ~37% and ~15% of them during tone presentation, respectively (Figures 4K and 4L). These results suggest that PPTg-to-VTA cholinergic and glutamatergic inputs may not be required for VTA DA cells to fire bursts to a reward-predictive tone. Instead, PPTg-to-VTA cholinergic and glutamatergic inputs are necessary to support neural activity of VTA non-DA neurons during tone presentation.
Figure 3.
Photoinhibition of PPTg-to-VTA Cholinergic or Glutamatergic Inputs Does Not Affect Cue-Induced Phasic Activity of Putative VTA DA Neurons
(A and B) Schematic of in vivo optetrode recording experiment targeting PPTg-to-VTA cholinergic (A) or glutamatergic (B) innervations.
(C–F) Top: raster plots of spikes of a representative example of putative DA unit from cholinergic (C and D) or glutamatergic (E and F) experimental groups in one recording session (50 trials). Half of the trials were randomly assigned to receive photoinhibition of the PPTg-to-VTA afferents (indicated by green bar) during tone presentation (indicated by orange bar) (D and F). The spikes of putative DA unit and time window of photoinhibition were synchronized to onset of 10-s tone. Bottom: averages over all the trials in corresponding treatments.
(G-J) Z score transformation of population data for individual tone-responsive DA units recorded from cholinergic (G and H; n = 12 from 5 mice) or glutamatergic (I and J; n = 18 from 3 mice) experimental group in corresponding treatments. Color bar represents Z-scored firing frequency.
(K-P) Photoinhibition of VTA cholinergic (K-M) or glutamatergic (N-P) afferents. (K and N) Average firing activity of DA units to 10-s tone in the absence (control, black line) and presence (Halo, green line) of photoinhibition. (L and O) Plot summarizes averages of tone-elicited phasic activity in control and Halo conditions (averaged between 50 and 200 ms after tone onset, paired t test, p = 0.66 in L and p = 0.49 in O). (M and P) Plot summarizes averages of firing activity during the CS in control and Halo conditions (averaged between 0.25 and 10 s after tone onset, paired t test, p = 0.78 in M and p = 0.01 in P). Error bars represent SEM.
Figure 4.
Photoinhibition of PPTg-to-VTA Cholinergic or Glutamatergic Inputs Significantly Decreases Firing Activity of Putative VTA non-DA Neurons
(A and B) Schematic of in vivo optetrode recording experiment targeting PPTg-to-VTA cholinergic (A) or glutamatergic (B) innervations.
(C-F) Top: raster plots of spikes from an example of a putative non-DA unit from cholinergic (C and D) or glutamatergic (E and F) experimental groups in one recording session (50 trials). Half of the trials were randomly assigned to receive photoinhibition of the PPTg-to-VTA afferents (indicated by green bar in D and F) during tone presentation (indicated by orange bar in C–F). The spikes of non-DA unit and time window of photoinhibition were synchronized to onset of 10-s tone. Bottom: averages over all the trials in corresponding treatments.
(G–J) Z score transformation of population data for individual putative non-DA units recorded from cholinergic (G and H; n = 142 from 5 mice) or glutamatergic (I and J; n = 164 from 3 mice) experimental groups in corresponding treatments. Color bar represents Z-scored firing frequency.
(K and L) Bar graphs summarize the modulation profile of firing activity of VTA non-DA units by photoinhibition of PPTg-to-VTA cholinergic (K) or glutamatergic (L) projections. Statistical significance is determined by Z score < −2 and p < 0.05, Wilcoxon signed rank test.
Photoinhibition of VTA Non-DA Neural Activity during Pavlovian Conditioning
On the basis of these results, we propose that VTA non-DA neurons, which are subjected to the regulation by PPTg excitatory inputs, are necessary to develop conditioned responding during Pavlovian conditioning. To test this hypothesis, we used an optogenetic approach to selectively inhibit VTA GABAergic or glutamatergic neuronal activity during CS presentation. We injected Cre-dependent eNpHR3.0-EYFP viral vectors and implanted fiber optics bilaterally in the VTA of VGatiCre/+ or VGlut2iCre/+ mice (Figures 5A and 5D; Figures S6 and S7). Control groups were littermates of respective mouse lines receiving bilateral EYFP-only viral injection and fiber optic implantation in the VTA (Figures 5A and 5D; Figures S6 and S7). Following recovery from surgery and robust expression of EYFP in the VTA, mice in both the control and Halo groups underwent the same Pavlovian conditioning described above (Figure 1), where they received green light illumination during tone presentation in each trial across training sessions.
Figure 5.
Photoinhibition of VTA GABAergic or Glutamatergic Neurons Dampens Cue-Reward Association
(A, D, and G) Schematic illustrations of experimental designs for targeting VTA GABAergic (A), glutamatergic (D), and dopamine (G) neurons, respectively.
(B, E, and H) Plots illustrate the development of conditioned responding to the CS in the control and Halo groups of VTA GABAergic (B), glutamatergic (E), and dopamine (H) inhibition experiments, respectively. (B), A two-way ANOVA (group × session) revealed neither interaction (F(4, 84) = 1.70, p = 0.1569) nor main effect of group (F(1, 21) = 3.28, p = 0.0845) (control group, n = 14; Halo group, n = 9). However, there was a significant main effect of session (F(4, 84) = 13.83, p < 0.0001). (E) A two-way ANOVA (group × session) demonstrated a significant interaction (F(4,88) = 7.66, p < 0.0001) and significant main effects of group (F(1, 22) = 14, p = 0.0011) and session (F(4, 88) = 15.73, p < 0.0001) (control group, n = 12; Halo group, n = 12). (H) A two-way ANOVA (group × session) revealed neither interaction (F(4, 72) = 0.74, p = 0.5672) nor main effect of group (F(1, 18) = 3.45, p = 0.0797) (control group, n = 10; Halo group, n = 10). However, there was a significant main effect of session (F(4, 72) = 16.97, p < 0.0001).
(C, F, and I) The distribution of head entries during the CS and after food pellet delivery across training sessions in VTA GABAergic (C), glutamatergic (F) and dopamine (I) inhibition experiments, respectively. Data points represent normalized head entries (HEs) in 2-s bins by subtracting the averaged head entries during a 10-s pre-CS. The changes of head entries distribution over training sessions are estimated by mixed-effects linear regression models. Error bars represent SEM. Asterisks denote significant difference in change of HE rate in the respective time windows between control and Halo groups, referenced to the first session.
(*p < 0.05; **p < 0.01; ***p < 0.001).
Although photoinhibition of VTA GABAergic neural activity during CS tended to delay the acquisition of conditioned responding compared to its control group, the difference between the control and Halo groups did not reach statistical significance (Figure 5B). However, by analyzing the temporal distribution of HEs around CS across training sessions, we found that the Halo group showed temporally uncoupled HEs following CS offset/food delivery since day 2 (Figure 5C, sessions 2–5; Table S2). Moreover, compared to the control group, the Halo group also showed a significantly lower increase of HEs following CS onset since day 3 (Figure 5C, sessions 3–5; Table 2).
Table 2.
Summary Statistics for Distribution of Head Entries during the CS across Training Sessions in VTA Manipulation Experiments
| Control | Halo | Comparison between Control and Halo |
|||||
|---|---|---|---|---|---|---|---|
| Session | Slope Mean (SEM) |
p Value of Comparison to Session 1 |
Slope Mean (SEM) |
p Value of Comparison to Session 1 |
Z Value | p Value | |
| VTA GABAergic manipulation |
1 | 0.01 (0.18) | — | 0.62 (0.31) | — | — | — |
| 2 | 0.46 (0.17) | 0.161 | 0.14 (0.31) | 0.339 | −1.64 | 0.102 | |
| 3 | 0.87 (0.18) | 0.008 | −0.20 (0.38) | 0.098 | −2.97 | 0.003 | |
| 4 | 1.86 (0.29) | <0.001 | 0.83 (0.33) | 0.669 | −2.89 | 0.004 | |
| 5 | 1.80 (0.28) | <0.001 | 0.63 (0.41) | 0.973 | −3.13 | 0.002 | |
| VTA glutamatergic manipulation |
1 | 0.28 (0.17) | — | −0.35 (0.17) | — | — | — |
| 2 | 0.35 (0.15) | 0.821 | −0.27 (0.19) | 0.798 | 0.02 | 0.985 | |
| 3 | 0.84 (0.24) | 0.071 | 0.27 (0.22) | 0.048 | 0.10 | 0.917 | |
| 4 | 1.87 (0.20) | <0.001 | −0.02 (0.26) | 0.287 | −2.87 | 0.004 | |
| 5 | 2.78 (0.31) | <0.001 | 0.25 (0.24) | 0.052 | −4.31 | <0.001 | |
| VTA dopaminergic manipulation |
1 | −0.19(0.14) | — | −0.28 (0.19) | — | — | — |
| 2 | 0.41 (0.14) | 0.07 | 0.18 (0.15) | 0.253 | −0.27 | 0.786 | |
| 3 | 0.67 (0.22) | 0.01 | 0.54 (0.21) | 0.041 | −0.07 | 0.946 | |
| 4 | 1.47 (0.29) | <0.001 | 1.09 (0.34) | 0.001 | −0.53 | 0.594 | |
| 5 | 1.77 (0.31) | <0.001 | 1.05 (0.42) | 0.001 | −1.22 | 0.222 | |
Photoinhibition of VTA glutamatergic neural activity significantly abolished conditioned responding learning (Figure 5E). Further temporal analysis of HEs around the CS showed temporally uncoupled HEs with respect to the CS in the Halo group and confirmed the learning deficit (Figure 5F; Table 2). Since day 2, the Halo group showed neither elevated HEs nor a time-dependent decrease of HEs following CS offset/food delivery as compared to control group (Figure 5F, sessions 2–5; Table 2; Table S2). Moreover, the Halo group failed to show a time-dependent increase of HEs following CS onset at any stage of training (Figure 5F, sessions 1–5; Table 2). The difference between the control and Halo groups in the temporal distribution of HEs during CS presentation reached statistical significance on days 4 and 5 (Figure 5F, sessions 4 and 5; Table 2).
Previous studies have shown that VGlut2 or VGat is expressed by a subpopulation of VTA DA neurons (Koos etal., 2011, Tritsch etal., 2014). It is therefore conceivable that cross-inactivation of some VTA DA neurons may account for the above observed behavioral deficits. To examine this possibility, we included an additional experiment in which VTA DA neural activity in THiCre/+ mice during CS presentation was inhibited using the same optogenetic approach (Figure 5G; Figures S6 and S8). We found that photoinhibition treatment onto VTA DA neurons during CS did not result in significant disruption in acquiring conditioned responding in the Halo group (Figures 5H and 5I). In addition, the Halo and control groups showed similar patterns of temporal distribution of HEs during the CS (Figure 5I; Table 2). Taken together, these results suggest that the neuronal activity of VTA GABAergic and glutamatergic neurons during CS-US presentations is necessary to develop temporally coupled conditioned responding during Pavlovian conditioning.
Discussion
In the present study, by taking advantage of reversible neural inhibition in both cell-type- and pathway-specific manners provided by optogenetics, we demonstrate that PPTg cholinergic and glutamatergic inputs to the VTA are necessary for developing conditioned responding toward the cue during Pavlovian appetitive conditioning. Further, we reveal through in vivo optetrode recordings that PPTg cholinergic and glutamatergic inputs to the VTA may not be required for VTA DA cells to fire burst activity to the reward-predictive cue. Instead, the PPTg excitatory inputs are necessary to maintain VTA non-DA neuron firing activity. Lastly, we show that VTA GABAergic and glutamatergic neural activity during cue presentation is critical in the development of conditioned responding.
PPTg-to-VTA Excitatory Inputs Are Necessary to Develop Conditioned Responding
Focusing on relevant stimulus makes behaviors efficient and helps to achieve optimal energy balance. The context in which learning takes place contains a variety of stimuli activating sensory modalities. By pairing with reward delivery, the contextual stimuli can elicit reward-seeking behavior on its own (Urcelay and Miller, 2014). In our Pavlovian cue-reward learning paradigm, mice quickly learn to expect food pellet reward from the food receptacle in the context, as demonstrated by an increased HE rate in the food receptacle. Across the training sessions, we observed that mice developed anticipatory approach responses to the reward-predictive tone. Repeated pairings between tone presentation and food pellet delivery provide the mice precise timing information to accurately predict food delivery. The established temporal association helps the mice to focus their attention and efforts specifically during and immediately after tone appearance, which is reflected in biased temporal distribution of HEs toward tone and food delivery. PPTg forms functionally distinct innervations to the midbrain (Oakman et al., 1995, Watabe-Uchida et al., 2012, Wilson et al., 2009). Previous studies have shown that PPTg lesion results in attention deficit (Cyr et al., 2015, Inglis et al., 2001) as well as failure to develop conditioned responding to the reward-predictive cue (Inglis et al., 2000), suggesting that PPTg is involved in relaying sensory information for stimulus-reward associative learning. Here, our study has selectively targeted PPTg projections to the VTA and shown that acquisition of conditioned anticipatory responding in Pavlovian conditioning is differentially dependent on phenotypically distinct efferents from the PPTg. We have observed that selective inhibition of PPTg-to-VTA excitatory and inhibitory inputs caused opposite changes in conditioned responses (Figures 2B and 2E versus Figure 2H; Figures 2C and 2F versus Figure 2I). Although the VTA receives substantial GABAergic inputs from the PPTg (Good and Lupica, 2009), our behavioral results suggest the contribution of PPTg-to-VTA GABAergic input in acquisition of cue-reward association is minimal. Nevertheless, we cannot rule out the possibility of incomplete photoinhibition or lack of sensitivity in employed behavioral paradigm to detect its functional contribution.
On the other hand, we show that PPTg-to-VTA excitatory inputs are required during cue-reward learning. VTA receives cholinergic inputs from the PPTg and laterodorsal tegmental nucleus (LDTg) (Oakman et al., 1995), which are both made up of heterogeneous populations of cells (Wang and Morales, 2009). Previous pharmacological approaches of targeting ACh receptor signaling in the VTA cannot distinguish the differential roles of PPTg and LDTg. In addition, lesion studies targeting PPTg or LDTg can neither rule out the contribution of non-cholinergic neurons nor link to its downstream target regions. Our study takes advantage of the optogenetic approach to help examine the functional contribution of cholinergic inputs from PPTg to the VTA in stimulus-reward learning. Nicotinic acetylcholine receptors (nAChR) activated by endogenous acetylcholine are expressed not only in VTA neurons (Jones and Wonnacott, 2004, Klink et al., 2001) but also in glutamatergic axonal terminals in the VTA (Jones and Wonnacott, 2004). Thus, endogenous acetylcholine released by PPTg cholinergic terminals can increase neural excitability of VTA neurons via somatodendritic nAChR activation (Pidoplichko et al., 1997) directly and can potentiate glutamate neurotransmission indirectly by presynaptic nAChR activation (Mansvelder and McGehee, 2000). This suggests PPTg-to-VTA cholinergic input may have a broad impact on global glutamatergic transmission in the VTA. In line with this notion, based on the severe learning deficits shown in the cholinergic photoinhibition group in our study, PPTg-to-VTA cholinergic input appears to play a more important role than glutamatergic input in appetitive Pavlovian conditioning.
Moreover, the present study uncovers that PPTg-to-VTA glutamatergic input is involved in cue-reward learning process through regulating VTA non-DA neural activity. NMDA receptor has been shown to be a key mediator in synaptic plasticity of VTA DA neurons (Bonci and Malenka, 1999) and infusion of NMDA receptor antagonist D-AP5 into the VTA blocks cue-reward association activity (Stuber et al., 2008, Zellner and Ranaldi, 2010). Although failure of NMDA receptor-dependent synaptic potentiation onto VTA DA neurons was thought to underlie the D-AP5-resulted behavioral deficit, this notion is not supported by NMDA receptor conditional knockout studies, in which genetically engineered mice without NMDA receptor subunit 1 (NR1) in DA neurons are still able to acquire Pavlovian cue-reward association (James et al., 2015, Parker et al., 2010, Parker et al., 2011, Wang et al., 2011). Therefore, to reconcile the discrepancy between the pharmacological and conditional knockout studies, we, and others, provide an alternative perspective that D-AP5 infusion in the VTA affects excitatory neural transmission in VTA non-DA neurons, and thereafter impact cue-reward learning (Luo et al., 2010, Steffensen et al., 1998). Our study supports this hypothesis with the following findings: first, PPTg-to- VTA cholinergic and glutamatergic inhibition affect cue-reward learning; second, PPTg-to-VTA cholinergic and glutamatergic inhibition decrease VTA non-DA neural activity; and last, VTA non-DA neural inhibitions recapitulate cue-reward learning deficit. Further studies will be required to examine the functional regulation of NMDA receptors in VTA non-DA neurons and its role in cue-reward learning.
PPTg-to-VTA Excitatory Inputs Do Not Mediate Tone-Elicited VTA DA Bursts
Lesion of PPTg impairs stimulus-reward associative learning (Inglis et al., 2000) and the capability to regulate burst activity of DA neurons has been thought to underlie the behavioral role of PPTg in reward-related learning (Diederich and Koch, 2005, Floresco et al., 2003, Lokwan et al., 1999, Pan and Hyland, 2005). However, given that Pavlovian cue-reward learning is not solely dependent on DA phasic activity (Flagel et al., 2011, James et al., 2015, Parker et al., 2010, Wang et al., 2011, Zweifel et al., 2009), whether PPTg is involved in cue-reward learning through regulating DA burst activity remains elusive. In the present study, we showed that PPTg-to-VTA excitatory afferents inputs are necessary to develop conditioned responding in appetitive Pavlovian conditioning. Nevertheless, we did not observe a reduction of tone-elicited burst activity of VTA DA neurons when PPTg cholinergic or glutamatergic inputs to the VTA were optically inhibited, suggesting that direct excitatory inputs from PPTg to VTA do not mediate cue-elicited DA burst activity. The discrepancy between the current results and previous studies might be accounted for by the techniques used to study the regulatory roles of PPTg on VTA DA neural activity. In the present study, optogenetic manipulation is employed to selectively inhibit PPTg-to-VTA excitatory inputs, but spares other PPTg efferents. In previous studies, the regulatory function of PPTg to VTA DA neurons is interpreted through pharmacological manipulation directly applied to PPTg (Coizet et al., 2003, Floresco et al., 2003, Pan and Hyland, 2005), which has two major limitations: first, lack of targeting specific PPTg efferents; second, the inability to rule out possible regulation by PPTg efferents other than PPTg-to-VTA projections. For example, PPTg also sends significant projections to substantia nigra pars compacta (SNpc). Those SNpc-projecting neurons relay reward-related signals to SNpc and help SNpc DA neurons encode motivation value and salience (Holmstrand and Sesack, 2011, Hong and Hikosaka, 2014, Lerner et al., 2015). Moreover, PPTg cholinergic neurons also project to the superior colliculus (SC) (Beninato and Spencer, 1986), which is capable of regulating both DA and non-DA neuronal activity in the SNpc/VTA (Coizet et al., 2003). The fact that PPTg neurons non-contingently respond to reward-related sensory cues implicates that other brain nuclei might directly mediate the cue-elicited burst activity of VTA DA neurons (Pan and Hyland, 2005). Since our in vivo recording experiments were performed after the mice had acquired cue-reward association, we cannot rule out the possible involvement of PPTg-to-VTA excitatory inputs in assisting other VTA afferents to develop DA burst activity to the reward-predictive cue during the acquisition phase of Pavlovian conditioning. Further chronic recording of neural activity from the same population of VTA neurons during acquisition phase of cue-reward learning while manipulating PPTg-to-VTA afferents will be required to conclusively understand whether PPTg excitatory afferents are engaged in generating cue-elicited VTA DA bursts.
PPTg-to-VTA Inputs Support VTA Non-DA Neural Activity, which Is Necessary to Develop Conditioned Responding
Aided by precise temporal and reversible control of neural activity afforded by optogenetic technique, we reveal that it is during cue presentation that the disturbance in VTA non-DA neural activity introduced by PPTg-to-VTA photoinhibition dampens the development of anticipatory approach responding. Previous studies have indicated that DA signaling is selectively required in learning a sign-tracking but not goal-tracking conditioned responding (Flagel et al., 2011, Saunders and Robinson, 2012). Since we employ an ‘invisible’ tone cue as a CS in present Pavlovian conditioning paradigm and the speaker is located apart from the food port, the observation that mice increase food port approach responses during CS presentation suggests that the mice mainly acquire goal-tracking conditioned responding. Consistent with previous study (Flagel et al., 2011), photoinhibition of VTA DA neurons during the CS did not result in a learning deficit in acquiring a goal-tracking conditioned responding. On the contrary, photoinhibition of VTA GABAergic or glutamatergic neurons causes significant learning deficits. Therefore, the result from VTA DA inhibition experiment not only rules out the possibility that subsets of TH-coexpressing GABAergic and glutamatergic neurons contribute to observed learning deficit in VTA GABAergic and glutamatergic inhibition experiments, but also further strengthens the conclusion that VTA non-DA neurons are critical mediators for cue-reward associative learning. Furthermore, our study opens up a possibility that VTA non-DA neurons, which are subjected to PPTg regulation, may be involved in the DA-independent neural mechanism underlying goal-tracking behaviors.
Despite the fact that both GABAergic and glutamatergic neurons are present in the VTA (Fields et al., 2007, Yamaguchi et al., 2007), their roles in stimulus-reward learning have been less explored. Activation of VTA GABAergic neurons can inhibit DA neural activity, presumably through local inhibition (Eshel et al., 2015, van Zessen et al., 2012), which, however, does not result in unequivocal behavioral phenotypes. An earlier study shows that optical activation of VTA GABAergic neurons during the cue does not affect anticipatory conditioned responding or reward consummatory behavior (van Zessen et al., 2012). It is actually during the reward consumption that VTA GABAergic stimulation decreases reward consummatory responses, but spares anticipatory conditioned responding (van Zessen et al., 2012). On the other hand, a recent study shows that optical activation of VTA GABAergic neurons decreases expectation-like changes of DA responses and, thereafter, decreases anticipatory conditioned responding (Eshel et al., 2015). The study further shows that bilateral optical inhibition of VTA GABAergic neurons results in an increase of DA phasic burst to reward, which contributes to reward-prediction error calculation (Eshel et al., 2015). In our electrophysiological study, we observed a small, but significant, increase in VTA DA baseline activity while optically inhibiting PPTg-to-VTA glutamatergic inputs, suggesting possible disinhibition of feed-forward inhibition from local GABAergic interneurons. Nevertheless, the disinhibition from GABAergic interneurons by PPTg-to-VTA glutamatergic photoinhibition does not appear to play a significant role in affecting DA burst activity. In addition, unlike the studies discussed above, which apply optical manipulation in well-trained mice, we applied photoinhibition during acquisition phase of cue-reward learning. We found that inhibiting VTA GABAergic neural activity during the CS not only significantly decreased time-dependent increase of HEs to the CS, which recapitulates the behavioral deficits revealed by PPTg-to-VTA cholinergic or glutamatergic inhibition, but also showed significantly less approach responses after reward delivery, which is not shown by PPTg-to-VTA cholinergic or glutamatergic inhibition. These results raise a possibility that a subpopulation of VTA GABAergic neurons is preferentially regulated by PPTg glutamatergic afferent inputs during cue-reward learning, and also more involved in perceiving the temporal relationship between cue and reward delivery.
The functional roles of VTA glutamatergic neurons are less well understood. VTA glutamatergic neurons establish local as well as extrinsic projections (Dobi et al., 2010, Hnasko et al., 2012, Morales and Root, 2014). A recent study suggests that the activation of VTA glutamatergic interneurons is rewarding through acting on mesoaccumbens DA neurons (Wang et al., 2015). From our electrophysiological study, we did not observe a decrease of VTA DA baseline or burst activity while optically inhibiting PPTg-to-VTA excitatory inputs, which rules out PPTg modulation onto VTA DA neurons through VTA glutamatergic interneurons. In our behavioral study, photoinhibition of VTA glutamatergic neurons during the CS completely abolished the acquisition of cue-reward association. Given that we did not observe a learning deficit by inhibiting VTA DA neurons, it is conceivable that disturbance of VTA glutamatergic neural activity during CS-US presentations may interfere with the functional integrity of downstream brain regions and hinder the development of anticipatory conditioned responses during Pavlovian learning. For example, VTA glutamatergic neurons form functional synapses in the ventral pallidium (Hnasko et al., 2012), whose activity is necessary for reward-related behaviors as well as motivation (Smith and Berridge, 2005, Smith et al., 2009). Further studies will be required to understand how VTA non-DA projection neurons engage their downstream brain regions during Pavlovian conditioning.
Our current study provides direct evidence indicating that VTA non-DA neurons are critical mediators for learning cue-reward association, and they are subjected to regulation by excitatory afferents from subcortical PPTg. This finding provides new perspectives on the functional roles of VTA non-DA neurons in appetitive Pavlovian conditioning.
Experimental Procedures
The study was approved by the Animal Care and Use Committee of the National Institute on Drug Abuse (NIDA) Intramural Research Program, and all procedures were conducted in accordance with National Research Council’s Guide for the Care and Use of Laboratory Animals.
Animals
Wild-type male C57BL/6J mice (2–3 months old, Jackson Laboratory) were used. Selective expression of Cre recombinase in glutamatergic neurons in the VTA and PPTg was achieved by using male VGlut2-ires-Cre (Slc17a6tm2(cre)Lowl/J, referred to as VGlut2iCre/+) mice (2–3 months old, Jackson Laboratory). PPTg cholinergic neurons and PPTg/VTA GABAergic neurons were targeted by using male ChAT-ires-Cre (B6;129S6-Chattm2(cre)Lowl/J, referred as ChATiCre/+) mice and VGat-ires-Cre (Slc32a1tm2(cre)Lowl/J, referred to as VGatiCre/+) mice, respectively (2–3 months old, Jackson Laboratory). VTA DA neurons were targeted using male tyrosine hydroxylase (TH)-ires-Cre mice (referred to as THiCre/+; Lindeberg et al., 2004). All transgenic mice were backcrossed with C57/BL/6J and bred at the NIDA animal facility. For behavioral experiments, mice are bilaterally injected with Cre-dependent virus and implanted with fiber optics, the details of which are described in Supplemental Experimental Procedures.
Pavlovian Cue-Reward Associative Learning
The behavioral experiments were conducted during the mice’s dark cycle. Mice were kept on a restricted diet of 2.5 g/day (dustless precision pellets, 0.5 g/pellet, BioServ) for 5 days prior to behavioral training. During the 2 days of habituation, mice were fed an additional 0.4 g of reward pellets (20 mg/pellet, 5TUL, TestDiet) in home cages, in addition to their 2.5-g restricted diet, before entering standard mouse operant chambers (ENV-307W, Med Associates) for 40 min of exploration. During the training period, the daily 2.5-g restricted diet was maintained after mice finished each training session. Each CS-US pairing started from the presentation of a 10 s tone (CS) followed by delivery of a 20-mg reward pellet (US) to the food port. The CS-US pairing was presented 40 times with variable ITI (80–120 s) in each session. Mice received five sessions of training, separated by 24 hr. The number of HEs into the food receptacle during CS presentation and ITI was obtained by infrared HE detector in the food receptacle and used to evaluate learning process.
In Vivo Recording
Following behavioral training, an optetrode assembly was constructed and then implanted in the VTA. 1–2 weeks after optetrode implantation, the electrophysiological recordings were started while the mice were performing Pavlovian conditioning task in the operant chamber. 50 CS-US pairings were present in each recording session, with variable ITI (80–120 s), and approximately half of the trials were randomly assigned to receive optical stimulation (532 nm, 10 mW) during tone presentation. Details of recording procedures and data analysis are described in Supplemental Experimental Procedures.
Supplementary Material
Highlights.
We study how VTA afferent inputs modulate appetitive Pavlovian conditioning
PPTg-to-VTA excitatory inputs are required for proper cue-reward association
PPTg-to-VTA excitatory inputs support non-DA neural activity in vivo
VTA non-DA neural activity is necessary for appetitive Pavlovian conditioning
Acknowledgments
This work was supported by the Intramural Research Program at National Institute on Drug Abuse. J.-H.T was supported by MOST 103-2911-I-038-501 from the Ministry of Science and Technology, Taiwan. The authors thank Stephanie Goddard and Christina Hatch for technical assistance and Roy Wise, Ross McDevitt, and Hugo Tejeda for critical reading of the manuscript.
References
- Beninato M, Spencer RF A cholinergic projection to the rat superior colliculus demonstrated by retrograde transport of horseradish peroxidase and choline acetyltransferase immunohistochemistry J. Comp. Neurol, 253 (1986), pp. 525–538 [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area J. Neurosci, 19 (1999), pp. 3723–3730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coizet V, Comoli E, Westby GW, Redgrave P Phasic activation of substantia nigra and the ventral tegmental area by chemical stimulation of the superior colliculus: an electrophysiological investigation in the rat Eur. J. Neurosci, 17 (2003), pp. 28–40 [DOI] [PubMed] [Google Scholar]
- Cyr M, Parent MJ, Mechawar N, Rosa-Neto P, Soucy JP, Clark SD, Aghourian M, Bedard MA Deficit in sustained attention following selective cholinergic lesion of the pedunculopontine tegmental nucleus in rat, as measured with both post-mortem immunocytochemistry and in vivo PET imaging with [18F]fluoroethoxybenzovesamicol Behav Brain Res, 278 (2015), pp. 107–114 [DOI] [PubMed] [Google Scholar]
- Diederich K, Koch M Role of the pedunculopontine tegmental nucleus in sensorimotor gating and reward-related behavior in rats Psychopharmacology (Berl.), 179 (2005), pp. 402–408 [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons J. Neurosci, 30 (2010), pp. 218–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Bukwich M, Rao V, Hemmelder V, Tian J, Uchida N Arithmetic and local circuitry underlying dopamine prediction errors Nature, 525 (2015), pp. 243–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement Annu. Rev Neurosci, 30 (2007), pp. 289–316 [DOI] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PE, Akil H A selective role for dopamine in stimulus-reward learning Nature, 469 (2011), pp. 53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission Nat. Neurosci, 6 (2003), pp. 968–973 [DOI] [PubMed] [Google Scholar]
- Geisler S, Wise RA Functional implications of glutamatergic projections to the ventral tegmental area Rev Neurosci, 19 (2008), pp. 227–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Zahm DS Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions J. Comp. Neurol, 490 (2005), pp. 270–294 [DOI] [PubMed] [Google Scholar]
- Good CH, Lupica CR Properties of distinct ventral tegmental area synapses activated via pedunculopontine or ventral tegmental area stimulation in vitro J. Physiol, 587 (2009), pp. 1233–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Floresco SB, Goto Y, Lodge DJ Regulation of firing of dopaminergic neurons and control of goal-directed behaviors Trends Neurosci, 30 (2007), pp. 220–227 [DOI] [PubMed] [Google Scholar]
- Grillner P, Berretta N, Bernardi G, Svensson TH, Mercuri NB Muscarinic receptors depress GABAergic synaptic transmission in rat midbrain dopamine neurons Neuroscience, 96 (2000), pp. 299–307 [DOI] [PubMed] [Google Scholar]
- Hamani C, Stone S, Laxton A, Lozano AM The pedunculopontine nucleus and movement disorders: anatomy and the role for deep brain stimulation Parkinsonism Relat. Disord, 13 (Suppl 3) (2007), pp. S276–S280 [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH Ventral tegmental area glutamate neurons: electrophysiological properties and projections J. Neurosci, 32 (2012), pp. 15076–15085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmstrand EC, Sesack SR Projections from the rat pedunculopontine and laterodorsal tegmental nuclei to the anterior thalamus and ventral tegmental area arise from largely separate populations of neurons Brain Struct. Funct, 216 (2011), pp. 331–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O Pedunculopontine tegmental nucleus neurons provide reward, sensorimotor, and alerting signals to midbrain dopamine neurons Neuroscience, 282 (2014), pp. 139–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis WL, Olmstead MC, Robbins TW Pedunculopontine tegmental nucleus lesions impair stimulus--reward learning in autoshaping and conditioned reinforcement paradigms Behav Neurosci, 114 (2000), pp. 285–294 [DOI] [PubMed] [Google Scholar]
- Inglis WL, Olmstead MC, Robbins TW Selective deficits in attentional performance on the 5-choice serial reaction time task following pedunculopontine tegmental nucleus lesions Behav. Brain Res, 123(2001), pp. 117–131 [DOI] [PubMed] [Google Scholar]
- James AS, Pennington ZT, Tran P, Jentsch JD Compromised NMDA/glutamate receptor expression in dopaminergic neurons impairs instrumental learning, but not Pavlovian goal tracking or sign tracking(1,2,3) eNeuro, 2 (2015) ENEURO.0040–14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones IW, Wonnacott S Precise localization of alpha7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral tegmental area J. Neurosci, 24 (2004), pp. 11244–11252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klink R, de Kerchove d’Exaerde A, Zoli M, Changeux JP Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei J. Neurosci, 21 (2001), pp. 1452–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tecuapetla F, Tepper JM Glutamatergic signaling by midbrain dopaminergic neurons: recent insights from optogenetic, molecular and behavioral studies Curr. Opin. Neurobiol, 21 (2011), pp. 393–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC Input-specific control of reward and aversion in the ventral tegmental area Nature, 491 (2012), pp. 212–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Shilyansky C, Davidson TJ, Evans KE, Beier KT, Zalocusky KA, Crow AK, Malenka RC, Luo L, Tomer R, Deisseroth K Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits Cell, 162 (2015), pp. 635–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Soderstrom S, Ebendal T Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus Genesis, 40 (2004), pp. 67–73 [DOI] [PubMed] [Google Scholar]
- Liu CL, Gao M, Jin GZ, Zhen X GABA neurons in the ventral tegmental area responding to peripheral sensory input PLoS ONE, 7 (2012), p. e51507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W Responses of monkey midbrain dopamine neurons during delayed alternation performance Brain Res, 567 (1991), pp. 337–341 [DOI] [PubMed] [Google Scholar]
- Lokwan SJ, Overton PG, Berry MS, Clark D Stimulation of the pedunculopontine tegmental nucleus in the rat produces burst firing in A9 dopaminergic neurons Neuroscience, 92 (1999), pp. 245–254 [DOI] [PubMed] [Google Scholar]
- Luo Y, Good CH, Diaz-Ruiz O, Zhang Y, Hoffman AF, Shan L, Kuang SY, Malik N, Chefer V.l., Tomac AC, et al. NMDA receptors on non-dopaminergic neurons in the VTA support cocaine sensitization PLoS ONE, 5 (2010), p. e12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS Long-term potentiation of excitatory inputs to brain reward areas by nicotine Neuron, 27 (2000), pp. 349–357 [DOI] [PubMed] [Google Scholar]
- Matsui A, Jarvie BC, Robinson BG, Hentges ST, Williams JT Separate GABA afferents to dopamine neurons mediate acute action of opioids, development of tolerance, and expression of withdrawal Neuron, 82 (2014), pp. 1346–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W Preferential activation of midbrain dopamine neurons by appetitive rather than aversive stimuli Nature, 379 (1996), pp. 449–451 [DOI] [PubMed] [Google Scholar]
- Morales M, Root DH Glutamate neurons within the midbrain dopamine regions Neuroscience, 282 (2014), pp. 60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakman SA, Faris PL, Kerr PE, Cozzari C, Hartman BK Distribution of pontomesencephalic cholinergic neurons projecting to substantia nigra differs significantly from those projecting to ventral tegmental area J. Neurosci, 15 (1995), pp. 5859–5869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WX, Hyland BI Pedunculopontine tegmental nucleus controls conditioned responses of midbrain dopamine neurons in behaving rats J. Neurosci, 25 (2005), pp. 4725–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JG, Zweifel LS, Clark JJ, Evans SB, Phillips PE, Palmiter RD Absence of NMDA receptors in dopamine neurons attenuates dopamine release but not conditioned approach during Pavlovian conditioning Proc. Natl. Acad. Sci. USA, 107 (2010), pp. 13491–13496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JG, Beutler LR, Palmiter RD The contribution of NMDA receptor signaling in the corticobasal ganglia reward network to appetitive Pavlovian learning J. Neurosci, 31 (2011), pp. 11362–11369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA Nicotine activates and desensitizes midbrain dopamine neurons Nature, 390 (1997), pp. 401–404 [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses Eur. J. Neurosci, 36 (2012), pp. 2521–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Romo R Dopamine neurons of the monkey midbrain: contingencies of responses to stimuli eliciting immediate behavioral reactions J. Neurophysiol, 63 (1990), pp. 607–624 [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake J. Neurosci, 25 (2005), pp. 8637–8649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC Ventral pallidum roles in reward and motivation Behav Brain Res, 196 (2009), pp. 155–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ Electrophysiological characterization of GABAergic neurons in the ventral tegmental area J. Neurosci, 18 (1998), pp. 8003–8015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg EE, Keiflin R, Boivin JR, Witten IB, Deisseroth K, Janak PH A causal link between prediction errors, dopamine neurons and learning Nat. Neurosci, 16 (2013), pp. 966–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Klanker M, de Ridder B, Bowers MS, Joosten RN, Feenstra MG, Bonci A, Reward-predictive cues enhance excitatory synaptic strength onto midbrain dopamine neurons Science, 321 (2008), pp. 1690–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SR, Badurek S, Dileone RJ, Nashmi R, Minichiello L, Picciotto MR GABAergic and glutamatergic efferents of the mouse ventral tegmental area J. Comp. Neurol, 522 (2014), pp. 3308–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritsch NX, Oh WJ, Gu C, Sabatini BL Midbrain dopamine neurons sustain inhibitory transmission using plasma membrane uptake of GABA, not synthesis eLife, 3(2014), p. e01936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urcelay GP Miller RR The functions of contexts in associative learning Behav Processes, 104 (2014), pp. 2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD Activation of VTA GABA neurons disrupts reward consumption Neuron, 73 (2012), pp. 1184–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Morales M Pedunculopontine and laterodorsal tegmental nuclei contain distinct populations of cholinergic, glutamatergic and GABAergic neurons in the rat Eur. J. Neurosci, 29 (2009), pp. 340–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Li F, Wang D, Xie K, Wang D, Shen X, Tsien JZ NMDA receptors in dopaminergic neurons are crucial for habit learning Neuron, 72 (2011), pp. 1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HL, Qi J, Zhang S, Wang H, Morales M Rewarding effects of optical stimulation of ventral tegmental area glutamatergic neurons J. Neurosci, 35 (2015), pp. 15948–15954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N Whole-brain mapping of direct inputs to midbrain dopamine neurons Neuron, 74 (2012), pp. 858–873 [DOI] [PubMed] [Google Scholar]
- Wilson D.l., MacLaren DA, Winn P Bar pressing for food: differential consequences of lesions to the anterior versus posterior pedunculopontine Eur. J. Neurosci, 30 (2009), pp. 504–513 [DOI] [PubMed] [Google Scholar]
- Wise RA Dopamine, learning and motivation Nat. Rev Neurosci, 5 (2004), pp. 483–494 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M Glutamatergic neurons are present in the rat ventral tegmental area Eur. J. Neurosci, 25 (2007), pp. 106–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin R, French ED A comparison of the effects of nicotine on dopamine and non-dopamine neurons in the rat ventral tegmental area: an in vitro electrophysiological study Brain Res. Bull, 51 (2000), pp. 507–514 [DOI] [PubMed] [Google Scholar]
- Zellner MR, Ranaldi R How conditioned stimuli acquire the ability to activate VTA dopamine cells: a proposed neurobiological component of reward-related learning Neurosci. Biobehav. Rev, 34 (2010), pp. 769–780 [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu Y, Chen X Carbachol induces burst firing of dopamine cells in the ventral tegmental area by promoting calcium entry through L-type channels in the rat J. Physiol, 568 (2005), pp. 469–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Daras M, Kim MJ, Mizumori SJ, Paladini CA, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior Proc. Natl. Acad. Sci. USA, 106 (2009), pp. 7281–7288 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.