The inability of the adult cardiomyocyte (CM) to readily divide in homeostasis and after injury contributes to the high morbidity and mortality of heart disease. It is well documented that adult CMs renew at a rate of less than 1% per year during homeostasis and have little capacity to increase their renewal rate following cardiac injury (1,2). Unsurprisingly, this has generated a large community of investigators interested in studying the CM cell cycle with the translational goal of creating therapies capable of stimulating CM renewal in patients with heart disease. Regardless of the experimental approach, it is essential to rigorously characterize CM division events when investigating CM proliferation. Current methods include immunofluorescence studies of the M-phase markers aurora B kinase (AURKB) and phospho-histone H3 (pHH3) that are widely used as markers of CM proliferation (3). Another approach, rigorous but labor intensive, is stereology in which all CMs in the heart are manually counted (1,2). In this issue of Circulation Research, Hesse et al provide data showing that positioning of the AURKB+ midbody and physical distance between daughter nuclei are novel markers of the dividing CM (4).
Clearly identifying CM division has been a continued challenge in cardiac regeneration research largely because cell cycle re-entry is not synonymous with CM cellular division. In fact, CMs undergo many cell cycle variants other than division regularly throughout both development and disease (Figure 1A). These variants include polyploidization, where CMs prematurely exit the cell cycle after S phase, and binucleation, in which CMs progress through mitosis and karyokinesis but fail to complete cytokinesis. Both cell cycle variants progress through DNA synthesis and therefore will show incorporation of nucleotides analogs (EdU, BrdU) and Ki67 expression. Furthermore, both cellular division and binucleation progress through mitosis and therefore demonstrate pHH3 expression. The ambiguity of cell cycle re-entry and authentic CM division has led to disagreements in the literature concerning CM proliferation during mouse heart development and after cardiac injury (5,6). While stereology is a reliable method to count CM and thereby provide evidence of proliferation, more direct methods of measuring CM division are needed.
Figure 1:
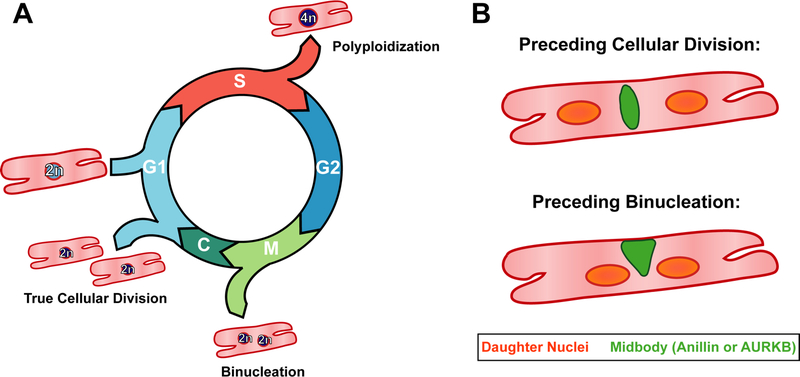
A) The postnatal cardiomyocyte is capable of progressing through multiple cell cycle variants. If the CM exits following DNA synthesis, a process known as polyploidization, it remains mononucleated and contains a tetraploid (4n) nucleus. Furthermore, CMs can complete mitosis but not cytokinesis, leading to the formation of two diploid (2n) nuclei, known as binucleation. True cellular division, the goal of cardiac regenerative therapies, only occurs if the cardiomyocyte is able to fully complete cytokinesis, resulting in two daughter CMs.
B) Hesse et al demonstrate that there are two major criteria for differentiating cellular division and binucleation: 1. The midbody positioning, here depicted in green, differs between the two events. Specifically, the midbody is found to be located symmetrically between the two daughter nuclei if the cell will complete cytokinesis and divide, but asymmetrically if instead the cardiomyocyte will binucleated. The authors demonstrate the midbody can be visualized by either anillin or AURKB immunofluorescence. 2. The distance between the daughter nuclei varies, with the distance between the nuclei being greater preceding cellular division than binucleation.
Previous work in neonatal rat CM culture systems demonstrated that two critical components for cytokinesis, AURKB and anillin, were differentially localized within the contractile ring and midbody of CMs undergoing division and binucleation (7). Hesse et al build upon this previous observation through the use of live-imaging technologies to study mouse CM division and binucleation in both in vitro and in vivo systems.
Hesse et al use a neonatal CM culture in vitro system, culturing CMs from a double transgenic Myh6-H2B-mCherry/CAG-eGFP-anillin transgenic mouse created previously by the group (8,9). This transgenic mouse expresses both the mCherry-H2B fusion protein to label CM nuclei and an eGFP-tagged anillin protein that is differentially located throughout the cell cycle. Both fluorophores are bright enough to be captured through live imaging, allowing for real-time observation of cell cycle activity in plated CMs.
Using this approach, they were able to stimulate cell cycle re-entry through treatment with miR-199 and p21 knockdown with siRNA, two previously published methods of inducing of CM proliferation (10,11). While treatments increased the number of both CM binucleation and division events as expected, more interesting is the observation that the localization of the midbody (marked by eGFP-anillin fusion protein) always predicts whether the CM will undergo binucleation or a true cellular division. Specifically, a symmetrical midbody, where anillin localization is detected exactly between the two nuclei, indicated CM division was to follow. Alternatively, an asymmetric midbody, where anillin localization is seen at the periphery of the cleavage furrow, indicated that only binucleation would occur.
The transgenic CMs were then fixed and the cellular location of AURKB was investigated through immunofluorescence. Each midbody identified by eGFP-anillin also stained positive for AURKB. The co-localization of both proteins at the midbody indicated that AURKB localization was also capable of differentiating between CM division and binucleation.
Hesse et al next investigate whether the midbody positioning is also predictive of CM cell cycle fate in vivo. To do this, the group generated another transgenic allele, Myh6-eGFP-anillin, to restrict the eGFP-tagged anillin expression only to CMs. Live imaging of both neonatal ventricular and atrial CMs was performed using acute heart slices and two-photon microscopy. Both binucleation and division events were captured in real time and anillin localization was again found to be symmetrical in CMs undergoing division and asymmetrical when undergoing binucleation. Additionally, the distance between the daughter nuclei, when the midbody is present, is consistently greater in cellular division than binucleation. Immunofluorescent labeling of AURKB in heart sections demonstrates similar results, both in terms of midbody positioning and distance between CM daughter nuclei (Figure 1B).
Through the use of the endogenous midbody reporter, eGFP-anillin, and live imaging, Hesse et al demonstrate that AURKB expression does not only indicate CM division, but also CM binucleation. Importantly, they were also able to establish AURKB + midbody symmetry and distance between daughter nuclei as criteria for differentiating these two cell cycle events. Specifically, their data leads the group to recommend co-labeling of the midbody with AURKB, CM nuclear lamina with PCM-1, and the cellular membrane with wheat germ agglutinin to quantify unequivocal CM division events in heart tissue from any regenerative model.
Importantly, it should be noted that the amount of binucleation events in both the in vitro and in vivo systems studied here were significant and often outnumbered the CM undergoing authentic cellular division. Furthermore, the ratio between the two events depended upon the specific treatment and developmental stage of the CMs. Both of these findings highlight the importance of quantifying authentic CM division events separately from binucleation when investigating CM proliferation.
Moving forward, the work of Hesse et al challenges the widely used quantification of AURKB + CMs as an assay for proliferation. Instead, this work suggests that more careful analysis must be done when performing AURKB immunofluorescence, taking into account localization of the midbody and daughter nuclei to separately categorize binucleation and division events. Complementary to the approach described in this work, and stereology to count CMs, is the use of the Mosaic Analysis with Double Markers (MADM) mouse model, which is a method of clonal analysis capable of unequivocally labeling CM division events. In the MADM system, the N- and C-terminus of RFP and GFP are located on reciprocal alleles, such that activation of Cre recombinase will allow for formation of recombinant alleles and fluorophore expression. If cell division is occurring during Cre-mediated recombination, there is a potential for the recombinant alleles to segregate into separate daughter cells, creating separate GFP and RFP labeled progeny, whereas a binucleation event would only result in co-expression of the fluorophores (12).
The MADM system has successfully been used in the heart, utilizing the inducible CM-specific Myh6CreERT2 allele to investigate postnatal CM division (13). A clear drawback of this system is the requirement of three alleles (one Cre allele and the two MADM alleles) into a single mouse to perform the experiment, even before the genetic alleles of interest are incorporated, which will require extensive mouse breeding schemes. However, if the regenerative therapy can be introduced through viral delivery, such as adenovirus or adeno-associated virus, then the MADM system can be used more conveniently to investigate CM division (14).
The criteria outlined in this issue of Circulation Research by Hesse et al will allow investigators studying CM proliferation to immediately identify unequivocal CM division in their model of interest. As novel regulators of the CM cell cycle are discovered, it is critically important that these new methodologies become the standard to rigorously differentiate authentic division events from CMs exhibiting premature cell cycle exit.
Acknowledgements:
Baylor College of Medicine Medical Scientist Training Program and Baylor Research Advocates for Student Scientists (BRASS).
Sources of Funding:
J.F.M. was supported by LeDucq Foundation Transatlantic Networks of Excellence in Cardiovascular Research Award 14CVD01, NIH Grants DE023177, HL127717, HL13084 and HL118761, and the Vivian L. Smith Foundation.
Footnotes
Disclosures:
None
References:
- 1.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leone M, Magadum A, Engel FB. Cardiomyocyte proliferation in cardiac development and regeneration: a guide to methodologies and interpretations. Am J Physiol Heart Circ Physiol. 2015;309:H1237–50. [DOI] [PubMed] [Google Scholar]
- 4.Hesse M, Doengi M, Becker A, Kimura K, Voeltz N, Stein V, Fleischmann BK. Midbody Positioning and Distance Between Daughter Nuclei Enable Unequivocal Identification of Cardiomyocyte Cell Division in Mice. Circ Res. Current issue. [DOI] [PubMed] [Google Scholar]
- 5.Naqvi N, Li M, Calvert JW, et al. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alkass K, Panula J, Westman M, Wu TD, Guerquin-Kern JL, Bergmann O. No Evidence for Cardiomyocyte Number Expansion in Preadolescent Mice. Cell. 2015;163:1026–36. [DOI] [PubMed] [Google Scholar]
- 7.Engel FB, Schebesta M, Keating MT. Anillin localization defect in cardiomyocyte binucleation. J Mol Cell Cardiol. 2006;41:601–12. [DOI] [PubMed] [Google Scholar]
- 8.Hesse M, Raulf A, Pilz GA, et al. Direct visualization of cell division using high-resolution imaging of M-phase of the cell cycle. Nat Commun. 2012;3:1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raulf A, Horder H, Tarnawski L, Geisen C, Ottersbach A, Röll W, Jovinge S, Fleischmann BK, Hesse M. Transgenic systems for unequivocal identification of cardiac myocyte nuclei and analysis of cardiomyocyte cell cycle status. Basic Res Cardiol. 2015;110:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eulalio A, Mano M, Dal Ferro M, Zentilin L, Sinagra G, Zacchigna S, Giacca M. Functional screening identifies miRNAs inducing cardiac regeneration. Nature. 2012;492:376–81. [DOI] [PubMed] [Google Scholar]
- 11.Di Stefano V, Giacca M, Capogrossi MC, Crescenzi M, Martelli F. Knockdown of cyclin-dependent kinase inhibitors induces cardiomyocyte re-entry in the cell cycle. J Biol Chem. 2011;286:8644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121:479–92. [DOI] [PubMed] [Google Scholar]
- 13.Ali SR, Hippenmeyer S, Saadat LV, Luo L, Weissman IL, Ardehali R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proc Natl Acad Sci U S A. 2014;111:8850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mohamed TMA, Ang YS, Radzinsky E, Zhou P, Huang Y, Elfenbein A, Foley A, Magnitsky S, Srivastava D. Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell. 2018;173:104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]


