Abstract
Background:
Polybrominated diphenyl ethers (PBDEs) exist extensively in the environment and human beings. PBDE concentrations are higher in children than adults. A previous study found that prenatal PBDE exposure was associated with decreased reading skills in children; however, evidence is limited on the potential impact of childhood exposure to PBDEs. The study examined the association between childhood PBDE exposures and reading ability in children at ages 5 and 8 years.
Methods:
The study included 230 children from an ongoing prospective pregnancy and birth cohort study, the Health Outcomes and Measures of Environment (HOME) Study, conducted in Cincinnati, Ohio. Children’s serum concentrations of eleven PBDE congeners were measured at 1, 2, 3, 5, and 8 years. The Woodcock-Johnson Tests of Achievement - III and the Wide Range Achievement Test - 4 were administered to assess children’s reading skills at ages 5 and 8 years, respectively. We used multiple informant models to examine the associations between repeated measures of PBDEs and reading scores at ages 5 and 8 years. We also estimated the βs and 95% CIs of the association of PBDE measure at each age by including interaction terms between PBDE concentrations and child age in the models.
Results:
All childhood BDE-153 concentrations were inversely associated with reading scores at 5 and 8 years, but associations were not statistically significant after covariate adjustment. For example, a 10-fold increase in BDE-153 concentrations at ages 3 and 5 years was associated with a −5.0 (95% confidence interval (CI): −11.0, 1.0) and −5.5 (95% CI: −12.5, 1.4) point change in Basic Reading score at age 5 years, respectively. Similarly, the estimates for Brief Reading score at age 5 years were −4.5 (95% CI: −10.5, 1.5) and −5.2 (95% CI: −12.2, 1.7) point changes, respectively. Serum concentration of BDE-47, −99, −100, and Sum4PBDEs (sum of BDE-47, 99, 100, and 153) at every age were inversely associated with reading scores at ages 5 and 8 years in unadjusted analyses. While the adjusted estimates were much attenuated and became non-significant, the direction of most of the associations was not altered.
Conclusion:
Our study has shown a suggestive but non-significant trend of inverse associations between childhood PBDE serum concentrations, particularly BDE-153, and children’s reading skills. Future studies with a larger sample size are needed to examine these associations.
Keywords: Polybrominated diphenyl ethers, childhood, serum concentration, reading ability
1. Introduction
Polybrominated diphenyl ethers (PBDEs) are a class of chemical flame retardants used in a large number of consumer products, including furniture, electronics, plastics, polyurethane foams, and carpet padding. Despite the phase out of Penta- and Octa-BDEs from the US market since 2004, and subsequently Deca-BDEs since 2013, PBDEs exist extensively in the environment, wildlife, house dust, and human beings, largely due to historically high production volumes, long half-lives, the bioaccumulation of many congeners (Hites 2004; Johnson-Restrepo 2008; Kodavanti et al. 2005; Sjodin et al. 2003; Thuresson et al. 2006), and the presence of PBDEs in many older household items. PBDEs have been detected in maternal serum, breast milk, cord serum, and placenta worldwide; however, the concentrations have been one order of magnitude higher in the US than in Asia or Europe (Frederiksen et al. 2009; U.S.EPA 2010). Serum PBDE concentrations in children are two to three times higher than in their mothers (Fischer et al. 2006; Toms et al. 2009; Vuong et al. 2017a), likely from multiple exposure pathways including placental transfer, breast milk, and hand-to-mouth behaviors of dust contaminated surfaces.
Concerns have been raised about the potential developmental neurotoxicity of PBDEs. Neonatal exposure to PBDEs has been shown to have neurotoxic effects on rodents, including changes in spontaneous behavior, impaired memory and learning, and abnormal motor activity (Buratovic et al. 2014; Viberg et al. 2003; Viberg et al. 2006; Zhang et al. 2013). In human studies, prenatal and postnatal PBDE exposures have been associated with poorer executive function (Sagiv et al. 2015; Vuong et al. 2016), more attention problems (Cowell et al. 2015; Eskenazi et al. 2013; Roze et al. 2009; Sagiv et al. 2015) and externalizing behavior problems (Braun et al. 2017b; Chen et al. 2014; Hoffman et al. 2012; Vuong et al. 2017c), and reduced adaptive skills in children (Ding et al. 2015; Gascon et al. 2011; Shy et al. 2011), although the results have not been entirely consistent across studies. A recent systematic review, which included a meta-analysis of four prospective cohort studies, provided supporting evidence that prenatal PBDE exposure was associated with reduced Intelligence Quotients (IQs) in children (Lam et al. 2017).
Reading ability, a specified cognitive process, is the basis and prerequisite of learning. Continuing development of reading ability into school age is crucial for a child’s academic performance and social adaptation. Reading skills essentially consist of two fundamental components including decoding (word recognition) and comprehension (Hoover and Gough 1990; Perfetti 2017). The two components are highly correlated and also dissociable. Both of them involve many of the same underlying cognitive and linguistic abilities, such as working memory, processing speed, and receptive/expressive vocabulary (Catts et al. 1999; Christopher et al. 2012; Durand et al. 2013). However, decoding stems from primary linguistic abilities including phonological awareness and alphabet knowledge, while comprehension requires the integration of these linguistic skills with higher-order cognitive processes, such as working memory (Kovachy et al. 2015). Child’s reading performance has been shown to be sensitive to environmental toxicants such as tobacco smoke and lead (Fried et al. 1997; Lanphear et al. 2005; Yolton et al. 2005). Further, animal studies supported an association between prenatal and postnatal PBDE exposure and impairments in learning and memory (Viberg et al. 2003; Viberg et al. 2006; Zhang et al. 2013), and several human studies also reported impaired language development (Ding et al. 2015; Eskenazi et al. 2013; Herbstman et al. 2010) and other poorer cognitive performance including full scale IQ, executive function, and visual spatial learning in children (Chen et al. 2014; Vuong et al. 2017a; Vuong et al. 2017b). However, few studies evaluated the association between PBDE exposure and child’s reading ability. Only our previous study reported that a 10-fold increase in the sum of four major PBDE congeners (BDE-47, −99, - 100, and −153) in maternal serum at 16 ± 3 weeks of gestation was associated with a 6.2-point decrease (95% Confidence interval (CI): –11.7, –0.6) in reading composite scores in children at age 8 years (Zhang et al. 2017), while the association between childhood PBDE exposure and reading ability remains to be examined.
Development of human brain continues after birth, and neural development including synapse pruning and myelination extends from the embryonic period through puberty (Rice and Barone 2000), which makes the brain vulnerable to insult of toxicants throughout childhood. Since serum PBDE concentrations are several-fold higher in children than adults (Toms et al. 2008; Toms et al. 2009), this study aimed to examine the association between early childhood PBDE concentrations and reading ability at ages 5 and 8 years in a US birth cohort. Based on previous findings regarding language development and other cognitive outcomes, we hypothesized that higher postnatal PBDE exposures are associated with impaired reading readiness at age 5 years as well as reading proficiency at age 8 years. In addition, child sex may modify the relationship with PBDE exposure (Sagiv et al. 2015; Vuong et al. 2017b; Vuong et al. 2017c). Therefore, we additionally examined possible effect modification by child sex.
2. Methods
2.1. Study population
The present study was based on an ongoing prospective pregnancy and birth cohort study, the Health Outcomes and Measures of the Environment (HOME) Study, conducted in Cincinnati, Ohio. In the HOME Study, 468 pregnant women were enrolled between 2003 and 2006, at 16±3 gestational weeks. At delivery, 390 singleton births remained enrolled and were followed up at ages 1, 2, 3, 4, 5, and 8 years. Information on the study population, recruitment criteria, and follow-up visits of the HOME Study are described in detail elsewhere (Braun et al. 2017a). The present study included 230 children who had at least one measurement of serum PBDEs at ages 1–8 years and one assessment of reading ability at ages 5 or 8 years. The HOME Study protocols have been approved by the Institutional Review Boards of Cincinnati Children’s Hospital Medical Center and the Centers for Disease Control and Prevention (CDC). All pregnant women participants gave their written informed consent for themselves and their children before enrollment and at subsequent study visits.
2.2. Postnatal serum PBDEs
Children’s blood samples were collected at each follow-up visit at 1, 2, 3, 4, 5, and 8 years. After separating serum from whole blood, the serum samples were stored at −80 °C until shipment to CDC for environmental chemical assays. We measured PBDE concentrations in residual serum samples collected at ages 1, 2, and 3 years and all available serums samples at ages 5 and 8 years. We did not measure child PBDE concentrations at age 4 years because the lowest number of subjects were followed up at age 4 years and funds were limited. In addition, the long half-lives of PBDEs and the reduction of hand-to-mouth behavior at age 4 years (compared with toddlers) made the measurement at this age not essential. Eleven PBDE congeners (BDE-17, −28, −47, −66, −85, −99, −100, −153, −154, −183, and −209) were measured using gas chromatography/isotope dilution high-resolution mass spectrometry (Jones et al. 2012). Detailed information on sample preparation, measurement, quality control, and limits of detection (LODs) have been described in detail previously (Vuong et al. 2017a). We calculated PBDE concentrations after serum lipid (defined as total cholesterol and triglycerides) standardization (ng/g lipid). If a congener concentration was below the LOD, the value was replaced with LOD divided by the square root of two (Hornung and Reed 1990). In the present study, we only analyzed data for four PBDE congeners (BDE-47, −99, −100, and −153) and their sum (Sum4PBDEs) because their detection frequencies at each follow-up age were higher than 95%, and they accounted for more than 90% of all tested congeners.
Among 230 children who completed at least one reading assessment at ages 5 or 8 years, 93 (40.4%), 76 (33.0%), 76 (33.0%), 164 (71.3%), and 191 (83.0%) had serum samples available at 1, 2, 3, 5, and 8 years, respectively, which were assayed for PBDE concentrations. A total of 27.8%, 33.5%, 7.8%, 11.7–13.5%, and 17.4–19.1% of children had 1, 2, 3, 4, and 5 PBDE samples during five follow-ups, respectively (Table S1). We observed positive correlations of each congener’s concentrations among ages 1, 2, 3, and 5 years with statistical significance. The concentration of BDE-153 at age 8 years was positively correlated with those at age 1, 2, 3, and 5 years with statistical significance. However, the concentrations of other congeners at 8 years were positively correlated with those at ages 3 and 5 years, but not at ages 1 and 2 years (Table S2). The availability of serum samples for measurement of PBDEs was limited at early ages. Thus, Multiple Imputations (MI) were performed to estimate and fill in the missing congener concentrations in each follow-up period. Detailed information on the MI method for postnatal PBDEs in the HOME Study has been described elsewhere (Vuong et al. 2017c). In brief, MI was conducted using the Markov Chain Monte Carlo method with 100 imputed datasets generated. Based on recommendations by Graham et al. (Graham et al. 2007), since the highest percentage of missing congener concentrations was 67% in the study, conducting 100 imputations was sufficient to achieve plausible values in place of the missing values with acceptable power falloff. Variables in imputation models included all available childhood PBDE concentrations, maternal serum PBDE concentrations, maternal blood lead concentrations, maternal serum polychlorinated biphenyl concentrations, marital status, breast milk feeding history, household income, Home Observation for Measurement of the Environment inventory score (HOME score, measured at 1-year home visit by study staff) (Caldwell and Bradley 1984), child’s full scale IQ (assessed by the Wechsler Intelligence Scale for children-IV) (Wechsler 2004), and externalizing behavior (assessed by the Behavioral Assessment System for Children-2) (Reynolds et al. 2011) at age 8 years considering their relationships with childhood PBDEs concentrations. MI was used only for filling in the missing concentrations of PBDE congeners but not the covariates with missing values.
2.3. Reading ability assessment
At age 5 years, trained examiners administered the Woodcock-Johnson Tests of Achievement- III (WJ- III) (Mather et al. 2001) to assess children’s reading skills, yielding three subtest scores and two composite scores. Letter-Word Identification measures reading decoding in which recognized letters and words are accessed from the mental lexicon and recoded phonologically. Word Attack measures grapheme-to-phoneme translation of pseudo words. Passage Comprehension is a complex, conceptually driven processing task that measures the ability to produce the mental representations provided by the text during the process of reading (Woodcock et al. 2004). The Basic Reading score comprises Letter-Word Identification and Word Attack subtests, measuring sight vocabulary and the ability to apply phonic and structural analysis skills. The Brief Reading score is composed of Letter-Word Identification and Passage Comprehension subtests, measuring reading concepts and readiness.
The Wide Range Achievement Test - 4 (WRAT-4) (Sayegh et al. 2014), including Word Reading and Sentence Comprehension subtests, was administered at age 8 years to assess children’s abilities to read words and comprehend sentences. Word Reading tests children’s abilities to recognize and name letters, and pronounce printed words. The Sentence Comprehension subtest measures children’s reading comprehension. In this analysis, we focused on the Reading Composite score, which is composed of the Word Reading and Sentence Comprehension subtests.
Both WJ-III and WRAT-4 are measures with outstanding reliability and validity that have been widely used as norm-references measures of achievement. One-year test-retest reliabilities of the Brief and Basic Reading composites in the WJ-III are r=.89 and r=.92, respectively, for children aged 4–7 years. Alternate-form one-month delayed-retest reliability of the Reading Composite in the WRAT-4 for children aged 8 years is r=0.91 (Wilkinson and Robertson 2006; Woodcock et al. 2004). Raw scores from both tests are converted into standard scores with a mean of 100 and a standard deviation of 15. Examiners were trained in proper use of the assessments and completed practice exams until they reached proficiency. They were then reassessed every 6 months to ensure continued accuracy in administration and scoring. All examiners were blind to exposure status at the time of assessment.
2.4. Statistical analyses
Multiple informant models were used to examine the associations between postnatal PBDE concentrations at different ages and children’s reading skills (Horton et al. 1999; Litman et al. 2007). The model is a modified version of Generalized Estimating Equation (Sanchez et al. 2011) and takes advantage of repeated measurements of postnatal PBDE concentrations. Thus, we could estimate the βs and 95% CIs of the association between serum PBDE concentrations and reading ability at each age by including interaction terms between PBDE concentrations (continuous) and child age (categorical). We ran multiple informant models in each of the 100 imputed datasets and obtained 100 estimations. The final regression estimation was based on Rubin’s Rules (Rubin 1987), by averaging parameter estimates and combining the within- and between-dataset variances for standard error of the estimation. The equation that was used to combine the variances is presented in Supplementary File (Figure S1).
Because the constructs measured at age 5 years with the WJ-III and age 8 years with the WRAT-4 are similar but not identical, and because we expected to measure reading readiness at age 5 years and reading ability at age 8 years, we maintained these as two independent tests. Using 100 imputed datasets, we performed multiple informant model analyses to estimate the associations: 1) between concentrations of each congener (BDE-47, 99, 100, 153) and Sum4PBDEsat ages 1, 2, 3, and 5 years and composite reading skills (including Basic Reading score and Brief Reading score) assessed by the WJ-III at age 5 years, as well as the subtest scores of Letter-Word Identification, Passage Comprehension, and Word Attack; and 2) between concentrations of each congener and Sum4PBDEs available during ages 1–8 years and the Reading Composite score assessed by the WRAT-4 at age 8 years, as well as subtest scores of Word Reading and Sentence Comprehension. Due to the right-skewness of PBDE concentrations, the concentrations were log10 transformed in the analyses. In multiple informant models, we included the interaction items between PBDE concentrations and ages (years) to examine whether the associations between PBDE concentrations and reading scales were modified by ages (years). The significance level of the interaction terms was set at p < 0.10.. All interaction items for the composite scores were not statistically significant (p > 0.10), however, we still listed the estimates for each congener by age of exposure to show the individual association. We additionally added 3-way interaction terms of sex, PBDE concentrations, and age (years), as well as all 2-way terms in the models to examine possible effect modification by child sex. We selected a list of covariates a priori from literature as they were potential risk factors for child’s neurobehavioral development or PBDE concentrations. Then, we identified potential confounders based on their significant associations with both childhood PBDEs concentrations and reading skills. We adjusted for the following variables in the final analyses: maternal age, education, race, IQ [assessed by Wechsler Abbreviated Scale of Intelligence (Wechsler 1999)], household-income, parity, marital status, maternal serum cotinine concentrations (indicating maternal smoking status), depressive symptoms [assessed by the Beck Depression Inventory-II during pregnancy (Beck et al. 1996)], child’s sex, and HOME inventory score. All covariates were categorical except maternal IQ and maternal serum cotinine concentrations.
Average concentrations for each congener at ages 1, 5, and 8 years were similar between the original (non-imputed) dataset and the imputed datasets, while concentrations at ages 2 and 3 years in the imputed datasets were slightly higher than those in the original dataset (TableS3). We compared prenatal PBDE concentrations and concentrations at age 5 years between those with and those without PBDE measures at age 2 or 3 years and found that children without PBDE concentrations at age 2 or 3 years had higher geometric means (GM) during the prenatal period and at 5 years of age (TableS4). The higher concentrations in the imputed datasets at ages 2 and 3 years were more likely due to differential missingness of children with higher prenatal and age 5 years’ concentrations, not from imputation-related issues. Despite this, we conducted sensitivity analyses that included only the imputed PBDE concentrations at 1 and 5 years for Basic Reading and Brief Reading, and at 1, 5, and 8 years for Reading Composite using multiple informant model to examine whether the results changed.
Still, to examine whether the results from the original dataset and the imputed datasets differed, we conducted sensitivity analyses using the original dataset to perform separate multiple linear regression models (by age of exposure assessment). Because of small sample sizes of PBDE concentrations at ages 1–3 years in the original dataset, we mainly analyzed the associations of PBDE exposure biomarkers at ages 5 and 8 years in the sensitivity analyses. Also, based on the original dataset, we performed Generalized Additive Models (GAMs) to examine whether a non-linear relationship existed between PBDE exposure biomakers at ages 5 and 8 years and reading skills.
In additional sensitivity analyses, we further adjusted for prenatal blood lead levels, prenatal polychlorinated biphenyl (PCB) concentrations, breast milk feeding of the index child, and prenatal Sum4PBDEs concentrations, individually, in multiple informant models to examine if estimates of PBDE exposure biomarkers changed. We also compared the characteristics of children and their mothers between those included in the study and those excluded (n=160).
All above-mentioned statistical analyses except effect measure modification were two two-sided with p value < 0.05 as significant. SAS version 9.4 (SAS Institute Inc., Cary, NC) was used to perform MI, multiple informant models, and multiple linear regression analyses, and R software version 3.4.1 (R Development Core Team) was used to conduct GAMs and generate corresponding graphs. The statistical analyses were two-sided with P value < 0.05 as significant.
3. Results
Table 1 presented the concentrations of PBDE and reading scores at ages 5 and 8 years by maternal characteristics in the imputed datasets. The geometric mean (range) of serum Sum4BDE concentrations was 111.2 (9 – 3,167) ng/g lipid at age 1 year, increased to 130.9 (5 – 7,134) ng/g lipid at age 2 years, and then decreased with age to 97.1 (4 – 10,452), 62.8 (6 – 1,037), and 42.8 (4 – 752) ng/g lipid at ages 3, 5, and 8 years, respectively (Table 1). Each PBDE congener’s LOD, detection rate and concentration distribution in non-imputed and imputed datasets are shown in Supplemental Table S3.
Table 1.
Childhood PBDE concentrations in serum (ng/g lipid) and reading scores at ages 5 and 8 years by maternal and child characteristics using the imputed datasets
| Childhood Sum4PBDEs# (GM ± GSD) |
WJ-III scores at 5 years (Mean ± SD) |
WRAT-4 score at 8 years (Mean ± SD) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 1 year | 2 years | 3 years | 5 years | 8 years | N | Basic Reading | Brief Readmg | N | Reading Composite |
|
| All participants | 230 | 111.2 ± 2.3 | 130.9 ± 2.5 | 97.1 ± 2.5 | 62.8 ± 2.2 | 42.8 ± 2.2 | 185 | 109.9 ± 18.0 | 104.2 ± 17.2 | 199 | 108.0 ± 15.6 |
| Age at delivery (years) | |||||||||||
| <25 | 60 | 101.4 ± 2.1* | 180.6 ± 2.1* | 113.1 ± 2.2 | 62.3 ± 2.2 | 39.3 ±2.2 | 42 | 102.1 ± 16.0* | 99.0 ± 14.9* | 55 | 99.9 ± 14.7 |
| 25–34 | 136 | 117.1 ± 2.4 | 128.7 ± 2.5 | 101.5 ± 2.5 | 63.4 ± 2.2 | 47.5 ± 2.1 | 114 | 111.3 ± 17.8 | 104.9 ± 17.5 | 113 | 109.6 ± 15.0 |
| >35 | 34 | 106.1 ± 2.2 | 79.5 ± 2.3 | 62.2 ± 2.4 | 61.1 ± 2.4 | 33.8 ± 2.3 | 29 | 115.7 ± 18.8 | 108.6 ± 18.1 | 31 | 116.2 ± 13.7 |
| Race/ethnicity− | |||||||||||
| Non-Hispanic White | 135 | 113.8 ± 2.3 | 104.9 ± 2.3* | 79.7 ± 2.3* | 61.3 ± 2.2 | 43.3 ± 2.2 | 112 | 112.4 ± 18.3* | 105.9 ± 18.0* | 118 | 114.0 ± 12.3 |
| Non-Hispanic Black and Others |
95 | 107.5 ± 2.4 | 179.1 ± 2.4 | 128.5 ± 2.5 | 64.9 ± 2.3 | 42.1 ± 2.2 | 73 | 106.0 ± 17.0 | 101.5 ± 15.7 | 81 | 99.2 ± 15.8 |
| Maternal education− | |||||||||||
| High school or less | 60 | 118.5 ± 2.4 | 196.2 ± 2.4* | 140.0 ± 2.5* | 68.6 ± 2.3 | 41.5 ± 2.1 | 42 | 101.0 ± 16.7* | 97.0 ± 15.2* | 53 | 98.3 ± 15.0* |
| Some college or 2-year college |
63 | 122.9 ± 2.1 | 155.1 ± 2.3 | 105.0 ± 2.3 | 70.8 ± 2.3 | 48.3 ± 2.2 | 53 | 105.7 ± 16.8 | 99.7 ± 15.9 | 56 | 103.9 ± 14.8 |
| Bachelor’s | 65 | 94.7 ± 2.3 | 94.4 ± 2.4 | 75.5 ± 2.4 | 57.3 ± 2.2 | 40.6 ± 2.3 | 56 | 113.5 ± 17.6 | 109.4 ± 16.1 | 54 | 115.9 ± 13.0 |
| Graduate or professional | 42 | 111.6 ± 2.4 | 94.4 ± 2.3 | 75.5 ± 2.4 | 53.4 ± 2.1 | 40.2 ± 2.0 | 34 | 116.4 ± 16.1 | 111.5 ± 18.5 | 36 | 116.6 ± 10.1 |
| Maternal parity− | |||||||||||
| Nulliparous | 100 | 95.5 ± 2.2* | 109.9 ± 2.4* | 82.9 ± 2.4* | 58.7 ± 2.2 | 44.6 ± 2.3 | 100 | 113.7 ± 16.5* | 108.3 ± 16.5* | 88 | 110.5 ± 16.0 |
| Parity = 1 | 73 | 114.0 ± 2.2 | 140.2 ± 2.3 | 101.9 ± 2.3 | 63.2 ± 2.2 | 43.5 ± 2.1 | 73 | 108.8 ± 17.8 | 102.6 ± 16.1 | 60 | 106.3 ± 14.3 |
| Parity > 1 | 57 | 140.4 ± 2.4 | 163.1 ± 2.7 | 120.6 ± 2.7 | 70.2 ± 2.3 | 39.2 ± 2.2 | 57 | 104.2 ± 19.7 | 98.6 ± 18.5 | 51 | 105.5 ± 16.1 |
| Maternal smoking− | |||||||||||
| Non-smoking | 192 | 110.0 ± 2.3 | 120.8 ± 2.5 | 90.6 ± 2.4 | 62.8 ± 2.2 | 43.8 ± 2.2 | 163 | 111.0 ± 17.7* | 105.2 ± 17.4* | 169 | 110.3 ± 14.4* |
| Environmental tobacco Smoking |
20 | 114.7 ± 2.0 | 195.3 ± 1.9 | 138.5 ± 2.0 | 62.7 ± 2.0 | 47.3 ± 2.2 | 14 | 97.4 ± 21.1 | 92.7 ± 16.1 | 19 | 91.5 ± 17.2 |
| Active smoking | 18 | 120.6 ± 2.3 | 197.8 ± 2.4 | 136.9 ± 2.8 | 62.9 ± 2.5 | 29.9 ± 1.9 | 8 | 109.4 ± 10.3 | 102.6 ± 6.8 | 16 | 100.7 ± 14.6 |
| Family Income− | |||||||||||
| < S40.000 | 95 | 126.1 ± 2.3* | 198.3 ± 2.3* | 136.0 ± 2.4* | 70.6 ± 2.3* | 44.8 ± 2.2 | 67 | 102.7 ± 16.7* | 98.5 ± 15.5* | 88 | 99.5 ± 14.6* |
| $40,000-$79,999 | 77 | 121.9 ± 2.1 | 123.5 ± 2.2 | 92.0 ± 2.2 | 63.4 ± 2.1 | 43.2 ± 2.2 | 70 | 111.4 ± 17.5 | 105.2 ± 15.3 | 64 | 111.7 ± 13.3 |
| ≥ $80,000 | 58 | 80.0 ± 2.3 | 71.7 ± 2.3 | 60.0 ± 2.2 | 51.2 ± 2.2 | 39.1 ± 2.2 | 48 | 117.7 ± 17.0 | 110.7 ± 18.6 | 52 | 117.4 ± 12.5 |
| Maternal Depression− | |||||||||||
| Minimal/mild | 208 | 111.8 ± 2.4 | 131.5 ± 2.5* | 102.1 ± 2.5 | 62.4 ± 2.2 | 43.2 ± 2.1 | 168 | 110.4 ± 17.9 | 104.7 ± 17.4 | 183 | 108.3 ± 15 |
| Moderate/severe | 20 | 162.2 ± 2.8 | 216.7 ± 2.9 | 145.1 ± 3.2 | 65.5 ± 2.6 | 37.4 ± 2.7 | 15 | 109.7 ± 16.9 | 102.1 ± 13.0 | 19 | 105.9 ± 16.2 |
| HOME score - | |||||||||||
| ≥ 40 | 135 | 100.2 ± 2.3* | 106.4 ± 2.3* | 83.4 ± 2.3 | 60.0 ± 2.1 | 43.0 ± 2.1 | 117 | 113.5 ± 17.9* | 107.0 ± 17.6* | 114 | 113.6 ± 13.3* |
| 35–39 | 41 | 128.6 ± 2.3 | 182.4 ± 2.7 | 128.6 ± 2.9 | 72.8 ± 2.7 | 47.2 ± 2.5 | 28 | 108.2 ± 16.4 | 102.7 ± 16.8 | 39 | 104.3 ± 15.6 |
| < 35 | 38 | 132.0 ± 2.2 | 178.0 ± 2.3 | 116.6 ± 2.4 | 68.8 ± 2.2 | 41.7 ± 1.9 | 32 | 101.2 ± 14.8 | 98.5 ± 14.0 | 32 | 95.9 ± 15.4 |
| Marital status− | |||||||||||
| Married/living with partner |
171 | 100.7 ± 2.3* | 105.3 ± 2.3* | 79.1 ± 2.2* | 57.0 ± 2.1* | 42.4 ± 2.1 | 142 | 112.5 ± 17.7* | 106.0 ± 17.5* | 143 | 111.2 ± 14.2* |
| Not married, living alone | 59 | 148.1 ± 2.2 | 246.3 ± 2.3 | 175.7 ± 2.5 | 82.9 ± 2.5 | 43.9 ± 2.3 | 43 | 101.2 ± 16.4 | 101.2 ± 14.7 | 56 | 99.6 ± 16.0 |
| Child Sex | |||||||||||
| Male | 105 | 115.7 ± 2.3 | 131.3 ± 2.3 | 94.5 ± 2.4 | 60.3 ± 2.3 | 43.0 ± 2.3 | 82 | 109.4 ± 17.3 | 104.2 ± 16.0 | 92 | 107.9 ± 15.3 |
| Female | 125 | 107.5 ± 2.3 | 130.6 ± 2.6 | 99.3 ± 2.5 | 64.9 ± 2.1 | 42.7 ± 2.1 | 103 | 110.3 ± 18.7 | 104.1 ± 18.3 | 112 | 108.0 ± 16.0 |
Abbreviations: GM, geometric mean; GSD, geometric standard deviation; SD, standard deviation
Sum4PBDEs: sum of BDE-47, BDE-99, BDE-100, and BDE-153
P<0.05for differences in Sum4PBDE concentrations reading scores between the categories of maternal and child characteristics.
Children whose mothers were unmarried/living alone or had household income <$40,000 per year during pregnancy had significantly higher Sum4PBDEs concentrations at ages 1, 2, 3, and 5 years, and had lower reading scores assessed at both 5 and 8 years. Children of mothers having parity >1 at enrollment had significantly higher Sum4PBDEs concentrations at ages 1, 2, and 3 years, and also had lower reading scores at 5 years. Children of non-white mothers or of mothers with less than a Bachelor’s degree had significantly higher Sum4PBDEs concentrations at ages 2 and 3 years, and also had lower reading scores at both ages 5 and 8 years. Maternal IQ was inversely correlated with Sum4PBDEs concentration in children at ages 2 and 3 years (rage2 = −0.283 and rage3 = −0.250, p < 0.05), and positively correlated with reading scores at ages 5 and 8 years (rbasic reading = 0.386, rbrief reading = 0.308, and rreading composite = 0.537, p<0.0001).
Maternal age at delivery and HOME score were also related to child Sum4PBDEs concentrations at ages 1 and 2 years. Children of mothers younger than 25 years at delivery had lower Basic Reading scores at age 5 years and Composite Reading scores at age 8 years. Children with HOME scores < 35 had lower reading scores at both ages 5 and 8 years. Children of mothers with moderate/severe depression during pregnancy had higher Sum4PBDEs concentrations at age 2 years.
3.1. Childhood PBDE exposures and reading skills in the imputed datasets
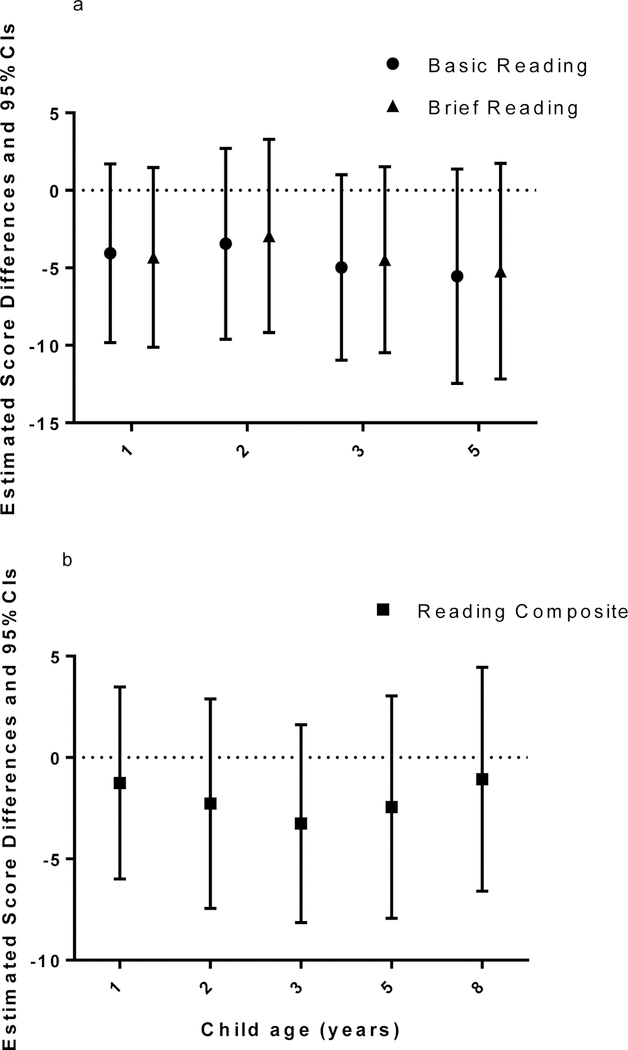
Table 2 presents the estimated score differences in reading skills by 10-fold increases in BDE-47, −99, −100, −153, and Sum4PBDEs using the imputed datasets. All childhood BDE-153 concentrations were inversely associated with child’s reading scores at 5 and 8 years in multiple informant models but the associations did not reach statistical significance after covariate adjustment (Table 2 and Figure 1). A 10-fold increase in BDE-153 concentrations during ages 1–3, 5 years was associated with a 3.5–5.5 point decrease in Basic Reading score at age 5 years, as illustrated by the estimate for exposure at ages 3 years (β: −5.0, 95% confidence interval (CI): - 11.0, 1.0) and 5 years (β: −5.5, 95% CI: −12.5, 1.4). Similarly, the estimates for Brief Reading score were −4.5 (95% CI: −10.5, 1.5) and −5.2 (95% CI: −12.2, 1.7) points lower per 10-fold increase in BDE-153 concentration at ages 3 and 5 years, respectively (Table 2 and Figure 1a).
Table 2.
Estimated score differences and 95% CIs in children’s reading scores at ages 5 and 8 years by 10-fold increases in childhood PBDE concentrations (ng/g lipid) in multiple informant models using the imputed datasets
| PBDEs | WJ−III reading scores at 5 years (β (95% CI), n=185) |
WRAT−4 Reading Composite score at 8 years ( β (95% CI), n=199) |
||||
|---|---|---|---|---|---|---|
| Basic Reading | Brief Reading | |||||
| Unadjusted | Adjusted* | Unadjusted | Adjusted* | Unadjusted | Adjusted* | |
| BDE−47 | ||||||
| 1 year | −2.5 (−10.5, 5.4) | 1.4 (−5.7, 8.5) | −1.9 (−9.4, 5.6) | 1.7 (−5.5, 8.9) | −0.8 (−7.7, 6.0) | 1.0 (−4.5, 6.5) |
| 2 years | −5.7 (−12.9, 1.4) | −0.3 (−6.8, 6.3) | −4.4 (−11.2, 2.4) | 0.6 (−6.0, 7.1) | −8.1 (−14.5, −1.6) # | −1.1 (−6.6, 4.5) |
| 3 years | −4.8 (−11.8, 2.3) | −0.3 (−6.7, 6.1) | −4.0 (−10.8, 2.8) | 0.4 (−6.2, 6.9) | −6.7 (−13.0, −0.5) # | −1.5 (−6.9, 3.9) |
| 5 years | −3.3 (−9.9, 3.3) | −0.8 (−6.9, 5.3) | −3.6 (−9.9, 2.7) | −0.8 (−7.0, 5.3) | −1.5 (−6.9, 4.0) | −0.5 (−5.2, 4.2) |
| 8 years | - | - | - | - | 0.0 (−5.5, 5.5) | −0.9 (−5.8, 4.0) |
| BDE−99 | ||||||
| 1 year | −4.1 (−10.9, 2.7) | −0.3 (−6.5, 6.0) | −3.7 (−10.0, 2.6) | −0.4 (−6.5, 5.6) | −2.8 (−9.0, 3.3) | −0.2 (−5.2, 4.8) |
| 2 years | −4.7 (−10.8, 1.3) | 0.7 (−5.2, 6.6) | −3.5 (−9.2, 2.3) | 1.2 (−4.7, 7.1) | −7.6 (−13.1, −2.0) # | −0.5 (−5.3, 4.2) |
| 3 years | −5.0 (−10.8, 0.8) | −0.3 (−5.9, 5.3) | −4.2 (−9.7, 1.4) | 0.1 (−5.6, 5.7) | −7.0 (−12.6, −1.5) # | −1.2 (−5.9, 3.4) |
| 5 years | −4.1 (−10.1, 2.0) | −1.5 (−7.1, 4.1) | −4.1 (−9.9, 1.7) | −1.6 (−7.2, 4.1) | −2.5 (−7.7, 2.8) | −0.8 (−5.3, 3.7) |
| 8 years | - | - | - | - | −2.4 (−7.4, 2.6) | −1.0 (−5.6, 3.5) |
| BDE−100 | ||||||
| 1 year | −5.3 (−13.2, 2.6) | −2.2 (−9.2, 4.8) | −4.7 (−12.0, 2.6) | −2.0 (−8.9, 5.0) | −2.6 (−9.7, 4.6) | −0.4 (−6.0, 5.3) |
| 2 years | −6.5 (−13.4, 0.5) | −1.6 (−8.1, 4.9) | −5.3 (−11.7, 1.2) | −0.7 (−7.1, 5.7) | −8.0 (−13.9, −2.1) # | −0.7 (−5.8, 4.5) |
| 3 years | −6.9 (−13.4, −0.3) # | −2.5 (−8.7, 3.7) | −5.8 (−12.0, 0.3) | −1.7 (−7.9, 4.5) | −8.8 (−14.4, −3.1) # | −1.8 (−6.7, 3.1) |
| 5 years | −5.7 (−12.5, 1.2) | −2.3 (−8.7, 4.1) | −5.9 (−12.4, 0.7) | −2.4 (−8.9, 4.1) | −5.0 (−10.8, 0.8) | −1.0 (−6.2, 4.1) |
| 8 years | - | - | - | - | −1.6 (−7.2, 3.9) | −0.1 (−5.1, 4.9) |
| BDE−153 | ||||||
| 1 year | −5.9 (−12.2, 0.4) | −4.1 (−9.8, 1.7) | −6.0 (−12.0, −0.1) # | −4.3 (−10.1, 1.5) | −1.8 (−7.5, 3.9) | −1.3 (−6.0, 3.5) |
| 2 years | −8.0 (−14.6, −1.4) # | −3.5 (−9.6, 2.7) | −7.2 (−13.5, −0.9) # | −2.9 (−9.2, 3.3) | −8.5 (−14.4, −2.6) # | −2.3 (−7.4, 2.9) |
| 3 years | −9.9 (−16.3, −3.5) # | −5.0 (−11.0, 1.0) | −9.0 (−16.8, −2.9) # | −4.5 (−10.5, 1.5) | −10.1 (−15.5, −4.6) # | −3.3 (−8.1, 1.6) |
| 5 years | −10.1 (−17.4, −2.8) # | −5.5 (−12.5, 1.4) | −9.9 (−16.8, −2.9) # | −5.2 (−12.2, 1.7) | −7.4 (−13.7, −1.1) # | −2.4 (−7.9, 3.0) |
| 8 years | - | - | - | - | −4.3 (−10.7, 2.1) | −1.1 (−6.6, 4.5) |
| Sum4PBDEs | ||||||
| 1 year | −5.3 (−13.6, 2.9) | −0.1 (−7.6, 7.4) | −4.6 (−12.3, 3.0) | 0.0 (−7.6, 7.5) | −2.9 (−9.9, 4.1) | −0.0 (−5.9, 5.9) |
| 2 years | −8.1 (−15.4, −0.7) # | −0.6 (−7.5, 6.4) | −6.3 (−13.2, 0.7) | 0.4 (−6.6, 7.4) | −11.6 (−17.9, −5.3) | −1.7 (−7.4, 3.9) |
| 3 years | −7.7 (−14.7, −0.6) # | −1.2 (−7.8, 5.5) | −6.5 (−13.2, 0.2) | −0.5 (−7.1, 6.2) | −10.7 (−16.7, −4.6) | −2.4 (−7.8, 2.9) |
| 5 years | −6.1 (−13.4, 1.2) | −1.8 (−8.6, 4.9) | −6.1 (−13.0, 0.8) | −1.7 (−8.5, 5.2) | −4.9 (−11.1, 1.4) | −1.6 (−6.9, 3.8) |
| 8 years | - | - | - | - | −2.3 (−8.6, 4.0) | −1.1 (−6.6, 4.5) |
Adjusted for maternal age, education, race, IQ, household income, parity, marital status, maternal serum cotinine, maternal depression, child sex, and HOME score.
P <0.05
Figure 1.
Estimated score differences and 95% CIs in WJ-III reading scores at age 5 years (a, n=185) and WRAT-4 Reading Composite scores at age 8 years (b, n=199) by a 10-fold increase in childhood serum BDE-153 concentrations using the imputed datasets. Adjusted for maternal age, education, race, IQ, household income, parity, marital status, maternal serum cotinine, maternal depression, child sex, and HOME score in multiple informant models.
A 10-fold increase in serum BDE-153 concentrations at age 3 years was associated with a −3.3 point change (95% CI: −8.1, 1.6) in Reading Composite score at 8 years, while the estimates were smaller for exposure at other ages (Table 2 and Figure 1b).
For BDE-47, −99, −100, and Sum4PBDEs, all unadjusted estimates were inversely associated with reading scores at ages 5 and 8 years, showing statistical significance in the associations of BDE-100 concentrations at age 2 years and Sum4PBDEs at both ages 2 and 3 years with Basic Reading score. All individual congeners and Sum4PBDE concentrations at ages 2 and 3 years showed an unadjusted associated 6.7 – 8.8 point decrease in Reading Composite scores with statistical significance. All estimates were attenuated and not statistically significant after adjustment for covariates, but the directions of most of the associations remained unchanged.
We also examined the subtest scores of WJ-III (Letter-word identification, Passage comprehension, and Word attack; Table S5) and WRAT-4 (Word reading and Sentence comprehension; Table S6), and the adjusted estimates showed similar patterns to the corresponding composite scores.
We repeated the analyses by adding interaction terms between sex, PBDE concentrations, and age (years) into the models. P-values for all three-way interaction terms and two-way interaction terms between sex and PBDEs were > 0.10, suggesting there was no significant effect measure modification by sex (Table S7). In multiple informant models only using the imputed PBDE concentration at 1 and 5 years for Basic Reading and Brief Reading and at 1, 5, and 8 years for Reading Composite scores, the results were very similar with those in the main analyses (Table S8).
3.2. Childhood PBDE exposure and reading skills in non-imputed, original dataset
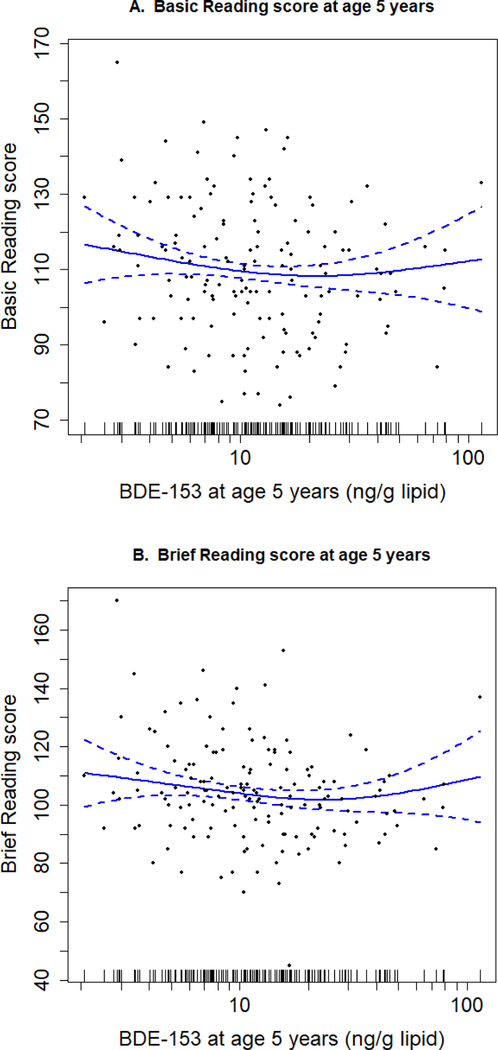
In the GAMs, there were inverse associations between BDE-153 at 5 years and Basic Reading and Brief Reading scores (Figure 2A and 2B) in the original dataset, suggesting that non-linear relationship were not observed. In the analyses of other PBDEs and reading scores at ages 5 and 8 years, non-linear relationships were again not observed (Figure S2-S5). In separate multivariable linear regression analyses using the original dataset, the unadjusted results showed very similar patterns as those in the imputed analyses (Table S9). All congeners except BDE-47 at age 1 year were inversely associated with reading scores, though associations were not statistically significant. The adjusted estimates of each congener at ages 5 and 8 years were essentially unchanged compared with those in imputed analyses (Table S9). For example, a 10-fold increase in BDE-153 concentration at age 5 years was associated with a −5.0 (95% CI - 13.8, 3.8) and −5.7 (95% CI −14.3, 3.0) point change in Basic Reading and Brief Reading score, respectively, similar to the results from imputed datasets. However, the estimates at ages 1–3 years with WJ- III reading score changed and the pattern observed in the imputed datasets became much weaker in the original dataset. The power of studying the associations of PBDE concentrations at ages 1– 3 years in the original dataset was low (only 0.051 – 0.130, Table S10). The insufficient power may have limited our ability to get valid results after adjusting for covariates in the original dataset.
Figure 2.
Scatter plots of childhood serum BDE-153 concentrations and WJ-III reading scores at age 5 years with Generalized Additive Model curve fitting using the non-imputed, original dataset. (A.) Basic Reading score at 5 years (n=153); (B.) Brief Reading score at 5 years (n=154). Data points represent serum BDE-153 concentrations at age 5 years and corresponding reading score for each child. Solid lines indicate the natural cubic spline regression of the association after adjustment for maternal age, education, race, IQ, household income, parity, marital status, maternal serum cotinine, maternal depression, child sex, and HOME score. Dotted lines are 95% confidence intervals of the regression curve.
3.3. Other sensitivity analyses
We re-ran the main analyses by separately adjusting for prenatal blood lead levels, prenatal PCB concentrations, breast milk feeding of the index child, and prenatal Sum4PBDEs concentrations in the multiple informant models. The results did not change markedly (data not shown). Finally, mothers of children included in the current analysis were more likely to be unmarried and live alone compared with those excluded (25.7% vs. 13.6%). However, the differences in other characteristics including child sex, maternal age, race, education, depression, smoking during pregnancy, family income, HOME score, and prenatal Sum4PBDEs were not statistically significant in the two groups (Table S11).
4. Discussion
In this study, we observed a non-significant trend of inverse associations between childhood BDE-153 concentrations and children’s reading skills at ages 5 and 8 years. The associations were consistent at ages 1, 2, 3, and 5 years and independent of maternal PBDE, PCB, or lead exposure. The findings of other PBDE congeners also showed a generally consistent pattern of inverse associations, albeit non-significant, after adjusting for covariates. It is noteworthy that all estimates were not statistically significant after covariate adjustment due to the insufficient power even in the imputed datasets (Table S10). Further, data on PBDE concentrations at ages 1–3 years were imputed at a high percentage and the pattern of the associations at these ages were much weaker in the original dataset which was remarkably smaller in sample size. Additional studies with a larger sample size are needed to examine these associations.
To our knowledge, this is the first study of the associations of childhood PBDE exposure biomarkers at multiple ages and children’s reading ability. Only our previous study reported decreased reading composite scores associated with prenatal PBDE exposure. However, reading ability could be partly predicted by cognitive and early linguistic skills (Christopher et al. 2012; Durand et al. 2013). The epidemiological studies that have examined the association between postnatal PBDE exposure and child cognitive function and language development could corroborate our findings to some extent. In the study conducted in the New York City, children with sustained high concentrations of BDE-47, −99, or −100 during ages 2–9 years scored 5–8 points lower on tests of visual memory (Cowell et al. 2018). In the U.S. CHAMACOS study (Center for the Health Assessment of Mothers and Children of Salinas), the sum of BDE-47, −99, −100, and −153 concentrations in child serum at age 7 years was marginally associated with decreased verbal comprehension and processing speed assessed concurrently with exposure (Eskenazi et al. 2013). Another study conducted in Spain found child serum BDE-47 concentrations above LOD at age 4 years was associated with a −3.2 point (95% CI: −7.7, 1.3) change in verbal score assessed by the McCarthy Scales of Children’s Abilities at the same age, compared with concentrations below the LOD (Gascon et al. 2011). Children from homes with higher dust concentrations of PBDEs had lower verbal comprehension scores at age 6 years (Chevrier et al. 2016), which is also consistent with our findings. Also, in the HOME Study, postnatal exposure to PBDE congeners at multiple age was associated with decreased Full Scale IQ at 8 years (Vuong et al. 2017c), and there was a pattern of impairments in visual spatial learning among children with higher BDE-153 concentrations (Vuong et al. 2017a).
However, two other studies that examined the association between PBDE concentrations in breast milk and language domain scores showed contradictory results. One study in Taiwan (Chao et al. 2011), with a smaller sample size (n=70) and much lower PBDE concentrations, reported no association of each congener or ∑14PBDEs (BDE-28, −47, −99, −100, −153, −154, −183, −196, −197, −203, −206, −207, −208, and −209) and the language domain of the Bayley-III scale at 8–12 months, except that BDE-196 was significantly associated with increased language domain scores. However, BDE-196 was not measured in the HOME Study. Another U.S. study from North Carolina reported PBDE levels in breast milk collected at 3 months postpartum tended to be positively associated with language scores using Mullen Scales of Early learning at 3 years (Adgent et al. 2014). The difference in participants’ characteristics may explain the inconsistencies between the North Carolina study and ours. In that study, 90% of the participants were White, had higher educational achievement and higher household income, and had longer breastfeeding duration since recruitment criteria included >3 months of breastfeeding. The benefit from the longer duration of breastfeeding may mitigate the adverse effects of PBDEs; it is also possible that residual sociodemographic factors may contribute to the positive associations identified in that study.
Our study showed that BDE-153 exhibited the most significantly adverse associations with reading skills, which may be explained by the different toxicokinetics of BDE-153 from BDE-47, −99, or −100. BDE-153 has the lowest excretion rate among the four congeners (Staskal et al. 2006) and appears to be accumulated in lipophilic tissues more effectively (Bramwell et al. 2016). Particularly, it exhibits a higher bioaccumulation and binding affinity to brain tissue than other congeners, with the concentration in brain tissue being approximately 10 times higher than that of BDE-47 (Staskal et al. 2006). Moreover, a single dose of BDE-153 administered to neonatal mice or rats caused long-lasting neurobehavioral abnormalities in adults, particularly in the domain of motor activity and cognitive function such as impaired learning and memory abilities (Viberg et al. 2003; Zhang et al. 2013). Also, we previously reported that prenatal exposure to BDE-153 was significantly associated with decreased reading scores in the HOME Study children (Zhang et al. 2017). In this report, we identified the association with early childhood BDE-153 exposure biomarkers. Overall, the findings from experimental and epidemiological studies provide evidence that the findings in the present study are biologically plausible.
The underlying mechanisms of PBDEs’ neurotoxic effects are still not fully understood. Disturbance of thyroid hormone (TH) homeostasis has been postulated to be one potential mechanism. In animal studies, PBDE exposure postnatally disrupted the thyroid hormone homeostasis (de-Miranda et al. 2016; Driscoll et al. 2009). In epidemiological studies, alterations of thyroid hormone levels have been observed among children with higher PBDE concentrations (Jacobson et al. 2016; Xu et al. 2014). Thyroid hormones are critically involved in brain development. A subtle change of thyroid hormone could result in structural alterations in the brain and long-lasting cognitive function decrement in rat models (Gilbert and Sui 2006; Hasegawa and Wada 2013). In humans, prenatal and postnatal hypothyroidism was associated with decreased cognitive function and impaired motor development that persisted into the adulthood (Porterfield 1994). Thus, the disruption of thyroid hormone homeostasis after childhood exposure to PBDE may affect the brain development related to reading ability. Another potential mechanism is that PBDEs may affect the brain directly. PBDEs and their hydroxylated metabolites may directly affect brain cells by inducing alteration of cell migration and differentiation into neurons and oligodendrocytes (Schreiber et al. 2010), interfering calcium signaling and protein kinase C pathways in neurons, and causing oxidative stress that results in DNA damage and cell apoptosis (Costa et al. 2014; Dingemans et al. 2011; Herbstman and Mall 2014). Further, postnatal exposure to PBDE including BDE-153 could affect the GABA and glutamate neurotransmitter systems located in frontal cortex (Bradner et al. 2013), and the cholinergic nicotinic receptors in hippocampus (Viberg et al. 2003). The frontal cortex has been involved in several cognitive functions and in some neurological disorders such as autism and dyslexia (Berbel et al. 2014; Bradner et al. 2013), while the hippocampus plays a fundamental role in learning and memory process. The GABA, glutamate, and cholinergic systems are important in maintaining the physiological functions of the brain. The alterations of these neurotransmitter systems could disrupt the excitation-inhibition neuronal activity and may lead to deficits in cognitive processes (Bradner et al. 2013).
The most important strengths of the study are the longitudinal design and multiple assessments of postnatal BPDE exposure, which is different from previous studies which only assessed exposure once or at the same time as the outcome assessment. We observed consistent associations between BDE-153 concentrations and reading skills assessed at both ages 5 and 8 years. In addition, we adjusted for a number of covariates such as maternal IQ and education, smoking during pregnancy, household income, and HOME score. We also took into consideration prenatal co-exposure to PBDEs, PCBs or lead to rule out explanations from other chemical exposures.
Still, our findings should be interpreted in light of several limitations. Attrition occurred during follow-up with only 59% of the initial participants remaining in the study. However, the included children had similar sociodemographic characteristics except maternal marital status and importantly, similar prenatal concentrations of sum4PBDEs, to those excluded. The loss to follow-up seemed to be non-differential, which alleviated our concerns to some extent regarding selection bias. Due to missing postnatal PBDE concentrations mostly at ages 1–3 years, multiple imputation was performed to estimate the missing values. The GMs of PBDE concentrations in imputed datasets were similar to those in the original dataset, except for slightly higher concentrations at 2 and 3 years of age in the imputed datasets. This pattern is more likely due to differential missingness of children with higher prenatal and age 5 years PBDE serum concentrations, not from imputation-related issues. The repeated multiple informant models based on imputed datasets excluding imputations for PBDE concentrations at ages 2 and 3 years showed similar results as in table 2. Moreover, we performed separate multivariable linear regression models using the original dataset to examine the associations. Similar results were observed for exposure at ages 5 and 8 years, while the pattern for exposure at ages 1–3 years became much weaker. The latter could be due to the much small sample size (n=61–76) and insufficient power. Our sample size was modest and models may have been underpowered to detect existing underlying associations. In the study, we found a consistent pattern of inverse associations between BDE-153 concentrations and reading skills across each exposure year, showing the entire childhood period may be susceptible. However, it was possible that the small sample size limited our ability to detect the particular association of each exposure year since the interaction test may need larger sample size. Furthermore, the long-lives of PBDEs during childhood may also restrict our ability to examine the association of each exposure year. Further, although we adjusted for some potential risk factors, such as maternal characteristics, child sex, HOME scores, it is possible that the results were still confounded by unmeasured confounders.
We did not adjust for multiple comparisons, however, we observed a consistent pattern of inverse associations between PBDE concentrations and reading ability. The pattern was less likely to be obliterated by multiple comparisons. Finally, we did not measure hydroxylated PBDE metabolite (OH-PBDEs) concentrations in the study. OH-PBDEs, having more structural resemblance to thyroxine and being able to bind thyroid hormone transport proteins (Dingemans et al. 2011; Li et al. 2010), may play more important roles in neurotoxic effects than PBDEs.
5. Conclusion
Our study suggests that childhood serum PBDE concentrations, particularly BDE-153, may be inversely associated with children’s reading skills. All estimates did not reach statistical significance after covariates adjustment and additional studies with a larger sample size are needed to examine these associations. Nevertheless, our findings highlight the potential impact of postnatal exposure to PBDEs, particularly BDE-153.
Supplementary Material
Highlights.
Child’s serum concentrations of PBDEs were measured at ages 1, 2, 3, 5, and 8 years
WJ-III was used to measure reading ability at age 5 years, and WRAT-4 at 8 years
Multiple informant model was used to examine the repeated measures of PBDEs
There was a pattern of inverse associations of BDE-153 exposure with reading scores
The pattern was consistent across each exposure year of BDE-153
Acknowledgments
This work was supported by grants from the National Institute of Environmental Health Sciences and the US Environmental Protection Agency (NIEHS P01 ES11261, R01 ES020349, R01 ES 024381, R01 ES014575, T32ES010957, P30ES006096; EPA P01 R829389). Drs. Chen and Miao also received support from the National Natural Science Foundation of China (NSFC 21628701). The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the U.S. Department of Health and Human Services.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: none
References
- Adgent MA, Hoffman K, Goldman BD, Sjodin A, Daniels JL (2014) Brominated flame retardants in breast milk and behavioural and cognitive development at 36 months Paediatric and perinatal epidemiology 28:48–57 doi: 10.1111/ppe.12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK (1996) Beck Depression Inventroy-II Psychological Corporation, San Antonio, TX [Google Scholar]
- Berbel P, Navarro D, Roman GC (2014) An evo-devo approach to thyroid hormones in cerebral and cerebellar cortical development: etiological implications for autism Frontiers in endocrinology 5:146 doi: 10.3389/fendo.2014.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradner JM, Suragh TA, Caudle WM (2013) Alterations to the circuitry of the frontal cortex following exposure to the polybrominated diphenyl ether mixture, DE-71 Toxicology 312:48–55 doi: 10.1016/j.tox.2013.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramwell L, Glinianaia SV, Rankin J, Rose M, Fernandes A, Harrad S, Pless-Mulolli T (2016) Associations between human exposure to polybrominated diphenyl ether flame retardants via diet and indoor dust, and internal dose: A systematic review Environment international 92–93:680–694 doi: 10.1016/j.envint.2016.02.017 [DOI] [PubMed] [Google Scholar]
- Braun JM et al. (2017a) Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study International journal of epidemiology 46:24 doi: 10.1093/ije/dyw006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM et al. (2017b) Prenatal environmental chemical exposures and longitudinal patterns of child neurobehavior Neurotoxicology 62:192–199 doi: 10.1016/j.neuro.2017.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buratovic S, Viberg H, Fredriksson A, Eriksson P (2014) Developmental exposure to the polybrominated diphenyl ether PBDE 209: Neurobehavioural and neuroprotein analysis in adult male and female mice Environmental toxicology and pharmacology 38:570–585 doi: 10.1016/j.etap.2014.08.010 [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH (1984) Home observation for measurement of the environment University of Arkansas at Little Rock; Little Rock, [Google Scholar]
- Catts HW, Fey ME, Zhang X, Tomblin JB (1999) Language basis of reading and reading disabilities: Evidence from a longitudinal investigation Scientific studies of reading 3:331–361 [Google Scholar]
- Chao HR, Tsou TC, Huang HL, Chang-Chien GP (2011) Levels of breast milk PBDEs from southern Taiwan and their potential impact on neurodevelopment Pediatric research 70:596–600 doi: 10.1203/PDR.0b013e3182320b9b [DOI] [PubMed] [Google Scholar]
- Chen A et al. (2014) Prenatal polybrominated diphenyl ether exposures and neurodevelopment in U.S. children through 5 years of age: the HOME study Environmental health perspectives 122:856–862 doi: 10.1289/ehp.1307562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevrier C et al. (2016) Childhood exposure to polybrominated diphenyl ethers and neurodevelopment at six years of age Neurotoxicology 54:81–88 doi: 10.1016/j.neuro.2016.03.002 [DOI] [PubMed] [Google Scholar]
- Christopher ME et al. (2012) Predicting word reading and comprehension with executive function and speed measures across development: a latent variable analysis Journal of experimental psychology General 141:470–488 doi: 10.1037/a0027375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, de Laat R, Tagliaferri S, Pellacani C (2014) A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity Toxicology letters 230:282–294 doi: 10.1016/j.toxlet.2013.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ et al. (2015) Prenatal exposure to polybrominated diphenyl ethers and child attention problems at 3–7 years Neurotoxicology and teratology 52:143–150 doi: 10.1016/j.ntt.2015.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ et al. (2018) Associations between prenatal and childhood PBDE exposure and early adolescent visual, verbal and working memory Environment international 118:9–16 doi: 10.1016/j.envint.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de-Miranda AS, Kuriyama SN, da-Silva CS, do-Nascimento MS, Parente TE, Paumgartten FJ (2016) Thyroid hormone disruption and cognitive impairment in rats exposed to PBDE during postnatal development Reproductive toxicology (Elmsford, NY) 63:114–124 doi: 10.1016/j.reprotox.2016.05.017 [DOI] [PubMed] [Google Scholar]
- Ding G et al. (2015) Association between prenatal exposure to polybrominated diphenyl ethers and young children’s neurodevelopment in China Environmental research 142:104–111 doi: 10.1016/j.envres.2015.06.008 [DOI] [PubMed] [Google Scholar]
- Dingemans MM, van den Berg M, Westerink RH (2011) Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system Environmental health perspectives 119:900–907 doi: 10.1289/ehp.1003035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll LL, Gibson AM, Hieb A (2009) Chronic postnatal DE-71 exposure: effects on learning, attention and thyroxine levels Neurotoxicology and teratology 31:76–84 doi: 10.1016/j.ntt.2008.11.003 [DOI] [PubMed] [Google Scholar]
- Durand VN, Loe IM, Yeatman JD, Feldman HM (2013) Effects of early language, speech, and cognition on later reading: a mediation analysis Frontiers in psychology 4:586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B et al. (2013) In utero and childhood polybrominated diphenyl ether (PBDE) exposures and neurodevelopment in the CHAMACOS study Environmental health perspectives 121:257–262 doi: 10.1289/ehp.1205597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer D, Hooper K, Athanasiadou M, Athanassiadis I, Bergman A (2006) Children show highest levels of polybrominated diphenyl ethers in a California family of four: a case study Environmental health perspectives 114:1581–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederiksen M, Vorkamp K, Thomsen M, Knudsen LE (2009) Human internal and external exposure to PBDEs--a review of levels and sources International journal of hygiene and environmental health 212:109–134 doi: 10.1016/j.ijheh.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Fried PA, Watkinson B, Siegel LS (1997) Reading and language in 9- to 12-year olds prenatally exposed to cigarettes and marijuana Neurotoxicology and teratology 19:171–183 [DOI] [PubMed] [Google Scholar]
- Gascon M, Vrijheid M, Martinez D, Forns J, Grimalt JO, Torrent M, Sunyer J (2011) Effects of pre and postnatal exposure to low levels of polybromodiphenyl ethers on neurodevelopment and thyroid hormone levels at 4 years of age Environment international 37:605–611 doi: 10.1016/j.envint.2010.12.005 [DOI] [PubMed] [Google Scholar]
- Gilbert ME, Sui L (2006) Dose-dependent reductions in spatial learning and synaptic function in the dentate gyrus of adult rats following developmental thyroid hormone insufficiency Brain research 1069:10–22 doi: 10.1016/j.brainres.2005.10.049 [DOI] [PubMed] [Google Scholar]
- Graham JW, Olchowski AE, Gilreath TD (2007) How many imputations are really needed? Some practical clarifications of multiple imputation theory Prevention science : the official journal of the Society for Prevention Research 8:206–213 doi: 10.1007/s11121-007-0070-9 [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Wada H (2013) Developmental hypothyroidism disrupts visual signal detection performance in rats Physiology & behavior 112–113:90–95 doi: 10.1016/j.physbeh.2013.02.019 [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Mall JK (2014) Developmental Exposure to Polybrominated Diphenyl Ethers and Neurodevelopment Current environmental health reports 1:101–112 doi: 10.1007/s40572-014-0010-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbstman JB et al. (2010) Prenatal exposure to PBDEs and neurodevelopment Environmental health perspectives 118:712–719 doi: 10.1289/ehp.0901340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hites RA (2004) Polybrominated diphenyl ethers in the environment and in people: a meta-analysis of concentrations Environmental science & technology 38:945–956 [DOI] [PubMed] [Google Scholar]
- Hoffman K, Adgent M, Goldman BD, Sjodin A, Daniels JL (2012) Lactational exposure to polybrominated diphenyl ethers and its relation to social and emotional development among toddlers Environmental health perspectives 120:1438–1442 doi: 10.1289/ehp.1205100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WA, Gough PB (1990) The simple view of reading Reading and writing 2:127–160 [Google Scholar]
- Hornung RW, Reed LD (1990) Estimation of average concentration in the presence of nondetectable values Applied occupational and environmental hygiene 5:46–51 [Google Scholar]
- Horton NJ, Laird NM, Zahner GE (1999) Use of multiple informant data as a predictor in psychiatric epidemiology International Journal of Methods in Psychiatric Research 8:6–18 [Google Scholar]
- Jacobson MH et al. (2016) Serum polybrominated diphenyl ether concentrations and thyroid function in young children Environmental research 149:222–230 doi: 10.1016/j.envres.2016.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Restrepo B (2008) Exposure to and Bioaccumulation of Brominated Flame Retardants in Humans and Marine Wildlife: Comparison to Patterns of Chlorinated Contaminants State University of New York at Albany [Google Scholar]
- Jones R, Edenfield E, Anderson S, Zhang Y, Sjödin A (2012) Semi-automated extraction and cleanup method for measuring persistent organic pollutants in human serum Organohalogen Compounds 74:97–98 [Google Scholar]
- Kodavanti PR, Ward TR, Ludewig G, Robertson LW, Birnbaum LS (2005) Polybrominated diphenyl ether (PBDE) effects in rat neuronal cultures: 14C-PBDE accumulation, biological effects, and structure-activity relationships Toxicological sciences : an official journal of the Society of Toxicology 88:181–192 doi: 10.1093/toxsci/kfi289 [DOI] [PubMed] [Google Scholar]
- Kovachy VN, Adams JN, Tamaresis JS, Feldman HM (2015) Reading abilities in school-aged preterm children: a review and meta-analysis Developmental medicine and child neurology 57:410–419 doi: 10.1111/dmcn.12652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J et al. (2017) Developmental PBDE Exposure and IQ/ADHD in Childhood: A Systematic Review and Meta-analysis Environmental health perspectives 125:086001 doi: 10.1289/ehp1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP et al. (2005) Low-level environmental lead exposure and children’s intellectual function: an international pooled analysis Environmental health perspectives 113:894–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F et al. (2010) Hormone activity of hydroxylated polybrominated diphenyl ethers on human thyroid receptor-beta: in vitro and in silico investigations Environmental health perspectives 118:602–606 doi: 10.1289/ehp.0901457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman HJ, Horton NJ, Hernández B, Laird NM (2007) Incorporating missingness for estimation of marginal regression models with multiple source predictors Statistics in medicine 26:1055–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather N, Wendling BJ, Woodcock RW (2001) Essentials of WJ III [TM] Tests of Achievement Assessment. Essentials of Psychological Assessment Series ERIC, [Google Scholar]
- Perfetti CA (2017) The representation problem in reading acquisition. In: Reading acquisition Routledge, pp 145–174 [Google Scholar]
- Porterfield SP (1994) Vulnerability of the developing brain to thyroid abnormalities: environmental insults to the thyroid system Environmental health perspectives 102 Suppl 2:125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW, Vannest KJ (2011) Behavior assessment system for children (BASC). In: Encyclopedia of clinical neuropsychology Springer, pp 366–371 [Google Scholar]
- Rice D, Barone S Jr. (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models Environmental health perspectives 108 Suppl 3:511–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF (2009) Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age Environmental health perspectives 117:1953–1958 doi: 10.1289/ehp.0901015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB (1987) Multiple Imputation for Nonresponse in Surveys John Wiley & Sons, Inc., New York [Google Scholar]
- Sagiv SK et al. (2015) Prenatal and childhood polybrominated diphenyl ether (PBDE) exposure and attention and executive function at 9–12 years of age Neurotoxicology and teratology 52:151–161 doi: 10.1016/j.ntt.2015.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez BN, Hu H, Litman HJ, Tellez-Rojo MM (2011) Statistical methods to study timing of vulnerability with sparsely sampled data on environmental toxicants Environmental health perspectives 119:409–415 doi: 10.1289/ehp.1002453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayegh P, Arentoft A, Thaler NS, Dean AC, Thames AD (2014) Quality of education predicts performance on the Wide Range Achievement Test-Word Reading subtest Archives of Clinical Neuropsychology 29:731–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber T et al. (2010) Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: evidence for endocrine disruption Environmental health perspectives 118:572–578 doi: 10.1289/ehp.0901435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy CG, Huang HL, Chang-Chien GP, Chao HR, Tsou TC (2011) Neurodevelopment of infants with prenatal exposure to polybrominated diphenyl ethers Bulletin of environmental contamination and toxicology 87:643–648 doi: 10.1007/s00128-011-0422-9 [DOI] [PubMed] [Google Scholar]
- Sjodin A, Patterson DG Jr., Bergman A (2003) A review on human exposure to brominated flame retardants--particularly polybrominated diphenyl ethers Environment international 29:829–839 doi: 10.1016/s0160-4120(03)00108-9 [DOI] [PubMed] [Google Scholar]
- Staskal DF, Hakk H, Bauer D, Diliberto JJ, Birnbaum LS (2006) Toxicokinetics of polybrominated diphenyl ether congeners 47, 99, 100, and 153 in mice Toxicological sciences : an official journal of the Society of Toxicology 94:28–37 doi: 10.1093/toxsci/kfl091 [DOI] [PubMed] [Google Scholar]
- Thuresson K, Hoglund P, Hagmar L, Sjodin A, Bergman A, Jakobsson K (2006) Apparent half-lives of hepta- to decabrominated diphenyl ethers in human serum as determined in occupationally exposed workers Environmental health perspectives 114:176–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toms LM, Harden F, Paepke O, Hobson P, Ryan JJ, Mueller JF (2008) Higher accumulation of polybrominated diphenyl ethers in infants than in adults Environmental science & technology 42:7510–7515 [DOI] [PubMed] [Google Scholar]
- Toms LM, Sjodin A, Harden F, Hobson P, Jones R, Edenfield E, Mueller JF (2009) Serum polybrominated diphenyl ether (PBDE) levels are higher in children (2–5 years of age) than in infants and adults Environmental health perspectives 117:1461–1465 doi: 10.1289/ehp.0900596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S.EPA (2010) An Exposure Assessment of Polybrominated Diphenyl Ethers (Pbde) (Final) U.S. Environmental Protection Agency, Washington, DC, [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P (2003) Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice Toxicology and applied pharmacology 192:95–106 [DOI] [PubMed] [Google Scholar]
- Viberg H, Johansson N, Fredriksson A, Eriksson J, Marsh G, Eriksson P (2006) Neonatal exposure to higher brominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice Toxicological sciences : an official journal of the Society of Toxicology 92:211–218 doi: 10.1093/toxsci/kfj196 [DOI] [PubMed] [Google Scholar]
- Vuong AM et al. (2017a) Prenatal and postnatal polybrominated diphenyl ether exposure and visual spatial abilities in children Environmental research 153:83–92 doi: 10.1016/j.envres.2016.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM et al. (2017b) Childhood polybrominated diphenyl ether (PBDE) exposure and executive function in children in the HOME Study International journal of hygiene and environmental health doi: 10.1016/j.ijheh.2017.10.006 [DOI] [PMC free article] [PubMed]
- Vuong AM et al. (2016) Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school-age children Environmental research 147:556–564 doi: 10.1016/j.envres.2016.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong AM et al. (2017c) Childhood polybrominated diphenyl ether (PBDE) exposure and neurobehavior in children at 8 years Environmental research 158:677–684 doi: 10.1016/j.envres.2017.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1999) Wechsler abbreviated intelligence scale San Antonio: The Psychological Corporation [Google Scholar]
- Wechsler D (2004) Wechsler Preschool and Primary Scale of Intelligence The Psychological Corporation, San Antonio, TX, [Google Scholar]
- Wilkinson GS, Robertson GJ (2006) Wide Range Achievement Test-Fourth Edition Lutz, FL: Psychological Assessment Resources [Google Scholar]
- Woodcock RW, McGrew KS, Mather N, Schrank F (2004) Woodcock-Johnson R III Itasca, IL: The Riverside Publishing Company, [Google Scholar]
- Xu X, Liu J, Zeng X, Lu F, Chen A, Huo X (2014) Elevated serum polybrominated diphenyl ethers and alteration of thyroid hormones in children from Guiyu, China PloS one 9:e113699 doi: 10.1371/journal.pone.0113699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton K, Dietrich K, Auinger P, Lanphear BP, Hornung R (2005) Exposure to environmental tobacco smoke and cognitive abilities among U.S. children and adolescents Environmental health perspectives 113:98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Li X, Nie J, Niu Q (2013) Lactation exposure to BDE-153 damages learning and memory, disrupts spontaneous behavior and induces hippocampus neuron death in adult rats Brain research 1517:44–56 doi: 10.1016/j.brainres.2013.04.014 [DOI] [PubMed] [Google Scholar]
- Zhang H et al. (2017) Prenatal PBDE and PCB Exposures and Reading, Cognition, and Externalizing Behavior in Children Environmental health perspectives 125:746–752 doi: 10.1289/ehp478 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.